Abstract
The development of selective reactions that utilize easily available and abundant precursors for the efficient synthesis of amines is a longstanding goal of chemical research. Despite the centrality of amines in a number of important research areas, including medicinal chemistry, total synthesis and materials science, a general, selective, and step-efficient synthesis of amines is still needed. In this work we describe a set of mild catalytic conditions utilizing a single copper-based catalyst that enables the direct preparation of three distinct and important amine classes (enamines, α-chiral branched alkylamines, and linear alkylamines) from readily available alkyne starting materials with high levels of chemo-, regio-, and stereoselectivity. This methodology was applied to the asymmetric synthesis of rivastigmine and the formal synthesis of several other pharmaceutical agents, including duloxetine, atomoxetine, fluoxetine, and tolterodine.
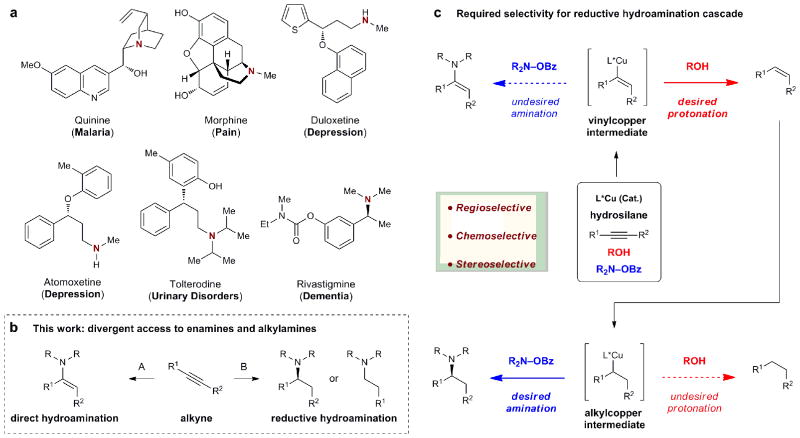
Complex organic molecules play a crucial role in the study and treatment of disease. The extent to which they can be utilized in these endeavors depends on the efficient and selective chemical methods for their construction1. Amines are widely represented in biologically active natural products and medicines2 (a small selection of which are shown in Fig. 1a). Consequently, the selective assembly of amines from readily available precursors is a prominent objective in chemical research3. There are a number of powerful methods that address this challenge including metal-catalyzed cross-coupling4,5, nucleophilic addition to imines6, C–H nitrogen insertion7, and enzymatic methods8,9. However, the direct production of amines from simple olefins or alkynes represents a highly attractive alternative, given the abundance and accessibility of these starting materials. For this reason, the addition of nitrogen and hydrogen across carbon–carbon multiple bonds (hydroamination) has long been pursued as a means to access amines10-12. While much progress has been made, a generally effective strategy to achieve chemo-, regio-, and enantioselective hydroamination of simple alkenes or alkynes remains elusive.
Figure 1. Bioactive amines, the synthesis of amines from alkynes, and the reductive hydroamination cascade strategy.
a, Representative alkaloids and drugs demonstrating the ubiquitous nature of amines in bioactive organic molecules. b, Catalytic hydroamination of alkynes to generate three product classes. c. Contrasting reactivities of vinyl- and alkylcopper intermediates required to achieve a reductive hydroamination cascade.
We became interested in developing hydroamination reactions of alkynes as a convenient and powerful means of accessing aminated products (Fig. 1b). Reactions that employ alkynes as starting materials are synthetically versatile, since alkynes can be prepared by a variety of strategies, including Sonogashira coupling13, nucleophilic addition of metal acetylides14, and homologation of carbonyl groups15. In addition, one or both π-bonds of alkynes may be utilized, further increasing their flexibility as starting materials. For these reasons, the hydrofunctionalization of alkynes has recently become an active area of research16-22. We23 as well as Hirano and Miura24 recently detailed catalyst systems for the asymmetric hydroamination of styrenes that operate through addition of a catalytic copper hydride species25-32 to a carbon–carbon double bond followed by carbon-nitrogen bond formation using an electrophilic nitrogen source33-36. We surmised that we could apply this approach to the selective functionalization of alkynes wherein alkyne hydrocupration would give a stereodefined vinylcopper intermediate. We anticipated that, in analogy to our previous work, direct interception of this intermediate would potentially enable the stereoselective formation of enamines (Fig. 1b, A). Enamines are versatile intermediates in organic synthesis and while catalytic methods have been developed for their synthesis by alkyne hydroamination, control of the regio- and stereochemistry of enamine formation is nontrivial16.
In addition to enamine synthesis, we speculated on the possibility that conditions could be developed to convert alkynes to enantioenriched α-branched alkylamines and/or linear alkylamines in one synthetic operation (Fig. 1b, B). Such cascade processes are highly desirable in organic synthesis, since potentially difficult workup and isolation steps can be avoided, and the generation of chemical waste is minimized37. In particular, we envisioned a scenario in which the starting alkyne is initially reduced to a transiently-formed alkene, which would then undergo hydroamination to afford the alkylamine product. If successful, this approach would be particularly attractive due to the ease and low cost of the Sonogashira process for the preparation of alkyne starting materials relative to the cross coupling of stereodefined vinylmetal reagents or other routes used to access geometrically pure alkenes for hydroamination. We were aware that, mechanistically, the vinyl- and alkylcopper intermediates in the proposed process are required to react in a highly chemoselective manner (Fig. 1c). Specifically, the vinylcopper species formed upon hydrocupration of the alkyne would need to be selectively intercepted by the proton source in the presence of the aminating reagent to furnish the intermediate alkene while the alkylcopper species formed upon hydrocupration of this alkene would need to selectively engage the electrophilic nitrogen source in the presence of a proton donor to ultimately furnish the desired alkylamine product. While both steps (i.e., alkyne semireduction38-40 and alkene hydroamination23) are well precedented, the ability to achieve the desired selectivity in one pot via a cascade sequence has never been demonstrated41,42. Herein, we report mild and scalable conditions for the highly chemo-, regio-, and stereoselective synthesis of enamines (“direct hydroamination”) and alkylamines (“reductive hydroamination”) products from alkynes, employing a single copper catalyst system.
Results and discussion
Direct hydroamination: development and scope
To assess the feasibility of the outlined alkyne hydroamination (Fig. 1b, A), we treated 1,2-diphenylacetylene (1a) with N,N-dibenzyl-O-benzoylhydroxylamine (2a, 1.2 equiv.) and an excess of diethoxymethylsilane (3) in the presence of 2 mol % copper acetate and a range of phosphine ligands. A number of ligands could be used to perform the direct hydroamination reaction, and the resulting enamine 4a was efficiently produced as a single geometric isomer, as determined by 1H NMR analysis (Table 1, entries 1–4). Although copper catalysts based on 2,2′-bis(diphenylphosphino)-1,1′-binaphthalene (BINAP, L1), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos, L2) or 4,4′-bi-1,3-benzodioxole-5,5′-diylbis(diphenylphosphane) (SEGPHOS, L3) were effective in this context, the catalyst based on 5,5′-bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole (DTBM-SEGPHOS, L4) was found to be the most efficient and generally applicable. We then evaluated the substrate scope of this reaction and, as shown in entries 5–9, a diverse range of aryl-substituted internal alkyne substrates could be converted to the corresponding (E)-enamines 4 with complete stereoselectivity (4b–4e; 80–99%). Notably, sterically hindered amines, which were problematic substrates for previously reported hydroamination reactions of alkynes43, could be successfully transformed using the current conditions (4b and 4d). More importantly, direct hydroamination of unsymmetrical internal alkynes occurred with excellent regioselectivity (4c–4e; >19:1). In addition, we found that a 1,2-dialkylacetylene was left intact under these conditions (4e) and pharmaceutically important heterocycles, including morpholine (4c), thiophene (4d), piperidine (4e), and pyrimidine (4e) were well-tolerated. While the direct hydroamination of terminal alkynes to construct monosubstituted enamines was not successful, the current method represents a rare example of a highly regio- and stereoselective hydroamination of internal alkynes for the construction of dialkyl enamines43.
Table 1. Optimization and scope of copper-catalyzed direct hydroamination of aryl alkynes.
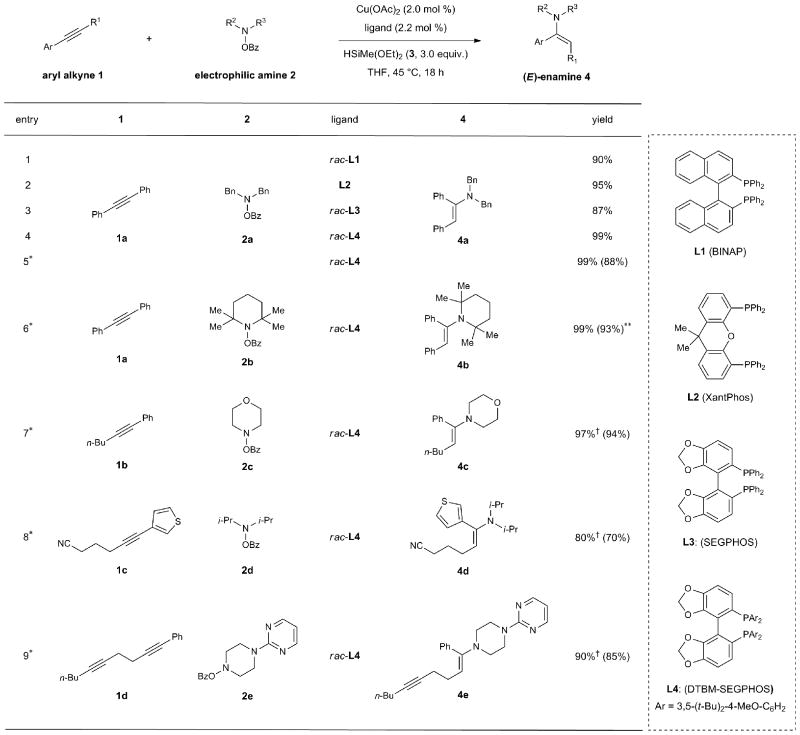
|
Conditions: 1a (0.2 mmol), 2a (0.24 mmol), 3 (0.6 mmol), Cu(OAc)2 (2.0 mol %), ligand (2.2 mol %), THF (1 M), 45 °C, 18 h. Yields and stereoselectivities were determined by 1H NMR analysis using 1,1,2,2-tetracholoroethane as an internal standard. In all cases only (E)-enamine products were observed.
Conditions: 1 (1.0 mmol), 2 (1.2 mmol), 3 (3.0 mmol), Cu(OAc)2 (2.0 mol %), rac-L4 (2.2 mol %), THF (1 M), 45 °C, 18 h. Isolated yields of products after reduction are given in parentheses (average of two runs).
Isolated yield of enamine after purification by flash column chromatography.
Major regioisomers are shown; in all cases, <5% of the minor regioisomer was observed as determined by 1H NMR analysis of the crude enamine product.
Reductive hydroamination: development and scope
As previously described, we were hopeful that the addition of a protic additive could divert this reaction from direct alkyne hydroamination to the outlined reductive hydroamination by selective protonation of the formed vinylcopper intermediate (Fig. 1c). Indeed, inclusion of methanol as an additive under the reaction conditions in Table 1 resulted in formation of the desired reductive hydroamination product 5a in moderate yield, along with a significant amount of enamine 4a (18%) and stilbene (17%) as side products (Table 2, entry 1). Fortunately, an evaluation of alcohol additives revealed that ethanol was a suitable proton source, which minimized the formation of these side products to afford benzylamine 5a in excellent yield and high enantioselectivity (entry 2, 92% yield, 89% e.e.). Interestingly, in contrast to the direct alkyne hydroamination protocol for enamine formation, L4 was uniquely able to perform the reductive hydroamination cascade reaction: reaction utilizing copper catalysts based on L1, L2 or L3 provided only enamine 4a in high yields even in the presence of ethanol (entries 4–6). We attribute the success of the catalyst system based on L4 to the ability of the CuH species to hydrocuprate alkynes and alkenes more rapidly. In contrast, the hydrocupration of alkynes occurred less efficiently when L1-L3 were employed, resulting in the consumption of the alcohol additive by the CuH before alkyne hydrocupration could take place. Therefore, only the enamine product was obtained in these cases. In addition, we found that arylacetylenes could also undergo reductive hydroamination, although in the case of these substrates, isopropanol was a superior protic additive (entry 8).
Table 2. Optimization of reductive hydroamination.
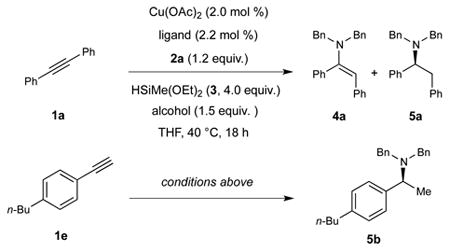
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | substrate | ligand | alcohol | yield 4a | yield 5a or 5b (e.e.) |
| 1 | 1a | (R)-L4 | MeOH | 18% | 60% (89% e.e.) |
| 2 | 1a | (R)-L4 | EtOH | 2% | 92% (89% e.e.) |
| 3 | 1a | (R)-L4 | i-PrOH | 2% | 83% (89% e.e.) |
| 4 | 1a | (R)-L1 | EtOH | 83% | 0 |
| 5 | 1a | L2 | EtOH | 95% | 0 |
| 6 | 1a | (R)-L3 | EtOH | 80% | 0 |
| 7 | 1e | (R)-L4 | EtOH | --* | 78% (99% e.e.) |
| 8** | 1e | (R)-L4 | i-PrOH | --* | 83% (99% e.e.) |
Conditions: 1a or 1e (0.2 mmol), 2a (0.24 mmol), 3 (0.8 mmol), alcohol (0.3 mmol), Cu(OAc)2 (2.0 mol %), ligand (2.2 mol %), THF (1 M), 40 °C, 18 h. Yields were determined by gas chromatography using dodecane as an internal standard. Enantiomeric excesses (e.e.) were determined by high-performance liquid chromatography (HPLC) analysis using chiral stationary phases.
The corresponding enamines were not observed.
Conditions: 1e (1.1 mmol), 2a (1.0 mmol), 3 (4.0 mmol), i-PrOH (1.2 mmol), Cu(OAc)2 (2.0 mol %), (R)-L4 (2.2 mol %), THF (1 M), 40 °C, 18 h.
Under the optimized set of reaction conditions, a range of chiral benzylamine derivatives could be prepared in moderate to high yield (61–85%) with very high levels of enantioselectivity (≥97% e.e., Table 3). These mild catalytic conditions tolerated a range of common functional groups including ethers (5c, 5h), alcohols (5i), aryl halides (5e, 5f), pyridines (5d), indoles (5g), acetals (5j), and ketals (5m, 5n). Moreover, a reaction conducted on 10 mmol scale proceeded efficiently in the presence of 1 mol % catalyst, furnishing the product in undiminished yield and enantioselectivity (5j). The applicability of new synthetic methods to the late-stage modification of complex natural products is a highly desirable feature, as analogs of bioactive molecules can be prepared without the need for de novo synthesis. Accordingly, readily available alkynes derived from the natural products δ-tocopherol and estrone were subjected to asymmetric reductive hydroamination conditions to afford aminated products with good yields and excellent, catalyst-controlled diastereoselectivities (d.r.: 99:1, 5k–5n). It is noteworthy that in all reductive hydroamination reactions employing aryl-substituted alkynes, the amination products were delivered with exclusive Markovnikov regioselectivity, with C–N bond formation occurring adjacent to the aryl group.
Table 3. Scope of copper-catalyzed reductive hydroamination of aryl alkynes.
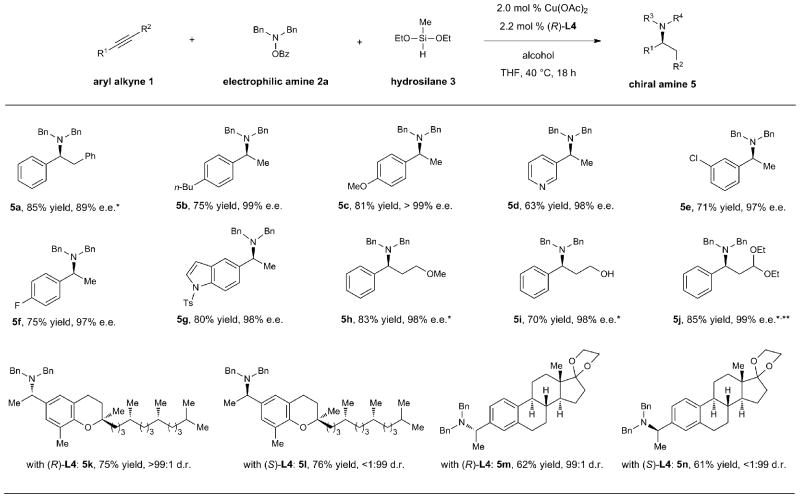
|
Conditions: 1 (1.1 mmol), 2 (1.0 mmol), 3 (4.0 mmol), i-PrOH (1.2 mmol), Cu(OAc)2 (2.0 mol %), (R)-L4 (2.2 mol %), THF (1 M), 40 °C, 18 h. Isolated yields are reported (average of two runs). Enantiomeric excesses (e.e.) and diastereomeric ratios (d.r.) were determined by HPLC analysis. *Using EtOH instead of i-PrOH. **Reaction performed on 10 mmol scale using 1 mol % of catalyst. See Supplementary Information (SI) for details.
In addition to aryl-substituted alkynes, we found that terminal aliphatic alkynes readily participate in catalytic reductive hydroamination to deliver alkylamines (Table 4). In contrast to aryl-substituted alkynes, anti-Markovnikov regioselectivity was observed when simple alkylacetylene substrates were used, giving rise to linear tertiary amines in high yields (71–88% yield). We note that it was crucial to use a slight excess of isopropanol compared to the alkylacetylene substrate in the case of terminal alkyne substrates, probably due to deactivation of the catalyst through formation of a copper acetylide species when the amount of isopropanol was insufficient40. It is noteworthy that this methodology could be applied to a substrate bearing an unprotected secondary amine to provide 1,3-diamine 6a in high yield. Furthermore, alkynol silyl ethers were suitable substrates for the current method. Upon reductive hydroamination and silyl deprotection, 1,3-amino alcohol products were prepared in good yields (6f, 6g). An enantioenriched 1,3-amino alcohol could be generated from the optically active alkynol silyl ether (98% e.e.) without erosion of enantiomeric excess (6g, 98% e.e.). The use of the current reductive hydroamination methodology, in conjunction with well-developed methods for the asymmetric synthesis of alkynols14, represents an attractive approach for the synthesis of these biologically relevant compounds.
Table 4. Reductive hydroamination of alkylacetylenes.
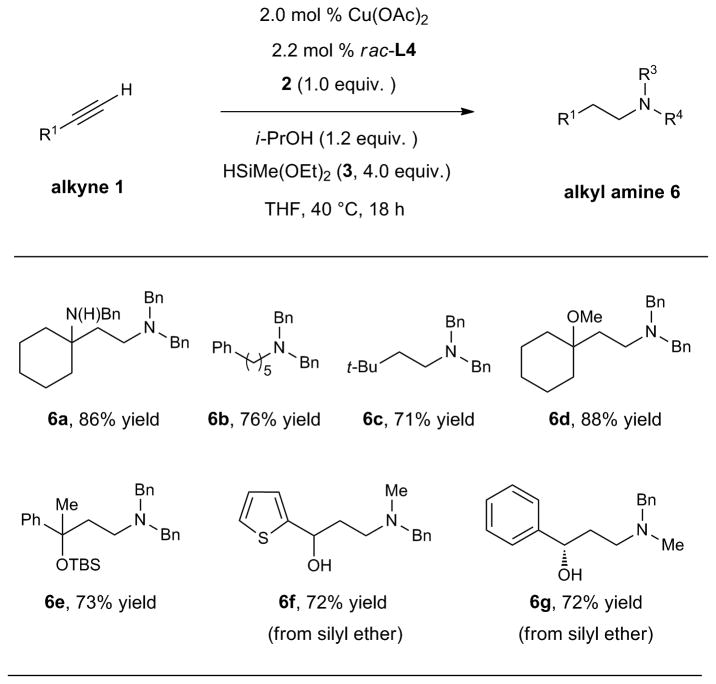
|
Conditions: 1 (1.1 mmol), 2 (1.0 mmol), 3 (4.0 mmol), i-PrOH (1.2 mmol), Cu(OAc)2 (2.0 mol %), rac-L4 (2.2 mol %), THF (1 M), 40 °C, 18 h. Isolated yields are reported (average of two runs). see SI for details.
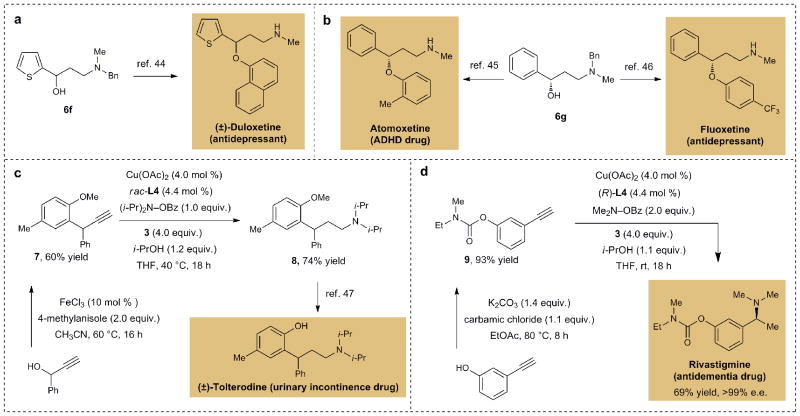
Application to drug synthesis
The functionalized amines obtained by the cascade hydroamination developed in this study have broad utility. To illustrate this point, 1,3-amino alcohol 6f can be readily transformed to the antidepressant drug duloxetine following the literature procedure44 (Fig. 2a). Moreover, 1,3-amino alcohol 6g is a key intermediate for the synthesis of the attention deficit hyperactivity disorder (ADHD) drug atomoxetine45, as well as the antidepressant drug fluoxetine46 (Fig. 2b). Furthermore, diisopropylamine 8 could be prepared by reductive hydroamination of readily synthesized alkyne 7 (Fig. 2c). Tolterodine, a drug used for symptomatic treatment of urinary incontinence, could be prepared by demethylation of 847. Our method was also applied towards a new two-step synthesis of rivastigmine, a drug that is used to treat dementia (Fig. 2d). Condensation of commercially available reagents gave carbamate 9, which upon asymmetric reductive hydroamination furnished the target in enantiopure form and good chemical yield. These syntheses exemplify the potential utility of the current hydroamination method for the rapid and efficient construction of medicinally relevant molecules.
Figure 2. Concise routes to drugs through cascade reductive hydroamination of alkynes.
a, Hydroamination product 6f as a key synthetic precursor for duloxetine synthesis; b, Hydroamination product 6g as a known synthetic precursor to atomoxetine and fluoxetine; c, Rapid synthesis of 8, a known precursor to tolterodine; d, Two-step synthesis of rivastigmine.
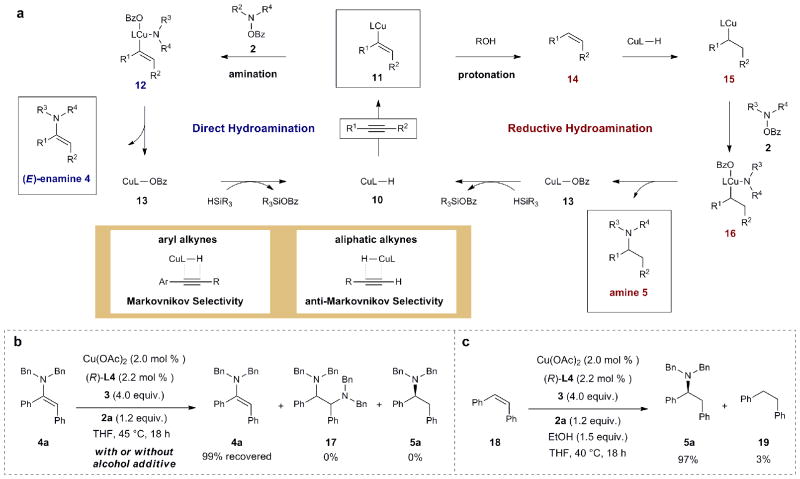
Mechanistic discussion
The proposed mechanisms for the formation of enamines and alkylamines (shown in Fig. 3a) both commence with syn-selective Cu–H addition to the alkyne substrate to give vinylcopper species 11. In the absence of a proton source (alcohol), direct interception by electrophilic amine 2 (likely via oxidative addition/reductive elimination) would produce the (E)-enamine product 4 and copper benzoate complex 13 that, upon transmetalation with hydrosilane, would regenerate the active CuH species 10. On the other hand, in the presence of alcohol, direct protonation of the vinylcopper intermediate 11 could afford the cis disubstituted alkene 14. A hydroamination similar to that we have previously reported via alkylcopper species 15 and 16 would deliver the alkylamine 5. As previously detailed, these amination protocols both provide products in excellent regioselectivity; aryl-substituted internal alkynes (and alkenes) proceed through a Markovnikov selective hydrocupration event and terminal aliphatic alkynes (and alkenes) undergo anti-Markovinikov hydrometalation. This regioselectivity is in accord with the results that were previously reported for copper-catalyzed alkyne semireduction39,40 and hydroamination23. We rationalize the Markovnikov regioselectivity observed for aryl alkynes as arising from the electronic stabilization of the vinyl- or alkylcopper species by an adjacent aryl group. In contrast, we attribute the anti-Markovnikov selectivity observed in the case of terminal aliphatic alkynes to steric effects (i.e., formation of the less substituted vinyl- or alkylcopper species).
Figure 3. Proposed catalytic cycles and mechanistic experiments.
a, Both direct hydroamination and reductive hydroamination are proposed to proceed through vinylcopper intermediate 11. In the absence of alcohol additive, electrophilic amination occurs directly to give the enamine product (left cycle). In the presence of alcohol additive, protonation of 11 affords alkene 14, which undergoes further hydrocupration and electrophilic amination to furnish the alkylamine product (right cycle). Internal aryl alkynes and terminal aliphatic alkynes undergo hydrocupration with Markovnikov and anti-Markovnikov regioselectivity, respectively. b, Enamine 4a is inert to direct hydroamination conditions (no alcohol additive), thus accounting for the lack of overreduction or diamination products observed under these conditions. Enamine 4a is also inert to reductive hydroamination conditions (alcohol additive present). Enamine 4a is thus unlikely to be an intermediate in the reductive hydroamination reaction. c, In the presence of ethanol as a proton source and electrophilic amination reagent 2a, cis-stilbene selectively undergoes hydroamination rather than reduction. Hence, the presence of alcohol does not interfere with the hydroamination of proposed alkene intermediate 14, allowing the reductive hydroamination cascade to proceed efficiently to afford the alkylamine product.
We conducted mechanistic experiments to gain a better understanding of the factors that result in the high selectivities observed in our system. Subjecting enamine 4a to the conditions developed for either alkyne hydroamination or reductive hydroamination resulted in no observed reaction (Fig. 3b). The inertness of the enamine under these conditions accounts for the exclusive formation of monoamination product in the case of alkyne hydroamination. In addition, these experiments suggest that alkyne hydroamination followed by enamine reduction is not occurring in the case of reductive hydroamination. Furthermore, we subjected cis-stilbene (18) to the hydroamination conditions in the presence of 1.5 equiv ethanol (Fig. 3c). Although a small amount of 1,2-diphenylethane (19, 3% yield) was formed, presumably as a result of protonation of the alkylcopper intermediate48, hydroamination adduct 5a was generated as the predominant product (97% yield). This result suggests that amination of the alkylcopper species 15 occurs selectively in the presence of a proton source. Combined, the results of these experiments are in agreement with our original hypothesis that vinylcopper species 11 and alkylcopper species 15 undergo selective protonation and amination respectively, thereby allowing the desired cascade reaction to proceed as designed.
Conclusion
In conclusion, we have developed catalytic conditions that allow for the controlled construction of enamines or alkylamines from alkynes and electrophilic amine sources. The products from these complementary systems were obtained with uniformly high levels of regio- and stereocontrol. Both catalytic processes operate through the formation of a vinylcopper intermediate, the product being determined by the presence or absence of an alcohol additive. The development of a protocol for the direct conversion of alkynes to alkylamines is especially notable, given the ease of access to requisite substrates and the demonstrable applicability of this method to the rapid synthesis of a number of pharmaceutical agents. Beyond the broad utility of this new protocol, we anticipate that this cascade strategy will motivate the design of other cascade processes for the more efficient synthesis of valuable targets.
Methods
A typical procedure for the copper-catalyzed reductive hydroamination of alkynes 1 is as follows (all reactions were set up on the benchtop using standard Schlenk technique). An oven-dried screw-top reaction tube equipped with a magnetic stir bar was charged with Cu(OAc)2 (3.6 mg, 0.02 mmol, 2 mol %) and (R)-L4 (26 mg, 0.022 mmol, 2.2 mol %). The reaction tube was sealed with a screw-cap septum, then evacuated and backfilled with argon (this process was repeated a total of three times). Anhydrous THF (0.5 mL) and hydrosilane 3 (0.64 mL, 4.0 mmol, 4.0 equiv.) were added sequentially via syringe. The resulting mixture was stirred at room temperature (rt) for 15 min and the color of the mixture changed from blue to orange. A second oven-dried screw-top reaction tube equipped with a stir bar was charged with alkyne substrate 1a (178 mg, 1.0 mmol, 1.0 equiv.) and hydroxylamine ester 2a (381 mg, 1.2 mmol, 1.2 equiv.). The reaction tube was sealed with a screw-cap septum, and then evacuated and backfilled with argon (this process was repeated a total of three times). Anhydrous THF (0.5 mL) and EtOH (88 μL, 1.5 mmol, 1.5 equiv.) were added, followed by dropwise addition of the catalyst solution from the first vial to the stirred reaction mixture at rt. The reaction mixture was then heated at 40 °C for 18 h. After cooling to rt, the reaction was quenched by addition of EtOAc and a saturated aqueous solution of Na2CO3. The phases were separated, the organic phase was concentrated, and product 5a was purified by flash column chromatography. The enantiomeric excesses (e.e.) of the products were determined by HPLC analysis using chiral stationary phases. All new compounds were fully characterized (see Supplementary Information).
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health for financial support (GM58160). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge Dr. Shaolin Zhu (MIT), Phillip J. Milner (MIT), and Dr. Michael Pirnot (MIT) for insightful discussions. The authors thank Dr. Yiming Wang (MIT), and Prof. Nathan T. Jui (Emory University) for help with the preparation of this manuscript.
Footnotes
Reprints and permissions information is available at www.nature.com/reprintsandpermissions/.
Author contributions: S.-L.S. and S.L.B. designed the project, analysed the data and wrote the manuscript. S.-L.S. performed the experiments.
Additional information: Supplementary information and chemical compounds information accompany this paper at www.nature.com/naturechemistry.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Trost BM. Selectivity: the key to synthetic efficiency. Science. 1983;219:245–250. doi: 10.1126/science.219.4582.245. [DOI] [PubMed] [Google Scholar]
- 2.Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 3rd edn. Wiley; 2008. [Google Scholar]
- 3.Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley; 2010. [Google Scholar]
- 4.Surry DS, Buchwald SL. Biaryl phosphane ligands in palladium-catalyzed amination. Angew Chem Int Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surry DS, Buchwald SL. Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide. Chem Sci. 2011;2:27–50. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robak MT, Herbage MA, Ellman JA. Synthesis and applications of tert-butanesulfinamide. Chem Rev. 2010;110:3600–3740. doi: 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- 7.Roizen JL, Harvey ME, Du Bois J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc Chem Res. 2012;45:911–922. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon RC, Grischek B, Zepeck F, Steinreiber A, Belaj F, Kroutil W. Regio- and stereoselective monoamination of diketones without protecting groups. Angew Chem Int Ed. 2012;51:6713–6716. doi: 10.1002/anie.201202375. [DOI] [PubMed] [Google Scholar]
- 9.Höhne M, Kühl S, Robins K, Bornscheuer UT. Efficient asymmetric synthesis of chiral amines by combining transaminase and pyruvate decarboxylase. ChemBioChem. 2008;9:363–365. doi: 10.1002/cbic.200700601. [DOI] [PubMed] [Google Scholar]
- 10.Hultzsch KC. Catalytic asymmetric hydroamination of non-activated olefins. Org Biomol Chem. 2005;3:1819–1824. doi: 10.1039/b418521h. [DOI] [PubMed] [Google Scholar]
- 11.Hannedouche J, Schulz E. Asymmetric hydroamination: a survey of the most recent developments. Chem Eur J. 2013;19:4972–4985. doi: 10.1002/chem.201203956. [DOI] [PubMed] [Google Scholar]
- 12.Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]
- 13.Chinchilla R, Nájera C. Recent advances in Sonogashira reactions. Chem Soc Rev. 2011;40:5084–5121. doi: 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]
- 14.Trost BM. & Weiss, A. H. The enantioselective addition of alkyne nucleophiles to carbonyl groups. Adv Synth Catal. 2009;351:963–983. doi: 10.1002/adsc.200800776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habrant D, Rauhala V, Koskinen AMP. Conversion of carbonyl compounds to alkynes: general overview and recent developments. Chem Soc Rev. 2010;39:2007–2017. doi: 10.1039/b915418c. [DOI] [PubMed] [Google Scholar]
- 16.Severin R, Doye S. The catalytic hydroamination of alkynes. Chem Soc Rev. 2007;36:1407–1420. doi: 10.1039/b600981f. [DOI] [PubMed] [Google Scholar]
- 17.Alonso F, Beletskaya IP, Yus M. Transition-metal-catalyzed addition of heteroatom–hydrogen bonds to alkynes. Chem Rev. 2004;104:3079–3160. doi: 10.1021/cr0201068. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X. Recent advances in catalytic sequential reactions involving hydroelement addition to carbon–carbon multiple bonds. Chem Rev. 2013;113:6864–6900. doi: 10.1021/cr400082n. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Herzon SB. Regioselective reductive hydration of alkynes to form branched or linear alcohols. J Am Chem Soc. 2012;134:17376–17379. doi: 10.1021/ja307145e. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Herzon SB. Temporal separation of catalytic activities allows anti-Markovnikov reductive functionalization of terminal alkynes. Nature Chem. 2014;6:22–27. doi: 10.1038/nchem.1799. [DOI] [PubMed] [Google Scholar]
- 21.Zeng M, Li L, Herzon SB. A highly active and air-stable ruthenium complex for the ambient temperature anti-Markovnikov reductive hydration of terminal alkynes. J Am Chem Soc. 2014;136:7058–7067. doi: 10.1021/ja501738a. [DOI] [PubMed] [Google Scholar]
- 22.Uehling MR, Rucker RP, Lalic G. Catalytic anti-Markovnikov hydrobromination of alkynes. J Am Chem Soc. 2014;136:8799–8803. doi: 10.1021/ja503944n. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Niljianskul N, Buchwald SL. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J Am Chem Soc. 2013;135:15746–15749. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miki Y, Hirano K, Satoh T, Miura M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew Chem Int Ed. 2013;52:10830–10834. doi: 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]
- 25.Deutsch C, Krause N, Lipshutz BH. CuH-catalyzed reactions. Chem Rev. 2008;108:2916–2927. doi: 10.1021/cr0684321. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney WS, Stryker JM. Hydride-mediated homogeneous catalysis. Catalytic reduction of α,β-Unsaturated Ketones Using [(Ph3P)CuH]6 and H2. J Am Chem Soc. 1989;111:8818–8823. [Google Scholar]
- 27.Lipshutz BH, Keith J, Papa P, Vivian R. A convenient, efficient method for conjugate reductions using catalytic quantities of Cu(I) Tetrahedron Lett. 1998;39:4627–4630. [Google Scholar]
- 28.Appella DH, Moritani Y, Shintani R, Ferreira EM, Buchwald SL. Asymmetric conjugate reduction of α,β-unsaturated esters using a chiral phosphine–copper catalyst. J Am Chem Soc. 1999;121:9473–9474. [Google Scholar]
- 29.Moritani Y, Appella DH, Jurkauskas V, Buchwald SL. Synthesis of β-alkyl cyclopentanones in high enantiomeric excess via copper-catalyzed asymmetric conjugate reduction. J Am Chem Soc 2000. 2000;122:6797–6798. [Google Scholar]
- 30.Yun J, Buchwald SL. One-pot synthesis of enantiomerically enriched 2,3-disubstituted cyclopentanones via copper-catalyzed 1,4-reduction and alkylation. Org Lett. 2001;3:1129–1131. doi: 10.1021/ol015577f. [DOI] [PubMed] [Google Scholar]
- 31.Jurkauskas V, Buchwald SL. Dynamic kinetic resolution via asymmetric conjugate reduction: enantio- and diastereoselective synthesis of 2,4-dialkyl cyclopentanones. J Am Chem Soc. 2002;124:2892–2893. doi: 10.1021/ja025603k. [DOI] [PubMed] [Google Scholar]
- 32.Rainka MP, Aye Y, Buchwald SL. Copper-catalyzed asymmetric conjugate reduction as a route to novel β-azaheterocyclic acid derivatives. Proc Natl Ac Sci U S A. 2004;101:5821–5823. doi: 10.1073/pnas.0307764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdik E, Ay M. Electrophilic amination of carbanions. Chem Rev. 1989;89:1947–1980. [Google Scholar]
- 34.Barker TJ, Jarvo ER. Developments in transition-metal-catalyzed reactions using electrophilic nitrogen sources. Synthesis. 2011;24:3954–3964. [Google Scholar]
- 35.Berman AM, Johnson JS. Copper-catalyzed electrophilic amination of diorganozinc reagents. J Am Chem Soc. 2004;126:5680–5681. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]
- 36.Campbell MJ, Johnson JS. Mechanistic studies of the copper-catalyzed electrophilic amination of diorganozinc reagents and development of a zinc-free protocol. Org Lett. 2007;9:1521–1524. doi: 10.1021/ol0702829. [DOI] [PubMed] [Google Scholar]
- 37.Xu PF, Wang W. Catalytic Cascade Reactions. Wiley; 2014. [Google Scholar]
- 38.Daeuble JF, McGettigan C, Stryker JM. Selective reduction of alkynes to cis-alkenes by hydrometallation using [(Ph3P)CuH]6. Tetrahedron Lett. 1990;31:2397–2400. [Google Scholar]
- 39.Semba K, Fujihara T, Xu T, Terao J, Tsuji Y. Copper-catalyzed highly selective semihydrogenation of non-polar carbon–carbon multiple bonds using a silane and an alcohol. Adv Synth Catal. 2012;354:1542–1550. [Google Scholar]
- 40.Whittaker AM, Lalic G. Monophasic catalytic system for the selective semireduction of alkynes. Org Lett. 2013;15:1112–1115. doi: 10.1021/ol4001679. [DOI] [PubMed] [Google Scholar]
- 41.Field LD, Messerle BA, Wren SL. One-pot tandem hydroamination/hydrosilation catalyzed by cationic iridium(I) complexes. Organometallics. 2003;22:4393–4395. [Google Scholar]
- 42.Heutling A, Pohlki F, Bytschkov I, Doye S. Hydroamination/hydrosilylation sequence catalyzed by titanium complexes. Angew Chem Int Ed. 2005;44:2951–2954. doi: 10.1002/anie.200462250. [DOI] [PubMed] [Google Scholar]
- 43.Hesp KD, Stradiotto M. Stereo- and regioselective gold-catalyzed hydroamination of internal alkynes with dialkylamines. J Am Chem Soc. 2010;132:18026–18029. doi: 10.1021/ja109192w. [DOI] [PubMed] [Google Scholar]
- 44.Jozsef B, et al. Process for preparation of duloxetine and intermediates. International patent WO 2008078124. 2008 Jul 3;
- 45.Wu F, Chen G, Yang X. Process for preparation of propylamine derivatives and application in manufacturing tomoxetine. Chinese patent CN1948277. 2007 Apr 18;
- 46.Bhandari K, Srivastava S, Shanker G, Nath C. Substituted propanolamines and alkylamines derived from fluoxetine as potent appetite suppressants. Bioorg Med Chem. 2005;13:1739–1747. doi: 10.1016/j.bmc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Paras NA, Simmons B, MacMillan DWC. A process for the rapid removal of dialkylamino-substituents from aromatic rings. Application to the expedient synthesis of (R)-tolterodine. Tetrahedron. 2009;65:3232–3238. [Google Scholar]
- 48.Rupnicki L, Saxena A, Lam HW. Aromatic heterocycles as activating groups for asymmetric conjugate addition reactions: enantioselective copper-catalyzed reduction of 2-alkenylheteroarenes. J Am Chem Soc. 2009;131:10386–10387. doi: 10.1021/ja904365h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.