Abstract
Selective degeneration of dopaminergic neurons in the substantia nigra underlies the basic motor impairments of Parkinson's disease (PD). Curcumin has been used for centuries in traditional medicines in India. Our aim is to understand the efficacy of genotropic drug curcumin as a neuroprotective agent in PD. Analysis of different developmental stages in model organisms revealed that they are characterized by different patterns of gene expression which is similar to that of developmental stages of human. Genotropic drugs would be effective only during those life cycle stages for which their target molecules are available. Hence there exists a possibility that targets of genotropic compounds such as curcumin may not be present in all life stages. However, no reports are available in PD models illustrating the efficacy of curcumin in later phases of adult life. This is important because this is the period during which late-onset disorders such as idiopathic PD set in. To understand this paradigm, we tested the protective efficacy of curcumin in different growth stages (early, late health stage, and transition phase) in adult Drosophila flies. Results showed that it can rescue the motor defects during early stages of life but is ineffective at later phases. This observation was substantiated with the finding that curcumin treatment could replenish depleted brain dopamine levels in the PD model only during early stages of life cycle, clearly suggesting its limitation as a therapeutic agent in late-onset neurodegenerative disorders such as PD.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease and affects approximately 1% of the population over age 50.1 Selective degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) underlies the basic motor impairments of PD. It has been postulated that free radicals, other reactive oxygen species (ROS), and reactive nitrogen species (RNS) derived from dopamine metabolism and auto-oxidation, nitric oxide reactions, lipid peroxidation, impaired mitochondrial function, and alterations in defense endogenous anti-oxidant systems may all lead to oxidative and nitrosative stress contributing to a progressive loss of DA neurons.2 Further epidemiological studies point to a relationship between increased cases of PD and exposure to environmental factors, such as agricultural agents, pesticides, and herbicides like paraquat (PQ) and rotenone.3–5 PQ has been considered a key risk factor for PD due to its structural similarity with 1-methyl-4-phenylpyridinium (MPP+), the active form of PD-inducing agent, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).6 Studies have shown that PQ penetrates the blood–brain barrier (BBB) and degenerates the DA neurons.7 It has been also shown that PQ accumulates in subcellular organelles such as mitochondria and inhibits mitochondrial complex I by producing superoxide anions and other redox products leading to PD.8 Hence, it is important to understand the relationship between exposure to herbicides and the onset of PD to develop therapeutics to this neurodegenerative disorder.
Curcumin is the main active component of turmeric, a yellow compound originally isolated from the plant Curcuma longa L. It is a member of the curcuminoid family and has been used for centuries as a spice and food additive and in traditional medicines, primarily in India; however, it is also used in other parts of Asia, including China. Curcumin has been associated with anti-oxidant, anti-inflammatory, anti-cancer, anti-viral, and anti-bacterial activities as indicated by over 6000 citations.9–12 In addition, over 100 clinical studies have been carried out with curcumin.13 Curcumin has also been shown to be neuroprotective in multiple model systems of PD. Curcumin reduces α-synuclein–induced cytotoxicity in a PD cell model.14 Furthermore, Liu et al. have shown that curcumin protects against A53T α-synuclein–induced toxicity in a PC12-inducible cell model for PD.15 Lee et al. have revealed that curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Park et al.15–17 have shown that curcumin improves mobility defects of Drosophila exposed to acute PQ toxicity. All of these studies highlight the efficacy of the curcumin as a therapeutic agent in mitigating the burden of PD.
The adult life span of Drosophila consists of a health span, a transition phase, and a senescent span.18 Analysis of these life stages in model organisms has revealed that they are characterized by different patterns of gene expression, the pattern of which is similar to that of the equivalent life stages of human. It was shown that there is significant change (about 23%) in genome-wide transcript profiles with age in Drosophila.19 Genotropic drugs would be effective only during those life cycle stages when their target molecules are available.20 Therefore, it is possible that targets of genotropic compounds such as curcumin may not be present in all life stages, which is an interesting paradigm. However, no reports are available regarding the efficacy of curcumin in PD models during later phases of adult life. This is very important because this is the period during which late-onset disorders such as idiopathic PD set in. To understand this idea, we tested the protective efficacy of curcumin in two different time points in health span (at 4–5 and 30 days) and one time point in transition phase (at 55 days) in Drosophila. Results showed that curcumin can rescue the behavioral symptoms and associated depletion in brain dopamine levels only during the health span of adult life. Our findings illustrate the limitation of curcumin as a therapeutic agent in late-onset neurodegenerative disorders such as PD.
Materials and Methods
Chemicals
PQ and curcumin were purchased from Sigma (St. Louis, MO). Whatman filter paper no.1 was used in disc-feeding experiments. Different concentrations of PQ and curcumin were prepared in 5% sucrose solution, and 250 μL of control and PQ solution was placed on filter paper. Twenty-five flies were placed per single vial. Flies were transferred to fresh vial with freshly prepared solutions at every 24 hr.
Animals and culture medium
We used male Oregon K flies (obtained from National Drosophila Stock Centre of University of Mysore, Mysore, Karnataka, India) of D. melanogaster in the present study. The flies were raised at 22°C±2°C and fed on a standard culture medium made of sucrose, yeast, agar agar, and propionic acid.21
Longevity assay
Newly eclosed flies were transferred to fresh culture medium. Twenty-five flies were placed in each vial with 3 mL of medium. All of the flies were transferred to fresh medium every third day and mortality, if any, was noted. Transfer continued until all the flies had died.
Negative geotaxis assay
A negative geotaxis assay (climbing assay) was performed as described by Botella et al.22 In brief, individual flies were dropped into the plastic tubes and allowed to acclimatize for 2 min. Then the fly was tapped to the bottom or the tube and the height it climbed in 12 sec was recorded. The experiment was repeated three times with each fly, and a minimum of 10 flies were scored for each group.
Paraquat resistance assay
To explore the effect of oxidative stress in Drosophila, males aged 4–5 days (up to this age window they were fed on normal culture medium, explained above) were transferred to a glass vials (30 mm×10 mm) containing different concentrations of PQ (2.5, 5,10, 15, 20, and 40 mM). Filter paper was soaked with different concentrations of PQ in 5% sucrose solution. The survival rate of the flies was observed and recorded at every 24 hr. Because it was established that starvation leads to alteration in cell survival pathways, flies were not starved before switching to PQ or curcumin or a combination of both. For further studies, 10 mM of PQ was employed.
Assay of curcumin toxicity and feeding
Four- to 5-day-old Drosophila males were fed on different concentrations of curcumin (25 μM to 50 mM). Mortality was recorded every 24 hr for 10 days. The concentrations at which no mortality was observed during this period were selected for further study to understand the protective efficacy of this molecule. Control flies were fed on 5% sucrose.
Curcumin co- and pre-treatment protocol
In the co-treatment regime, flies were fed with PQ (10 mM) and a combination of PQ (10 mM) and curcumin (100 μM, 500 μM, 1 mM, 1.5 mM, and 2 mM). Control flies were fed with 5% sucrose only. In the pre-treatment regime, flies were fed with curcumin for 5 days, then switched to PQ (10 mM). For the PQ treatment, flies were fed on 5% sucrose for 5 days and then transferred to PQ. Control flies were fed with 5% sucrose only. The protocol remained the same in treating flies belonging to both the stages, i.e., health span and transition phase.
Dopamine estimation with high-performance liquid chromatography
Ten heads of 5- and 55-day-old flies were homogenized in 100 μL of 0.1 M phosphate-buffered saline (pH 7.4). Homogenate was centrifuged at 6000 rpm for 10 min at 4°C. Supernatant was taken and protein quantification was performed simultaneously. A total of 50 μL of the supernatant was dissolved in an equal volume of 5% trichloroacetic acid (TCA) and centrifuged at 5000 rpm for 10 min at 4°C. Supernatant was stored at −80°C until dopamine estimation was performed using high-performance liquid chromatography (HPLC).
Results
Survivability of Drosophila
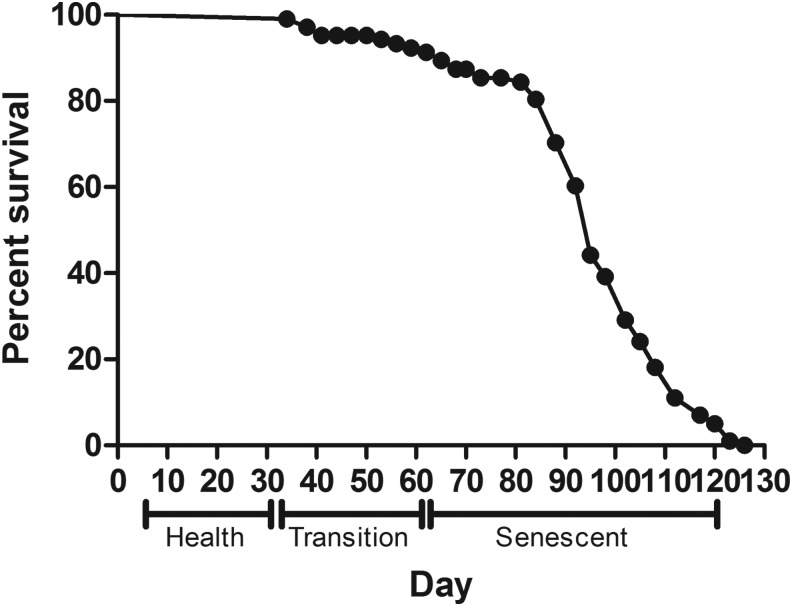
The adult life span of Drosophila consists of a health span, a transition phase, and a senescent span.18 The adult health span is distinct as the point of time when there are no natural deaths. The adult transition period is acknowledged by a slight decline in adult survival and defined by the time period wherein about 10% deaths occur (about 90% survival). The adult senescent period is defined by the steady decline of the survival curve and distinguished by the window between the end of the transition period and the maximum longevity duration (in animal studies maximum life span is typically taken to be the mean life span of the most long-lived 10% of a given cohort). Oregon K male flies were grown on normal culture medium, and survival was recorded until all the flies died. Results showed that health span extended up to 30 days; the transition phase is 31–60 days of adult span and senescent span is 61–120 days. The maximum life span was found to be 121 days, whereas the median life span was 95 days (Fig. 1).
FIG. 1.
Survival proportions of Oregon K male flies on regular culture medium. Flies were grown on normal culture medium, and survival was recorded until all of the flies died. Health span extends from day 4/5 to 30 days; the transition phase is 31–60 days of adult span, and the senescent span is 61–120 days. Maximum life span is 121 days and median life span is 95 days.
Drosophila is susceptible to PQ in a concentration-dependent manner
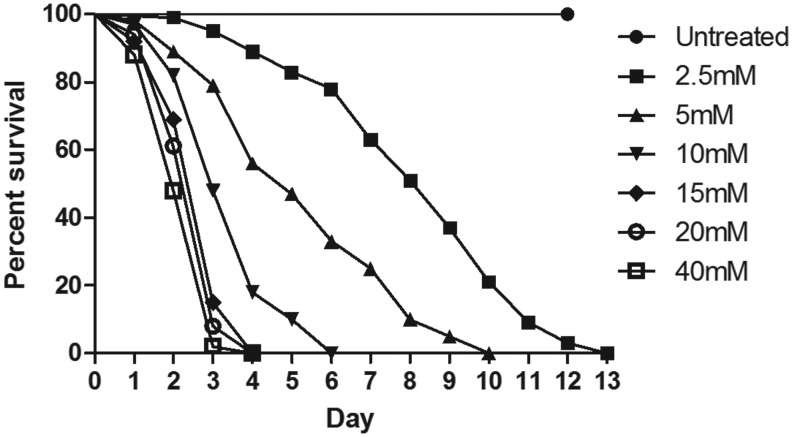
To understand Drosophila susceptibility to PQ, male Oregon K flies (4–5 days old) were exposed to different concentrations (2.5, 5, 10, 15, 20, 40 mM) of PQ. For each concentration, 100 flies (25 flies/vial) were transferred to vials with filter paper that was soaked with different concentrations of PQ in 5% sucrose solution. A 5% sucrose solution was fed for control (untreated) flies. The survival of flies was observed and recorded at every 24 hr until 100% flies were dead in the most diluted concentration of PQ solution, i.e., 2.5 mM (Fig. 2).
FIG. 2.
Concentration- and time-dependent mortality of Drosophila melanogaster (Oregon K) exposed to paraquat (PQ). Adult male flies (4–5 days) were exposed to six different concentratins of PQ (2.5, 5, 10, 15, 20, 40 mM). PQ exposure induced concentration-dependent lethality. Comparison of survival curves reveals that the response difference among different tested concentrations was significant (log-rank [Mantel–Cox test, p<0.0001]).
Comparison of survival curves showed that the response difference among all the tested concentrations was significant (log-rank [Mantel–Cox] test, p<0.0001). The survival rates at the exposed concentrations of 2.5, 5, 10, 15, 20, and 40 mM after 72 hr were 95%, 79%, 48%, 15%, 8%, and 2%, respectively. This indicates that a PQ concentration over 10 mM in the method adopted was highly toxic to the flies. While modeling PD-related pathophysiology in animal systems, including Drosophila, it is important to circumvent the toxin concentration that kills the animal. It is possible that at this point animals can die because of organismal failure due to toxin well before the dopaminergic neurons degenerated. A high concentration of PQ also generates ROS, which is characteristic of PQ toxicity; however, at that level of exposure, the anti-oxidant markers the researcher tries to estimate may not be relevant to the DA degeneration! Hence our choice was to expose the flies to 10 mM PQ and characterize the behavioral, biochemical, cytological, and molecular markers associated with PD at a 24-hr time point, where only 1%–2% mortality was observed.
Assessing curcumin toxicity
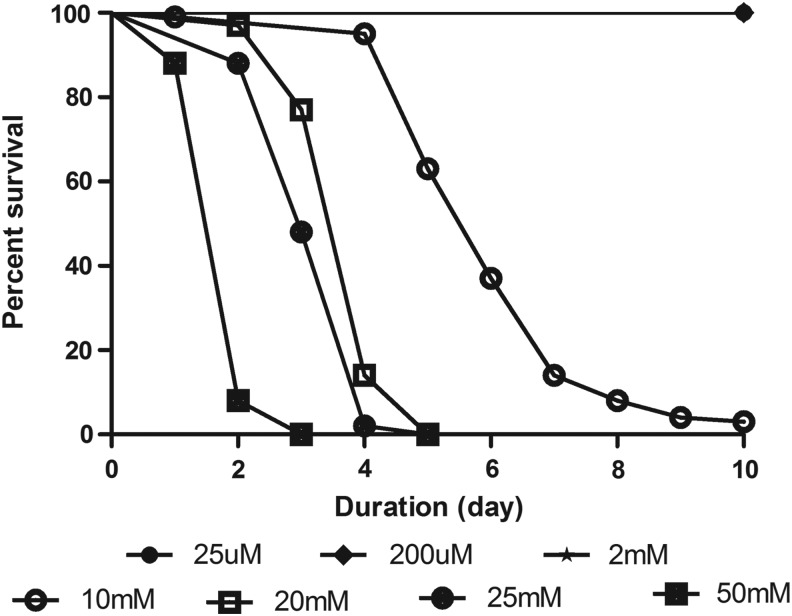
Contrary to reports of curcumin therapeutic properties, concentration-dependent curcumin toxicity has been reported in animal models.23 Therefore, we first tested a range of curcumin concentrations from 25 μM to 50 mM on 4- to 5-day-old male Drosophila for 10 days to assess potential deleterious effects. We observed that feeding of 2.5 mM curcumin or higher concentration affected the viability, whereas concentrations lower to that showed no observable toxicity (Fig. 3). All subsequent experiments employed curcumin concentrations of 2 mM or less to avoid drug-related toxicity.
FIG. 3.
Assessing curcumin toxicity. Effect of increasing concentrations of curcumin (25 μM to 50 mM) for 10 days on survival of adult male flies (4–5 days). Feeding of 2.5 mM or higher concentration affected viability, whereas concentrations lower than that exhibited no observable toxicity. Data were collected every 24 hr for each group.
Curcumin rescues flies against PQ-induced mobility defects during health span (4- to 5-day-old) under co- and pre-treatment regimens
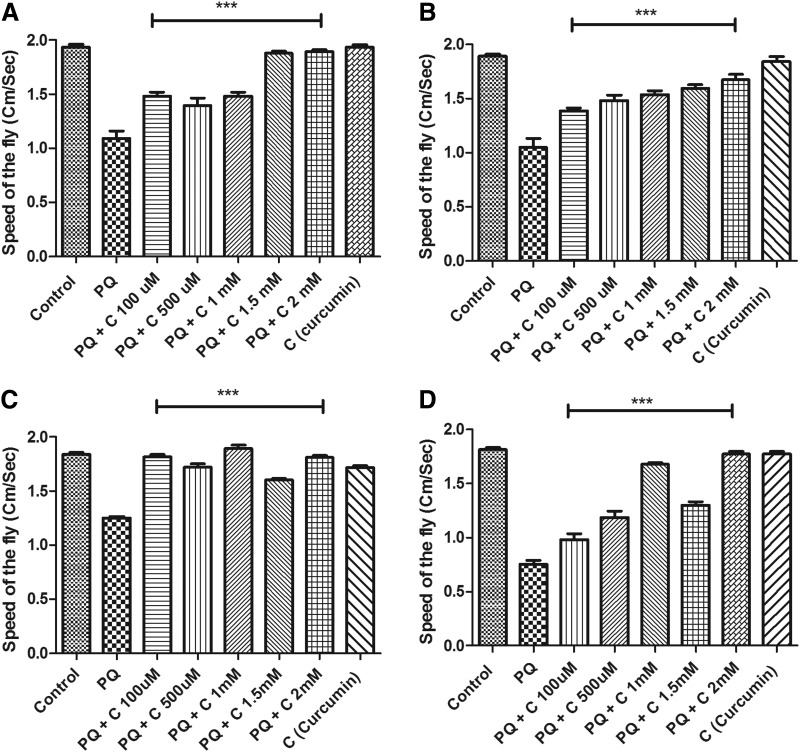
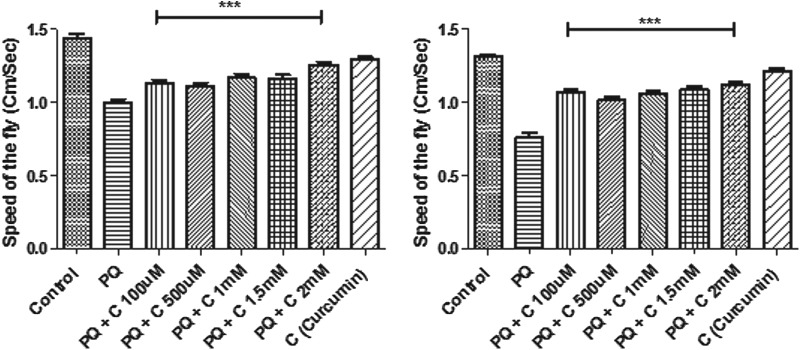
To evaluate whether curcumin could rescue the mobility defects induced by PQ, similar to those observed by Inamdar et al.,24 we employed a negative geotaxis assay, which is a perceptive indicator of the onset of dopaminergic neuron linked movement dysfunction. After 24 hr of PQ feeding, Drosophila exhibited resting tremors and bradykinesia, which are characteristic clinical symptoms associated with PD in human patients. Some of the flies tried to climb on the wall, they but they failed to hold their grip and slipped to the bottom. Some of them also exhibited an over-active/restless tendency as observed by their wing flipping (fast up and downward waving of wings). Climbing speed decreased to 56% when compared to controls. In contrast, when PQ was fed with curcumin (co-feeding regime), movement defects were not apparent, and the speed of the fly was significantly improved when compared to PQ-treated flies (Fig. 4A). Similar speed was recorded in control and curcumin only–fed flies, suggesting curcumin per se feeding has no influence on mobility. Co-feeding of curcumin also rescued the mobility defects even at 48 hr of exposure to PQ (Fig. 4B).
FIG. 4.
Curcumin (C) protects against paraquat (PQ)-induced mobility defects in 4- to 5-day-old flies under co-treatment and pre-treatment regimes as tested in a negative geotaxis assay. The distance a male fly climbs in 12 sec after 24 hr (A) and 48 hr (B) of exposure to 10 mM PQ or 10 mM PQ with different concentrations of curcumin was assayed. Feeding of curcumin alone showed no effect on speed of the fly, whereas ingestion of PQ alone adversely affected mobility. PQ and C co-feeding resulted in significant improvement in mobility performance. In the pre-treatment regime, curcumin protects against PQ-induced mobility defects after 24 hr (C) and 48 hr (D) in a negative geotaxis assay, suggesting that the rescue effect of curcumin is not due to antagonistic interaction with PQ (one-way analysis of variance [ANOVA] followed by Newman–Keuls multiple comparison test showed that the protective efficacy of curcumin in the co-treatment regime is highly significant compared to the PQ-treated group). (***) p<0.0001.
It is essential to prove that the protective effect curcumin conferring is not due to antagonistic interaction with PQ. To disprove this possibility, the pre-treatment regime was adhered to, in which flies were fed with curcumin for 5 days and then they were switched to PQ treatment. In the pre-treatment regime, curcumin also improved PQ-induced mobility defects significantly as was observed in co-treatment regime (Fig. 4C, D).
Curcumin rescues against PQ-induced mobility defects in 30-day-old Drosophila
Before checking the efficacy in the transition phase, it is essential to understand curcumin efficacy over a wide window of health span. To ascertain this, we tested the protective efficacy of curcumin in 30-day-old Drosophila, and results showed that it can rescue the climbing defects during this phase of life span (Fig. 5).
FIG. 5.
Curcumin (C) protects against paraquat (PQ)-induced mobility defects (in co-treatment regime) in 30-day-old flies after 24 hr (A) and 48 hr (B) exposure to PQ (***) p<0.0001 compared to the PQ-treated group.
Curcumin fails to rescue against Paraquat induced mobility defects during transition phase
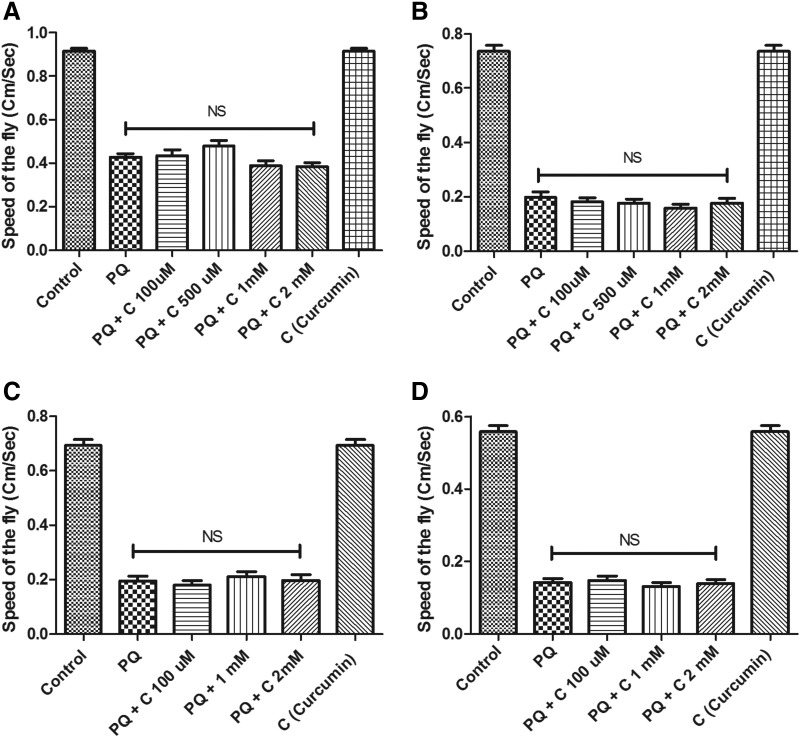
We tested curcumin efficacy in the age group of the transition phase (55 days). With far-reaching limitation of therapeutic efficacy in a neurodegenerative disorder like PD, which shows an average age of onset about 60 years, curcumin failed to rescue the climbing defects in negative geotaxis assay in both co- and pre-treatment regimen (Fig. 6) (method of treatment in both the stages, i.e., health span and transition stage is same).
FIG. 6.
Curcumin's failure to rescue paraquat (PQ)-mediated mobility defects during the transition phase. Curcumin (C) fails to protect against PQ-induced mobility defects in 55-day-old flies (transition phase) in the co-treatment regime after 24 hr (A) and 48 hr (B) of exposure and in the pre-treatment regime after 24 hr (C) and 48 hr (D) exposure to PQ. NS, lack of significance between the PQ-treated group and rescue groups.
Estimation of dopamine in Drosophila head tissue extracts
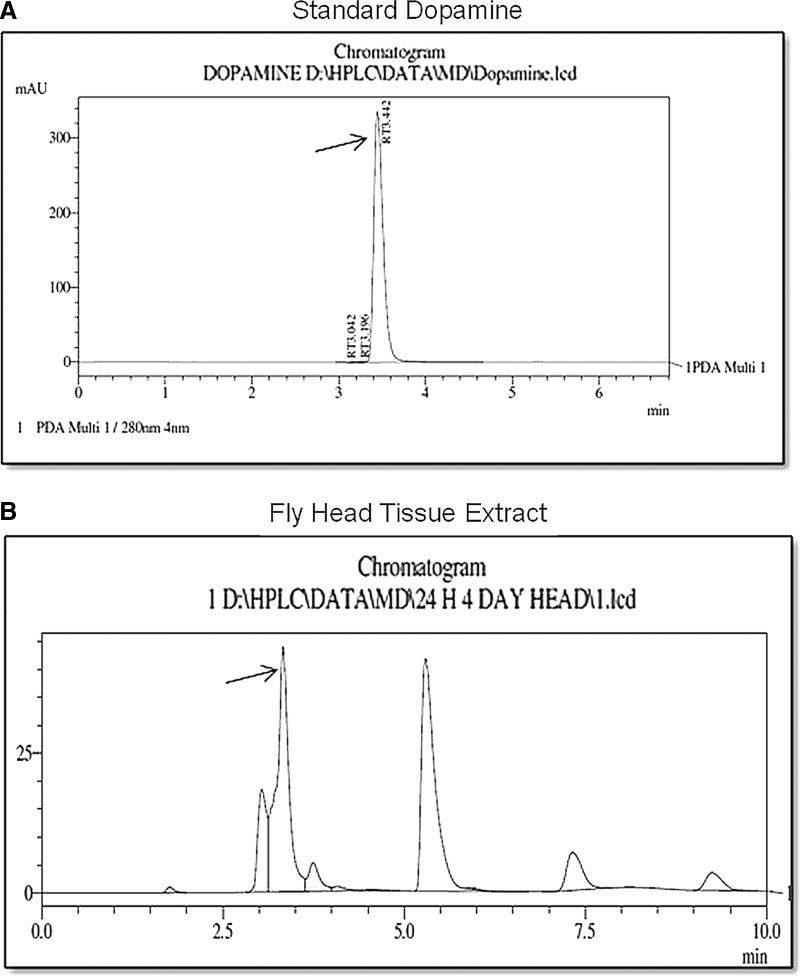
Quantification of head dopamine level was done with HPLC (Fig. 7). The chromatogram of HPLC run for standard dopamine revealed its retention time during 3.196–3.442 min, which is evident from the clear peak at the mentioned time window (Fig. 7A). The chromatogram of the run for Drosophila head tissue extracts exhibits a peak during the observed time window for standard dopamine, confirming that this is the signal picked up for dopamine (Fig. 7B).
FIG. 7.
Quantification of brain dopamine level with high-performance liquid chromatography (HPLC). Chromatogram of standard dopamine showing a retention time between 3.196 and 3.442 min (A) and chromatogram for Drosophila head tissue extract, showing a peak during the observed time window for standard dopamine (B) (peak for dopamine is pointed with an arrow in both the panels). Peaks can be seen (at 5 min and 7.5 min) much away from retention time window observed for standard dopamine that amount to artifact.
Curcumin replenishes diminished dopamine levels upon PQ exposure in Drosophila during health span but fails in the transition phase
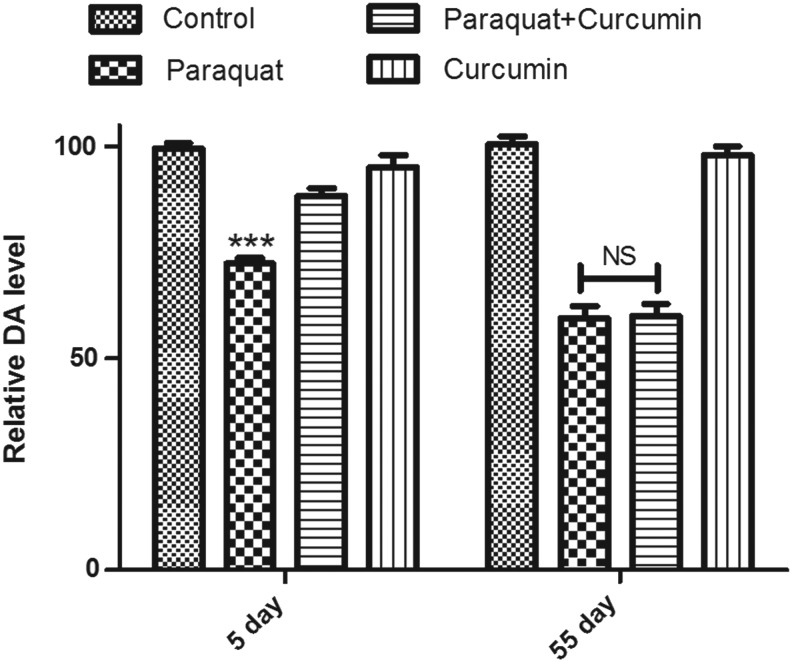
In this study, curcumin failed to improve PQ-induced mobility defects in the transition-phase flies. Hence we asked whether failing to improve the climbing phenotype during the transition phase is due to curcumin's failure in replenishing brain dopamine levels. To confirm this phenomenon, we estimated dopamine levels in head tissue extracts of control flies and flies treated with PQ, PQ and curcumin, and curcumin alone. We observed that deplenished brain dopamine levels were replenished on curcumin treatment during health span but failed during the transition phase (Fig. 8). This suggests that curcumin efficacy could be stage specific in late-onset diseases such as PD. This observation highlights its limitation as a therapeutic agent in PD. We are trying to understand if developmental feeding can rescue the levels of dopamine and mobility defects rather than stage-specific treatment (as was done here), which will have significant implication in understanding the efficacy of curcumin in treating PD.
FIG. 8.
Quantification of dopamine levels in head tissue. Relative dopamine levels in 5-day (health span) and 55-day (transition phase) fly brains exposed to 10 mM paraquat (PQ) for 24 hr. (***) p<0.0001 compared to control and curcumin alone fed. NS, lack of significance between toxin-treated group and rescue group, i.e., PQ+curcumin).
Discussion
Epidemiological studies suggest a relationship between exposure to environmental toxins such as PQ and onset of PD in later stages of life, and this may contribute as a cause of sporadic PD that constitutes more than 90% of PD cases. Our results show that Drosophila is susceptible to the neurotoxicant PQ in a concentration-dependent manner. Our specific interest is to understand the pathophysiology associated with PD before the occurrence of death and at the point of exhibiting disease phenotype such as mobility defects, thus we chose a 10 mM concentration of PQ (at 24 hr of exposure, only 1%–2% of mortality was observed). However, assays were also performed at later time point (48 hr) to understand the reinforcing protective efficacy of neuroprotectant.
Workers have reported concentration-dependent curcumin toxicity in animal models.23 To avoid this negative effect, we conducted a prolonged experiment with an array of curcumin concentrations and observed that feeding 2.5 mM or higher concentration affects the viability of Drosophila. Hence both in co- and pre-treatment regimen experiments of present study, concentrations lower than 2.5 mM were administered to avoid drug-related toxicity (Fig. 3).
The adult life span of Drosophila can be categorized into health span, transition, and senescent spans.18 The adult health span is distinct as the point of time when there are no natural deaths. The adult transition period is acknowledged by slight decline in adult survival and defined by the time period wherein about 10% deaths occur (about 90% survival). The adult senescent period is defined by the steady decline of the survival curve and distinguished by the window between the end of the transition period and the maximum longevity duration (in animal studies maximum life span is typically taken to be the mean life span of the most long-lived 10% of a given cohort).20 On the basis of survival proportions and longevity studies of the Oregon K strain of Drosophila, up to 30 days of adult life span is considered as the health span period, 31–60 days as the transition phase, and 61–120 days of life span is classified as the senescent span (Fig. 1). To understand the efficacy of therapeutic molecules in PD models, several workers either co-treat or pre-treat young animals with these molecules for a few days. They determine whether the molecule is protecting toxin-mediated DA neuronal toxicity by assessing behavioral markers such as mobility defects, biochemical markers such as levels of brain dopamine levels, or cytological markers such as degeneration of dopaminergic neurons. Park et al.17 have shown the efficacy of curcumin against acute concentration (20 mM) of PQ in enhancing survival rate, improving climbing defects.
PD causes progressive neurodegeneration, and most of the cases are late onset in nature. Different stages of the life span have different patterns of gene expression. As shown by Pletcher et al.,19 there exists significant change of about 23% in genome-wide transcript profiles with age in Drosophila, suggesting that targets of genotropic compounds such as curcumin may well not be present in all life stages. These results indicate that such genotropic compounds may have stage-specific positive effects in one stage of life span but may exert neutral or negative effects in another stage of the adult life span. Soh et al.20 have shown that curcumin increases longevity when administered in the developmental or health span stages, and surprisingly it exerts negative effect when administered over the entire adult life span or over the later stages of life, such as the transition and senescent phases, revealing a lack of genetic targets in certain stages of life cycle and suggesting its limitation with reference to a long-life phenotype.
The average age of onset of PD is about 60 years (according to www.ninds.nih.gov/disorders/parkinsons disease). Both prevalence and incidence increase with advancing age; the rates are very low in people under 40 years and rise among people in their 70s and 80s. To understand the efficacy of curcumin in ameliorating motor symptoms associated with PQ exposure, we tested efficacy in the age group of the transition phase. Our results illustrate that curcumin ameliorates PQ-induced mobility defects in Drosophila during health span (both during the 4- to 5-day and 30-day period of adult life span) in both the co- and pre-treatment regimens at 24 hr of treatment, at which time no observable mortality occurs. Curcumin improves mobility defects caused by PQ at 48 hr of treatment too, suggesting its reinforcing protective efficacy. However, curcumin fails to improve the locomotory defects in 55 days (transition phase), suggesting its limitation as a therapeutic agent in a neurodegenerative disorder such as PD.
PQ treatment leads to a depletion of brain dopamine levels in Drosophila (Fig. 8). Diminished dopamine levels are responsible for mobility defects exhibited by flies and also symptoms such as bradykinesia, akinesia, and rigidity that are characteristic clinical symptoms associated with DA degeneration in human PD patients.24
Quantification of brain dopamine levels through HPLC reveals that during health span there is significant replenishment of dopamine levels in curcumin co-treated flies; however, the failure of restoration of DA levels during transition stage fly brain emphatically suggests that genotropic drugs such as curcumin do not have target genes during this stage. Soh et al.20 have shown that curcumin feeding to adult Drosophila during only the health span results in a significantly extended health span with increased median and maximum life span. However, feeding curcumin during the transition phase results in a marginal decrease in the median life span. Interestingly, feeding during the senescent phase has no influence on functional longevity, suggesting that an extended longevity phenotype is induced by feeding curcumin in stage-specific manner. Their gene expression data supplementarily illustrates that stage-specific feeding affects certain pathways such as target of rapamycin (TOR), indicating that curcumin is a genotropic nutraceutical that acts through developmental stage-specific target pathways. This clearly emphasizes the fact that curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila.
We are wondering if multiple phenotypes such as functional longevity and dopaminergic neuronal protection and resulting improvement in motor abilities share common or overlapping genetic pathways. We are working to decipher genetic targets of curcumin in multiple phases of adult life stages of the fly that may provide exciting insights into probable therapeutic targets for PD. We are also working with multiple regimen treatment protocols to understand if levels of genetic targets can be sustained beyond the health span phase by adhering to these methods, which will have far-reaching interesting positive consequences in understanding therapeutic efficacy of curcumin subsequently in reducing the burden of PD. We are making efforts to understand if curcumin can be a prophylactic molecule with reference to life stages other than health span in PD model.
However, issues relating to bioavailability of curcumin are a matter of concern because they differ between the fly and humans. At the same time, it is fundamental to note the fact of the mechanistic similarity of curcumin's action, because workers have shown that the curcumin's genetic targets remain similar among nematode roundworms, the fruit fly Drosophila, and mouse, suggesting an evolutionarily conserved molecular mechanism(s) of its action.10
This is the first report to illustrate the fact that curcumin has limitations as a therapeutic agent in idiopathic PD, and its efficacy is limited only to the health span of adult organisms.
Acknowledgments
This research is supported by the Department of Biotechnology (DBT), Ministry of Science and Technology, India (R&D grant no. BT/249/NE/TBP/2011, 25-4-2012). We thank Dr. Dhanya, Technical Officer, and Mrs. SriRanjini, Senior Research Fellow (ICMR) at CFTRI, Mysore, for their help in running HPLC for dopamine estimation in fly head extracts, and Ms. Zevelo and Mr. Ayaz for helping with fly head dissections. Support rendered by Prof. K. VijayRaghavan, NCBS (TIFR), Bengaluru, India, as a consultant for the project is acknowledged. S.C.Y. thanks Mrs. Carol MacDonald, Scotland, UK, for reviewing the manuscript.
Part of this work was presented at the 3rd Scottish Drosophila Conference (organized by The Genetics Society, UK), held at Wolfson Medical School, University of Glasgow, Glasgow, UK on December 6, 2013, and in National Seminar on Metabolomics—A New Frontier in Natural Products Research, held at North Eastern Hill University (NEHU), Shillong, Meghalaya, India, May 23–24, 2014.
Author Disclosure Statement:
No competing financial interests exist.
References
- 1.Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, Sanges G, Stenroos ES, Pho LT, Schaffer AA, Lazzarini AM, Nussbaum RL, Duvoisin RC. Mapping of a gene for Parkinson's disease to chromosome 4q21–q23. Science 1996;274:1197–1199 [DOI] [PubMed] [Google Scholar]
- 2.Tapias V, Cannon JR, Greenamyre JT. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease. Neurobiol Aging 2014;35:1162–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology 1998;50:1346–1350 [DOI] [PubMed] [Google Scholar]
- 4.Vanacore N, Nappo A, Gentile M, Brustolin A, Palange S, Liberati A, Di Rezze S, Caldora G, Gasparini M, Benedetti F, Bonifati V, Forastiere F, Quercia A, Meco G. Evaluation of risk of Parkinson's disease in a cohort of licensed pesticide users. Neurol Sci 2002;23:2:S119–S120 [DOI] [PubMed] [Google Scholar]
- 5.Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 2004;318:225–241 [DOI] [PubMed] [Google Scholar]
- 6.Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson's disease. Cell Death Differ 2010;1115–1125 [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res 2003;976:243–252 [DOI] [PubMed] [Google Scholar]
- 8.Yumino K, Kawakami L, Tamura M, Hayashi T, Nakamura M. Paraquat- and diquat-induced oxygen radical generation and lipid peroxidation in rat brain microsomes. J Biochem 2002;131:565–570 [DOI] [PubMed] [Google Scholar]
- 9.Mythri RB, Bharath MMS Curcumin: A potential neuroprotective agent in Parkinson's disease. Curr Pharm Des 2012;18:91–99 [DOI] [PubMed] [Google Scholar]
- 10.Shen LR, Parnell LD, Ordovas JM, Lai CQ. Curcumin and aging. Biofactors 2013;39:133–140 [DOI] [PubMed] [Google Scholar]
- 11.Maruta H. Herbal therapeutics that block the oncogenic kinase PAK1: A practical approach towards PAK1-dependent diseases and longevity. Phytother Res 2014;28:656–672 [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Bang SM, Lee JW, Cho KS. Evaluation of traditional medicines for neurodegenerative diseases using Drosophila models. Evid Based Complement Alternat Med 2014;2014:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahdeo P, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of Curcumin: The golden pigment from golden spice. Cancer Res Treat 2014;46:2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang MS, Boddapati S, Emadi S, Sierks MR. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson's disease cell model. BMC Neurosci 2010;30:11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Yu Y, Li X, Ross CA, Smith WW. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol Res 2011;63:439–444 [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M. Curcumin extends life span, improves health span and modulates the expression of age associated aging genes in Drosophila melanoasger. Rejuvenation Res 2010;13:561–570 [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Jung JW, Ahn YJ, Kwon HW. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic Biochem Physiol 2012;104:118–225 [Google Scholar]
- 18.Arking R, Novoseltseva J, Hwangho DS, Novoseltsev V, Lane M. Different age specific demographic profiles are generated in the same normal-lived Drosophila strain by different longevity stimuli. J Gerontol A Biol Sci Med Sci 2002;57:B390–B398 [DOI] [PubMed] [Google Scholar]
- 19.Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol 2002;12:712–723 [DOI] [PubMed] [Google Scholar]
- 20.Soh JW, Marowsky N, Nichols TJ, Rahman AM, Miah T, Sarao P, Khasawneh R, Unnikrishnan A, Heydari AR, Silver RB, Arking R. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Exp Gerontol 2013;48:229–239 [DOI] [PubMed] [Google Scholar]
- 21.Luckinbill IS, Arking R, Clare M, Cirocco W, Buck S. Selection of delayed senescence in Drosophila melanogaster. Evolution 1984;38:996–1003 [DOI] [PubMed] [Google Scholar]
- 22.Botella JA, Ulschmid JK, Gruenewald C, Moehle C, Kretzschmar D, Becker K, Schneuwly S. The Drosophila carbonyl reductase sniffer prevents oxidative stress-induced neurodegeneration. Curr Biol 2004;14:782–786 [DOI] [PubMed] [Google Scholar]
- 23.Zhao HL, Song CH. and Chai OH. Negative effects of curcumin on liver injury induced by alcohol. Phytother Res 2012;26:1857–1863 [DOI] [PubMed] [Google Scholar]
- 24.Inamdar AA, Chaudhari A, O'Donnell The protective effect of minocycline in a paraquat induced Parkinson's disease model in Drosophila is modified in altered genetic backgrounds. Parkinson's Dis 2012;2012: http://dx.doi.org/10.1155/2012/938528 [DOI] [PMC free article] [PubMed]