Abstract
OBJECTIVES
Elucidating associations of specific inflammatory biomarkers with cognitive function in African Americans (AA) and European Americans (EA) with prevalent vascular risk factors could identify vascular-mediated effects on cognitive impairment.
DESIGN
Cross-sectional analysis using Generalized Estimating Equations to account for familial clustering; standardized β-coefficients, adjusted for age, sex, and education are reported.
SETTING
A community cohort study in Jackson, MS and Rochester, MN.
PARTICIPANTS
Genetic Epidemiology Network of Arteriopathy (GENOA)-Genetics of Microangiopathic Brain Injury (GMBI) Study.
MEASUREMENTS
We examined associations between inflammation [high-sensitivity C-reactive protein (CRP), interleukin (IL)-6, soluble tumor necrosis factor receptors 1 and 2 (sTNFR1, sTNFR2)] and cognitive function measures [global (G), processing speed (PS), language (L), memory (M), and executive function (EF)] in AA and EA (N=1965; age 26–95y, 64% women, 52% AA, 75% hypertensive).
RESULTS
In AA, higher sTNFR2 was associated with poorer cognition across all domains (G: −0.11, p=.009; PS: −0.11, p<.001; L: −0.08, p=.002; M: −0.09, p=.008; EF: −0.07, p=.032); sTNFR1 was associated with poorer PS (−0.08, p<.001) and with EF (−0.08, p=.008); higher CRP was associated with lower PS (−0.04, p=.024), and higher IL6 was associated with poorer EF (−0.07, p=.019). In EA, only higher sTNFR1 was associated with poorer PS (−0.05, p=.007). We did not find support for associations between cognition and sTNFR2, CRP or IL6 in EA.
CONCLUSION
In a population with heightened vascular risk, adverse associations between inflammation and cognitive function were especially apparent in AA, primarily involving markers of TNFα activity.
Keywords: inflammation, cognition, ethnicity
INTRODUCTION
Dementia affects approximately 5 million people in the United States, with Alzheimer disease (AD) constituting 60–80% of these cases and vascular cognitive impairment (VCI) accounting for most of the remainder. Vascular disease causes VCI and also amplifies the deleterious effects of AD pathology by lowering the threshold for cognitive impairment and augmenting the trajectory of cognitive decline1–3. A growing literature suggests that inflammation may contribute to the pathophysiology of both AD and vascular dementia (VaD).4 Furthermore, studies demonstrating that inflammation improves predictive ability of lipid markers in cardiovascular disease outcomes5 provide face validity for an inflammatory-mediated role in vascular disease of the brain, similar to that of other end organs.
Associations between cognitive function and interleukin-6 (IL6),6–9 tumor necrosis factor (TNF)-α and soluble TNF receptors (sTNFR),10–12 and C-reactive protein (CRP)8, 13–17 have been reported. Inflammation may be differentially involved in VaD versus AD and among different racial or ethnic groups. For example, TNFα,18 CRP and IL615 are higher in persons with VaD compared to AD and may be important risk factors for cognitive impairment in persons with increased cardiovascular risk factors. Although African Americans (AA) may bear a disproportionate burden of dementia compared to European Americans (EA),19 relatively few studies of inflammation and cognitive function have included AA.6, 7, 13 Inflammatory biomarker levels appear to differ between AA and EA20–22 and AA may exhibit exaggerated responses to inflammatory stimuli compared to EA.23 Additionally, some20, 21, 24, 25 but not all studies26 suggest levels and actions of inflammatory markers differ between EA and AA and may contribute differentially to the pathophysiology of dementia. The purpose of this study was to examine associations of CRP, IL6, and TNFα activity with cognitive function in a biracial cohort with prevalent cardiovascular risk factors, all of whom had either hypertension or two siblings with hypertension before age 60.
METHODS
Population
The Genetic Network of Arteriopathy (GENOA) study, begun in 1995, follows a well-characterized cohort of hypertensive individuals and their siblings recruited from Jackson, Mississippi (AA only) and Rochester, Minnesota (EA only; both sites: n =3,437 individuals; 66% female, 57% AA, age 28–91y, 52% obese at baseline). At least two members of each sibship had hypertension before age 60 at enrollment. Inflammatory markers were assayed at the second examination (GENOA Visit 2, 2000–2004). Coinciding with or following Visit 2, neurocognitive testing was conducted (The Genetics of Microangiopathic Brain Injury [GMBI], 2001–2006; hereafter included with Visit 2). Participants in Visit 2 and GMBI (n=2721) included 1239 individual EA (469 full-sibling pairs) and 1482 individual AA (626 full-sibling pairs). Of these, 162 (3 EA, 159 AA) were missing all inflammatory markers; ten self-reported a history of dementia and were excluded leaving 2549 participants. Cognitive data were available in 1965 (960 EA and 1005 AA) who comprise the analysis dataset including sensitivity analyses to address potential bias of missing data. Of these, 1857 (95%) had inflammatory biomarker data and comprise the completers data set.
Inflammatory Markers
At Visit 2, fasting blood samples were centrifuged for 10 min at 4°C, aliquoted in 0.5–1 mL volumes of EDTA plasma (serum for CRP), and stored at −80°C within 2 hours of venipuncture; frozen samples were shipped to the Mayo Clinic Immunochemical Core Laboratory, Rochester, MN overnight on dry ice. CRP assays were performed using immunoturbidometric assays (Diasorin, Inc, Stillwater, MN; inter-assay imprecision 1.8–2.6%; intra-assay imprecision 1.0–9.2%) and multiplex assays (SearchLight™, Pierce, Boston, MA) were used for IL6 and TNFsRs. TNF soluble receptor fractions show stability over time with longer half-lives than TNFα levels and have been validated as sensitive indicators of TNFα system activation.27, 28 Precision of the assays performed by SearchLightTM was retrospectively determined based on data derived from a blinded, internal plasma control sample. Algorithms were developed to reduce plate-to-plate variations in protein levels and all analyses used these normalized data.29
Cognitive Testing
Neurocognitive tests were offered to all participants in the same sequence using standardized protocols to assess global mental status, memory, language, processing speed, and executive function. All scores were ordered so that higher values reflect better cognition, and standardized coefficients were used to allow comparisons across measures.
Global Cognitive Function
Mini-Mental State Examination (MMSE, range 0 [worst] to 30 [best])30 was administered per protocol consistent with the Consortium for the Establishment of a Registry for Alzheimer’s Disease (CERAD) battery.31, 32
Processing Speed
Wechsler Adult Intelligence Scale Revised (WAIS-R) Digit Symbol Substitution Task (DSST) tests complex visual attention, sustained and focused concentration, response speed, and visuomotor coordination; Trail Making Test A (TMT-A) measures visual conceptual and visuomotor tracking, attention, sequencing, mental flexibility, visual search and motor function (nearest 0.01 second, maximum 4 minutes).32 Because higher (slower) times indicate poorer performance, for analyses, times were multiplied by -1 so that higher numbers represent better performance.
Memory
The Rey Auditory Verbal Learning Test (RAVLT, scored 0–15) assesses learning and memory utilizing multiple learning trials and a 30-minute delayed recall of 15 items on a list.32 The WAIS-III Incidental Learning Task allows continuation of the DSST until the third row of the test has been completed.33 After a 5-minute delay, the symbol pairs with free-recall32 is presented again.
Language
The FAS measures letter fluency; the participant must spontaneously produce words beginning with a specific letter (F, A, S) within 60 seconds.32 The Animal Naming Task measures category (animals) fluency.31
Executive Function
The TMT-B assesses attention, sequencing, mental flexibility, visual search and motor function using time and error counts.32 Times were multiplied by -1 so that higher scores represent better function.
Composite Cognitive Domain Measures
Composite measures for processing speed, memory, and language domains were constructed from two tests within each domain to reduce measurement error, floor and ceiling effects of individual tests.34–36 A standardized z-score was created for each measure, and then z-scores were averaged within a domain to create the composite.34–36 A factor-analytic combination method for constructing the domain scores yielded similar associations between inflammation and cognitive measures. (results available on request.)
Covariates
Blood pressure, measured three times in a seated, rested state with appropriately sized cuffs, was defined as the average of the 2nd and 3rd measurements. Hypertension was defined as a blood pressure >140/90, self-report, or anti-hypertensive medication use. Diabetes was defined as fasting glucose ≥126 mg/dl or random ≥200 mg/dl, self-report, or hypoglycemic medication use. Anti-anxiety, anti-depressant, hypnotic, sleep aids (OTC and prescription), and narcotic medications taken in the previous two weeks were classified as medications with potential to affect cognition. Never-smoker was defined as having never smoked more than 100 cigarettes. Height was measured by stadiometer and weight by electronic balance with participants wearing lightweight clothes. Body mass index (BMI) was calculated as weight (kilograms)/height2 (meters2).
Statistical Analysis
Associations between inflammatory markers and each cognitive domain were estimated using linear models fit with Generalized Estimating Equations (GEE) to account for familial clustering and Huber-White robust standard error estimates. Because inflammatory markers and cognitive function scores all use different measurement units, standardized outcomes and predictors were modeled to facilitate examinations across models. Thus, a beta coefficient of −0.5 is interpreted as a 0.5 standard deviation (SD) decrease in the cognitive score outcome being associated with a 1 SD increase in the inflammatory marker. Diagnostic lowess smoothers revealed linear relationships on the natural-scale, inflammatory markers were only mildly skewed, and estimates were resistant to any extreme value effects (see Figure 1), hence we present associations with standardized, non-log-transformed inflammatory markers. Primary adjusted models included age, education, and sex, and accounted for familial clustering. “Extended adjusted” models additionally included diabetes, hypertension, BMI, smoking, stroke history, alcohol, lipid-lowering medications, and CNS meds. Differences by race were examined using interaction terms, while acknowledging that race and site are aliased by design. We present results stratified by AA/EA as shorthand for AA (MS)/EA (MN) race(site) groups.
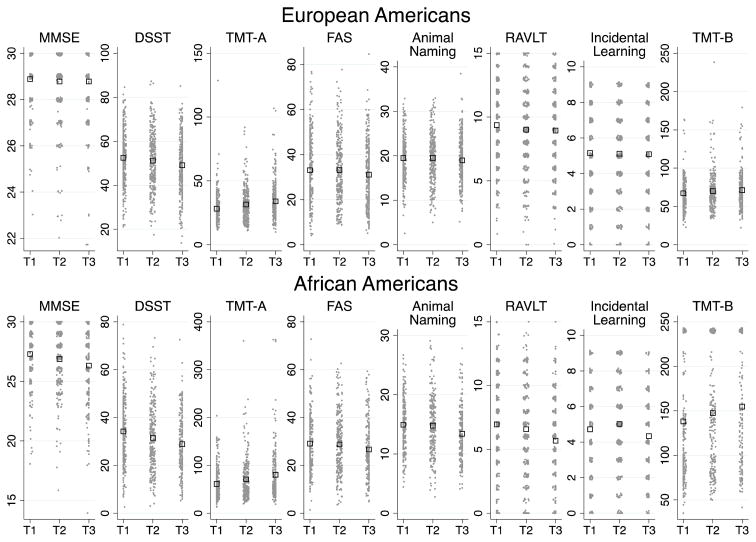
Figure 1. Raw cognitive score means across race-stratifieds TNFR2 inflammatory tertiles. Open squares indicate raw cognitive score means with individual, raw cognitive scores displayed as points within each tertile.
sTNFR2=soluble tumor necrosis factor receptor 2; MMSE=Mini-Mental State Examination; DSST=Digit Symbol Substitution Task; TMT-A=Trail Making Test A; RAVLT=Rey Auditory Verbal Learning Test; Incidental Learning - Wechsler Adult Intelligence Scale -III Incidental Learning Task; TMT-B - Trails Making Test-B; T1=Tertile 1; T2=Tertile 2; T3=Tertile 3
We evaluated characteristics of participants who had and who were missing cognitive data by race and conducted sensitivity analyses using weighted GEE to examine the robustness of findings after accounting for missing cognitive data. Analyses were performed using STATA 12 (College Station, TX).
RESULTS
Participant characteristics are shown for those with inflammatory marker data, stratified by race and presence/absence of cognitive data (Table 1). AA were older, had a higher proportion of women, diabetics, hypertensives, fewer never-smokers and higher mean BMI. In AA, CRP and IL6 were higher, sTNFR1 was lower, and sTNFR2 was similar to EA. MMSE scores ranged from 14 to 30, mean 27.9 (2.3). Fourteen AA (2.0%) and eight EA (1.1%) met race-specific criteria for cognitive impairment.37
Table 1.
Participant Characteristics by Race and Cognitive Data Status Reported as Mean (±SD) for Continuous or Frequency (%) for Categorical Variables
| Characteristic | European Americans | African Americans | ||
|---|---|---|---|---|
| With Cognitive Data (n=960) | Missing Cognitive Data (n=272) | With Cognitive Data (n=1005) | Missing Cognitive Data (n=474) | |
| Age, years | 59.2 (10.0) | 57.6 (10.9) | 62.9 (8.7) | 63.3 (10.9) |
| Women (%) | 565 (59) | 135 (50) | 707 (70) | 341 (72) |
| BMI (kg/m2) | 30.5 (6.0) | 31.6 (7.4) | 31.3 (6.3) | 32.4 (7.6) |
| Hypertension (%) | 706 (74) | 191 (70) | 789 (79) | 385 (81) |
| Diabetes (%) | 131 (14) | 51 (19) | 270 (27) | 164 (35) |
| Never-smoked (%) | 466 (49) | 142 (52) | 398 (40) | 196 (41) |
| Education (%) | ||||
| <12 years | 51 (7) | 15 (7) | 301 (36) | 213 (52) |
| 12 years | 402 (52) | 116 (53) | 279 (33) | 112 (28) |
| Some college | 139 (18) | 38 (17) | 18 (2) | 7 (2) |
| ≥College degree | 182 (23) | 51 (23) | 246 (29) | 75 (18) |
| sTNFR1 (pg/mL) | 1362 (681) | 1480 (947) | 1171 (622) | 1318 (754) |
| sTNFR2 (pg/mL) | 1953 (797) | 1932 (884) | 1878 (811) | 2065(976) |
| CRP (SI) | 40.4 (48.0) | 39.5 (48.4) | 53.9 (61.2) | 60.9 (63.6) |
| IL6 (SI) | 1.11 (0.87) | 1.23 (0.97) | 1.23 (0.87) | 1.35 (0.91) |
BMI=body mass index; sTNFR=soluble tumor necrosis factor receptor; CRP=high sensitivity C-reactive protein; IL6=interleukin-6
All cognitive data were missing in 271 (22%) EA and 421 (32%) AA. Among EA, missing data was associated with being younger (p=0.017), male sex (p=0.008), and diabetes (p=0.042). AA participants who were missing cognitive data had higher BMI (p=0.03), more diabetes (p=0.003), lower education level (p<0.001), and higher levels of all inflammatory markers: sTNFR1 (p=0.005), sTNFR2 (p=0.006), CRP (p=0.012), and IL6 (p=0.006) than AA with cognitive data (Table 1).
Mean race-stratified cognitive scores by inflammatory tertiles differed in AA compared to EA (Figure 1). For example, in AA, all cognitive measures were worse across tertiles of sTNFR2. In EA, only processing speed (p<0.001) and executive function (p=0.026) were associated with sTNFR2 (Table 2, Figure 1). In AA, sTNFR1 was also associated with cognitive domains (all p<.05), except for memory, while processing speed (p<.001), language (p=.011), EF (p<.001), and marginally memory (p=.097) in EA was associated with sTNFR1. CRP was associated with memory in EA (p=.043), but no associations were observed between cognition and CRP or IL6 in AA. IL6 was associated with PS (p<.001), language (p<.002), and EF (p=.034) in EA (Table 2, Figure 1).
Table 2.
Means of Cognitive Outcomes by Race-stratified sTNFR2 Tertiles
| Cognitive Outcomes | European Americans Mean (±SD) |
African Americans Mean (±SD) |
||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Tertile 1 [252 – 1554) | Tertile 2 [1544 – 2084) | Tertile 3 [2084 – 6290) | p-value* | Tertile 1 [286 – 1495) | Tertile 2 [1495 – 1995) | Tertile 3 [1995 – 6290) | p-value* | |
|
| ||||||||
| MMSE (0–30 points) | 28.9 (1.34) | 28.8 (1.58) | 28.8 (1.49) | 0.847 | 27.3 (2.30) | 26.9 (2.49) | 26.3 (2.87) | <0.001 |
| DSST (0–93) | 52.5 (12.1) | 51.2 (13.0) | 49.1 (12.8) | 0.006 | 34.2 (13.6) | 31.5 (13.4) | 28.8 (12.9) | <0.001 |
| TMT-A (seconds) | 28.2 (10.8) | 31.5 (12.7) | 34.0 (14.2) | <0.001 | 61.0 (31.1) | 70.9 (43.2) | 80.3 (54.4) | <0.001 |
| FAS (Number of words) | 33.1 (14.2) | 33.1 (14.1) | 31.1 (13.2) | 0.171 | 29.1 (11.5) | 28.7 (12.3) | 26.6 (11.7) | 0.052 |
| Animal Naming (Number of animals) | 19.5 (4.82) | 19.5 (5.41) | 19.0 (4.78) | 0.218 | 14.9 (4.30) | 14.8 (4.81) | 13.4 (4.23) | <0.001 |
| RAVLT (0–15) | 9.37 (3.11) | 9.01 (3.43) | 8.94 (3.29) | 0.254 | 6.95 (3.48) | 6.58 (3.58) | 5.68 (3.34) | <0.001 |
| Incidental Learning (0–93) | 5.15 (2.42) | 5.12 (2.48) | 5.09 (2.45) | 0.948 | 4.72 (2.52) | 5.02 (2.63) | 4.33 (2.46) | 0.023 |
| TMT-B (seconds) | 67.2 (20.3) | 70.0 (24.6) | 71.2 (22.2) | 0.121 | 138.1 (66.5) | 147.4 (65.3) | 154.7 (68.5) | 0.041 |
p-value for trends
sTNFR=soluble tumor necrosis factor receptor; MMSE=Mini-Mental State Examination; DSST=Digit Symbol Substitution Test; TMT-A=Trails Making Test-A; RAVLT=Rey Auditory Verbal Learning Test; Incidental Learning - Wechsler Adult Intelligence Scale -III Incidental Learning Task; TMT-B - Trails Making Test-B
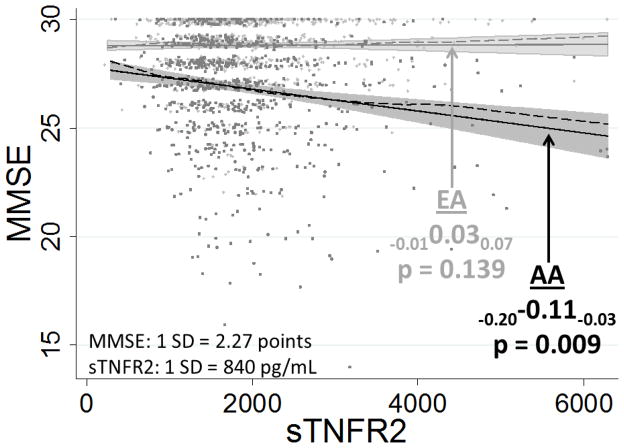
In adjusted models, every 1 SD increase in sTNFR2 was associated with a 0.11 SD lower MMSE score in AA (β=−0.11, (95% confidence interval (CI): −0.20, −0.03) p=0.009). The data did not support a similar association in EA (0.03 (−0.01, 0.07), 0.140); the interaction term supported a differential relationship by race/site (−0.14 (−0.24,−0.05) p=0.003, Figure 2, Table 3). Similar to the MMSE outcome, data supported inverse associations between sTNFR2 and all other cognitive domains for AA but not EA. (Table 3, eFigure) Each SD increase in sTNFR2 in AA was associated with poorer performance in processing speed (−0.11 (−0.16, −0.07), p<0.001), language (−0.08 (−0.13, −0.03), p=0.002), memory (−0.09 (−0.16, −0.02), p=0.008), and executive function (−0.07 (−0.13, −0.01), p=0.032). The inferences were the same in extended-adjusted models (data available on request).
Figure 2. Differential associations of sTNFR2 on Mini-Mental State Examination (MMSE) in African Americans (AA: dark gray) and European Americans (EA: light gray) from adjusted models. Solid lines are regression lines with shaded confidence bounds.
Dashed lines are LOWESS nonlinear smoothers (diagnostic check)
Race specific standard deviations (SDs), and standardized beta coefficients, are shown with subscripted lower and upper 95% confidence limits (lower confidence limit “LCL” and upper confidence limit “UCL”), displayed as LCLβUCL.
sTNFR=soluble tumor necrosis factor receptor
Table 3.
Relationships between Standardized Cognitive Domains and Standardized Inflammatory Markers in European Americans and African Americans
| Inflammatory Marker | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cognitive Domain |
sTNFR1 | sTNFR2 | CRP | IL6 | ||||
| European Americans |
African Americans |
European Americans |
African Americans |
European Americans |
African Americans |
European Americans |
African Americans |
|
| Global | 0.01 p=0.685 (−0.03,0.05) | −0.07 p=0.092 (−0.16,0.01) | 0.03 p=0.139 (−0.01,0.07) | −0.11 p=0.009 (−0.20,−0.03) | 0.02 p=0.329 (−0.02,0.06) | 0.01 p=0.713 (−0.05,0.08) | 0.03 p=0.219 (−0.02,0.07) | −0.00 p=0.946 (−0.09,0.09) |
| PS | −0.05 p=0.007 (−0.08,−0.01) | −0.08 p<0.001 (−0.12,−0.04) | −0.03 p=0.079 (−0.07,0.00) | −0.11 p<0.001 (−0.16,−0.07) | −0.03 p=0.109 (−0.08,0.01) | −0.04 p=0.024 (−0.07,−0.01) | −0.01 p=0.464 (−0.05,0.03) | −0.03 p=0.199 (−0.07,0.02) |
| Language | −0.03 p=0.356 (−0.08,0.03) | −0.04 p=0.074 (−0.09,0.00) | −0.02 p=0.560 (−0.07,0.04) | −0.08 p=0.002 (−0.13,−0.03) | −0.03 p=0.361 (−0.08,0.03) | −0.03 p=0.118 (−0.06,0.01) | −0.03 p=0.265 (−0.09,0.02) | −0.02 p=0.334 (−0.07,0.02) |
| Memory | −0.02 p=0.566 (−0.09,0.05) | 0.01 p=0.781 (−0.06,0.09) | 0.02 p=0.534 (−0.04,0.08) | −0.09 p=0.008 (−0.16,−0.02) | −0.01 p=0.676 (−0.06,0.04) | −0.02 p=0.459 (−0.07,0.03) | −0.02 p=0.379 (−0.08,0.03) | −0.02 p=0.643 (−0.08,0.05) |
| Executive Function | −0.01 p=0.635 (−0.05,0.03) | −0.08 p=0.008 (−0.14,−0.02) | −0.01 p=0.552 (−0.05,0.03) | −0.07 p=0.032 (−0.13,−0.01) | −0.02 p=0.245 (−0.06,0.02) | −0.03 p=0.230 (−0.08,0.02) | 0.02 p=0.363 (−0.02,0.06) | −0.07 p=0.019 (−0.12,−0.01) |
Adjusted for age, sex, education, and accounting for familial clustering
95% CI= 95% confidence interval
sTNFR=soluble tumor necrosis factor receptor; CRP=high sensitivity C-reactive protein; IL6=interleukin-6; Global=Global Cognition; PS=Processing Speed
Higher sTNFR1 in AA was associated with poorer processing speed (−0.08, 95%CI −0.12, −0.04, p<0.001) and executive function in adjusted models (eFigure, Table 3: −0.08 (−0.14, −0.02), p=0.008). The associations between sTNFR1 and MMSE or language in AA were similar in magnitude but did not reach statistical significance (MMSE: −0.07 (−0.16, 0.01), p=0.092; language: −0.04 (−0.09, 0.00), p=0.074). In AA, higher CRP was associated with poorer processing speed (−0.04 (−0.070, −0.010) p=0.020), and higher IL6 was associated with poorer executive function (−0.07, (−0.12, −0.01), p=0.019). (Table 3, eFigure).
In EA, sTNFR1 was only associated with processing speed, −0.05 (−0.08, −0.01) p=0.007; sTNFR2, IL6, and CRP were not statistically associated with any cognitive domain (eFigure, Table 3).
Sensitivity analyses using weighted GEE suggested that the reported results comparably or more conservatively estimate associations between inflammatory markers and cognitive function (eTable).
DISCUSSION
In a biracial cohort enriched with hypertension and among whom substantial proportions had other risk factors for cardiovascular disease, inflammatory markers were differentially associated with cognitive function in AA and EA. In AA, a biomarker of TNFα activity was associated with five domains of cognitive function, whereas IL6 and CRP were associated only with executive function and processing speed respectively; in EA, only markers of TNFα activity were associated with a single cognitive domain, processing speed. Although the cohort overall may be considered at high risk of cardiovascular disease, cardiovascular risk factors were also more prevalent in the AA than EA. These findings suggest that, in these young to old AA with hypertension or a strong family history of hypertension and prevalent cardiovascular risk factors, TNFα activity, and perhaps IL6 and CRP, may be important risk factors for cognitive dysfunction.
One explanation for these findings in this hypertensive-enriched population could involve inflammatory-mediated cerebrovascular disease. Cardiovascular risk factors, inflammation,5, 7, 13, 38, 39 brain structure abnormalities40 and poorer cognitive function,41, 42 particularly executive function41 and processing speed41 are interrelated. Mechanisms linking blood pressure to cognition are especially relevant for this study population and have been classified as functional (e.g. endothelial dysfunction/vascular dysregulation; altered blood flow including nocturnal dipping patterns; reduced amyloid clearance), structural (e.g. white matter hyperintensities, atrophy), pharmacologic (related to renin-angiotensin system), stroke-related, and other (including hypertension with insulin resistance/impaired insulin signaling centrally).43 AA are disproportionately burdened by cardiovascular disease44 and may exhibit heightened responses and greater endothelial dysfunction in response to inflammatory stimuli in vasculature, specifically TNFα pathways.23 Furthermore, TNFα upregulation has been observed in hypertensive patients,45 and more than 70% of this cohort was hypertensive while the remainder had at least two siblings with hypertension before age 60. Thus, inflammation potentially mediates cognitive decline through arteriosclerotic disease in the brain but may also adversely affect cognition through direct effects of inflammation on synaptic plasticity, neurogenesis, and neuromodulation that affect cognition. 46 However, elevated inflammatory biomarkers can occur for a number of reasons, and mechanisms explaining the link between inflammation and cognition in this cross-sectional cohort study could not be elucidated. However, these findings can be considered hypothesis generating in a hypertension-enriched sample of African American subjects.
These findings further complement and expand upon other studies linking inflammation and cognition4, 6–10, 13–18, 47 by reporting findings from a relatively large AA cohort across a broad age range. Studies in older adults demonstrate associations between higher TNFα activity and poorer cognitive function,10 higher TNFα activity in VaD patients compared to AD patients,18 and in either VaD or AD patients compared to controls.18, 48 In this young-to-old, cognitively unimpaired cohort, we observed associations between higher inflammation and poorer cognitive function in AA. These findings are supported by a longitudinal Swedish study that showed higher baseline sTNFR1 and sTNFR2 levels in persons with mild cognitive impairment who converted to dementia compared to both those with mild cognitive impairment who remained stable or to controls.12 A relationship between TNFα and executive function decline, but not other cognitive domains, was observed in the largely EA Framingham Offspring cohort.10 We observed associations with processing speed only in the GENOA EA. Differences in the cohorts’ prevalent cardiovascular risk factors, with the GENOA cohort having a greater burden, might explain some of the inconsistency. In addition, our analysis was cross-sectional and Framingham Offspring study was longitudinal.
Both Framingham Offspring and the GENOA cohorts are younger than many studies of inflammation and cognition. Findings in these younger cohorts are of particular interest since interventions to halt or delay cognitive decline may be more effective in earlier stages. It would also be of interest to see if interventions that target the conditions that cause inflammation are more successful than interventions that target existing inflammation.
Although the individual estimates of associations between standardized inflammatory biomarkers and standardized cognitive measures may appear small, across the sTNFR2 range, there was an average 2-point difference in MMSE, which is clinically meaningful. To additionally put the sTNFR2 effect into context, an increase in one standard deviation of sTNFR2 (β=−0.11) would be similar to a 5-year difference in age (age β=−0.02 per year); across the range of sTNFR2 from 252μg to 2690μg, this would be similar to 20 years of aging.
Some limitations warrant further discussion. We acknowledge that the differential results by race could be due to regional differences in the AA and EA populations, since study sites were race specific. Regardless, the relationships we report are of interest. In addition to potential race and site effects, the higher risk profile in AA (e.g. older age, more obesity, hypertension and diabetes) could contribute to differential findings. This explanation is of particular interest as it potentially identifies mechanisms that may be more relevant in populations with prevalent cardiovascular risk factors. Additionally, this study is limited to four inflammatory markers: CRP, sTNFR1 and 2, and IL6 even though other pro-inflammatory markers have been associated with cognition, including other interleukins and serum amyloid A.49, 50 However, the biomarkers in the current study are among those with biologic plausibility and some evidence in other studies, although mostly EA, that these biomarkers may be important in vascular and non-vascular cognitive impairment. We also acknowledge the non-specificity of inflammatory biomarkers and the influence of other conditions that cause increased inflammation could not be excluded. However, several comorbidities associated with inflammation, including cardiovascular disease and diabetes were included as covariates. In addition to adjusting for other potential confounders in parsimonious and extended adjusted models, we included standard approaches of stratification and conducted sensitivity analyses for potential informative missingness effects. The cognitive measures may not detect early decline; despite this, significant relationships were observed in this population. However, additional limitations include the cross-sectional design, limiting inferences of causality. Volunteer bias may limit generalizability to dissimilar populations, but the findings reported are among a few reporting such relationships in AA. Longitudinal studies could address some limitations by assessing temporal associations between inflammation and cognitive decline.
CONCLUSION
Inflammation is increasingly recognized as an important contributor to numerous health outcomes. Deleterious effects on cognitive function may be especially apparent in AA with heightened vascular risk. The associations between inflammation and decreased cognitive function across a broad age spectrum in cognitively unimpaired AA with vascular risk factors require further study to ascertain pathways through which inflammation, specifically TNFα activity, may erode cognitive function. In addition, studies targeting modifiable vascular risk factors and effects on inflammation and cognitive function in at-risk populations are needed.
Supplementary Material
Associations of standardized inflammatory markers with cognitive domains, adjusted for age, sex, education, and accounting for familial clustering. Regression coefficients with lower and upper confidence limits are shown. Results supported statistically are displayed with black lines versus gray lines.
sTNFR=soluble tumor necrosis factor receptor; CRP=high sensitivity C-reactive protein; IL6=interleukin 6; MMSE=Mini-Mental State Examination; PS=Processing Speed; EF=Executive Function
Acknowledgments
Funding Sources: This work was supported by NIH grants U01-HL054463, R01-HL87660, HL-81331, M01 RR00585.
Sponsor’s Role: Sponsor played no role in the design, methods, subject recruitment, data collections, analysis or preparation of paper.
Footnotes
Related Paper Presentations: American College of Physicians Abstract Day Poster Winner, American Heart Association Poster Presentation
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Concept and design (B. Gwen Windham, Michael Griswold, Thomas Mosley), acquisition of subjects/data (Thomas Mosley, Iftikhar Kullo, Patricia Peyser, Lawrence Bielak, Stephen Turner); analysis/interpretation of data (Seth Lirette, Brittany N. Simpson, Michael Griswold, B. Gwen Windham, Thomas Mosley, John Bridges); preparation of manuscript (B. Gwen Windham, Brittany Simpson, Michael Griswold, John Bridges, Iftikhar Kullo, Patricia Peyser, Seth Lirette, Thomas Mosley, Lawrence Bielak, Stephen Turner). All authors contributed significantly to the work in concept, design, data analysis/interpretation or preparation of the manuscript, have made critical reviews and revisions of the manuscript and have given written consent for manuscript publication.
References
- 1.Román GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13:S115–S123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 3.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H, Arai T, Kondo H, et al. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord. 2000;14 (Suppl 1):S47–53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, et al. Comparison of c-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Jenny NS, French B, Arnold AM, et al. Long-term assessment of inflammation and healthy aging in late life: The Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright CB, Sacco RL, Rundek TR, et al. Interleukin-6 is associated with cognitive function: The Northern Manhattan Study. J Stroke Cerebrovasc Dis. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 9.Helmy AA, Abdel Naseer MM, El Shafie S, et al. Role of interleukin 6 and alpha-globulins in differentiating Alzheimer and vascular dementias. Neurodegener Dis. 2012;9:81–86. doi: 10.1159/000329568. [DOI] [PubMed] [Google Scholar]
- 10.Jefferson AL, Massaro JM, Beiser AS, et al. Inflammatory markers and neuropsychological functioning: The Framingham Heart Study. Neuroepidemiology. 2011;37:21–30. doi: 10.1159/000328864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trollor JN, Smith E, Baune BT, et al. Systemic inflammation is associated with MCI and its subtypes: The Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30:569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- 12.Buchhave P, Zetterberg H, Blennow K, et al. Soluble TNF receptors are associated with abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2010;31:1877–1884. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 14.Laurin D, David Curb J, Masaki KH, et al. Midlife c-reactive protein and risk of cognitive decline: A 31-year follow-up. Neurobiol Aging. 2009;30:1724–1727. doi: 10.1016/j.neurobiolaging.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28:1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R, Schmidt H, Curb JD, et al. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 17.Marioni RE, Stewart MC, Murray GD, et al. Peripheral levels of fibrinogen, c-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosom Med. 2009;71:901–906. doi: 10.1097/PSY.0b013e3181b1e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuliani G, Ranzini M, Guerra G, et al. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. J Psychiatr Res. 2007;41:686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 20.Fisher G, Hyatt TC, Hunter GR, et al. Markers of inflammation and fat distribution following weight loss in African-American and white women. Obesity (Silver Spring) 2012;20:715–720. doi: 10.1038/oby.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll JF, Fulda KG, Chiapa AL, et al. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity. 2009;17:1420–1427. doi: 10.1038/oby.2008.657. [DOI] [PubMed] [Google Scholar]
- 22.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of c-reactive protein levels: A systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MD, Feairheller DL, Thakkar S, et al. Racial differences in tumor necrosis factor-alpha-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folsom AR, Aleksic N, Catellier D, et al. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 25.Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: The Mayo Clinic Proteomic Markers of Arteriosclerosis Study. PLoS ONE. 2010;5:e9065. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heald AH, Anderson SG, Ivison F, et al. C-reactive protein and the insulin-like growth factor (IGF)-system in relation to risk of cardiovascular disease in different ethnic groups. Atherosclerosis. 2003;170:79–86. doi: 10.1016/s0021-9150(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 27.Aderka D, Engelmann H, Shemer-Avni Y, et al. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine Cytokine Res. 1992;11:157–159. [PubMed] [Google Scholar]
- 28.Aderka D, Sorkine P, Abu-Abid S, et al. Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic tnf leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J Clin Invest. 1998;101:650–659. doi: 10.1172/JCI694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellington AA, Kullo IJ, Bailey KR, et al. Measurement and quality control issues in multiplex protein assays: A case study. Clin Chem. 2009;55:1092–1099. doi: 10.1373/clinchem.2008.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (cerad). Part i. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 32.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press; 1998. [Google Scholar]
- 33.Kaplan E, Fein D, Morris R, et al. Wais-r ni manual. San Antonio, Texas: The Psychological Corporation; 1991. [Google Scholar]
- 34.Chibnik LB, Shulman JM, Leurgans SE, et al. Cr1 is associated with amyloid plaque burden and age-related cognitive decline. Ann Neurol. 2011;69:560–569. doi: 10.1002/ana.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massaro JM, D’Agostino RB, Sr, Sullivan LM, et al. Managing and analysing data from a large-scale study on Framingham offspring relating brain structure to cognitive function. Stat Med. 2004;23:351–367. doi: 10.1002/sim.1743. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 37.Bohnstedt M, Fox PJ, Kohatsu ND. Correlates of mini-mental status examination scores among elderly demented patients: The influence of race-ethnicity. J Clin Epidemiol. 1994;47:1381–1387. doi: 10.1016/0895-4356(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 38.Fornage M, Chiang YA, O’Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: The Cardiovascular Health Study. Stroke. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright CB, Moon Y, Paik MC, et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40:3466–3471. doi: 10.1161/STROKEAHA.109.559567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 41.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Gorelick PB, Nyenhuis D, et al. American Society of Hypertension Writing G. Blood pressure and treatment of persons with hypertension as it relates to cognitive outcomes including executive function. J Am Soc Hyperten. 2012;6:309–315. doi: 10.1016/j.jash.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorffel Y, Latsch C, Stuhlmuller B, et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 46.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 47.van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: Results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarkowski E, Blennow K, Wallin A, et al. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19:223–230. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- 49.Jordanova V, Stewart R, Davies E, et al. Markers of inflammation and cognitive decline in an African-Caribbean population. Int J Geriatr Psychiatry. 2007;22:966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- 50.van den Biggelaar AHJ, Gussekloo J, de Craen AJM, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations of standardized inflammatory markers with cognitive domains, adjusted for age, sex, education, and accounting for familial clustering. Regression coefficients with lower and upper confidence limits are shown. Results supported statistically are displayed with black lines versus gray lines.
sTNFR=soluble tumor necrosis factor receptor; CRP=high sensitivity C-reactive protein; IL6=interleukin 6; MMSE=Mini-Mental State Examination; PS=Processing Speed; EF=Executive Function