Abstract
Background/Objectives
Polypharmacy is receiving increased attention as a potential problem for older persons, who frequently have multiple chronic conditions. The purpose of this study was to summarize evidence regarding the health outcomes associated with polypharmacy, defined as number of prescribed medications,among older community-dwelling persons.
Design
Systematic review of MEDLINE (OvidSP 1946 to May Week 3 2014).
Setting
Community
Participants
Observational studies examining health outcomes according to the number of prescription medications taken.
Measurements
Association of number of medications with health outcomes. Because of the importance of comorbidity as a potential confounder of the relationship between polypharmacy and health outcomes, articles were assessed regarding the quality of their adjustment for confounding.
Results
Of the total of 50 studies identified, the majority studies that were rated as “good” in terms of their adjustment for comorbidity demonstrated relationships between polypharmacy and a range of outcomes, including falls/fall outcomes/fall risk factors; adverse drug events, hospitalization, mortality, and measures of function and cognition. However, a number of these studies failed to demonstrate associations, as did a substantial proportion of those studies rated as “fair” or “poor.”
Conclusions
Data are mixed regarding the relationship between polypharmacy, considered in terms of number of medications, and adverse outcomes among community-dwelling older persons. Because of the challenge of confounding, randomized controlled trials of medication discontinuation may provide more definitive evidence regarding this relationship.
Keywords: Polypharmacy, observational studies, systematic review
INTRODUCTION
The majority of older persons have multimorbidity, the co-existence of multiple chronic conditions. In a study using 100% Medicare claims data from 2008(with 83.5% of beneficiaries age ≥ 65 years), 67% of all beneficiaries had two or more chronic conditions. The prevalence increased with age, to 81.5% for beneficiaries 85 years of age or older.1 A consequence of multimorbidity is the use of multiple medications. In a 2003 survey of Medicare beneficiaries, nearly 90% were taking at least one prescription medication, and, among these persons, 46% were taking five or more medications.2 The use of multiple medications has been given the namepolypharmacy, although there are other definitions for the term, such as use of inappropriate medications, and, even when defined according to number of medications,no consensus exists regarding the number of medications at which polypharmacy begins. There are concerns about the consequences of polypharmacy among persons with multimorbidity. Increasing the number of medications exponentially increases the number of combinations of medications, which, in turn, increases the risk of adverse drug reactions and drug-drug interactions.3One study that applied clinical practice guidelines to the care of an older patient with five common chronic co-existing conditions(hypertension, chronic heart failure, stable angina, atrial fibrillation, hypercholesterolemia, diabetes mellitus, osteoarthritis, chronic obstructive pulmonary disease, and osteoporosis)found that she would be prescribed twelve medications requiring a complex administration schedule, with the potential for multiple drug-drug and drug-disease interactions.4 Because data regarding the benefits and harms of medications have come largely from randomized controlled trials excluding persons with multimorbidity, the marginal benefits and harms associated with prescribing additional medications for persons who are already taking other medications are not known.5
While reviews of polypharmacy defined as the receipt of multiple medications have concluded that number of medications is associated with adverse outcomes among older adults,3,6 the full range of outcomes potentially associated with polypharmacy is not well understood. Several prior reviews of polypharmacy focused on the epidemiology of and interventions to reduce polypharmacy rather than on outcomes.6,7One review examining outcomes associated with polypharmacy used the key words “polypharmacy” or “multiple medications” as identifiers of studies examining the use of multiple medications.3 Our examination of key articles suggested that relevant studies were not uniformly indexed using these terms. We therefore sought to undertake a systematic review of the literature to examine the health outcomes associated with polypharmacy employing a number of strategies to identify studies that examined the use of multiple medications.
METHODS
Data Sources and Searches
The search was constructed to address the question, “Among community-living persons age 65 years and older receiving outpatient care, what are the clinical outcomes associated with polypharmacy related to medications taken for chronic conditions?” The following databases were searched for relevant studies with in the dates shown: MEDLINE (OvidSP 1946 to May Week 3 2014).The search strategies used synonymous free text words in addition to controlled vocabulary terms and to capture the concept of polypharmacy by identifying studies examining the prescription of 4 or more medications and/or drug burden in persons age 65 and older. The search strategy was not limited by study design or language of publication. The full strategy is shown in the appendix.
Study Selection
In the absence of consensus regarding the definition of polypharmacy, articles were included if they examined outcomes associated with a greater number of medications as compared to a lesser number, regardless of what these numbers were. Because multiple articles enrolled population-based studies regardless of site of residence, we included these articles, but excluded articles if the population consisted entirely of persons living in assisted living facilities or nursing homes. We excluded observational designs other than cohort or case-control studies (e.g. case series).Articles were excluded if they examined hospitalized patients and patients receiving emergency room care, as these represented selected cohorts. Articles were also excluded if they did not examine a health-related outcome; an example of such an exclusion was medication adherence. A final exclusion criterion was the prescription of inappropriate medications or number of medications in a specific medication class as the sole measure of polypharmacy, without also examining the number of medications prescribed. We did this because of our specific interest in examining the evidence regarding the relationship between number of medications and patient outcomes regardless of the appropriateness of each medication considered individually. We did not exclude studies of patients on possibly inappropriate medications if they had polypharmacy defined according to total number of medications.A total of 50 titles and/or abstracts were reviewed independently by three of the investigators to confirm uniformity in the process of excluding articles. The titles and/or abstracts of the remaining references were initially reviewed by one of two investigators, and articles that were not excluded were re-reviewed by a third investigator to achieve consensus regarding inclusion. Full text review was performed for these articles. The reference lists for the final set of articles identified by this search as well as for articles that did not meet inclusion criteria but provided reviews or conceptual discussions of polypharmacy were also examined to identify additional articles meeting inclusion criterion.
Data Extraction
For each of the included studies, two investigators independently extracted the following data elements: 1) study design; 2) study population; 3) measure of polypharmacy; 4) main findings. Disagreements were resolved through discussion. Many of the customary measures of quality utilized for systematic reviews that are designed to assess the risk of bias in randomized controlled trials, such as adequacy of randomization, blinding, and completion of outcome reporting, were not applicable to the observational studies included in the current review.8 Instead, we focused on the issue of confounding. Adjustment for chronic illnesses was particularly important to consider because of the possibility that the outcomes examined were associated with the multiple diseases for which medications were prescribed rather than the medications themselves. We therefore evaluated each study for the adequacy of adjustment for confounding, rating each study as “good,” “fair,” or “poor.”A rating of good was assigned if the study used specific assessments of comorbid conditions (e.g. comorbidity index, number of chronic conditions, presence or absence of multiple individual conditions).A rating of fair was assigned if the study did not assess a full complement of chronic conditions but did include other characteristics associated with health, such as age, self-reported health, and/or function. A rating of poor was assigned if the study did not include any of these adjustments or if the methods section did not explicitly describe how adjustment was done.
Data Synthesis and Analysis
We organized studies according to the outcome examined. Because of the heterogeneity in design, population, and definition of polypharmacy, we did not attempt to combine the results.
RESULTS
Identification of Articles
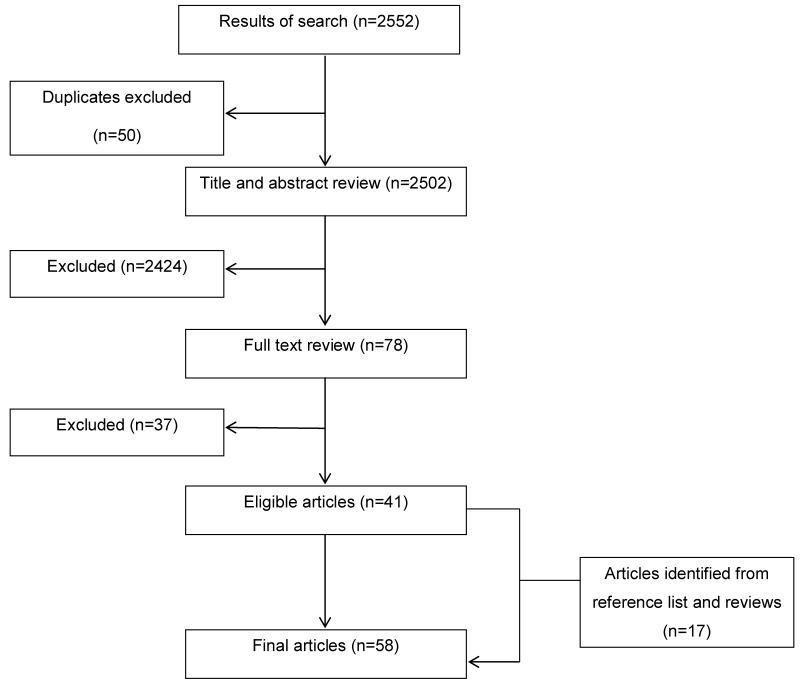
The literature search yielded 2,552 articles, including 50 duplicates. A total of 78 articles passed the title/abstract screening process. Full-text review resulted in the exclusion of 37 articles. Review of the reference lists for the remaining 41 articles and articles identified as not meeting inclusion criteria but providing a review of polypharmacy resulted in the identification of an additional17 articles, for a total of 58 (Figure).
Figure.
Summary of literature search and selection
Type of Studies Identified
All of the studies were observational, with the majority of these being cross-sectional or longitudinal cohort studies;9-62 the remainder of the observational studies were case-control.63-66 Many were population-based or enrolled participants from disease or medical practice registries and represented persons from multiple countries. The largest number of studies (23) examined falls or a fall-related outcome (e.g. dizziness, fear of falling, fracture) as the outcome of interest.9-30,63A total of 14 studies examined adverse drug events (ADEs), although these studies varied in how they measured ADEs, including self-report, identification by investigator, recognition by clinician, and reason for outpatient visit.31-44 Hospitalization and/or mortality were the outcomes examined in ten studies,10,15,45-51,65 and a total of 15 studies examined a variety of outcomes, including general markers of health as well as specific symptoms, function, cognition, and, in one study, risk of developing a new disease, Parkinson’s.9,15,52-62,64,66
Study Findings
Of the 23 studies that examined falls or fall-related outcomes, 14were rated as good in terms of adjustment for chronic conditions.9-22 Among these studies, 12 found at least one positive association between polypharmacy and the outcome of interest.9-15,17,18,20-22Of the 12, one study found an association between polypharmacy and falls among women but not among men,11and one found an association between polypharmacy and dizziness but not falls.14Several studies examining multiple categories of number of medications did not find associations between the use of one to two or two to three medications versus no medications and the outcome of interest, but did find associations for categories including a higher number of medications.9,17,22 In one study, the authors stratified the participants according to whether they were taking a medication that was individually found to be associated with falls, or a “risk” medication, and reported that polypharmacy was associated with falls only among those taking a risk medication.22 The remaining nine studies were assessed fair or poor in terms of adjustment for chronic conditions;23-30,63 among these studies, seven found at least one positive association between polypharmacy and the outcome of interest.23,24,26-29,63
A total of eight of the studies examining ADEs were rated as good in their adjustment for chronic conditions.31-38Of these, five found an association between polypharmacy and ADEs measured in a number of different ways, including self-report, chart review, and outpatient visit.31-34,38Of the five, one found a significant associated only for 14 or more medications.35Of the six studies rated as fair or poor in their adjustment, four found an association between number of medications and ADEs.39,41-43
Of the ten studies examining hospitalization and/or mortality, four were rated as good in their adjustment for comorbidity and associations were found between number of medications and all-cause hospitalization and/or mortality in three of the studies10,15,45 and flu-related hospitalization and mortality in the fourth.65 Of the six studies rated as fair or poor,three found associations between number of medications and mortality or hospitalization.46-48 A fourth study included older persons using insulin or sulfonylureas and found an association between number of medications and serious hypoglycemia resulting in an emergency room visit, hospitalization, or death.50
A total of 15 studies examined a broad range of outcomes, including weight loss, self-perceived health status, physical performance, function, cognition, symptoms, and disease development.9,15,52-62,64,66 Of the eleven studies rated as good regarding adjustment for comorbidities, all found an association between number of medications and at least one outcome of interest,9,15,52-58,64,66 although one study examining the outcomes of nutritional, functional, and cognitive status only found an association for 10 or more medications.54 The remaining studies, rated fair or poor, also found associations.59-62
DISCUSSION
This systematic review, designed to address the question of the outcomes associated with polypharmacy, demonstrated marked heterogeneity among studies in terms of definition of polypharmacy and outcomes studied. Because the number of medications cannot be assigned to patients, these studies are observational, and therefore the issue of confounding is particularly important, since individuals who take more medications are likely to have poorer health than those who take fewer. The studies in this review also varied widely in their approaches to and adequacy of adjustment for chronic conditions. However, the majority of studies involved large and often population-based cohorts, suggesting that the study cohorts were representative of persons taking multiple medications. The results of the studies were mixed, with some studies demonstrating an association of polypharmacy with falls, fall risk factors, and fall-related injury; adverse drug events; hospitalization; mortality; and a variety of measures of symptoms and physical and cognitive function, while other studies failed to find these associations.
Our expectation was that studies with less adequate adjustment for confounding would be more likely to demonstrate an association between polypharmacy and adverse outcomes, because polypharmacy is likely to be a marker of the patient’s underlying health status. The results of the review showed that, for falls and fall-related outcomes, a greater proportion of studies assessed as good in terms of adjustment for confounding demonstrated an association with polypharmacy as compared to studies assessed as fair or poor. For the other outcomes included in the review, some studies assessed as good as well as those assessed as fair or poor demonstrated associations while others did not. It is unclear why studies doing less adequate adjustment failed to demonstrate associations, but this finding highlights the multifactorial nature of the outcomes examined in the studies included in this review. To the extent that these negative studies reflect a lack of association between health status/chronic diseases and the outcomes examined, they support the conclusion that findings of associations between polypharmacy and the various outcomes reflect a true independent relationship.However, it may also be that even good adjustment for comorbidities cannot account for differences in health associated with differential receipt of medications. A study examining the relationship between the use of individual medications and mortality among older persons found that medications for which there is no evidence of mortality benefit, including non-steroidal anti-inflammatory agents and benzodiazepines, were nonetheless associated with a lower risk of death among a large cohort of older persons. Adjustment for comorbidity had little effect on this association, leading the authors to conclude that the prescription of these medications was a marker of better health status not captured by comorbidity, as physicians would be reluctant to prescribe these medications to their sickest patients.67
There is a burgeoning interest in polypharmacy as reflected in the literature. The number of studies indexed by this term in Medline increased three-fold in the last decade, from 77 articles 2002 to 286 in 2012. Moreover, multiple studies have been conducted with the aim of reducing polypharmacy, based on the hypothesis that polypharmacy is a risk factor for adverse outcomes.68-71 The results of this review provide mixed support for this hypothesis. In addition, one of the studies included in our review raises the question of whether the number of medications per se is associated with adverse outcomes or whether the number of medications is a marker for the use of individual medications with a well-established risk of causing adverse events, such as psychotropic agents72-74 and other medications established by expert consensus to be inappropriate for some or all older persons.75,76Our review identified a study finding that individuals who were prescribed a greater number of medications had a greater likelihood of taking a medication associated with fall risk after adjusting for age, gender, comorbidity, and disability, and that polypharmacy was a risk for falling only if it included one of these individual medications.22
In addition to the question of whether number of medications is merely a marker for the receipt of inappropriate medications, there is also the question of whether it is also a marker for under prescribing. In one study, approximately 40% of older veterans who were taking five or more medications were simultaneously taking one or more medications considered to be inappropriate and not taking a potentially beneficial medication.77This finding raises the possibility that, if there is a relationship between number of medications and adverse outcome, it may result, at least in part, from underuse of appropriate medications. The challenge, however, is a lack of data regarding which medications are appropriate for older persons with multiple chronic conditions. These patients are systematically excluded from participation in clinical trials, with the result that trials underestimate the harms these patients may experience from medications.78 In addition, a recent study demonstrated that, because of lower life expectancy, older persons with chronic kidney disease derive much less benefit from medications to prevent end-stage renal disease than do younger patients.79Taken together, these studies highlight the complexity of the relationship between medications and outcomes, suggesting that number of medications alone may not be sufficient indicator of the quality of a patient’s medication regimen.
Because of the issues regarding confounding and the complex relationship between medication regimens and outcomes, it is likely that a more definitive answer to the question of the outcomes associated with polypharmacy will require randomized controlled trials. The results of this systematic review provide sufficient preliminary evidence to support such trials. In addition, additional studies provide ancillary evidence of benefits of medication reduction. Several studies have examined on interventions to reduce inappropriate prescribing, including the use of unnecessary and inappropriate medications as well as underuse of medications. An early study utilizing a clinical pharmacist demonstrated a 25% reduction in the likelihood of an ADE, although this result did not reach statistical significance,80 and a more recent study of outpatient geriatric care demonstrated a significant 35% reduction in serious ADEs.81Additional studies have focused on medication reduction. A randomized controlled trial of a multifactorial falls prevention intervention targeting multiple risk factors included older persons taking four or more medications including at least one centrally acting antihypertensive, nitrate, diuretic, histamine blocker, or nonsteroidal anti-inflammatory drug who reported fatigue, dizziness. These participants received the targeted intervention of medication review.82,83 Overall, the intervention reduced the risk of falls by 31% and also reduced the likelihood of taking four or more medications (86% in the control group versus 63% in the intervention group.)82A second study examined the feasibility of reducing medications among 70 older community-dwelling adults in a pre- post- study design. This study resulted in discontinuation of 81% of medications for which a recommendation was made among 64 participants, of whom 88% reported improvement in their general health.84 Taken together, this evidence provides support for the efficacy of interventions to improve medication regimens. Whether, however, reduction in polypharmacyper se results in better patient outcomes is still unknown, and studies addressing this issue will face the challenge of disentangling the effects of eliminating high-risk medications from the effects of reducing the overall burden of medications that individually are not generally associated with harm.
This review has a number of limitations. First, because a number of health outcomes were common to many of the studies, it would be ideal to have a summary measure of the association of polypharmacy with these outcomes. However, the heterogeneity in study populations and in definitions of polypharmacy made direct comparisons among the studies highly challenging. Second, we found a sizeable proportion of the studies included in the review through the reference lists of other articles. This suggests that, while we attempted to make our search strategy as broad as possible, by using text words as well as formal subheadings, we may have missed other relevant articles.Third, the studies included in the review were heterogeneous in terms of the types of medications they examined. While some studies specified the exclusion of over-the-counter medications and/or medications prescribed for a short course to address an acute condition, other studies included these and still others did not provide sufficient data to determine which medications were included/excluded.
In summary, this systematic review addressing the question of the health outcomes associated with polypharmacy provides mixed evidence regarding these associations. While some articles that were assessed as good in terms of adjusting for comorbidities that likely confound the relationship between medication use and outcomes demonstrated an association between polypharmacy and falls, adverse drug events, hospitalization, and other outcomes, other such articles did not. More definitive evidence regarding these associations will require randomized controlled trials testing the effects of medication discontinuation.
Table.
Outcomes associated with polypharmacy*
| Outcome:FALLS/FRACTURES/DIZZINESS | ||||
|---|---|---|---|---|
| Author/ Year |
Study Design, Follow-up, & N |
Population | Measure of Polypharmacy |
Main Findings |
|
Agostini, 2004 |
Cohort: cross-sectional (weight loss); longitudinal 1 year (impaired balance) N= 885 |
Probability-sample single U.S. city, age 72+ years |
1-2, 3-4, 5+ vs. 0 meds |
Impaired balance: NS for 1-2 meds, OR (95% confidence interval) 1.72 (1.09, 2.71) for 3-4 meds, 1.80 (1.02, 3.19) for 5+ meds. |
|
Beer, 2011 |
Cohort: cross-sectional N= 4,260 |
Population-based single city Western Australia; males ages 65-83 years |
Continuous | Falls: OR 1.06 (1.02, 1.09) |
|
Campbell, 1989 |
Cohort: longitudinal 1 year N= 761 |
Population-based rural New Zealand community, age 70+ years |
1-3, 4+ vs. 0 meds |
Falls: Significant for women only. RR2.6 (1.2, 5.5) for 1-3 meds; RR 4.5 (1.9, 10.6) for 4+ meds |
| Clough- Gorr, 2008 | Cohort: longitudinal 1 year N= 1,644 |
Population-based three European cities, age 65+ years |
4+ vs. 0-3 meds | Falls or ever fallen: OR: 1.3 (1.0, 1.8) |
|
Fletcher, 2009 |
Cohort: cross-sectional N= 453 |
Participants in five Canadian fall prevention studies, mean age 80.7 years |
Continuous | 1 fall: OR 1.19 (1.09, 1.3) 2+falls: OR 1.21 (1.02, 1.43) |
|
Gassman, 2009 |
Cohort: cross-sectional and longitudinal 2 years N= 620 |
Population-based three German cities age 65+ years |
4+ vs 0-3 meds | Dizziness at baseline: OR 2.36 (1.62, 3.44); at follow-up: OR 1.60 (1.11, 2.32) Falls: NS |
|
Gnjidic, 2012 |
Cohort: longitudinal 2 years ISM,242 |
Population-based single Australian city, age 70+ years |
Continuous | Falls: OR 1.07 (1.03, 1.12) |
|
Gomez, 2011 |
Cohort: cross-sectional N= 1,692 |
Population-based four suburban/rural Columbian communities, age 60+ years |
4+ vs 0-3 meds | Dizziness: NS |
|
Huang, 2009 |
Cohort: longitudinal 5 years N= 46,946 |
HMO disease registry of patients with DM single U.S. city, age 18+ years |
2-3, 4-5, 6-7, >7 vs. 0-1 meds |
Falls: NS for 2-3 meds. OR 1.22 (1.04, 1.43) for 4-5 meds; OR 1.33 (1.12, 1.58) for 6-7 meds; OR 1.59 (1.34, 1.89) for >7 meds. |
|
Lawlor, 2003 |
Cohort: cross-sectional N= 4,050 |
General practice lists three U.K. towns, women, age 60-79 yrs |
Continuous | Falls: NS. |
|
Murphy, 2002 |
Cohort: cross-sectional N= 433 |
Probability-sample single U.S. city, age 72+ years |
5+ vs. 0-4 meds | Fear of falling/activity restriction: NS |
|
Tinetti, 2000 |
Cohort: cross-sectional N= 1,087 |
Probability-sample single U.S. city, age 72+ years |
5+ vs. 0-4 meds | Dizziness: RR 1.3 (1.01, 1.63) |
|
Vellas, 1998 |
Cohort: longitudinal 2 years N= 482 |
Volunteers from two prior U.S. studies, age 60+ years |
Continuous | Falls: RR 1.054 (1.001, 1.109) Injurious falls: RR 1.135 (1.022, 1.260) |
|
Ziere, 2005 |
Cohort: cross-sectional N= 6,928 |
Population-based Dutch city, age 55+ years |
3, 4+ vs. 0 meds | Falls: NS for 3 meds; OR 1.6 (1.1, 2.1) for 4+ meds. No increased risk if not on individual med associated with falls in adjusted model. |
|
| ||||
|
Curcio, 2006 |
Cohort: cross-sectional N= 1,668 |
Volunteers Columbian towns, age 60+ years |
4+ vs. 0-3 meds | Fear of falling: OR 1.56 (1.14, 2.14) |
| Jacqmin- Gadda, 1998 | Cohort: longitudinal 5 years N= 3,216 |
Population-based random sample two communities in France, age 65+ years |
>3 nonpsychotropic drugs vs. ≤3 |
Hip fractures: NS Non-hip fractures: OR 1.36 (1.04, 1.78) |
|
Kojima, 2012 |
Cohort: longitudinal 2 years N=172 |
Consecutive patients seen in single outpatient Japanese geriatric clinic, age 65+ years |
5+ versus 0-4 meds |
Falls: OR 4.50 (1.66, 12.2) |
| Kao, 2001 | Cohort: cross-sectional N= 262 |
Patients in single U.S. geriatric assessment center, age 60+ years |
3+ vs. 0-2 meds | Dizziness: NS |
|
Lord, 1994 |
Cohort: longitudinal 1 year N= 704 |
Population-based single Australian city, women age 65+ years |
4+ vs, 0-3 meds | Falls: RR 1.28 (1.03, 1.58) |
| Wu, 2013 | Cohort: cross-sectional N= 671 |
Random sample of persons participating in free health examination single Taiwanese city, age 55+ years |
4+ vs. 0-3 meds | Falls: OR 2.17 (1.18, 3.97) |
|
| ||||
|
Buatois, 2010 |
Cohort: longitudinal 25 months; N= 1,618 |
Persons presenting for senior medical checkup singe French city, age 65+ years |
4+ meds vs. 0-3 meds |
Falls: OR 1.66 (1.06, 2.60) |
| Lai, 2010 | Retrospective case-control N= 9,312 |
Population-based sample of Taiwanese residents, age 65+ years, age 65+ years |
2-4, 5-7, 8-9, 10+ vs. 0-1 meds |
Hip fracture: OR 1.65 (1.47, 1.83) for 2-4 meds, OR 3.21 (2.77, 3.73) for 5-7 meds, OR 5.54 (3.77, 8.13) for 8-9 meds, OR 8.42 (4.73, 15.0) for 10+ meds |
| Liu, 1995 | Cohort: longitudinal 1 year N= 100 |
Volunteers single Canadian city, mean age 83 years |
Continuous | Falls: NS |
| Outcome: ADVERSE DRUG REACTIONS or EVENTS | ||||
|---|---|---|---|---|
| Author/ Year |
Study Design, Follow-up, & N |
Population | Measure of Polypharmacy |
Main Findings |
| Calderon- Larranaga, 2012 |
Retrospective cohort: longitudinal 1 year |
Patients of 7 urban primary care centers single Spanish city age 14+ |
6+ versus 0-5 meds |
ADE coded in electronic medical record: OR 1.344 (1.106, 1.634) |
|
Chrischilles, 2007 |
N=79, 089 Cohort: longitudinal 1 year N= 689 | Population-based Iowa Medicare beneficiaries with mobility limitation, age 65+ |
4-6, 7-9, 10-13, 14-34 vs. 0-3 meds |
Self-reported ADE, unwanted reaction: increasing OR under unadjusted and 2 adjusted models. Only 14-34 meds w/ sig OR. |
| Field, 2004 | Cohort: longitudinal 1 year N= 30,397 |
years Patients of large multispecialty group practice single U.S. city, age 65+ years |
2-4,5-7, 8+ vs. 0-1 meds, |
Preventable drug-related injury: NS for 2-4 meds; OR 2.4 for 5-7 meds; OR 3.1 for 8+ meds. |
| Field, 2007 | Cohort: longitudinal 1 year N= 30,000 |
Patients of large multispecialty group practice single U.S. city, age 65+ years |
3-4, 5-6, 7+ vs. 0-2 meds |
Drug related injury: NS for 3-4 meds; OR 3.1 for 5-6 meds; OR 3.3 for 7+ meds. |
|
Gandhi, 2000 |
Retrospect cohort: longitudinal 1 year N= 2,248 |
Patients of 11 ambulatory practices single U.S. city, ages 20-75 years |
Continuous | Self-reported ADR and ADR in medical record: NS |
|
| ||||
| Green, 2007 | Retrospect cohort: cross-sectional. N= 405 |
Medicare managed care enrollee single U.S. city, age 65+ years |
Continuous | Self-reported ADE: NS (either as 5+ meds or continuous) |
|
Hutchinson, 1986 |
Cohort: longitudinal 1 year N= 1,026 | Patients of internal medicine practice single Canadian city, mean age 55 (±16) |
# drug courses 1, 2, 3-5, 6-10, 11 + |
Risk of ADE to newly-started med: NS |
| Sarkar, 2011 | Cohort: cross-sectional † |
Nationally representative U.S. probability sample, age 25+ years |
1-3, 4-5, 6-8 vs. 0 meds |
Outpt visit for ADE: OR 2.8 (1.66, 4.74) for 1-3 meds; OR 3.61 (1.92, 6.78) for 4-5 meds; OR 3.83 (2.2, 6.65) for 6-8 meds |
|
| ||||
|
Chrischilles, 1992 |
Cohort: cross-sectional N= 3,170 |
Probability sample 2 rural U.S. counties, age 65+ years |
Continuous | Self-reported ADR: OR 1.25 (1.18, 1.33) |
|
Schneider, 1992 |
Cohort: longitudinal 1 year N= 463 | Outpatients of geriatric and general medicine clinics in single U.S. city, age 70+ years |
Continuous | ADRs or symptoms linked to medication by clinician/investigator: NS |
|
| ||||
|
Bourgeois, 2010 |
Cohort: cross-sectional † |
Nationally representative U.S. probability sample, all ages |
3-4, 5+ vs. 0-2 meds |
Outpt/ER visit for ADE: OR 1.45 (1.25, 1.58) for 3-4 meds; OR 1.88 (1.58, 2.25) for 5+ meds |
|
Gandhi, 2003 |
Cohort: longitudinal 3 months N= 661 | Patients of four ambulatory practices U.S., age 18+ years |
Continuous | ADE/pt symptoms linked by investigator to med: for each additional med, mean # of ADEs per pt increased by 10% (6, 15%) |
|
Reason, 2012 |
Cohort: cross-sectional N=3132 |
Nationally representative Canadian probability sample age 65+ years |
5+ vs. 1-2, 3-4 meds |
Self-reported ADE requiring MD or ER visit: 5% for 1-2 med, 6% for 3-4 meds, 12% for 5+ meds (p=.0001) |
|
Veehof, 1999 |
Retrospective cohort: cross-sectional. N= 2,185 |
Patients of three general practices single Dutch city, age 65+ years |
2-3, 4-5, 5+ vs. 0-1 meds |
ADR as recognized by clinician: NS |
| Outcome: HOSPITALIZATION OR MORTALITY | ||||
|---|---|---|---|---|
| Author/ Year | Study Design, Follow-up, & N | Population | Measure of Polypharmacy | Main Findings |
| Beer, 2011 | Cohort: longitudinal 4.5 years N= 4,260 | Population-based single city Western Australia; males ages 65-83 years | Continuous | Hospital admission: HR 1.04 (1.03, 1.06) Cardiovascular events: HR 1.09 (1.06, 1.12) Mortality: HR 1.04 (1.00, 1.07) |
| Gnjidic, 2012 | Cohort: longitudinal 2 years N=1,242 | Population-based single Australian city, age 70+ years | Continuous | Mortality: OR 1.09 (1.04, 1.15) |
| Hak, 2001 | Case-control N= 315 | Population-based throughout Netherlands with chronic disease | Continuous | Flu hospitalization or mortality: OR 1.3 (1.1, 1.5) |
| Richardson 2011 | Cohort: longitudinal 18 years N= 12,423 | Population-based three urban and two rural communities Great Britain, age 65+ years | 5+ meds | 2-year mortality: HR 1.42 (1.28, 1.58) for men; HR 1.3 (1.19, 1.41) for women. |
|
| ||||
| Espino, 2006 | Cohort: longitudinal 8 years N= 1,823 | Probability sample 4 Southwest U.S. cities, Mexican Americans age 6599 years | >4 vs. 1 med | Mortality: HR 1.27 (1.04, 1.56) |
| Jensen, 2001 | Cohort: longitudinal 1 year, N= 386 | Members of single managed Medicare plan, age 65+ years | 3+ vs. 0-2 meds | Hospitalization: OR 3.79 (1.33, 10.90) |
| Jyrkka, 2009 | Cohort: longitudinal, measured at 2 time points, 9 years. N= 601/339 | Population-based single Finnish city, age 75+ years | 6-9, 10+ vs. 0-5 meds | Mortality: No association in 1st phase In 2nd phase, NS for 6-9 meds; OR 2.23 (1.21, 4.12) for 10+ meds. |
| Pozzi, 2010 | Cohort: longitudinal 4 years N=568 | Population-based single small town Italy, age 65+ years | 5+ meds | Mortality: NS |
| Shorr, 1997 | Retrospective cohort: Longitudinal 5 years N= 19,932 | Population-based Tennessee Medicaid enrollees, age 65+ years using insulin or sulfonylureas | 5+ vs. 0-4 meds | Serious hypoglycemia resulting in ER visit, hospitalization, or death: RR 1.3 (1.1, 1.5) |
|
| ||||
| Hershman 1995 | Cohort: longitudinal 2-10 years N= 488 | Volunteers single New York city, ages 75-85 years | Continuous | Mortality: NS |
| Outcome: MISCELLANEOUS | ||||
|---|---|---|---|---|
| Author/ Year |
Study Design & N | Population | Measure of Polypharmacy |
Outcome: Main Findings |
|
Agostini, 2004 |
Cohort: cross-sectional (weight loss); longitudinal 1 year (impaired balance) N= 885 |
Probability-sample single U.S. city, age 72+ years |
1-2, 3-4, 5+ vs. 0 meds |
Weight loss: NS for 1-2 meds, OR 1.96 (1.08, 3.54) for 3-4 meds, OR 2.78 (1.38, 5.60) for 5+ meds |
|
| ||||
| Fu, 2004 | Cohort: longitudinal 4 years N= 22,601 | Nationally representative probability sample of U.S. residents, age 65+ years |
Continuous | Self-perceived health status: Ordered probit model: total # meds p= .01 |
|
Gnjidic, 2012 |
Cohort: longitudinal 2 years ISM,242 | Population-based single Australian city, age 70+ years |
5+ vs. 0-5 meds. |
Frailty: OR 4.97 (3.04, 8.14) |
|
Gnjidic, 2012 |
Cohort: longitudinal 2 years ISM,242 | Population-based single Australian city, age 70+ years |
Continuous | Frailty: OR 1.13 (1.06, 1.21); ADL disability: OR 1.08 (1.00, 1.15); Cognitive impairment: NS |
|
Jyrkka, 2011 |
Cohort: longitudinal 3 years N= 294 | Population-based single Finnish city, age 75+ years |
6-9, 10+ vs. 0-5 meds |
Decline in MNA-SF: NS for 6-9 meds, p -.62 (- .27, -.98) for 10+ meds; Decline in IADL: p -.29 (-.10, -.47) for 6-9 meds, p -.53 (-.26, -.81) for 10+ meds; Decline in MMSE: NS for 6-9 meds, P -1.36 (-.63, -2.10) for 10+ meds. |
| Lai, 2012 | Case-control N=35,675 |
Population-based sample of Taiwanese residents, age 65+ years |
2-4, 5-9, 10+ vs. 0-1 meds |
Dementia: OR 1.28 (1.18, 1.38) for 2-4 meds, OR 1.34 (1.23, 1.46) for 5-9 meds, OR 1.56 (1.38, 1.76) for 10+ meds |
| Lai, 2011 | Case-control N= 14,135 |
Population-based sample of Taiwanese residents, age 65+ years |
2-4, 5-7, 8-9, 10+ vs. 0-1 meds |
Parkinson's disease: OR 1.53 (1.34, 1.75) for 2- 4 meds, OR: 2.08 (1.79, 2.42) for 5-7 meds, OR 2.64 (2.19, 3.18) for 8-9 meds, OR 2.95 (2.73, 3.59) for 10+ meds: |
|
Magaziner, 1989 |
Cohort: longitudinal 1 year N= 609 |
Population-based random sample single U.S. city, females age 65+ years |
Continuous | Decline in MMSE: NS; Increase in CES-D: p = .13 (p<01); Decline in IADL: p = .12 (p<001); Decline in PADL: NS. |
| Pugh, 2007 | Cohort: longitudinal 7 years N= 3,050 | Probability sample 4 Southwest U.S. cities, Mexican Americans age 65- 99 years |
5+ vs. 0-5 meds | Lower extremity functional limitation: regression estimate -.014 (p=.004). |
|
Rosso, 2013 |
Cohort: longitudinal 3 years N= 29,544 | Population-based from areas surrounding forty clinical centers in 24 US states and the District of Columbia |
5+ vs. 0-5 meds | Incident ADL disability: RR 1.95 (1.54, 2.46) |
| Starr, 2004 | Retrospective cohort: longitudinal 69 yrs (age 11 and 80) N= 478 |
Survivors of earlier population-based cohort single urban region Scotland, age 80 years |
Continuous | Change in IQ from age 11 to age 80: lower IQ scores w/ great number of meds (F=12.2, D= 001V |
|
| ||||
|
Monastero, 2006 |
Retrospective cohort: longitudinal 3-4 yrs N= 718 |
Population-based district of Stockholm, age 75+ years |
1-4, 5+ vs. 0 meds |
CIND: NS for 1-4 meds; OR 2.6 (1.1, 6.1) for 5+ meds. |
|
| ||||
|
Cohen, 1998 |
Cohort: cross-sectional N= 1,611 |
Population-based random sample electoral role of Australia, age 60+ years |
Continuous | Self-reported symptoms of postprandial & postural hypotension: OR 1.17 (1.05, 1.31) |
|
Kadam, 2011 |
Cohort: longitudinal 5 years N= 4,506 | Patients of six general practices England, age 50+ years |
5-7, 8-11, 12+ vs. 1-4 meds |
Worsening physical and mental health as measured by SF-12:: OR 1.55 (1.2, 2.1) for 5-7 meds; OR 2.25 (1.7, 3.1) for 8-11 meds; OR 2.91 (2.0, 4.2) for 12+ meds. |
|
Pilotto, 2005 |
Cohort: cross-sectional N= 5,515 |
General practice patients throughout Italy, age 65+ years |
0, 1-3, 4-6, 7+ meds |
Upper GI symptoms: Prevalence increased as # of meds increased in bivariate analysis (0, 1-3, 4-6, or 7+ meds) (p<.0001) |
NS = non-significant
OR = Odds ratio
HR = Hazard ratio
RR= Risk ratio
ADE = adverse drug event
ADR = adverse drug reaction
MNA-SF = Mini Nutritional Assessment Short-Form
IADL = Instrumental Activities of Daily Living
MMSE = Mini-Mental State Examination
PADL = Physical Activities of Daily Living
ADL = Activity of Daily Living
CIND = cognitive impairment not dementia
IQ = intelligence quotient
GI = gastrointestinal
The shading of the box indicates the quality of adjustment for chronic illness. No shading = good; light gray = fair; dark gray = poor. See text for details.
†Unit of analysis was ambulatory visit
ACKNOWLEDGMENTS
We would like to thank Janis Glover, MLS, for her assistance in conducting the Medline search. Views expressed are those of the authors and not necessarily those of the Dept of Veterans Affairs.
This research was supported by grant DF11-303 from the Donaghue Foundation and by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG21342 NIH/NIA).
Sponsor’s role: The sponsors had no role in the design, methods, data analysis, or preparation of the paper.
Appendix: Search strategy
| Step # | Search | Result |
|---|---|---|
| 1 | polypharmacy/ | 2344 |
| 2 | polypharmacy.tw. | 2703 |
| 3 | (drug adj1 burden).mo. | 70 |
| 4 | ((prescription* or medication( or polypharmacy) adj (number* or amount* or multiple*)).mp |
237 |
| 5 | ((four or five or six or seven or eight or nine or ten) adj1 (prescription* or medication* or polypharmacy)).mp |
440 |
| 6 | 1 or 2 or 3 or 4 or 5 | 4786 |
| 7 | limit 6 to humans | 4678 |
| 8 | limit 7 to ‘all aged (65 and over)’ | 2552 |
Conflict of Interest:
| Elements of Financial/Personal Conflicts | TRF | JO | VT | MKG | MT | DKM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | ||||||
| Grants/Funds | X | X | X | X | X | X | ||||||
| Honoraria | X | X | X | X | X | X | ||||||
| Speaker Forum | X | X | X | X | X | X | ||||||
| Consultant | X | X | X | X | X | X | ||||||
| Stocks | X | X | X | X | X | X | ||||||
| Royalties | X | X | X | X | X | X | ||||||
| Expert Testimony | X | X | X | X | X | X | ||||||
| Board Member | X | X | X | X | X | X | ||||||
| Patents | X | X | X | X | X | X | ||||||
| Personal Relationship | X | X | X | X | X | X | ||||||
Author contributions:
Concept and Design: TRF
Acquisition of data: TRF
Analysis and interpretation of data: JO’L, VT, MKG, MT, DKM
Preparation of manuscript: TRF, JO, VT, MKG, MT, DKM
REFERENCES
- 1.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 2.Safran DG, Neuman P, Schoen C, et al. Prescription drug coverage and seniors: Findings From A 2003 national survey. Health Aff (Millwood) 2005 doi: 10.1377/hlthaff.w5.152. [DOI] [PubMed] [Google Scholar]
- 3.Frazier SC. Health outcomes and polypharmacy in elderly individuals: an integrated literature review. J Gerontol Nurs. 2005;31:4–11. doi: 10.3928/0098-9134-20050901-04. [DOI] [PubMed] [Google Scholar]
- 4.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Bogardus ST, Jr., Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–4. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–51. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17:123–32. doi: 10.1111/j.1041-2972.2005.0020.x. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostini JV, Han L, Tinetti ME. The relationship between number of medications and weight loss or impaired balance in older adults. J Am Geriatr Soc. 2004;52:1719–23. doi: 10.1111/j.1532-5415.2004.52467.x. [DOI] [PubMed] [Google Scholar]
- 10.Beer C, Hyde Z, Almeida OP, et al. Quality use of medicines and health outcomes among a cohort of community dwelling older men: an observational study. Br J Clin Pharmacol. 2011;71:592–9. doi: 10.1111/j.1365-2125.2010.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol A Biol Sci Med Sci. 1989;44:M112–7. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 12.Clough-Gorr KM, Erpen T, Gillmann G, et al. Multidimensional geriatric assessment: back to the future preclinical disability as a risk factor for falls in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:314–20. doi: 10.1093/gerona/63.3.314. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher PC, Berg K, Dalby DM, et al. Risk factors for falling among community-based seniors. J Patient Saf. 2009;5:61–6. doi: 10.1097/PTS.0b013e3181a551ed. [DOI] [PubMed] [Google Scholar]
- 14.Gassmann K, Rupprecht R. Dizziness in an older community dwelling population: a multifactorial syndrome. J Nutr Health Aging. 2009;13:278–82. doi: 10.1007/s12603-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 15.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–95. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Gomez F, Curcio CL, Duque G. Dizziness as a geriatric condition among rural community-dwelling older adults. J Nutr Health Aging. 2011;15:490–7. doi: 10.1007/s12603-011-0050-4. [DOI] [PubMed] [Google Scholar]
- 17.Huang ES, Karter AJ, Danielson KK, et al. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med. 2009;25:141–6. doi: 10.1007/s11606-009-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–7. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy SL, Williams CS, Gill TM. Characteristics associated with fear of falling and activity restriction in community-living older persons. J Am Geriatr Soc. 2002;50:516–20. doi: 10.1046/j.1532-5415.2002.50119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132:337–44. doi: 10.7326/0003-4819-132-5-200003070-00002. [DOI] [PubMed] [Google Scholar]
- 21.Vellas BJ, Wayne SJ, Garry PJ, et al. A two-year longitudinal study of falls in 482 community-dwelling elderly adults. J Gerontol A Biol Sci Med Sci. 1998;53:M264–M74. doi: 10.1093/gerona/53a.4.m264. [DOI] [PubMed] [Google Scholar]
- 22.Ziere G, Dieleman JP, Hofman A, et al. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61:218–23. doi: 10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curcio C-L, Gomez F, Reyes-Ortiz CA. Activity restriction related to fear of falling among older people in the Colombian Andes mountains: are functional or psychosocial risk factors more important? J Aging Health. 2009;21:460–79. doi: 10.1177/0898264308329024. [DOI] [PubMed] [Google Scholar]
- 24.Jacqmin Gadda H. Risk factors for fractures in the elderly. Epidemiol. 1998;9:417. [PubMed] [Google Scholar]
- 25.Kao AC, Nanda A, Williams CS, et al. Validation of dizziness as a possible geriatric syndrome. J Am Geriatr Soc. 2001;49:72–5. doi: 10.1046/j.1532-5415.2001.49012.x. [DOI] [PubMed] [Google Scholar]
- 26.Kojima T, Akishita M, Nakamura T, et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatrics &Gerontology International. 2012;12:425–30. doi: 10.1111/j.1447-0594.2011.00783.x. [DOI] [PubMed] [Google Scholar]
- 27.Lord SR, Ward JA, Williams P, et al. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Aust J Public Health. 1993;17:240–5. doi: 10.1111/j.1753-6405.1993.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu TY, Chie WC, Yang RS, et al. Factors associated with falls among community-dwelling older people in Taiwan. Ann Acad Med Singapore. 2012;42:320–7. [PubMed] [Google Scholar]
- 29.Buatois S, Perret-Guillaume C, Gueguen R, et al. A simple clinical scale to stratify risk of recurrent falls in community-dwelling adults aged 65 years and older. Phys Ther. 2010;90:550–60. doi: 10.2522/ptj.20090158. [DOI] [PubMed] [Google Scholar]
- 30.Liu BA, Topper AK, Reeves RA, et al. Falls among older people: relationship to medication use and orthostatic hypotension. J Am Geriatr Soc. 1995;43:1141–5. doi: 10.1111/j.1532-5415.1995.tb07016.x. [DOI] [PubMed] [Google Scholar]
- 31.Calderon-Larranaga A, Poblador-Plou B, Gonzalez-Rubio F, et al. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well? Br J Gen Pract. 2012;62:e821–6. doi: 10.3399/bjgp12X659295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrischilles E, Rubenstein L, Van Gilder R, et al. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55:29–34. doi: 10.1111/j.1532-5415.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 33.Field TS, Gurwitz JH, Harrold LR, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc. 2004;52:1349–54. doi: 10.1111/j.1532-5415.2004.52367.x. [DOI] [PubMed] [Google Scholar]
- 34.Field TS, Mazor KM, Briesacher B, et al. Adverse drug events resulting from patient errors in older adults. J Am Geriatr Soc. 2007;55:271–6. doi: 10.1111/j.1532-5415.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007;5:31–9. doi: 10.1016/j.amjopharm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson TA, Flegel KM, Kramer MS, et al. Frequency, severity and risk factors for adverse drug reactions in adult out-patients: a prospective study. J Chronic Dis. 1986;39:533–42. doi: 10.1016/0021-9681(86)90198-0. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46:1517–33. doi: 10.1111/j.1475-6773.2011.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. A study of community-dwelling persons 65 years of age and older. Ann Intern Med. 1992;117:634–40. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 40.Schneider JK, Mion LC, Frengley JD. Adverse drug reactions in an elderly outpatient population. Am J Hosp Pharm. 1992;49:90–6. [PubMed] [Google Scholar]
- 41.Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901–10. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–64. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 43.Reason B, Terner M, Moses McKeag A, et al. The impact of polypharmacy on the health of Canadian seniors. Fam Pract. 2012;29:427–32. doi: 10.1093/fampra/cmr124. [DOI] [PubMed] [Google Scholar]
- 44.Veehof LJ, Stewart RE, Meyboom-de Jong B, et al. Adverse drug reactions and polypharmacy in the elderly in general practice. Eur J Clin Pharmacol. 1999;55:533–6. doi: 10.1007/s002280050669. [DOI] [PubMed] [Google Scholar]
- 45.Richardson K, Ananou A, Lafortune L, et al. Variation over time in the association between polypharmacy and mortality in the older population. Drugs Aging. 2011;28:547–60. doi: 10.2165/11592000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci. 2006;61:170–5. doi: 10.1093/gerona/61.2.170. [DOI] [PubMed] [Google Scholar]
- 47.Jensen GL, Friedmann JM, Coleman CD, et al. Screening for hospitalization and nutritional risks among community-dwelling older persons. Am J Clin Nutr. 2001;74:201–5. doi: 10.1093/ajcn/74.2.201. [DOI] [PubMed] [Google Scholar]
- 48.Jyrkka J, Enlund H, Korhonen MJ, et al. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26:1039–48. doi: 10.2165/11319530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Pozzi C, Lapi F, Mazzaglia G, et al. Is suboptimal prescribing a risk factor for poor health outcomes in community-dwelling elders? The ICARe Dicomano study. Pharmacoepidemiol Drug Saf. 2010;19:954–60. doi: 10.1002/pds.1997. [DOI] [PubMed] [Google Scholar]
- 50.Shorr RI, Ray WA, Daugherty JR, et al. INcidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681–6. [PubMed] [Google Scholar]
- 51.Hershman DL, Simonoff PA, Frishman WH, et al. Drug utilization in the old old and how it relates to self-perceived health and all-cause mortality: results from the Bronx Aging Study. J Am Geriatr Soc. 1995;43:356–60. doi: 10.1111/j.1532-5415.1995.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 52.Fu AZ, Liu GG, Christensen DB. Inappropriate medication use and health outcomes in the elderly. J Am Geriatr Soc. 2004;52:1934–9. doi: 10.1111/j.1532-5415.2004.52522.x. [DOI] [PubMed] [Google Scholar]
- 53.Gnjidic D, Hilmer SN, Blyth FM, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91:521–8. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 54.Jyrkka J, Enlund H, Lavikainen P, et al. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf. 2011;20:514–22. doi: 10.1002/pds.2116. [DOI] [PubMed] [Google Scholar]
- 55.Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health. 1989;1:470–84. [Google Scholar]
- 56.Pugh MJV, Palmer RF, Parchman ML, et al. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53:445–53. doi: 10.1159/000119460. [DOI] [PubMed] [Google Scholar]
- 57.Rosso AL, Eaton CB, Wallace R, et al. Geriatric syndromes and incident disability in older women: results from the women’s health initiative observational study. J Am Geriatr Soc. 2013;61:371–9. doi: 10.1111/jgs.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Starr JM, McGurn B, Whiteman M, et al. Life long changes in cognitive ability are associated with prescribed medications in old age. Int J Geriatr Psychiatry. 2004;19:327–32. doi: 10.1002/gps.1093. [DOI] [PubMed] [Google Scholar]
- 59.Monastero R, Palmer K, Qiu C, et al. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2007;15:60–9. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 60.Cohen I, Rogers P, Burke V, et al. Predictors of medication use, compliance and symptoms of hypotension in a community-based sample of elderly men and women. J Clin Pharm Ther. 1998;23:423–32. doi: 10.1046/j.1365-2710.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- 61.Kadam UT. Potential health impacts of multiple drug prescribing for older people: a case-control study. Br J Gen Pract. 2011;61:128–30. doi: 10.3399/bjgp11X556263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilotto A, Franceschi M, Vitale D, et al. Drug use by the elderly in general practice: effects on upper gastrointestinal symptoms. Eur J Clin Pharmacol. 2006;62:65–73. doi: 10.1007/s00228-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 63.Lai S-W, Liao K-F, Liao C-C, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore) 2010;89:295–9. doi: 10.1097/MD.0b013e3181f15efc. [DOI] [PubMed] [Google Scholar]
- 64.Lai S-W, Su L-T, Lin C-H, et al. Polypharmacy increases the risk of Parkinson’s disease in older people in Taiwan: a population-based study. Psychogeriatrics. 2011;11:150–6. doi: 10.1111/j.1479-8301.2011.00369.x. [DOI] [PubMed] [Google Scholar]
- 65.Hak E, Verheij TJ, van Essen GA, et al. Prognostic factors for influenza-associated hospitalization and death during an epidemic. Epidemiol Infect. 2001;126:261–8. doi: 10.1017/s0950268801005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai SW, Lin CH, Liao KF, et al. Association between polypharmacy and dementia in older people: a population-based case-control study in Taiwan. Geriatrics & Gerontology International. 2012;12:491–8. doi: 10.1111/j.1447-0594.2011.00800.x. [DOI] [PubMed] [Google Scholar]
- 67.Glynn RJ, Knight EL, Levin R, et al. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12:682–9. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Kroenke K, Pinholt EM. Reducing polypharmacy in the elderly. A controlled trial of physician feedback. J Am Geriatr Soc. 1990;38:31. doi: 10.1111/j.1532-5415.1990.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 69.Planton J, Edlund BJ. Strategies for reducing polypharmacy in older adults. J Gerontol Nurs. 2010;36:8–12. doi: 10.3928/00989134-20091204-03. [DOI] [PubMed] [Google Scholar]
- 70.Muir AJ, Sanders LL, Wilkinson WE, et al. Reducing medication regimen complexity: a controlled trial. J Gen Intern Med. 2001;16:77–82. doi: 10.1046/j.1525-1497.2001.016002077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bolton PG, Tipper SW, Tasker JL. Medication review by GPs reduces polypharmacy in the elderly: A quality use of medicines program. Austr J Primary Health. 2004;10:78–82. [Google Scholar]
- 72.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47:30–9. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 73.Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: Critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172–81. doi: 10.1093/gerona/62.10.1172. [DOI] [PubMed] [Google Scholar]
- 74.Cumming RG, Le Couteur DG. Benzodiazepines and risk of hip fractures in older people. CNS Drugs. 2003;17:825. doi: 10.2165/00023210-200317110-00004. [DOI] [PubMed] [Google Scholar]
- 75.Fick D, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 76.Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 77.Steinman MA, Seth Landefeld C, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516–23. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 78.Gurwitz JH. Polypharmacy: A new paradigm for quality drug therapy in the elderly? Arch Intern Med. 2004;164:1957–9. doi: 10.1001/archinte.164.18.1957. [DOI] [PubMed] [Google Scholar]
- 79.O’Hare AM, Hotchkiss JR, Kurella Tamura M, et al. Interpreting treatment effects from clinical trials in the context of real-world risk information: End-stage renal disease prevention in older adults. JAMA Intern Med. 2014;174:391–7. doi: 10.1001/jamainternmed.2013.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 81.Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. The Am J Med. 2004;116:394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 82.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–7. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 83.Tinetti ME, McAvay G, Claus E. Does Multiple risk factor reduction explain the reduction in fall rate in the Yale FICSIT trial? Am J Epidemiol. 1996;144:389–99. doi: 10.1093/oxfordjournals.aje.a008940. [DOI] [PubMed] [Google Scholar]
- 84.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648–54. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]