Abstract
Enhanced long-term survival rates of young women with cancer and advances in reproductive medicine and cryobiology have culminated in an increased interest in fertility preservation methods in girls and young women with cancer. Present data suggest that young patients with cancer should be referred for fertility preservation counselling quickly to help with their coping process. Although the clinical application of novel developments, including oocyte vitrification and oocyte maturation in vitro, has resulted in reasonable success rates in assisted reproduction programmes, experience with these techniques in the setting of fertility preservation is in its infancy. It is hoped that these and other approaches, some of which are still regarded as experimental (eg, ovarian tissue cryopreservation, pharmacological protection against gonadotoxic agents, in-vitro follicle growth, and follicle transplantation) will be optimised and become established within the next decade. Unravelling the complex mechanisms of activation and suppression of follicle growth will not only expand the care of thousands of women diagnosed with cancer, but also inform the care of millions of women confronted with reduced reproductive fitness because of ageing.
Introduction
Although cancer incidence peaks after the age of 50 years, thousands of reproductive-age women and girls are diagnosed with cancer every year.1 Reduction of cancer-related mortality remains the main objective of health-care providers, but quality of life in the increasing population of young women who have had cancer also merits attention. Driven by the increase in cancer survival rates, there is growing interest in the prevention of the loss of reproductive fitness in young women who have had cancer,2 either as a result of cancer or its treatment with gonadotoxic treatments. A large proportion of young women with cancer do not receive fertility preservation counselling for various reasons. Here, we discuss the present status of fertility preservation in women with cancer and focus on new developments on the horizon.
Fertility preservation counselling
Because of the huge emotional effect of a diagnosis of cancer and the immediate focus on effective cancer treatment, the potential gonadotoxic effects of cancer or its treatment are often not discussed at the time of diagnosis. Fertility preservation is a recent endeavour and many patients with cancer and health-care workers are unfamiliar with the rapidly advancing developments in fertility preservation research and their implementation in clinical practice. Although some fertility preservation techniques have become established and validated in the past decade, others are still regarded as experimental.3 Easy access and timely referral to fertility preservation counselling allow patients with cancer to better cope with the infertility that might arise from gonadotoxic treatment,4 but failure to define the precise long-term effect of cancer treatment on the reproductive potential of individual patients with cancer complicates the decision-making process. In the past 10 years, treatment guidelines3,5 and internet-based decision aids (eg, fertile hope and the oncofertility consortium) have been developed, which have raised awareness of fertility risk and fertility preservation among health-care professionals and patients with cancer. However, an estimated 30–50% of young women diagnosed with cancer might not be offered fertility preservation counselling before the start of cancer treatment.6,7 Not all patients are candidates for or want to pursue fertility preservation; thus, patients should also be informed about alternative options for having a family after cancer, such as oocyte donation and adoption.
Search strategy and selection criteria.
We searched Medline between Jan 1, 1990, and Dec 21, 2013, for reports published in English using combinations of the search terms “fertility preservation”, “female cancer”, “childhood cancer”, “gonadotoxic”, and “cancer treatment”. We mainly selected publications from the past 5 years, but did not exclude older, commonly referenced publications. We iteratively checked the reference lists of articles identified by this search strategy and selected those we considered relevant. Our initial reference list was shortened on the basis of comments from editorial and peer review.
The effect of cancer treatment on the ovary
Women who have had cancer are at an increased risk of early menopause and primary ovarian insufficiency as a result of ovarian follicle depletion, stromal fibrosis, and vascular injury after chemotherapy and radiotherapy.8 Early menopause has a negative effect on quality of life,9 and is associated with osteoporosis,10 cardiovascular disease,11 and psychosocial disorders such as depression.12 Even survivors in whom ovarian function resumes or is maintained after cancer treatment might face a shortened window of fertility.13
The extent of the damage to the ovary depends on the type and dose of chemotherapy,14 the radiotherapy dose, fractionation scheme, and irradiation field,15 and the ovarian reserve before treatment. Age is an important marker of ovarian reserve, as are the serum markers estradiol, inhibin B, follicle-stimulating hormone, and anti-Müllerian hormone (AMH).16 AMH has emerged as an especially strong predictor of ovarian function after chemotherapy.17 A rapid and substantial reduction of circulating AMH concentrations is noted in adults after the start of chemotherapy,18 and recent data suggest that AMH concentrations before the start of chemotherapy,17 and the reduction and recovery of AMH during and after chemotherapy, might predict the amount of ovarian damage.19 These data suggest a crucial role for AMH in the identification of patients who might benefit from fertility preservation and which approach will optimise future fecundity. However, in prepubertal and peripubertal girls, AMH concentrations should be interpreted with caution because they cannot unequivocally predict reproductive lifespan.20 Long-term follow-up data for AMH concentrations decades after cancer treatment are not yet available to establish the predictive potential of AMH with regards to reproductive lifespan in cancer survivors.8
Follicle depletion is the hallmark of ovarian damage and is most pronounced in women given alkylating agents, such as cyclophosphamide,19 and in those who receive total-body irradiation before haemopoietic stem cell transplantation or direct irradiation of the ovaries. The precise mechanism by which cyclophosphamide affects the primordial follicle pool is not entirely understood, but recent studies investigating the effect of cyclophosphamide in mouse ovaries suggest that cyclophosphamide results in activation rather than apoptosis of primordial follicles,21 via upregulation of the PI3K/PTEN/Akt signalling pathway. According to these data, cyclophosphamide causes apoptosis of larger growing follicles, with cyclophosphamide-induced primordial follicle activation ultimately resulting in follicle burnout.
Other chemotherapeutic drugs might directly damage the growing oocyte or the highly proliferative granulosa cells within the developing follicle. These drugs might also cause follicle depletion indirectly, by damaging growing follicles and enhancing recruitment of primordial follicles to deplete the follicle pool, or by altering the ovarian stroma.8 An understanding of the gonadotoxic mechanisms of chemotherapy is needed to design effective agents that protect against iatrogenic depletion of ovarian follicles in young patients with cancer.
Fertility preservation strategies
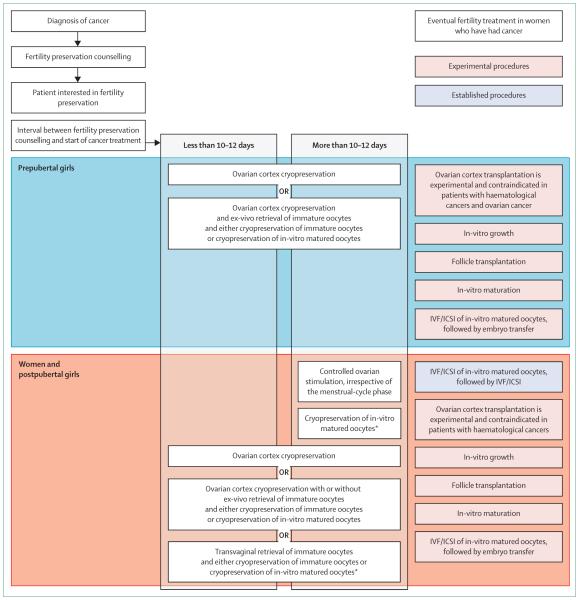
The assessment of fertility risk and the selection of an individualised strategy to optimise fecundity after cancer treatment are huge challenges and require intense cooperation between fertility preservation specialists, oncologists, other health-care workers, and the patient. Guidelines developed on the basis of systematic reviews and scientific literature analyses recommend fertility preservation approaches3,5 by patient age, cancer type, type of treatment, presence of a male partner or patient preference for the use of banked donor sperm, time available for fertility preservation intervention, and the likelihood of ovarian metastasis (figure). Established fertility preservation methods include oocyte and embryo cryopreservation, both derived from routine reproductive clinical practice, and ovarian transposition (oophoropexy), which can be offered to women undergoing pelvic irradiation.22 Ovarian suppression with gonadotropin-releasing hormone analogues during adjuvant chemotherapy induces a prepubescent state that restricts ovarian damage during chemotherapy through various hypothetical mechanisms,23 although data do not support the clinical validity of this approach.24
Figure.
Recommended fertility preservation approaches
*Or cryopreservation of embryos. IVF=in-vitro fertilisation. ICSI=intracytoplasmic sperm injection.
Ovarian cortex cryopreservation is deemed experimental, needs additional informed consent, and is typically offered to patients for whom established methods cannot be applied or a delay in cancer treatment is not possible. Whether ovarian biopsy (cortical strips) or unilateral oophorectomy should be done is uncertain. In follow-up studies,24 patients who underwent unilateral oophorectomy showed a significant number of spontaneous pregnancies, but removal of an entire ovary might be too aggressive and could further reduce the ovarian reserve, at least in some young patients.25 A systematic review of all available fertility preservation methods is beyond the scope of this review; rather, we aim to highlight important elements in the fertility preservation decision-making algorithm (figure).
Young patients with breast cancer represent a substantial proportion of patients referred for fertility preservation. Most patients with breast cancer, including those with early-stage disease, will ultimately receive adjuvant chemotherapy.26 The time interval between primary surgery and the start of chemotherapy—when fertility preservation treatment could be done—is based on the assumption that chemotherapy should be started as soon as possible. However, no published randomised controlled trials have investigated the association between a delay in chemotherapy and survival in patients with breast cancer. Data from a meta-analysis27 of seven independent studies that investigated overall survival in patients with operable primary breast cancer who received cyclophosphamide, methotrexate, and fluorouracil and anthracycline-based regimens suggested that overall survival decreased by 15% for every 4-week delay in initiation of chemotherapy. However, premenopausal women with breast cancer represent a distinct population with respect to responsiveness to adjuvant chemotherapy, and no studies are available to suggest an optimum interval before start of chemotherapy in this subpopulation of patients.28 Furthermore, oocyte or embryo cryopreservation typically needs controlled ovarian stimulation, the initiation of which has been timed to the phase of the menstrual cycle, and has been associated with a marked increase in circulating estradiol concentrations. As a result, many oncologists are hesitant to allow patients to undergo ovarian stimulation for oocyte or embryo cryopreservation, especially patients with hormone-responsive cancers. Novel methods for ovarian stimulation, such as co-treatment with the aromatase inhibitor letrozole, have been developed for patients with oestrogen receptor-positive breast cancer to mitigate the estradiol rise associated with ovarian stimulation. These alternative methods do not seem to result in a significant increase of breast cancer recurrence, although long-term follow-up studies are not yet available.29 A modification of the controlled ovarian stimulation protocol dissociates the timing of ovarian stimulation and the phase of the menstrual cycle; this approach is based on the recent finding that many waves of follicular development happen within one menstrual cycle,30 and that a cohort of antral follicles present during the late follicular phase or luteal phase are in early stages of development and might grow if exogenous gonadotropins are given. These findings have culminated in the random-onset ovarian stimulation protocol, which allows patients to start gonadotropin stimulation irrespective of menstrual cycle phase, with no impairment of oocyte yield and only a small increase of stimulation duration.31,32 This protocol has markedly reduced the time needed for ovarian stimulation and even offers the option of two consecutive cycles of stimulation before the start of chemotherapy, which should substantially enhance the number of mature oocytes available for IVF and embryo cryopreservation.33
Embryo cryopreservation has been done since the early years of assisted reproductive technology (1984), and vitrified-warmed embryo transfer in an artificial endometrial priming cycle is regarded as efficient as fresh embryo transfer.34 Moreover, embryo storage time does not affect livebirth rates after thawing.35 However, this method requires a male partner or the use of donor sperm, which introduces the potential for ethical and legal concerns about the fate of orphan embryos if the patient dies or if the patient and her partner separate. A patient who has become infertile after cancer treatment might have no other biological material cryopreserved except the frozen embryos generated with sperm from her ex-partner. Cryopreservation of mature oocytes can avoid these concerns and has emerged as an established method in recent years.36 Livebirth rates after transfer of embryos generated with vitrified-warmed oocytes are similar to those after intracytoplasmic sperm injection using fresh oocytes,37 although extrapolation of these results to the cancer patient population needs to be made with caution, and up to now, only three livebirths by cancer survivors have been reported after oocyte vitrification.38-40
Oocyte in-vitro maturation
In-vitro maturation of immature oocytes obviates the need for ovarian stimulation by use of gonadotropins before oocyte collection, and substantially reduces the delay of chemotherapy. Although cryopreservation of in-vitro matured oocytes is still regarded as experimental, the method is an emerging option for women who need to start chemotherapy soon after diagnosis and for prepubertal girls who cannot undergo ovarian stimulation. Although a large proportion of prepubertal girls have antral follicles on ultrasound, the true developmental potential of any immature oocytes retrieved is unknown.
At any stage of the menstrual cycle, a cohort of small antral follicles (0·9–6·0 mm) can be recorded. These follicles constitute a valuable source of oocytes for fertility preservation and are typically obtained through transvaginal aspiration. Clinical outcomes after in-vitro maturation are lower than those after conventional assisted reproductive technology.41 Although more than 2000 children have been born after fertilisation of fresh in-vitro maturation oocytes, very few livebirths have been reported with the use of in-vitro maturation oocytes that have been cryopreserved and thawed or warmed. Immature oocytes within small antral follicles can also be retrieved ex vivo at the time of ovarian cortex processing before cryopreservation,42-44 and then matured in vitro and cryopreserved. The first livebirth following this approach was recently reported.45 The fertilisation potential of in-vitro matured oocytes might be compromised by the cryopreservation process, and vitrification of mature oocytes is preferred over cryopreservation of immature oocytes.46 Research focused on reduction of the efficiency gap between in-vitro and in-vivo oocyte maturation is in progress, and harnessing the activity of factors from the TGF-β family,47 cAMP modulators,48,49 and EGF-like factors50 has shown promising results.
Advances in ovarian cortex transplantation
The birth of the first healthy girl after transplantation of cryopreserved human ovarian tissue happened nearly a decade after the initial work of Roger Gosden and David Baird in sheep.51-53 The present techniques for slow freezing of ovarian cortex by use of dimethyl sulfoxide as cryoprotectant are based on this pioneering research. So far, ovarian tissue grafting has yielded more than 30 healthy children after autotransplantation, mainly into the pedicle of the remaining ovary or onto peritoneal sites. Although restoration of fertility is the main aim of ovarian tissue transplantation, the mean duration of ovarian endocrine function after transplantation is about 5 years,54 which suggests that this procedure might also be used to delay menopause. Programmed slow freezing seems to result in substantial loss of both growing follicles and primordial follicles. Optimisation of the cryopreservation process and grafting technique should improve survival of the follicular reserve and reduce the need for multiple grafts in women who have had cancer.
Although the ovarian medulla, broad ligament, or ovarian fossa are intuitively deemed the best places for transplantation, there is insufficient evidence that these are better than distant heterotopic sites—eg, abdominal wall, forearm, or breast. Although resumption of endocrine function has been consistent54 and embryo development has arisen after heterotopic transplantation, only three pregnancies derived from oocytes aspirated from a heterotopic graft have been reported.55-57
The potential of revascularisation of the graft is perhaps the most important factor for success, because it establishes the survival rate of the follicle pool within the graft. The development of capillaries into the tissue from the bed of the graft happens within a few days.58 Careful preparation of the vascular bed, either mechanically or with vasoactive substances, might improve graft survival and prolong its functionality.
Cryopreservation of ovarian tissue: vitrification or slow freezing?
Advances in the specialty of cryobiology have improved the cryopreservation of complex multicellular structures and even whole organs. During the past decade, vitrification has gradually replaced slow programmed freezing for the cryopreservation of embryos and oocytes. Although slow freezing of ovarian cortex is still applied in most fertility preservation laboratories and has resulted in most of the livebirths after ovarian cortex transplantation, vitrification of ovarian tissue is an emerging focus of investigation,59 and the first livebirth after transplantation of vitrified-warmed tissue was recently reported.60 Compared with slow freezing, vitrification is associated with improved conservation of the ovarian follicular and stromal structures,61 and increased follicle survival rates,62,63 which should lead to improved function of the tissue after transplantation. However, the use of high concentrations of cryoprotectant chemicals and the ultra-rapid cooling rate, which requires direct contact with liquid nitrogen, have raised questions about the safety of this method. Comparative data for the efficiency of vitrification and slow freezing of ovarian cortex need to be validated in prospective randomised studies, with healthy livebirth rates as the primary endpoint.
When considering autotransplanation of cryopreserved ovarian cortex, avoidance of reintroduction of malignant cells via the tissue graft is paramount. In a recent review of 391 patients eligible for ovarian tissue cryopreservation,64 investigators noted metastases in ovarian tissue of 1·3% of all patients with cancer by use of light microscopy; patients with leukaemia had the highest risk of malignant cells within ovarian tissue grafts. In another study,65 malignant cell infiltration was recorded in 7% of tissue specimens from 422 patients by use of biochemical and histological detection methods including PCR and xenotransplantation. Although none of the patients had cancer recurrence after transplantation,54,65 robust methods to detect or prevent inclusion of cancer cells in transplanted ovarian tissue are key to enhancing the safety of ovarian tissue transplantation. In addition to in-vitro culture of isolated primordial follicles for transplantation, ovarian tissue purging has been used to eliminate malignant cells,66 and artificial ovaries of primordial follicles combined with disease-free stromal elements have been constructed.67,68
Primordial and preantral follicle culture
Strategies for culturing follicles ex vivo have been under development for many years, not only to address fundamental questions of follicle development, but also for applications in fertility preservation. Many methods for in-vitro growth of follicles are under development worldwide, showing the large interest in provision of next-generation assisted reproductive technology options for patients with cancer who have banked their ovarian tissue. Most follicles in the removed tissue are at primordial and preantral stages, and two-dimensional and three-dimensional follicle culture systems in mice and human beings have been developed (appendix). Primary follicles need several days in culture and the support of a dynamic somatic cell compartment; this architectural requirement led to the development of alginate and other biomaterials that mimic the natural ovarian structure. The alginate hydrogel system was tested in mice and has been successfully applied to rhesus monkey, baboon, and human follicles.69-71 Mature eggs and embryos have been derived in the non-human primate setting, whereas the terminal meiotic maturation of the human oocyte, its fertilisation, and transfer to a human recipient await development. The main advantage of this system is that follicle growth is undertaken completely in vitro, which produces a mature egg that can be fertilised. Thus, the embryo, which contains no somatic cells from the donor, can be transferred to the patient or to a gestational carrier without the risk of re-introducing cancer to the patient. However, this method does not restore endocrine function, and the patient will need endocrine management for pregnancy and the duration of her life.
The development of ovarian tissue and follicle culture systems is associated with several major challenges. The recent finding of abnormal oocyte morphology in a significant number of primordial oocytes in the ovarian cortex from prepubertal girls has raised some concern.72 The data suggest that a population of follicles from prepubertal patients with cancer who undergo ovarian cortex cryopreservation might harbour poor quality oocytes. Other challenges include the need to synchronously activate cultured primordial follicles from the dormant to the growing stage, incomplete knowledge of the physical and biochemical factors that support normal growth and differentiation across preantral stages and inhibit atresia, and the design of non-invasive assays of oocyte development within the cultured follicle. In the past decade, major advances have been made in molecular biochemistry and bioinformatics and the unravelling of the principal networks driving early follicular growth processes in vivo. The integration of biomolecular science, biophysics, and non-invasive analytical methods should vastly improve follicle culture systems, make easier the non-invasive assessment of oocyte nuclear and cytoplasmic development in cultured follicles, and lead to the identification of the molecular signature for oocyte competence.73
One intriguing and recent report60 of a healthy livebirth after in-vitro follicle activation merits further scrutiny. The fragmentation of ovaries from women with primary ovarian insufficiency triggers follicle growth, through disruption of Hippo signalling. After subsequent activation of the Akt signalling pathway and autografting, mature oocytes were obtained in eight patients, two of whom became pregnant. These data suggest that activation of residual dormant follicles is a feasible approach to achievement of future pregnancy in women who have had cancer and who are infertile because of ovarian follicle depletion.
Future preventive strategies to decrease the effect of gonadotoxic treatment
One of the most important new directions for fertility preservation is the development of mitigation strategies to reduce the off-target effects of chemotherapy or radiation treatment on the ovaries (table). These strategies will reduce the need to pursue more radical interventions such as ovariectomy, which might be especially difficult to consider in children with a cancer diagnosis.
Table 1.
Neoadjuvant pharmacotherapy to reduce the eff ects of chemotherapy on the ovaries
| Species | Efficacy | |
|---|---|---|
| Nano-encapsulation of arsenic trioxide treatment in mice with lymphoma74 |
Mouse | More active against lymphoma and less deleterious to ovarian function |
| c-Abl-TAp63 pathway inactivation by imatinib75,76 | Mouse | Decreased oocyte death induced by chemotherapy |
| Gonadotropin-releasing hormone agonist analogue triptorelin treatment77 |
Rat | Protected ovarian function from x-irradiation |
| Gonadotropin-releasing hormone agonist analogue triptorelin treatment during chemotherapy in young patient with breast cancer78,79 |
Human being | Decreased early menopause rate and prolonged ovarian function upon triptorelin treatment |
| S1P treatment in adult mice80 | Mouse | Reduced radiation-induced oocyte loss |
| Immunomodulator AS101 treatment in adult female mice21,81 |
Mouse | Increased DNA repair ability after irradiation, reduced follicle loss, and preserved fertility |
| S1P or FTY720 treatment before ovarian radiation82 |
Rhesus monkey | Livebirth and normal fertility |
| S1P treatment in human ovarian tissue xenograft in mouse82 |
Human being | Reduced radiation-induced primordial oocyte depletion in human ovarian cortical tissue |
One approach consists of encapsulation of the chemotherapeutic entity within a nanoparticle. By virtue of its size and the potential to functionalise the drug, a nanoparticulate vehicle can target a chemo-payload to the tumour.83 The method is predicated on the differential vascular density of solid tumours relative to other tissues and the ability to create a lethal dose in the cancer tissue with little or no appreciable uptake of the nanoparticles in other tissues.84-86 This idea has been tested with arsenic trioxide, a chemotherapy drug used in lymphoma but not in breast cancer because the effective dose is lethal.87 Nano-encapsulation of the drug was tested in a mouse model of breast cancer, and showed that the drug was effective in restricting the disease, but did not affect fertility.74 Nano-encapsulation strategies could be extended to any existing chemotherapy targeting solid tumours to restrict the effects of these powerful anticancer drugs on the gonads.
A second mitigation strategy is fairly controversial but is based on inhibition of the immediate early apoptotic effect of chemotherapies and radiation on primordial follicles. Primordial follicles die rapidly after drug delivery or radiation exposure because of the presence of a hyperactive cell suicide pathway, suggested by high expression levels of p63.88,89 As few as four double-strand breaks result in cellular apoptosis through this pathway.82 The tyrosine kinase inhibitor imatinib (Gleevec) blocks the kinase c-abl, which feeds the death pathway and, in the presence of cisplatin, can restrict primordial follicle death.75,76 The use of this drug or others that restrict cellular apoptosis would be attractive for preservation of follicles, especially in women diagnosed with cancer after the age of 38 years and who face an early menopause as a consequence of cancer treatment. Restriction of primordial follicle loss in this group of women ensures a longer time to ovarian failure. Furthermore, affected oocytes might be able to repair themselves if they are not immediately targeted for apoptosis, which provides an application for this treatment in young patients.
Sphingosine-1-phosphate (S1P) treatment reduces radiation-induced oocyte apoptosis.80 Proof-of-concept evidence was achieved with the livebirth of a monkey from a female that had been treated with a chemotherapeutic and the antiapoptotic factor S1P.82 Promising results have also been published for the immunomodulator AS101.21,81 More drug tests are necessary to assess the fertility preservation applications and endocrine effects of these interventions. Drugs that interfere with apoptosis might also be oncogenic; therefore, these strategies are deemed experimental and need more preclinical research in appropriate animal models.
Finally, gonadotropin-releasing hormone analogues have been used to block the reproductive axis to restrict chemotherapeutic-induced primordial follicle death. This treatment has been studied for many years in both non-randomised and randomised trials with inconsistent outcomes; so far, investigators have noted no improved livebirth rates after treatment with gonadotropin-releasing hormone analogues before chemotherapy.24 For now, with no good basic rationale for the effect, the use of gonadotropin-releasing hormone analogues for fertility preservation remains controversial.
Derivation of germ cells from stem cells
In the past 10 years, the ovaries of mice and human beings were found to contain not only the non-renewable oocyte-containing follicle pool established at birth,90 but also ovarian or oogonial stem cells. Oogonial stem cells can be collected and cultured and develop into oocytes under certain conditions.91,92 Present efforts are aimed at establishing whether the oocytes derived from the oogonial stem cells can be combined with support cells (granulosa and theca cells) to form follicles that will grow and produce mature oocytes that can be fertilised.93
Mouse oogonial stem cells have been cultured in vitro, GFP labelled, and transplanted back into recipient ovaries, leading to the production of GFP-positive fertilised oocytes, embryos, and live offspring.92,94 In human beings, early-stage follicle-like structures with granulosa cell-enclosed oocytes are formed when human-isolated oogonial stem cells are aggregated with dispersed adult human ovarian tissues. Furthermore, after transplantation of oogonial stem cell-containing human ovarian cortical tissues into immunodeficient mice, primordial and primary-like follicles can be detected, which suggests the possible reconstitution of follicles from the human oogonial stem cells.90 More research is needed to improve the efficacy of ovarian stem cell isolation and development into oocytes.
Induced pluripotent cell-derived stem cells might provide an additional route for fertility restoration. Primordial germ cell-like cells, which have been successfully induced from embryonic stem cells of male mice, undergo normal spermatogenesis and can be used for fertilisation to produce offspring.95 Recently, female primordial germ cell-like cells derived from female embryonic stem cells were used to reconstitute ovaries in vitro for transplantation back to mouse ovaries;96 the primordial germ cell-like cells produced meiotically competent oocytes that could be fertilised to produce viable offspring. Alternatively, the bone marrow and peripheral blood might be potential sources of ovarian germ cells capable of generating oocytes;97 however, these findings remain controversial because no ovulated oocyte derived from circulating germ cells has been confirmed.98
The use of native or in-vitro-derived germ cells raises the possibility that, for women and girls with cancer, the production of new oocytes to replace the ovarian reserve destroyed by cancer treatment might be possible. This approach would not only offer a way to restore fertility in young women who have had cancer, but also prevent premature ovarian failure and the loss of ovarian hormones that have far-reaching health benefits in cancer survivors of advancing age.99
Conclusion
Taken together, the specialty of oncofertility is growing with new technologies that are meeting patient needs. These promising developments represent a strong incentive to provide fertility preservation counselling, and the preservation, surveillance, and restoration of fertility are becoming integral parts of care for women who have had cancer. Nevertheless, many novel approaches are still experimental and need validation. Further translational and clinical research will be crucial for the establishment of patient-tailored interventions to maximise the likelihood of healthy offspring after cancer.
Acknowledgments
Fertility preservation research at UZ Brussel is supported by grants from the Research Foundation-Flanders (G.0343.13 to JS), the Flemish Agency for Innovation by Science and Technology (IWT/TBM/110680 to JS), and the Belgian Foundation against Cancer (Project HOPE, C69 to JS). Fertility preservation research at Northwestern University is supported by NIH/NICHD U54 grant to TKW. We thank Shuo Xiao for contributing the table to this article and Stacey Tobin for editorial support, and acknowledge the many important contributions to the entire specialty, but because of editorial restrictions, a maximum of 100 references were allowed.
Footnotes
This is the second in a Series of three papers about fertility preservation
Declaration of interests
We declare no competing interests.
Contributor Information
Michel De Vos, Centre for Reproductive Medicine, UZ Brussel, Brussels, Belgium.
Johan Smitz, Laboratory of Clinical Chemistry and Radioimmunology, UZ Brussel, Brussels, Belgium.
Teresa K Woodruff, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2010. National Cancer Institute; http://seer.cancer.gov/csr/1975_2010/ (accessed Sept 18, 2014) [Google Scholar]
- 2.Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94:1652–56. doi: 10.1016/j.fertnstert.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100:1214–23. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Mersereau JE, Goodman LR, Deal AM, Gorman JR, Whitcomb BW, Su HI. To preserve or not to preserve: how difficult is the decision about fertility preservation? Cancer. 2013;119:4044–50. doi: 10.1002/cncr.28317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loren AW, Mangu PB, Beck LN, et al. American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RA, Weddell A, Spoudeas HA, et al. Do doctors discuss fertility issues before they treat young patients with cancer? Hum Reprod. 2008;23:2246–51. doi: 10.1093/humrep/den252. [DOI] [PubMed] [Google Scholar]
- 7.Corney RH, Swinglehurst AJ. Young childless women with breast cancer in the UK: a qualitative study of their fertility-related experiences, options, and the information given by health professionals. Psychooncology. 2014;23:20–26. doi: 10.1002/pon.3365. [DOI] [PubMed] [Google Scholar]
- 8.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–35. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 9.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–17. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruning PF, Pit MJ, de Jong-Bakker M, van den Ende A, Hart A, van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61:308–10. doi: 10.1038/bjc.1990.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeanes H, Newby D, Gray GA. Cardiovascular risk in women: the impact of hormone replacement therapy and prospects for new therapeutic approaches. Expert Opin Pharmacother. 2007;8:279–88. doi: 10.1517/14656566.8.3.279. [DOI] [PubMed] [Google Scholar]
- 12.Carter J, Rowland K, Chi D, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97:90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–39. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meirow D, Biederman H, Anderson RA, Wallace WHB. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–39. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 15.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–44. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RA, Cameron DA. Pretreatment serum anti-müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–43. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 18.Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280–85. doi: 10.1016/j.rbmo.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–67. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 20.Fleming R, Kelsey TW, Anderson RA, Wallace WH, Nelson SM. Interpreting human follicular recruitment and antimüllerian hormone concentrations throughout life. Fertil Steril. 2012;98:1097–102. doi: 10.1016/j.fertnstert.2012.07.1114. [DOI] [PubMed] [Google Scholar]
- 21.Kalich-Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 22.Jadoul P, Squfflet J, Donnez J. Laparoscopic ovarian transposition before radiotherapy. In: Donnez J, editor. Atlas of Operative Laparoscopy and Hysteroscopy. Informa Healthcare; UK: 2007. pp. 349–353. [Google Scholar]
- 23.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–54. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 24.Turner NH, Partridge A, Sanna G, Di Leo A, Biganzoli L. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertain. Ann Oncol. 2013;24:2224–35. doi: 10.1093/annonc/mdt196. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt KT, Nyboe Andersen A, Greve T, Ernst E, Loft A, Yding Andersen C. Fertility in cancer patients after cryopreservation of one ovary. Reprod Biomed Online. 2013;26:272–79. doi: 10.1016/j.rbmo.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379:432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu K-D, Huang S, Zhang J-X, Liu G-Y, Shao Z-M. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:240–50. doi: 10.1186/1471-2407-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colleoni M, Bonetti M, Coates AS, et al. The International Breast Cancer Study Group Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. J Clin Oncol. 2000;18:584–90. doi: 10.1200/JCO.2000.18.3.584. [DOI] [PubMed] [Google Scholar]
- 29.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–35. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 30.Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69:1023–31. doi: 10.1095/biolreprod.103.017772. [DOI] [PubMed] [Google Scholar]
- 31.Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95:2125.e9–11. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–80. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 33.Turan V, Bedoschi G, Moy F, Oktay K. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril. 2013;100:1681. doi: 10.1016/j.fertnstert.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Similar ongoing pregnancy rates after blastocyst transfer in fresh donor cycles and autologous cycles using cryopreserved bipronuclear oocytes suggest similar viability of transferred blastocysts. Fertil Steril. 2010;93:319–21. doi: 10.1016/j.fertnstert.2009.07.966. [DOI] [PubMed] [Google Scholar]
- 35.Riggs R, Mayer J, Dowling-Lacey D, Chi T-F, Jones E, Oehninger S. Does storage time influence postthaw survival and pregnancy outcome? An analysis of 11,768 cryopreserved human embryos. Fertil Steril. 2010;93:109–15. doi: 10.1016/j.fertnstert.2008.09.084. [DOI] [PubMed] [Google Scholar]
- 36.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–08. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 37.Rienzi L, Romano S, Albricci L, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MK, Lee DR, Han JE, et al. Live birth with vitrified-warmed oocytes of a chronic myeloid leukemia patient nine years after allogenic bone marrow transplantation. J Assist Reprod Genet. 2011;28:1167–70. doi: 10.1007/s10815-011-9681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99:1994–99. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Fujino Y, Nakamura Y, Wakimoto E, Koike K, Ueda J, Matsumoto M. Pregnancy after the embryo transfer developed from unfertilized oocytes frozen for the fertility preservation before the chemotherapy of acute myeloid leukemia. Fertil Steril. 2013;100:S171. [Google Scholar]
- 41.Gremeau A-S, Andreadis N, Fatum M, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril. 2012;98:355–60. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 42.Fasano G, Moffa F, Dechène J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150. doi: 10.1186/1477-7827-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Revel A, Revel-Vilk S, Aizenman E, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92:458–63. doi: 10.1016/j.fertnstert.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Escribá MJ, Grau N, Escrich L, Novella-Maestre E, Sánchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. J Assist Reprod Genet. 2012;29:1261–65. doi: 10.1007/s10815-012-9860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasath EB, Chan MLH, Wong WHW, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–78. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 46.Brambillasca F, Guglielmo M-C, Coticchio G, Mignini Renzini M, Dal Canto M, Fadini R. The current challenges to efficient immature oocyte cryopreservation. J Assist Reprod Genet. 2013;30:1531–39. doi: 10.1007/s10815-013-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo CX, Gilchrist RB, Thompson JG, Lane M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23:67–73. doi: 10.1093/humrep/dem140. [DOI] [PubMed] [Google Scholar]
- 48.Nogueira D, Ron-El R, Friedler S, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod. 2006;74:177–84. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- 49.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011. doi: 10.1093/humrep/deq246. [DOI] [PubMed] [Google Scholar]
- 50.Zamah AM, Hsieh M, Chen J, et al. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25:2569–78. doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 52.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–71. doi: 10.1210/endo.140.1.6453. [DOI] [PubMed] [Google Scholar]
- 53.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 54.Donnez J, Dolmans MM, Pellicer A, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 55.Rosendahl M, Loft A, Byskov AG, et al. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod. 2006;21:2006–09. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- 56.Stern CJ, Toledo MG, Hale LG, Gook DA, Edgar DH. The first Australian experience of heterotopic grafting of cryopreserved ovarian tissue: evidence of establishment of normal ovarian function. Aust N Z J Obstet Gynaecol. 2011;51:268–75. doi: 10.1111/j.1479-828X.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 57.Stern CJ, Gook D, Hale LG, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod. 2013;28:2996–99. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 58.Van Eyck AS, Bouzin C, Feron O, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 59.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–86. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110:17474–79. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keros V, Xella S, Hultenby K, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 62.Herraiz S, Novella-Maestre E, Rodríguez B, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101:775–84. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Amorim CA, David A, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertil Steril. 2011;95:1094–97. doi: 10.1016/j.fertnstert.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 64.Dolmans MM, Jadoul P, Gilliaux S, et al. A review of 15 years of ovarian tissue bank activities. J Assist Reprod Genet. 2013;30:305–14. doi: 10.1007/s10815-013-9952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30:11–24. doi: 10.1007/s10815-012-9912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schröder CP, Timmer-Bosscha H, Wijchman JG, et al. An in vitro model for purging of tumour cells from ovarian tissue. Hum Reprod. 2004;19:1069–75. doi: 10.1093/humrep/deh244. [DOI] [PubMed] [Google Scholar]
- 67.Shikanov A, Zhang Z, Xu M, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labied S, Delforge Y, Munaut C, et al. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. Transplantation. 2013;95:426–33. doi: 10.1097/TP.0b013e318279965c. [DOI] [PubMed] [Google Scholar]
- 69.Tingen CM, Kiesewetter SE, Jozefik J, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–20. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu M, Barrett SL, West-Farrell E, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smitz J, Dolmans MM, Donnez J, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson RA, McLaughlin M, Wallace WHB, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vigone G, Merico V, Prigione A, et al. Transcriptome based identification of mouse cumulus cell markers that predict the developmental competence of their enclosed antral oocytes. BMC Genomics. 2013;14:380. doi: 10.1186/1471-2164-14-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahn RW, Barrett SL, Raja MR, et al. Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PLoS One. 2013;8:e58491. doi: 10.1371/journal.pone.0058491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–85. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 76.Kim SY, Cordeiro MH, Serna VA, et al. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20:987–97. doi: 10.1038/cdd.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camats N, García F, Parrilla JJ, Calaf J, Martín-Mateo M, Caldés MG. The GnRH analogue triptorelin confers ovarian radio-protection to adult female rats. Mutat Res. 2009;669:67–79. doi: 10.1016/j.mrfmmm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–76. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 79.Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–23. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 80.Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 81.Kalechman Y, Shani A, Albeck M, Sotnik Barkai I, Sredni B. Increased DNA repair ability after irradiation following treatment with the immunomodulator AS101. Radiat Res. 1993;136:197–204. [PubMed] [Google Scholar]
- 82.Zelinski MB, Murphy MK, Lawson MS, et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95:1440–45. doi: 10.1016/j.fertnstert.2011.01.012. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain KK. Nanotechnology-based drug delivery for cancer. Technol Cancer Res Treat. 2005;4:407–16. doi: 10.1177/153303460500400408. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Ahn R, Van den Bossche J, Thompson DH, O’Halloran TV. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther. 2009;8:1955–63. doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H, MacDonald RC, Li S, Krett NL, Rosen ST, O’Halloran TV. Lipid encapsulation of arsenic trioxide attenuates cytotoxicity and allows for controlled anticancer drug release. J Am Chem Soc. 2006;128:13348–49. doi: 10.1021/ja064864h. [DOI] [PubMed] [Google Scholar]
- 86.Chen H, Pazicni S, Krett NL, et al. Coencapsulation of arsenic- and platinum-based drugs for targeted cancer treatment. Angew Chem Int Ed Engl. 2009;48:9295–99. doi: 10.1002/anie.200903655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahn RW, Chen F, Chen H, et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin Cancer Res. 2010;16:3607–17. doi: 10.1158/1078-0432.CCR-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135:3–12. doi: 10.1530/REP-07-0054. [DOI] [PubMed] [Google Scholar]
- 89.Suh EK, Yang A, Kettenbach A, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–28. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 90.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–109. [Google Scholar]
- 91.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 92.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18:353–54. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- 94.Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–36. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–32. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–75. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- 97.Johnson J, Bagley J, Skaznik-Wikiel M, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 98.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–14. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 99.Dunlop CE, Telfer EE, Anderson RA. Ovarian stem cells—potential roles in infertility treatment and fertility preservation. Maturitas. 2013;76:279–83. doi: 10.1016/j.maturitas.2013.04.017. [DOI] [PubMed] [Google Scholar]