Abstract
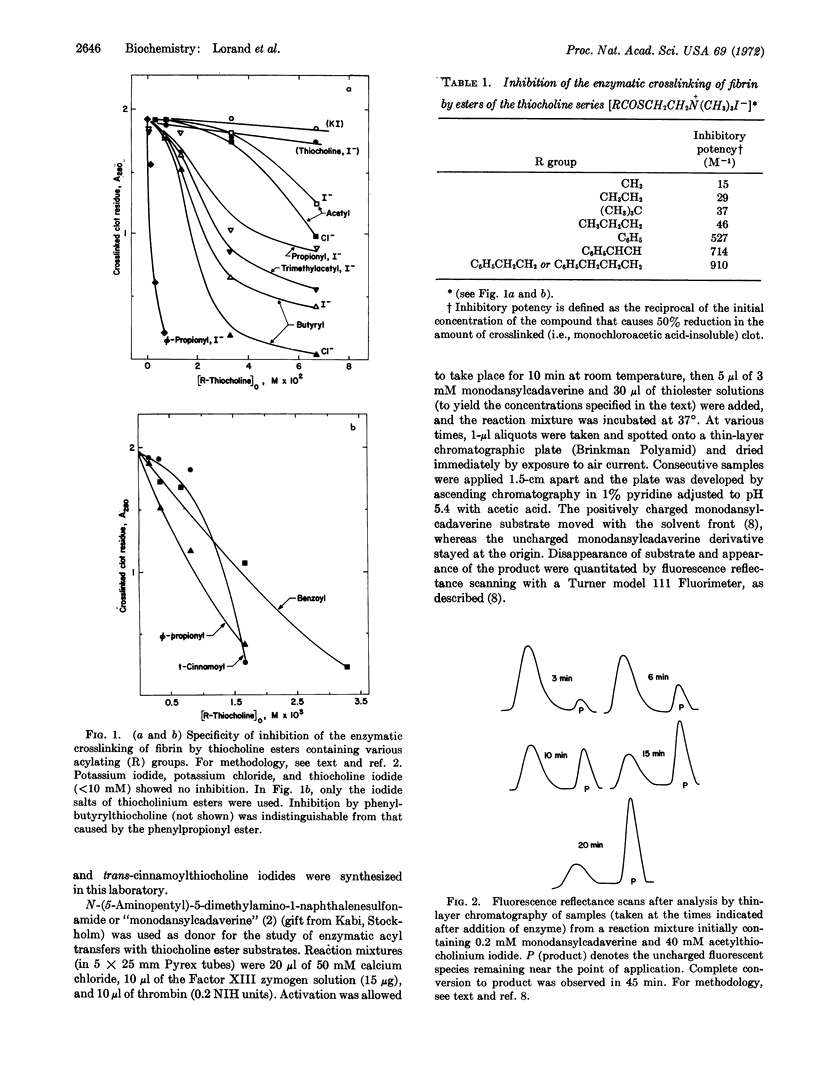
Esters of thiocholine were shown to inhibit the crosslinking of fibrin clots by the transamidating enzyme, fibrinoligase (thrombin-activated fibrin-stabilizing factor or activated Factor XIII). Inhibition depended on the nature of the acylating group with the phenylpropionyl, phenylbutyryl, and trans-cinnamoyl esters being most effective of the compounds tested so far.
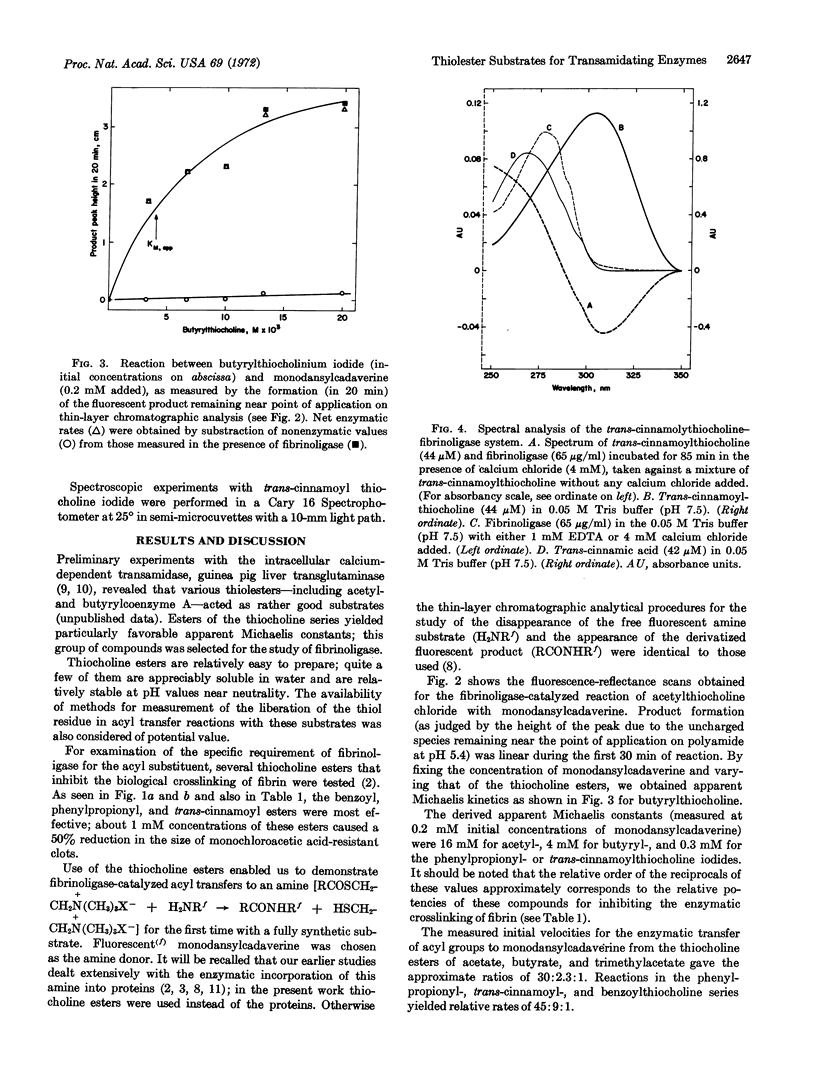
Use of the thiolesters made it possible for the first time to study the reactions of fibrinoligase in fully synthetic substrate systems. Enzyme-catalyzed acyl-group transfers from the thiol-esters to a fluorescent amine [N-(5-aminopentyl) - 5 - dimethylamino - 1 - naphthalenesulfonamide] could be readily demonstrated and measured.
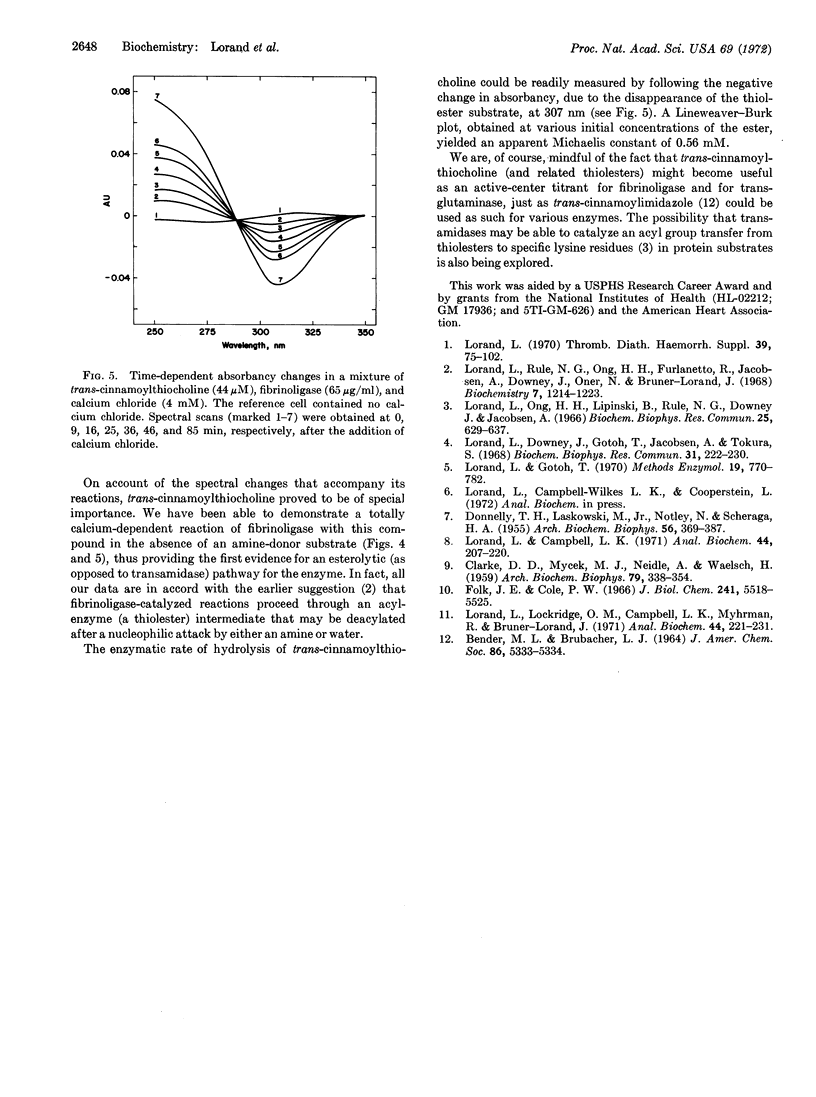
Trans-cinnamoylthiocholine reacted with fibrinoligase in a totally calcium-dependent manner in the absence of any added amine, thus providing the first evidence for an esterolytic pathway for this enzyme. Spectral qualities, as well as appreciable extent of solubility in water, would seem to make trans-cinnamoylthiocholine a specially suitable substrate for further studies.
Keywords: acyl group transfer, hydrolysis, transglutaminase, Factor XIII
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DONNELLY T. H., LASKOWSKI M., Jr, NOTLEY N., SCHERAGA H. A. Equilibria in the fibrinogen-fibrin conversion. II. Reversibility of the polymerization steps. Arch Biochem Biophys. 1955 Jun;56(2):369–387. doi: 10.1016/0003-9861(55)90258-7. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Mechanism of action of guinea pig liver transglutaminase. I. Purification and properties of the enzyme: identification of a functional cysteine essential for activity. J Biol Chem. 1966 Dec 10;241(23):5518–5525. [PubMed] [Google Scholar]
- Lorand L., Campbell L. K. Transamidating enzymes. I. Rapid chromatographic assays. Anal Biochem. 1971 Nov;44(1):207–220. doi: 10.1016/0003-2697(71)90362-9. [DOI] [PubMed] [Google Scholar]
- Lorand L., Downey J., Gotoh T., Jacobsen A., Tokura S. The transpeptidase system which crosslinks fibrin by gamma-glutamyle-episilon-lysine bonds. Biochem Biophys Res Commun. 1968 Apr 19;31(2):222–230. doi: 10.1016/0006-291x(68)90734-1. [DOI] [PubMed] [Google Scholar]
- Lorand L., Lockridge O. M., Campbell L. K., Myhrman R., Bruner-Lorand J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal Biochem. 1971 Nov;44(1):221–231. doi: 10.1016/0003-2697(71)90363-0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]


