Abstract
Objective: To assess existing reported human trials of Withania somnifera (WS; common name, ashwagandha) for the treatment of anxiety.
Design: Systematic review of the literature, with searches conducted in PubMed, SCOPUS, CINAHL, and Google Scholar by a medical librarian. Additionally, the reference lists of studies identified in these databases were searched by a research assistant, and queries were conducted in the AYUSH Research Portal. Search terms included “ashwagandha,” “Withania somnifera,” and terms related to anxiety and stress. Inclusion criteria were human randomized controlled trials with a treatment arm that included WS as a remedy for anxiety or stress. The study team members applied inclusion criteria while screening the records by abstract review.
Intervention: Treatment with any regimen of WS.
Outcome measures: Number and results of studies identified in the review.
Results: Sixty-two abstracts were screened; five human trials met inclusion criteria. Three studies compared several dosage levels of WS extract with placebos using versions of the Hamilton Anxiety Scale, with two demonstrating significant benefit of WS versus placebo, and the third demonstrating beneficial effects that approached but did not achieve significance (p=0.05). A fourth study compared naturopathic care with WS versus psychotherapy by using Beck Anxiety Inventory (BAI) scores as an outcome; BAI scores decreased by 56.5% in the WS group and decreased 30.5% for psychotherapy (p<0.0001). A fifth study measured changes in Perceived Stress Scale (PSS) scores in WS group versus placebo; there was a 44.0% reduction in PSS scores in the WS group and a 5.5% reduction in the placebo group (p<0.0001). All studies exhibited unclear or high risk of bias, and heterogenous design and reporting prevented the possibility of meta-analysis.
Conclusions: All five studies concluded that WS intervention resulted in greater score improvements (significantly in most cases) than placebo in outcomes on anxiety or stress scales. Current evidence should be received with caution because of an assortment of study methods and cases of potential bias.
Introduction
Anxiety disorders are widespread and disabling conditions with a lifetime prevalence of nearly 30% in the United States.1 As the most common mental disorder, anxiety presents an urgent problem that affects people of all ages. Anxiety is often accompanied by stress, which is the body's physiologic response to mental or physical threats. While brief exposure to the stress response is meant to be a beneficial coping mechanism, long-term stress is likely to result in the decline of overall health and the complication of existing diseases. Treatment protocols for the management of anxiety and the reduction of stress are continuously being sought to mitigate the effect of these prevailing health risks. Alternatives to benzodiazepines and other prescription medications are of great interest, with intentions to lessen exposure to harmful adverse effects affiliated with these drugs.
Withania somnifera (WS), widely known as ashwagandha, is an Ayurvedic herb that has recently gained recognition as a treatment for anxiety and stress in the United States. Although used as a broad-spectrum remedy in India for centuries, WS has only recently been under investigation in laboratory settings. WS is categorized as an anti-inflammatory,2,3 antioxidant herbal supplement.4 These hypothesized healing properties lad to widespread use of WS in Ayurvedic medicine, and it has been studied as a treatment for various health conditions.
Therapeutic implications for cancerous tumors4 as well as neurodegenerative diseases5 have been documented, for example. The herb is also classified as an adaptogen, which indicates its ability to regulate physiologic processes and thereby stabilize the body's response to stress.2 WS exerts an anxiolytic effect in animals and humans.5,6 One study has examined the effects of a standardized WS extract on chronic stress in rats exposed to a shock procedure; the researchers concluded that the rats treated with WS extract responded better to the induced chronic stress symptoms.7 In a similar investigation, WS increased stress tolerance among animals subjected to a cold water swimming stress test.8 WS has even proven to have effects on anxiety similar to those of standard benzodiazepines. After experiencing a series of anxiety-producing events, WS generated analogous effects compared with lorazepam in rats.9 The results of this particular study indicate that herbal supplementation is similarly effective in the management of anxiety as are standard prescription drugs, without the harmful adverse effects, in a rodent model.
As Ayurvedic practices, such as the administration of herbs, gradually acquire more support in primary care, the need to evaluate the use of herbal substances in the management of specific conditions becomes more acute.10 Several human clinical trials have been undertaken in light of the prospective results from WS research in animal models. Thus, the primary objective of the systematic review of the literature described here was to identify and evaluate all published evidence derived from human trials of WS as a treatment for anxiety and stress.
Materials and Methods
Search strategy
A medical librarian (V.Y.) performed the comprehensive literature searches of the following databases: PubMed, SCOPUS, and CINAHL. The PubMed search used the following terms: (“Ashwagandha” [Supplementary Concept] OR “Ashwagandha” [All Fields] OR “withania” [MeSH Terms] OR “withania” [All Fields]) AND (“humans” [MeSH Terms] AND English [lang]). This core search was then combined with more specific searches of mental health term combinations, such as “anxiety” and “behavior.” The SCOPUS search was conducted by using the following: TITLE-ABS-KEY(ashwagandha) OR TITLE-ABS-KEY(withania) AND TITLE-ABS-KEY(human) AND (mental health OR behavior OR mood OR anxiety) AND TITLE-ABS-KEY(clinical trial OR randomized controlled trial) AND (LIMIT-TO(LANGUAGE, “English”). CINAHL was searched by using the terms “ashwagandha” OR “withania” AND “human” with limiters of peer reviewed and exclusion of MEDLINE records. Additional efforts were performed through Google Scholar searches of “Ashwagandha anxiety disorders” and citation searching. General searches in Google and in the AYUSH Research Portal11–13 were performed to assess the extent of grey or unpublished literature on the topic.
Inclusion/exclusion criteria
Studies met inclusion criteria if categorized as human randomized controlled trials with a treatment arm that included WS as a method for anxiety or stress relief. Studies were excluded if results did not include outcomes on anxiety or stress. Also excluded were animal studies, as well as any systematic reviews or reports on the uses of WS. Finally, only studies published in the English language were considered.
Review of records
The records retrieved from the database searches were reviewed by the study team members to determine eligibility. Records were reviewed by one author (M.A.P.), screening titles and abstracts. If considered pertinent, a full-text review was then performed by three authors (M.A.P., K.L.N., C.P.M.), and a final decision of inclusion status was established through open discussion and consensus.
The studies that were ultimately selected for inclusion were reviewed in full, and the data of interest were extracted by one author (M.A.P.); the extracted data were then checked against the original texts by a second author (C.P.M.). The collected data from each study included a sample of participants, study duration, WS dosage/control method, outcome measures, study results, and threats to validity. The study team then compiled and assessed data from the included studies. Validity and risk of bias for each study were assessed by using the Cochrane Collaboration risk-of-bias tool.14
Results
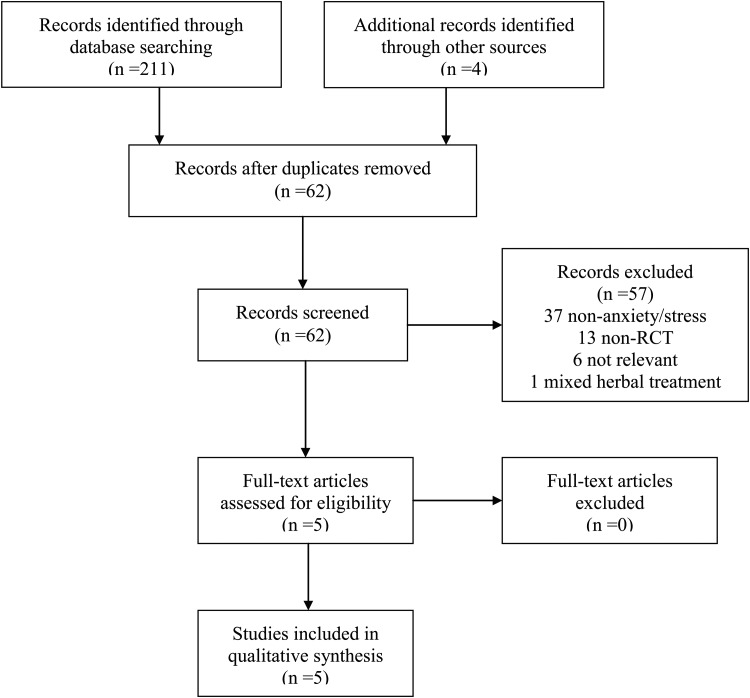
A total of 211 records were returned from the comprehensive database search. In addition, four records were identified through sources outside of the core search: two through reference list searching and two through general Google searching. No additional studies were identified via the AYUSH Research Portal. Upon elimination of duplicates, a total of 62 records were screened for eligibility. Figure 1 further depicts the selection process of the studies. After completion of screening, five human trials met inclusion criteria and were selected for the systematic review. Basic pharmacologic information15 is presented in Table 1, and clinical trial characteristics are displayed in Table 2. Each study is summarized below.
FIG. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of search results from systematic review.
Table 1.
Overview of Pharmacologic Details Presented in Each of the Five Studies Included in the Systematic Review
| Study | Type/form | Dose | Product composition | Brand/extract (manufacturer name) | Notes |
|---|---|---|---|---|---|
| Andrade et al., 200016 | Ethanolic extract; 250 mg tablets | Two 250-mg tablets, twice a day (1000 mg/d) | Not given | Not given | Dose adjustment after week 2; minimum of 2 and maximum of 10 tablets per day |
| Auddy et al., 200817 | Standardized WS root and leaf extract (WSE); 125-mg and 250-mg coded hard-gelatin capsules | One 125-mg capsule once a day (125 mg/d) One 125- mg capsule twice a day (250 mg/d) One 250-mg capsule twice a day (500 mg/d) |
Standardized to minimum of 8% withanolide glycosides and 32% oligosaccharides, maximum withaferin A | Sensoril® (Natreon, Inc.) Essentra® (NutraGenesis, LLC) |
Product derived from withaferin A and corresponding withanolide glycoside-predominant, genetically uniform chemotype, cultivated in central and northern provinces of India WS root and leaf material processed using water-based extraction protocol and assessed using high-performance thin-layer chromatography analysis |
| Cooley et al., 200918 | Herb WS prepared from the root (Swiss ashwagandha); 300-mg supplements | One 300-mg supplement twice a day (600 mg/d) | Standardized to 1.5% withanolides | Not given | None |
| Chandrasekhar et al., 201219 | High-concentration full-spectrum WS root extract capsules; 300-mg capsules | One 300-mg capsule twice a day (600 mg/d) | Standardized to at least 5% withanolide content | KSM-66 WS extract (Ixoreal Biomed) | Extract from Hyderabad, India Extract drawn only from roots of WS plant, no other parts Produced by unique extraction process, based on principles of “green chemistry,” without using alcohol or any synthetic solvents |
| Khyati and Ayup, 201420 | Granules made with dried root powder of WS; 4-g granules | One 4-g granule 3 times a day (12,000 mg/day) | 1 part WS roots, 1 part sugar, and water | Not given | Method of drug preparation: Dried WS root pulverized to fine powder Equal quantity of sugar syrup prepared by adding sufficient quantity of water in mild flame with constant stirring until syrup reaches tantumatvam (thread like) stage WS powder then added to sugar syrup and mixed thoroughly to prepare homogenous blend Blended mass sieved through a 40# sieve to obtain granule form, dried at ambient temperature • Prepared granules stored in airtight container in a cool dry place away from direct sunlight. |
WS, Withania somnifera.
Table 2.
Overview of Five Studies Included in the Systematic Review
| Study | Sample (n) | Duration | WS dosage | Control regimen | Outcome measures | Results | Risk of bias and threats to validity |
|---|---|---|---|---|---|---|---|
| Andrade et al., 200016 | 39 (15 female, 24 male) | 6 wk (42 d) | 1000 mg/d (250-mg per tablet); dose adjusted after wk 2 | Placebo twice per day | Primary: HAM Secondary: GRS, SAFTEE |
88.2% response in WS group vs. 50% in placebo group (p=0.026) | Risk of bias: high Inconsistent dosing; range of 2–10 tablets per day, small sample, short duration, 48.7% dropout rate |
| Auddy et al., 200817 | 130 (35 female, 95 male) | 60 d | 125 mg/d, 250 mg/d, 500 mg/d | Placebo twice per day | Primary: mHAM-A Secondary: biomarkers |
Significant dose-dependent decrease in mean sum mHAM-A score (p<0.001) | Risk of bias: unclear Reporting bias; conflicts of interest: 2 authors employed by funder (Natreon Inc.); 24.6% dropout rate |
| Cooley et al., 200918 | 81 (51 female, 30 male) | 12 wk (84 d) | 600 mg/d with counseling | PT: CBT+placebo | Primary: BAI Secondary: SF-36, FQ, MYMOP |
Final BAI scores decreased by 56.5% in NC group and 30.5% in PT group (p<.0001) Greater improvement in BAI for NC versus PT (p=0.003) |
Risk of bias: high Performance bias: care providers not blinded to participant distribution, lack of true control group, 21.0% dropout rate |
| Chandrasekhar et al., 201219 | 64 (23 female, 41 male) | 60 d | 600 mg/d after food and with water | Placebo twice per day | Primary: PSS Secondary: DASS, GHQ-28, cortisol |
Greater decrease in PSS (44.0% versus 5.5%; p<0.0001), GHQ-28 and DASS (p<0.0001), and cortisol (27.9% versus 7.9%; p=0.002) for WS versus placebo | Risk of bias: unclear Small sample size, 4.7% dropout rate |
| Khyati and Ayup, 201420 | 86 | 60 d | 12,000 mg/d taken with Anupana (milk) | Placebo thrice per day | Primary: HAM | Greater improvement in WS versus placebo in anxious mood (p<0.001) | Risk of bias: unclear Dropout rate not indicated |
Risk of bias assessed by using Cochrane Collaboration tool.
HAM, Hamilton Anxiety Scale; GRS, Global Rating Scale; SAFTEE, Systematic Assessment for Treatment Emergent Effects; mHAM-A, modified Hamilton Anxiety Scale; CBT, cognitive-behavioral therapy; BAI, Beck Anxiety Inventory; SF-36, Short Form 36; FQ, Fatigue Questionnaire; MYMOP, Measure Yourself Medical Outcomes Profile; NC, naturopathic care; PT, psychotherapy; PSS, Perceived Stress Scale; DASS, Depression Anxiety Stress Scale; GHQ-28, General Health Questionnaire 28.
Summary of trials
Participants in the experimental group of Andrade et al.16 were instructed to take a daily WS dose of 1000 mg, and the control group was prescribed a placebo tablet. Participants were assessed via the Hamilton Anxiety Scale (HAM) and the Global Rating Scale (GRS) at baseline, week 2, and week 6; a systematic assessment for treatment-emergent effects (SAFTEE) at weeks 2 and 6. Clinical response was coded as a reduction of HAM score to 12 or below, with an associated GRS (by both subject and rater) of not more than 1. At week 6, 15 of 17 intervention participants were classified as meeting criteria for response, with only 8 of 16 control participants showing response (p=0.026). Differences in raw score changes for all instruments (HAM, GRS, SAFTEE) tended to favor the experimental drug but were not significant at the 0.05 level in any case. The validity of results is questionable considering the small sample size (n=39), a dropout rate of nearly 50%, and a short study duration (6 weeks). Adverse effects were considered minor, and withdrawal of treatment was described as mild in severity, occurring in both the experimental and control groups.
Auddy et al.17 randomly assigned 130 participants into three treatment groups (125 mg once daily, 125 mg twice daily, and 250 mg twice daily) and one control group (placebo). Participants received treatment for 60 days and were assessed by a modified HAM in addition to various biomarkers. A significant dose-dependent improvement in all major outcome measures was observed when compared to a placebo control. This manufacturer-funded trial raises concerns of reporting bias by its authors, who are also employees of the company. Participants and dropouts reported no adverse or withdrawal effects.
Cooley and colleagues' study18 was composed of 81 participants distributed into the naturopathic care (NC) group or the psychotherapy (PT) group. Participants in the NC group received weekly counseling sessions from a naturopathic doctor and daily WS dosages of 600 mg, while the PT group received cognitive-behavioral therapy (CBT) sessions and placebo. Beck Anxiety Inventory (BAI) scores decreased significantly in the NC group compared with the PT group; mean changes in the BAI were −13.31 points in the NC group and −7.15 points in the PT group (p=0.004). Because the care providers could not be blinded to participant distribution, the results may be flawed by performance bias. Adverse effects did not differ between the two groups, and the participants indicated that all reported reactions were mild.
Chandrasekhar et al.19 enrolled 64 participants in a 60-day clinical trial that compared 600 mg of WS per day with placebo. Significant differences were found for all outcome measures, including scores on the Perceived Stress Scale (p<0.0001), the General Health Questionnaire (p<0.0001), and levels of cortisol in the bloodstream (p=0.0006). The validity of the study may have been compromised by a small sample size (n=64). The authors reported no serious adverse events; they describe WS as a safe and well-tolerated medicinal herb.
The treatment group in Khyati and Anup's study20 received the highest WS dose of all evaluated studies (12,000 mg/d) but resulted in mostly nonsignificant differences on HAM scores compared with the placebo group. The only item that WS participants scored significantly higher on was for “anxious mood” (p<0.001). No adverse drug reactions were reported during treatment.
Primary outcome measures of anxiety or stress varied among the selected trials. Three studies used versions of the HAM,16,17,20 one used the BAI,18 and the remaining used the Perceived Stress Scale.19 Analysis of primary outcome measures for most trials revealed significant differences between the WS groups and control groups. The primary outcomes for all studies were patient-reported measures of anxiety and stress. Secondary measures, such as additional questionnaires and biochemical markers, showed significant differences between intervention and control groups. All trials concluded that WS is a safe herbal supplement for general use because of the lack of severity and frequency of adverse effects.
Threats to validity
The study design of Cooley and colleagues' trial18 comparing NC and PT may have subjected the results to performance bias. Care providers were aware of the distribution of participants into the study groups. The naturopathic doctors (NC providers) and cognitive-behavioral therapists (PT providers) could not be blinded, which may have affected their counseling performance. The differences in therapy composition (NC versus PT) and lack of a true control group made it impossible to isolate the effects of each aspect of treatment. The internal validity for this particular study is therefore limited, and it cannot be claimed that the findings were due exclusively to administration of WS.
Auddy et al.17 were funded by Natreon Inc. to perform a trial on the company's patented WS extract with trade names of Essentra and Sensoril. The circumstances present a conflict of interest for the authors and may have heightened their incentive to selectively report only the findings that would promote the herbal products of Natreon Inc. Recognition that two of the authors are also employees for the WS manufacturing company further increases the possibility of reporting bias.
Small sample sizes are a concern for some studies; notably those of Andrade et al. (n=39)16 and Chandrasekhar et al. (n=64).19 In addition, three trials had dropout rates above 20%.16–18 Most trials discussed are methodologically flawed and underpowered. The proclaimed results should therefore be interpreted with caution. The current evidence is insufficient to declare a definitive conclusion on the efficacy of WS. However, the herbal supplement has shown therapeutic potential, and further study is warranted to affirm its standing as a treatment for anxiety and stress relief.
Discussion
This systematic review aimed to collect and assess data from human randomized controlled trials on the effectiveness of WS as a treatment for anxiety and stress. Study design and outcomes varied widely among the five selected studies. The general finding among these studies was that WS produced favorable results when compared with a placebo. The one study that approached, but failed to achieve, significance for its primary outcome measure had the shortest trial duration and smallest sample size.16 The remaining four trials showed significant differences between WS and placebo when examining anxiety and stress relief outcomes.
However, the strength of trial results may be very limited by factors of potential bias. The methods of Cooley et al.18 prevented blinding of the care providers, allowing greater chance for performance bias. Suggestion of reporting bias in Auddy et al.17 was indicated by conflicts of interest between the authors and the company funding the trial. Another factor to consider in evaluating the results is that the primary outcomes for all included studies were classified as patient-reported measures. Future studies may benefit from adding blinded diagnostic interviews to gain non–patient-rated information as a comparison. Additionally, the use of such biomarkers as salivary amylase and serum cortisol levels would provide further objective measures and differentiation between the studied populations. None of the studies attained a low risk-of-bias rating according to Cochrane criteria,14 and the mildly favorable outcomes reported in this review should be understood in the context of an unclear, and probably moderate to high, risk of bias across these studies.
None of the trials in this review reported significant adverse effects of WS. All effects reported by participants were mild and did not differ in duration or severity when compared with results in the placebo groups. The conclusion that WS is a safe herbal supplement for general use agrees with findings from a recent evaluation of the tolerability and safety of WS in human participants.21 Additional research is needed to determine standardization of WS supplements and dosage recommendations.
Limitations
One major limitation of this review is the shortage of studies included for assessment. A Google Scholar search and a general Google search were performed, as was a search with appropriate terms in the AYUSH Research Portal, which covers Indian Ayurvedic literature. These were done in addition to the main database searches with the aim of extending the field of review into the grey literature, but only one further study was revealed (via Google). All five trials that were considered had been implemented outside of the United States: four in India and one in Canada. Therefore, additional trials may exist in other countries but may be inaccessible, not yet published, or written in a language other than English.
Other limitations to the review include the deficiency of qualifying trials and the diversity of methods and outcomes among them. Performing a meta-analysis was highly impractical, which ultimately limited the ability to sufficiently compare study results. The studies used different dosages of WS, different control methods, and different methods of assessment and did not report findings consistently across the group. The findings should be interpreted with caution because of the great degree of variability in WS administration routines. Search efforts should be expanded in future literature reviews to strengthen the collection of evidence for this subject. In addition, this review began as a clinical inquiry, in the context of Western (U.S.) medical practice, and we focused on the use of WS in Western medical practice. Given the resources available to us, as well as the heterogeneity visible even in the small number of studies identified, the authors chose not to introduce even greater heterogeneity into the review by incorporating Ayurvedic equivalent disease categories, non-Western formulations, and so forth. In addition, the protocol was not entered into any registry of systematic reviews before its inception. A full-scope review (i.e., one including a larger dedication of resources to exhaustively search additional terms, Ayurvedic databases,12,13,22,23 and other sources) should be registered in advance with a systematic review registry, such as the Cochrane Collaboration14 or PROSPERO.24
Any future review effort might also consider expanding the inclusion criteria. WS may be included in multiherbal supplements, and study designs that did not include a control group were excluded. Our inclusion/exclusion criteria and search methods may have excluded articles which may nevertheless add additional information to the picture. One example of such an excluded article would be the single-arm study of the WS-containing herbal combination product OCTA©, which was conducted by Seely and Singh roughly a decade ago.25 A broader-scope review may also be able to address additional issues, such as herb-drug and herb-herb interactions, which are also of clinical significance. The studies included in this review simply reported upon adverse events but did not delve into interactions.
The review was precipitated by the fact that WS has anecdotally been appearing more in Western practice contexts, where it is unfortunately difficult for a physician with allopathic or osteopathic training to interact with a physician trained in Ayurvedic medicine. In an Indian context, Ayurvedic physicians follow clearly defined guidelines, and research on Ayurvedic medications is often conducted in consultation with an Ayurvedic physician. The articles that were included were not thoroughly assessed for, and generally did not discuss, this aspect of study design. While the current study team did not include an Ayurvedic physician, it did include a family physician with additional board certification in integrative medicine, along with a PhD-level researcher with experience in designing studies and in conducting reviews, a medical librarian, and a public health student with interests in complementary and alternative medicine.
A final limit is the lack of a meta-analysis. The initial hope was that the extracted data would be more amenable, but this intention was abandoned as soon as the set of included articles became apparent. With four different outcome measures and five different dose/formulation/control intervention combinations, it was quickly apparent that a meta-analysis would not be possible.
Clearly, the systematic review must be considered within limits. However, despite the limitations listed above, this review appears to have systematically identified all controlled trials of WS for anxiety that have been published in indexed Western medical journals by mid-2014.
Conclusions
The present review revealed a limited number of human clinical trials testing WS as a treatment for anxiety and stress. The range of study design and outcome measures, as well as the identified sources of bias, should be considered while analyzing the given findings. Those that qualified for inclusion offered somewhat promising but early, and possibly biased, results. Most studies concluded with significant improvement in symptoms for the WS group when compared to a variety of controls, including placebo and psychotherapy. While WS appears to alleviate these prevalent conditions in these limited controlled trials, additional research in larger samples and in more clinical contexts is essential to validate its therapeutic capabilities for widespread use.
Acknowledgments
This study was partially funded by Health Resources and Services Administration AAU grant D54HP05462 (C.P.M, principal investigator). The authors are grateful for the input and feedback of Dr. John Epling, chair, Department of Family Medicine, SUNY Upstate Medical University.
Author Disclosure Statements
No competing financial interests exist.
References
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602 [DOI] [PubMed] [Google Scholar]
- 2.Provino R. The role of adaptogens in stress management. Aust J Med Herbal 2010;22:41–49 [Google Scholar]
- 3.Alramadhan E, Hanna MS, Hanna MS, et al. Dietary and botanical anxiolytics. Med Sci Monit 2012;18:RA40–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra L-C, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern Med Rev 2000;5:334–346 [PubMed] [Google Scholar]
- 5.Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med 2011;8(5 Suppl):208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, part 2: a review of clinical studies with supporting preclinical evidence. CNS Drugs 2013;27:301–19 [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Muruganandam A. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav 2003;75:547–555 [DOI] [PubMed] [Google Scholar]
- 8.Archana R, Namasivayam A. Antistressor effect of Withania somnifera. J Ethnopharmacol 1998;64:91–93 [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine 2000;7:463–9 [DOI] [PubMed] [Google Scholar]
- 10.Sharma H, Chandola HM, Singh G, Basisht G. Utilization of Ayurveda in health care: an approach for prevention, health promotion, and treatment of disease. Part 2—Ayurveda in primary health care. J Altern Complement Med 2007;13:1135–50 [DOI] [PubMed] [Google Scholar]
- 11.Ayush Research Portal [homepage on Internet]. Online document at: http://ayushportal.nic.in/, accessed August26, 2014
- 12.Narahari SR, Aggithaya MG, Suraj KR. A protocol for systematic reviews of Ayurveda treatments. Int J Ayurveda Res 2010;1:254–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narahari SR, Aggithaya MG, Suraj KR. Conducting literature searches on Ayurveda in PubMed, Indian, and other databases. J Altern Complement Med. 2010;16:1225–1237 [DOI] [PubMed] [Google Scholar]
- 14.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Online document at: http://handbook.cochrane.org/, accessed August26, 2014
- 15.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301 [PMC free article] [PubMed] [Google Scholar]
- 17.Auddy B, Hazra J, Mitra A, Abedon B, Ghosal S. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. J Am Neutraceut Assoc 2008;11:50–56 [Google Scholar]
- 18.Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS One 2009;4:e6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med 2012;34:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khyati S, Anup T. A randomized double blind placebo controlled study of ashwagandha on generalized anxiety disorder. Int Ayurvedic Med J 2013;1:1–7 [Google Scholar]
- 21.Raut AA, Rege NN, Tadvi FM, et al. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med 2012;3:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narahari SR, Ryan TJ, Bose KS, Prasanna KS, Aggithaya GM. Integrating modern dermatology and Ayurveda in the treatment of vitiligo and lymphedema in India. Int J Dermatol 2011;50:310–334 [DOI] [PubMed] [Google Scholar]
- 23.Narahari SR, Ryan TJ, Aggithaya MG, Bose KS, Prasanna KS. Evidence-based approaches for the Ayurvedic traditional herbal formulations: toward an Ayurvedic CONSORT model. J Altern Complement Med 2008;14:769–776 [DOI] [PubMed] [Google Scholar]
- 24.University of York Centre for Review and Dissemination. PROSPERO. International Prospective Register of Systematic Reviews [homepage on Internet]. Online document at: http://www.crd.york.ac.uk/PROSPERO/, accessed August26, 2014
- 25.Seely D, Singh R. Adaptogenic potential of a polyherbal natural health product: report on a longitudinal clinical trial. Evid Based Complement Alternat Med. 2007;4:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]