Abstract
Despite advances in the understanding of its molecular pathophysiology, pancreatic cancer remains largely incurable, highlighting the need for novel therapies. We developed a chimeric antigen receptor (CAR) specific for prostate stem cell antigen (PSCA), a glycoprotein that is overexpressed in pancreatic cancer starting at early stages of malignant transformation. To optimize the CAR design, we used antigen-recognition domains derived from mouse or human antibodies, and intracellular signaling domains containing one or two T cell costimulatory elements, in addition to CD3zeta. Comparing multiple constructs established that the CAR based on human monoclonal antibody Ha1-4.117 had the greatest reactivity in vitro. To further analyze this CAR, we developed a human pancreatic cancer xenograft model and adoptively transferred CAR-engineered T cells into animals with established tumors. CAR-engineered human lymphocytes induced significant antitumor activity, and unlike what has been described for other CARs, a second-generation CAR (containing CD28 cosignaling domain) induced a more potent antitumor effect than a third-generation CAR (containing CD28 and 41BB cosignaling domains). While our results provide evidence to support PSCA as a target antigen for CAR-based immunotherapy of pancreatic cancer, the expression of PSCA on selected normal tissues could be a source of limiting toxicity.
Introduction
Pancreatic cancer remains the 4th leading cause of cancer-related deaths in the United States despite being the 10th most frequently diagnosed malignancy (Siegel et al., 2012). Most patients present with locally advanced or metastatic disease at diagnosis and are therefore not eligible for surgical resection. In addition, pancreatic cancer cells tend to be intrinsically resistant to chemo- and radiotherapy. The standard of care is gemcitabine-based chemotherapy, which reduces morbidity but does not induce a proven survival benefit. Median survival is currently estimated to be 6–8 months (Cartwright et al., 2008).
The lack of curative treatment options for patients with advanced disease has prompted researchers to assay diverse experimental approaches. Several active immunotherapy clinical trials have been conducted, including vaccination with peptides derived from tumor-associated antigens or with peptide-loaded dendritic cells, but these trials failed to provide evidence of clinical responses (Koido et al., 2011). In order to expand the repertoire of molecular targets for immunotherapy of pancreatic cancer, we generated and characterized a set of chimeric antigen receptors (CARs) directed to prostate stem cell antigen (PSCA). PSCA is a 123 amino acid glycophosphatidylinositol-anchored surface glycoprotein of unknown function (Saeki et al., 2010) initially described to be highly expressed in prostate tumors, with low basal expression in prostate epithelium, urinary bladder, kidney, esophagus, stomach, and placenta (Gu et al., 2000). Later studies demonstrated that it was overexpressed in a variety of human malignancies, including pancreatic cancer, but remained undetectable in healthy pancreas (Argani et al., 2001). A recently published randomized phase II clinical trial showed that administration of a PSCA-specific antibody in combination with gemcitabine improved the 6-month survival rate over gemcitabine alone, though there was not a significant difference in progression-free or overall survival between the two groups (Wolpin et al., 2013).
Herein we report the development and characterization of potent anti-PSCA CARs entirely derived from molecules of human origin. We further demonstrated that these human antibody-based CARs had superior surface expression and increased reactivity than a mouse antibody-derived counterpart, and elicited significant in vivo antitumor activity in a humanized mouse model of pancreatic cancer.
Materials and Methods
Gene expression analysis
Commercial RNA from normal pancreas and pancreatic ductal adenocarcinoma were purchased from Origene (CR560779, CR560781, CR560929, CR56131, CR561392, CR561533, CR560122, and CR560156; Rockville, MD). Normal tissue and tumor cDNA arrays were purchased from Clontech (MTC panels I and II; Mountain View, CA) and Origene, respectively. PSCA and mesothelin (MSLN) mRNA expression was analyzed using TaqMan primer/probe sets (Applied Biosystems, Foster City, CA). Total RNA was isolated using RNeasy Mini Kit (Qiagen, Germantown, MD) and cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix for quantitative reverse transcription polymerase chain reaction (qRT-PCR; Invitrogen, Carlsbad, CA) following the manufacturer's instructions. PSCA and MSLN mRNA expression was analyzed using TaqMan primer/probe sets Hs00245879_m1 and Hs04177224_g1, respectively (Applied Biosystems). Normal tissue and tumor cDNA arrays were purchased from Clontech (MTC panels I and II) and Origene, respectively.
A custom-designed PSCA CAR-specific TaqMan primer/probe set was used for the analysis of copies of transgene in persisting splenocytes: forward primer, CACCGTGACCGTGTCCTC; reverse primer, CTCTGGGTCAGCTGGATGTC; probe, CCGCTGCCTCCACCGC. Human PSCA (Thermo Fisher Scientific, Inc., Waltham, MA) and MSLN (Clontech SC110135) cDNA clones were utilized for the generation of standard curves.
Cell lines and primary human lymphocytes
LNCaP, DU145, HPAC, NorP1, Panc 02.03, Panc 02.13, and T3M4 cell lines were purchased from American Type Culture Collection (Manassas, VA), and cultured as instructed. Primary lymphocytes from healthy donors were cultured in AIM-V medium (Invitrogen) as described (Zhao et al., 2009).
Retroviral constructs and analysis
Ha1-4.117-based single-chain fraction variable (scFv) cDNA was derived from a human hybridoma producing a PSCA-specific antibody (Gudas, 2012). DNA was synthesized by BlueHeron (Bothell, WA), using an optimization algorithm for codon usage in humans, and cloned into NcoI and XhoI sites of pMSGV1-28Z and pMSGV1-28-BBZ vectors. A CD28-containing second-generation CAR against MSLN was generated based on previously published sequences (Li et al., 2004; Carpenito et al., 2009) and cloned into MSGV1 retroviral vector. Evaluation of interferon-gamma (IFNg) secretion by CAR-transduced T cells was performed as described. Flow cytometry analyses were performed using a FACSCanto II flow cytometer with FACSDiva software (BD Biosciences, San Jose, CA), and analyzed using FlowJo software (Tree Star, Ashland, OR). CARs were stained with biotinylated Protein-L (GenScript, Piscataway, NJ) and phycoerythrin (PE)-conjugated streptavidin (BD Bioscience), as previously described (Zheng et al., 2012). Antihuman CD3 (FITC- or APC-H7-conjugated, clone SK7) was purchased from BD Biosciences. Unless otherwise stated, peripheral blood lymphocytes (PBL) were transduced with 1:2 dilution of viral supernatant of bm2B3-28BBZ CAR or 1:4 dilution of viral supernatant of Ha1-4.117-based CARs.
Mouse model
For the mouse adoptive cell transfer (ACT) experiments, naïve CD8+lymphocytes were isolated from cryopreserved apheresis samples using the naïve CD8+T cell isolation kit (human; Miltenyi Biotec, Auburn, CA), following manufacturer's instructions. Briefly, 5×108 peripheral blood mononuclear cells were first depleted of non-naïve cells and subsequently enriched for CD8+ cells by positive selection. Lymphocytes were stimulated with anti-CD3/-CD28 beads (Dynabeads; Invitrogen) and transduced on days 2 and 3 poststimulation. Four- to six-week-old NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) female mice (The Jackson Laboratory, Bar Harbor, ME) were used for adoptive cell transfer experiments. All procedures were approved by National Cancer Institute animal care and use committee. Briefly, each mouse received a subcutaneous injection of 2×106 HPAC cells resuspended in 100 μl of 1×HBSS with 1% Matrigel. When tumors became palpable, at approximately day 15, mice were tagged and randomized into five groups, and received the indicated treatments. Human PBLs, resuspended in 500 μl of HBSS, were injected intravenously into tumor-bearing mice. In both experiments, the number of CAR-transduced CD8 T cells administered to each mouse was equalized based on flow cytometry analysis of CAR expression. In one of the experiments, mice received three daily intraperitoneal administrations of recombinant human IL-2 (Proleukin; Prometheus, San Diego, CA) at 220,000 IU in 500 μl of phosphate buffered saline (PBS). Tumor growth was monitored three times per week by a blinded observer. At the end of the experiment, spleens were harvested and filtered through a 70 μm nylon strainer. The resulting single-cell suspension was incubated in ACK Lysing Buffer (Biosource, Rockville, MD) and resuspended in PBS.
Immunohistochemistry
Excised tumors were embedded in OCT medium (Sakura Finetek USA, Inc., Torrance, CA) and frozen. Cryosections were prepared by American Histolabs Inc. (Gaithersburg, MD) and fixed with acetone before staining. PSCA staining was performed using a peroxidase-based kit as instructed by manufacturer (EnVision+System-HRP [DAB]; DAKO, Carpinteria, CA). Monoclonal mouse antihuman PSCA antibody 1G8 was kindly provided by Dr. Robert E. Reiter, University of California–Los Angeles. For fibroblast activation protein (FAP) staining, FAP-specific antibody FAP5 (Ostermann et al., 2008) or mouse IgG2a isotype control antibodies were biotinylated (EZ-Link NHS PEG4-biotin; Pierce, Rockford, IL) and used at approximately 2 μg/ml. Sections were developed with ABC reagent (VectorLabs, Burlingame, CA) and DAB substrate (Dako). FAP5 antibody was kindly provided by Pilar Garin-Chesa (Boehringer Ingelheim, Vienna, Austria). Samples were counterstained with a 50% Mayer's hematoxylin–50% Harris hematoxylin solution (Sigma-Aldrich, St. Louis, MO). Images were acquired using an Eclipse E400 microscope (Nikon Instruments Inc, Melville, NY) coupled to a Nuance Multispectral Imaging System VIS camera (PerkinElmer, Waltham, MA).
Statistical analysis
Comparisons between groups were performed using Mann–Whitney's U-test. p-Values were calculated using Prism 5.04 (GraphPad Software). Tumor growth statistics were calculated using the Wilcoxon rank-sum test, based on linear slopes of the tumor growth curves at each data point. p-Values of 0.05 or lower were considered statistically significant.
Results
PSCA mRNA expression in tumors, pancreatic cancer cell lines, and normal tissue
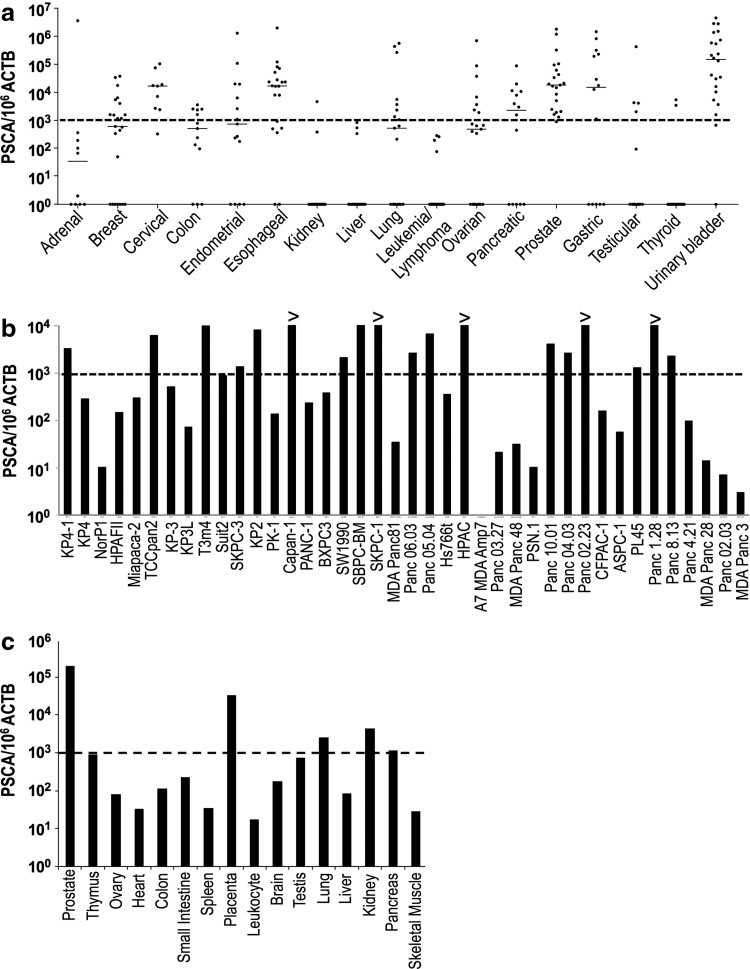
To evaluate the pattern of expression of PSCA in normal and malignant tissues, we characterized the levels of mRNA expression using qRT-PCR. First, we evaluated PSCA expression in a cDNA array derived from 437 tumor samples of diverse histologies. With a threshold of 1000 copies of PSCA per 106 copies of beta-actin (ACTB) mRNA defined as the criterion for positivity, qPCR analysis showed that the majority of specimens of pancreatic, prostatic, and urinary bladder tumors were positive for PSCA, similar to previous reports (Gu et al., 2000; Argani et al., 2001; Elsamman et al., 2006) (Fig. 1a). Interestingly, unlike a previous report (Bahrenberg et al., 2000), our results indicate that esophageal and gastric tumors were also positive for PSCA, with median expression levels comparable to those of prostate and pancreatic cancer (Fig. 1a). Moreover, positive expression of PSCA was observed in cervical tumors (Fig. 1a).
FIG. 1.
PSCA expression in tumor and normal tissues. (a) Quantitative RT-PCR analysis of PSCA expression in 437 tumor samples of 18 different histologies. Results shown for each individual sample (dots) grouped by cancer histology. Horizontal lines in each dataset represent median expression value for a given histology. Dotted line marks expression level of thousand copies of PSCA per million copies of ACTB. (b) Quantitative RT-PCR analysis of PSCA expression in 40 cell lines derived from pancreatic cancer. PSCA expression higher than a thousand copies per million copies of ACTB (dotted line) was found in 18 cell lines. “>” means higher than the upper limit of the standard curve. (c) Quantitative RT-PCR results for a panel of cDNA derived from human normal tissues. ACTB, beta-actin; PSCA, prostate stem cell antigen; RT-PCR, reverse transcription polymerase chain reaction.
We next analyzed RNA samples from 40 cell lines derived from pancreatic adenocarcinomas. As shown in Fig. 1b, 18 out of 40 cell lines demonstrated PSCA mRNA levels greater than 1000 copies per million copies of ACTB, with SKPC-1, HPAC, Panc 02.03, and Panc 8.13 having greater than 10,000 copies of PSCA per million copies of ACTB.
When we analyzed a panel of cDNA from 15 normal tissues, we found that normal prostate and placenta tissues expressed high levels of PSCA mRNA, while normal lung, kidney, and pancreas expressed a much lower amount of the transcript. PSCA expression in other normal tissues remained below 1000 copies per million copies of ACTB (Fig. 1c). These results suggest that PSCA has restricted expression in normal tissues and is highly overexpressed in a variety of human malignancies, including pancreatic cancer.
Superior antitumor activity of an anti-PSCA CAR derived from human antibody Ha1-4.117
We sought to develop a fully human anti-PSCA CAR in order to circumvent potential antimouse immune responses shown to arise in trials using mouse antibody-based CARs (Kershaw et al., 2006; Lamers et al., 2006). We designed a CAR containing the antigen-recognition domain from Ha1-4.117, a human antibody described to be highly specific in its recognition of PSCA (Gudas, 2012). Two versions of this CAR were generated; a second-generation CAR containing costimulatory molecule CD28, linked to the zeta-chain of CD3, and a third-generation design with CD28 and 4-1BB costimulatory domains linked to CD3-zeta (Fig. 2a). In addition, we generated an anti-PSCA CAR derived from humanized mouse antibody bm2b3 (Fig. 2a) (Leyton et al., 2008, 2009). These CAR sequences were inserted into retroviral vectors and used to transduce human PBL. As shown in Fig. 2b, Ha1-4.117-derived CARs were more efficiently expressed in human T cells than the bm2b3-derived CAR, and more abundantly than a control anti-Her2/neu CAR (Fig. 2b).
FIG. 2.
Human monoclonal antibody-based anti-PSCA CARs demonstrate superior expression and reactivity. (a) Schematic representation of three different anti-PSCA CAR designs. bm2B3-28BBZ is a third-generation CAR containing an antigen-recognition domain derived from a humanized mouse monoclonal antibody (bm2B3) and a signaling domain containing CD28, 4-1BB (CD137), and CD3-zeta moieties. Ha1-4.117-28Z and Ha1-4.117-28BBZ are second- and third-generation CARs, respectively, containing an antigen-binding domain derived from fully human Ha1-4.117 antibody. They differ in their signaling domain, composed of a CD28 plus CD3-zeta moiety in Ha1-4.117-28Z or CD28 plus 4-1BB (CD137) plus CD3-zeta in Ha1-4.117-28BBZ. (b) FACS analysis of CAR expression in primary human T cells. OKT3-stimulated peripheral blood mononuclear cells were transduced twice with retroviral vectors encoding the indicated CAR constructs. A Herceptin-based anti-Her2/neu CAR was used as a positive control. SA-PE: cells incubated with secondary staining (streptavidin-PE) alone as isotype control. (c) Anti-PSCA CARs endow human T lymphocytes with specific reactivity against PSCA-expressing targets. In vitro-transduced T cells were cocultured O/N with the indicated targets. IFNg release was measured in culture supernatants by enzyme-linked immunosorbent assay. Bar charts show results from representative experiments (values represent the average of duplicates) for different donors. Target cells: LNCaP (prostate cancer), LNCaP-PSCA and DU145-PSCA (prostate cancer lines engineered to express PSCA), HPAC and PANC-1 (pancreatic cancer), 624mel (melanoma), U251-vIII (glioma), NHDF-A2 (normal fibroblasts), SKOV3 (ovarian cancer), and H1299-A2 (lung cancer). White bars, bm2B3-CD28BBZ CAR; gray bars, Ha1-4.117-28Z CAR; black bars, Ha1-4.117-28BBZ CAR; dotted bars, GFP control; horizontal-line bars, mock transduced control. Ab, antibody; CAR, chimeric antigen receptor; FACS, fluorescence-activated cell sorting; IFNg, interferon-gamma.
Specific recognition of PSCA-expressing target cells was tested in coculture experiments (Fig. 2c). Antigen-specific IFNg release was observed for PSCA-expressing prostate cancer lines LNCaP-PSCA and DU145-PSCA, but not PSCA-negative LNCaP. Similarly, strong recognition of PSCA-expressing pancreatic cancer line HPAC was observed, whereas PSCA-negative lines PANC-1, 624mel, U251-vIII, NHDF-A2, SKOV3, and H1299-A2 were not recognized by PSCA CAR-transduced lymphocytes. These results demonstrated that the potent reactivity conferred by these CARs is highly antigen-specific (Fig. 2c). In four of four donors evaluated, the Ha1-4.117-based CARs induced stronger release of IFNg than the murine bm2b3-derived counterpart.
PSCA CARs induce greater reactivity against pancreatic cancer cell lines than MSLN CARs
MSLN is overexpressed in pancreatic cancer and mesothelioma cells and is currently being tested as a target in a CAR-based immunotherapy trial for pancreatic cancer (NCT01583686; www.clinicaltrials.gov). We compared the mRNA expression levels of PSCA and MSLN in pancreatic cancer samples and demonstrated that PSCA mRNA is expressed at a 10-fold higher level than MSLN mRNA (Fig. 3a). Moreover, the difference in expression between normal pancreatic tissue and pancreatic ductal adenocarcinoma samples was higher for PSCA (1000-fold) than for MSLN (10-fold), suggesting that PSCA-targeted treatments may be more selective for pancreatic cancer cells than MSLN-targeted counterparts (Fig. 3a).
FIG. 3.
PSCA and MSLN as targets for pancreatic cancer immunotherapy. (a) Quantitative RT-PCR analysis of PSCA and MSLN expression in samples of human PDA and normal tissues. Quantitation was performed in parallel for both genes in order to compare their expression in malignant and healthy tissue. (b) Surface expression of anti-PSCA and anti-MSLN CARs. Histograms showing results of protein-L staining, analyzed by FACS, of primary human T lymphocytes engineered to express the two versions of Ha1-4.117-based anti-PSCA CARs or the anti-MSLN CAR. GFP was used as a control of transduction. (c) IFNg release upon coculture of human primary T cells transduced with the indicated CARs against PSCA or MSLN. Horizontal-line bars, GFP-transduced control T cells; black bars, Ha1-4.117-28Z CAR; white bars, Ha1-4.117-28BBZ CAR; gray bars, anti-MSLN CAR; dotted bars, mock transduced control. Expression of PSCA and MSLN shown as copies of mRNA per million copies of ACTB mRNA. MSLN, mesothelin; PDA, pancreatic ductal adenocarcinoma.
We then compared the transduction efficiency of the second- and third-generation Ha1-4.117-derived anti-PSCA CARs to the anti-MSLN CAR currently used in clinical trial (NCT01583686; www.clinicaltrials.gov) and observed a greater transduction efficiency by the anti-PSCA CARs (85% and 87% vs. 29%, respectively, using protein L staining) (Fig. 3b). When human T lymphocytes expressing anti-PSCA or anti-MSLN CARs were cocultured against pancreatic cancer cell lines Panc 02.03, Panc 4.21, Panc 02.13, and KP4-1, the PSCA CAR-transduced cells released higher amounts of IFNg (Fig. 3c). Based on our observations of a much greater differential gene expression of PSCA in pancreatic cancer versus normal pancreatic tissues, along with increased transduction efficiency of the PSCA CAR vector, ACT targeting PSCA using Ha1-4.117-CAR transduced T cells may result in a more specific antitumor response than that achieved by targeting MSLN with the anti-MSLN CAR currently being tested in a clinical trial.
PSCA CAR-transduced human CD8+T cells induce potent antitumor effects in vivo
Finally, we sought to evaluate whether human lymphocytes expressing PSCA-specific CARs could mediate an antitumor response in vivo. To that end, we established a murine model of adoptive cell transfer consisting of immunodeficient NSG mice engrafted with established HPAC pancreatic cancer cells followed by intravenous infusion of CAR-engineered human CD8+ cells.
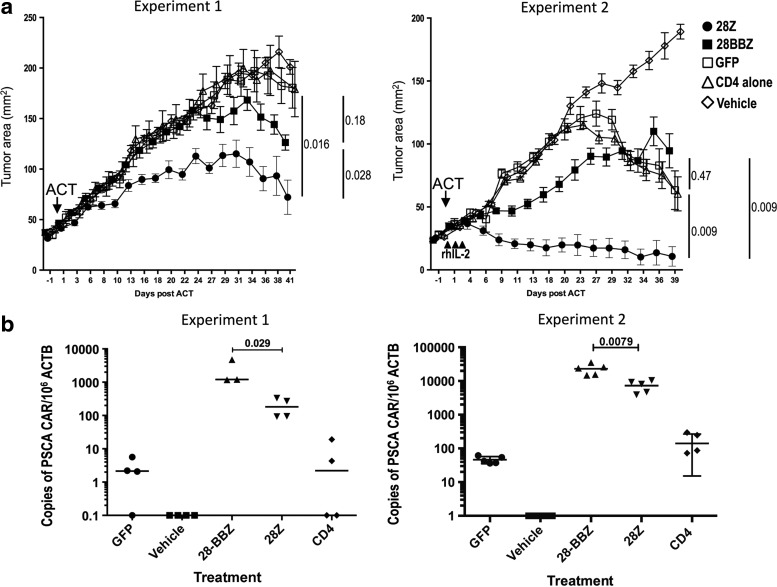
Two experiments were performed following the design shown in Supplementary Fig. S1 (Supplementary Data are available online at www.liebertpub.com/hum). HPAC tumors were implanted in subcutaneous tissue 2 weeks before ACT. Naïve human CD8+lymphocytes were retrovirally transduced with either a GFP control vector or the second- or third-generation PSCA CAR vectors. Transduced lymphocytes were administered together with untransduced CD4+cells to provide homeostatic cytokines as previously described (Gattinoni et al., 2011). In the first experiment, the group receiving cells transduced with the second-generation CAR experienced a significant reduction in tumor growth as compared with a control group treated with GFP-transduced lymphocytes (p=0.016) and with the group treated with the third-generation CAR (p=0.028; Fig. 4a). The group treated with the third-generation CAR was not distinguishable from the GFP control group (p=0.18). In the second experiment, mice received three intraperitoneal injections of rhIL-2, in addition to CD8+cells or control treatments. Under these conditions, the group treated with the second-generation CAR experienced a reduction in the size of the tumors, with two of five treated mice demonstrating tumor eradication. The tumor growth in mice treated with the second-generation CAR was significantly different from that of the groups that received the third-generation CAR (p=0.009) and the control group receiving GFP-transduced cells (p=0.009; Fig. 4a). The third-generation CAR-treated group was indistinguishable from the GFP control group, indicating that in this model the addition of a 4-1BB moiety results in a reduction of the antitumor effect induced by CAR containing a CD28 signaling domain.
FIG. 4.
PSCA CARs effectively treat established tumors in NSG mice. (a) Growth curves after treatment with CAR-expressing human T cells or controls, in two independent experiments. Tumor area expressed as mean of five tumor bearing mice±SEM. Filled circles, PSCA-28Z CAR; filled squares, PSCA-28BBZ CAR; open squares, GFP; open triangles, untransduced CD4 alone; open diamonds, vehicle alone. Arrows indicate day of adoptive cell transfer; arrowheads indicate administration of rhIL-2 intraperitoneal. (b) Number of CAR transgene in genomic DNA of splenocytes, obtained at the end of the treatment, analyzed by quantitative PCR. Undetectable expression results were assigned a value of 0.1. NSG, NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ.
At the end of the experiments, splenocytes were isolated and screened for the presence of human CD3+ cells by flow cytometry. Human lymphocytes were observed in all groups except for mice treated with vehicle alone (Supplementary Fig. S2). Persistence of gene-modified lymphocytes was analyzed by PCR for the integrated proviral DNA in splenocytes. In both experiments, the groups treated with the third-generation CAR had a significantly higher number of CAR-transgene DNA than the groups treated with the second-generation CAR. The mean numbers of integrated copies per 106 copies of ACTB in the PSCA-28BBZ and PSCA-28Z groups were 2379 versus 198 (p=0.029) in the first experiment, and 23,104 versus 7307 (p=0.0079) in the second experiment (Fig. 4b). A similar trend was observed for the number of integrated CAR cDNA in lymph node-derived T cells (Supplementary Fig. S3). These results suggest that, in this model, a higher persistence of CAR-transduced cells does not correlate with better antitumor response.
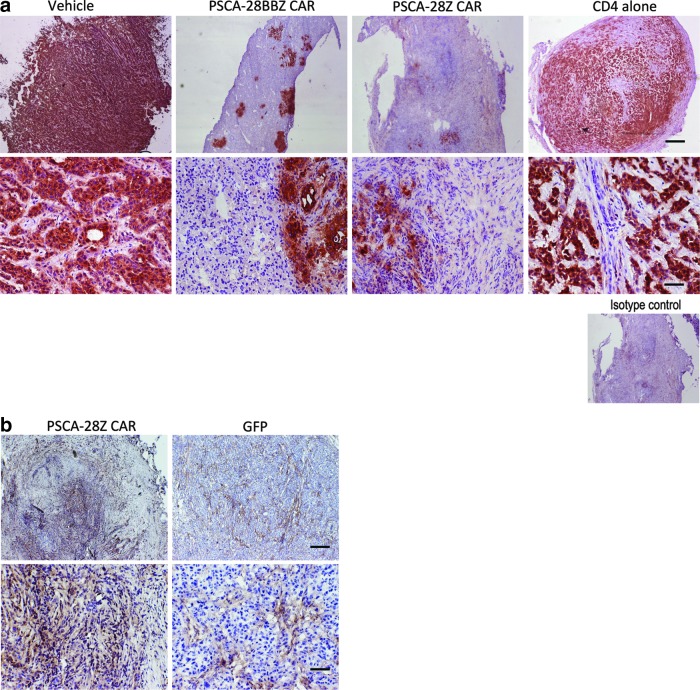
Residual tumor masses from experiment 1 were subject to immunohistochemistry. A reduction of PSCA expression was observed in the CAR-transduced T cell-treated groups, but not in control groups, supporting the notion of a target-specific antitumor effect (Fig. 5a). We also stained residual tumor masses for FAP, a protein expressed in tumor-associated stromal cells (Ostermann et al., 2008). PSCA staining and FAP staining were mutually exclusive (Supplementary Fig. S4), and while FAP expression was restricted to stromal bands in untreated tumors, the vast majority of cells remaining in treated tumor masses stained positive for FAP (Fig. 5b), suggesting that the treated residual masses were comprised predominantly of stromal cells.
FIG. 5.
Immunohistochemistry staining of PSCA CAR-treated tumors. (a) PSCA expression in remaining tumors after treatment. Immunohistochemistry of tumor sections showing strong PSCA staining in control groups and greatly reduced staining in PSCA CAR-treated mice. Scale bars represent 2000 μm in the top row and 200 μm in the bottom row. (b) Immunohistochemical analysis of FAP in PSCA-28Z CAR-treated tumor and control GFP-treated tumor. Scale bars represent 1000 μm in the top row and 200 μm in the bottom row. FAP, fibroblast activation protein.
Discussion
Recent advances in the understanding of the molecular events underlying pancreatic cancer pathophysiology have permitted the identification of genetic alterations associated with specific stages of malignant transformation. In a proposed linear progression model, PSCA upregulation is an early event, detected in premalignant pancreatic intraepithelial neoplasias (PanIN), while MSLN upregulation occurs later and is present only in fully transformed cells that have acquired metastatic potential (Feldmann et al., 2007). Thus, elimination of MSLN-expressing cells may result in reduction of tumor burden but might fail to eliminate PanINs, whereas targeting PSCA-expressing cells might also eliminate premalignant neoplastic cells, thus reducing the likelihood of relapse. Furthermore, we demonstrated that anti-PSCA CARs have superior surface expression and induce higher reactivity than an anti-MSLN CAR currently being tested in a clinical trial, suggesting that PSCA-targeted adoptive cell transfer therapies may be more efficient than MSLN-targeted adoptive cell transfer therapies in inducing antitumor responses in pancreatic cancer patients.
Several CARs have been tested in vitro and/or in animal models as potential immunotherapy agents for pancreatic cancer, targeting CEA (Chmielewski et al., 2012), EGFR (Zhou et al., 2013), CD24, or Her2/neu (Maliar et al., 2012). A report by Morgenroth et al. (2007) described a first-generation CAR against PSCA based on mouse monoclonal antibody 7F5. This design was shown to induce in vitro cytotoxicity in a mouse CTL cell line when exposed to PSCA-expressing targets as a model of prostate cancer, but no data on human primary T cells was reported (Morgenroth et al., 2007). More recently, by using a 2b3-based first-generation CAR, Katari et al. (2011) showed that CAR-transduced human T cells were able to recognize and lyse PSCA-expressing cells, but did not show any evidence of in vivo antitumor effect. The same group showed later, using a CAPAN1 intraperitoneal xenograft model in SCID mice, that intraperitoneal administration of T cells expressing the 2b3-derived CAR resulted in selection of PSCA tumor cells in vivo but failed to control tumor growth (Anurathapan et al., 2014).
In the present study, we describe the development of anti-PSCA CARs based on the fully human antibody Ha1-4.117. We demonstrate that Ha1-4.117-based CARs presented superior surface expression in human T cells and conferred higher target recognition capabilities than a CAR based on bm2b3 (a variant of 2b3 with enhanced affinity) (Leyton et al., 2009), without compromising antigen specificity. This design is expected to prevent the development of an immune response directed to the remaining murine immunoglobulin regions present in 2b3-based CARs. The majority of CARs utilized in current clinical trials contain scFv fragments derived from murine monoclonal antibodies, which may lead to the induction of humoral immune responses in the host as a reaction to the xenogeneic proteins. For instance, the development of anti-CAR immune responses in metastatic renal cell cancer patients treated with a murine anti-CAIX CAR was analyzed in detail, and it was postulated that T cell function and persistence may have been negatively impacted by this effect (Lamers et al., 2011). Most concerning, it was recently reported that repeated administration of murine CAR-engineered T cells led to development of antimouse antibody responses, associated with anaphylaxis and cardiac arrest in one subject (Maus et al., 2013). The use of a human antibody such as Ha1-4.117 instead of mouse antibodies such as 2b3 would prevent this type of reactions.
To our knowledge, this is the first report of an in vivo antitumor response using PSCA-targeted intravenous ACT approaches against established pancreatic tumors. This antitumor effect was not limited to a delay in tumor growth, but involved tumor shrinkage with replacement by stromal cells or even complete regression of two tumors when IL-2 was administered following ACT. After the submission of this article, Hillerdal et al. (2014) reported the use of a 1G8-based CAR against xenografts derived from melanoma cells engineered to express PSCA, as a model for prostate cancer. In their experimental setting, mice received three intravenous infusions of CAR-transduced cells, starting 1 day after subcutaneous injection of the tumor cells. The other two doses were administered 7 and 14 days later. This treatment resulted in a slight delay in tumor implantation in the CAR-treated group, but all tumors grew exponentially after the last infusion of CAR-T cells (Hillerdal et al., 2014). In contrast, our results showed that a single administration of human T cells transduced with a second-generation Ha1-4.117-based CAR could mediate growth delay and regression of established tumors.
Our results indicate that third-generation CARs containing a 4-1BB signaling domain in addition to a CD28 moiety may endow human T cells with a survival advantage over those expressing second-generation CARs containing only the CD28 costimulatory domain. This advantage, however, did not correlate with an enhanced antitumor effect. A similar phenomenon was described by Hombach et al. (2013), who described that cytokine-induced killer (CIK) cells expressing a second-generation CAR containing CD28-CD3zeta domains presented superior antitumor efficacy than CIK expressing a third-generation CD28-Ox40-CD3zeta CAR. In that setting, the detrimental effect of the additional costimulatory domain was attributed to increased activation-induced cell death in CIK cells expressing the third-generation CAR (Hombach et al., 2013). On the other hand, other authors postulate that third-generation CARs containing both CD28 and 4-1BB outperform second-generation CARs containing only CD28, as a general rule (Tammana et al., 2010; Zhong et al., 2010). The discrepancy between our results and previously published reports imply that the optimal CAR design might need to be empirically determined for each individual target. Interestingly, in our current clinical trials, we have not observed any correlation between transgene persistence in blood and clinical response (Robbins et al., 2011; Morgan et al., 2013).
Our results on PSCA mRNA expression in some normal tissues, together with a recent report showing PSCA expression in normal pancreas islet cells (Ono et al., 2012), are a concern for potential on-target/off-tumor toxicity, as has been observed in other CAR trials. On-target/off-tumor toxicity has been documented in patients treated for renal cell carcinoma (targeting CAIX) (Lamers et al., 2006), colorectal cancer (targeting Her2/neu) (Morgan et al., 2010), and B cell malignancies (targeting CD19) (Brentjens et al., 2010; Kochenderfer et al., 2012). Nevertheless, given the paucity of truly tumor-specific antigens to target, the outstanding target-specific reactivity conferred by our CARs suggests that PSCA deserves further consideration as a clinically relevant antitumor target in pancreatic cancer patients with otherwise no treatment options. Strategies involving the use of complementary CARs (Kloss et al., 2013) or suicide gene safety switches (Di Stasi et al., 2011) may be devised to capitalize on the potent target recognition of this CAR, avoiding the toxicity related to recognition of normal tissues.
Supplementary Material
Acknowledgments
We would like to thank Arnold Mixon, Shawn Farid, and Dave Jones for technical support. This project was supported in part by a generous contribution from the Milstein Family Foundation and by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, Bethesda, MD.
Author Disclosure Statement
No competing financial interests exist.
References
- Anurathapan U., Chan R.C., Hindi H.F., et al. (2014). Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol. Ther. 22, 623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argani P., Rosty C., Reiter R.E., et al. (2001). Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 61, 4320–4324 [PubMed] [Google Scholar]
- Bahrenberg G., Brauers A., Joost H.G., and Jakse G. (2000). Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem. Biophys. Res. Commun. 275, 783–788 [DOI] [PubMed] [Google Scholar]
- Brentjens R., Yeh R., Bernal Y., et al. (2010). Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 18, 666–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenito C., Milone M.C., Hassan R., et al. (2009). Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA 106, 3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright T., Richards D.A., and Boehm K.A. (2008). Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control 15, 308–313 [DOI] [PubMed] [Google Scholar]
- Chmielewski M., Hahn O., Rappl G., et al. (2012). T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 143, 1095–1107.e2 [DOI] [PubMed] [Google Scholar]
- Di Stasi A., Tey S.K., Dotti G., et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsamman E., Fukumori T., Kasai T., et al. (2006). Prostate stem cell antigen predicts tumour recurrence in superficial transitional cell carcinoma of the urinary bladder. BJU Int. 97, 1202–1207 [DOI] [PubMed] [Google Scholar]
- Feldmann G., Beaty R., Hruban R.H., and Maitra A. (2007). Molecular genetics of pancreatic intraepithelial neoplasia. J. Hepatobiliary Pancreat. Surg. 14, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Lugli E., Ji Y., et al. (2011). A human memory T cell subset with stem cell-like properties. Nat Med. 17, 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Thomas G., Yamashiro J., et al. (2000). Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 19, 1288–1296 [DOI] [PubMed] [Google Scholar]
- Gudas J., Jakobovits A., Jia X., et al. (2012). Antibodies and Related Molecules That Bind to PSCA Proteins (Agensys, Inc., Santa Monica, CA: ) [Google Scholar]
- Hillerdal V., Ramachandran M., Leja J., and Essand M. (2014). Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer 14, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach A.A., Rappl G., and Abken H. (2013). Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation.” Mol. Ther. 21, 2268–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katari U.L., Keirnan J.M., Worth A.C., et al. (2011). Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 13, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw M.H., Westwood J.A., Parker L.L., et al. (2006). A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss C.C., Condomines M., Cartellieri M., et al. (2013). Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 31, 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N., Dudley M.E., Feldman S.A., et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koido S., Homma S., Takahara A., et al. (2011). Current immunotherapeutic approaches in pancreatic cancer. Clin. Dev. Immunol. 2011, 267539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers C.H., Sleijfer S., Vulto A.G., et al. (2006). Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24, e20–e22 [DOI] [PubMed] [Google Scholar]
- Lamers C.H., Willemsen R., Van Elzakker P., et al. (2011). Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 117, 72–82 [DOI] [PubMed] [Google Scholar]
- Leyton J.V., Olafsen T., Lepin E.J., et al. (2008). Humanized radioiodinated minibody for imaging of prostate stem cell antigen-expressing tumors. Clin. Cancer Res. 14, 7488–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton J.V., Olafsen T., Sherman M.A., et al. (2009). Engineered humanized diabodies for microPET imaging of prostate stem cell antigen-expressing tumors. Protein Eng. Des. Sel. 22, 209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Verschraegen C.F., Mendoza J., and Hassan R. (2004). Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 24, 1327–1335 [PubMed] [Google Scholar]
- Maliar A., Servais C., Waks T., et al. (2012). Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143, 1375–1384.e1–e5 [DOI] [PubMed] [Google Scholar]
- Maus M.V., Haas A.R., Beatty G.L., et al. (2013). T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 1, 26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A., Yang J.C., Kitano M., et al. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A., Chinnasamy N., Abate-Daga D., et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth A., Cartellieri M., Schmitz M., et al. (2007). Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate 67, 1121–1131 [DOI] [PubMed] [Google Scholar]
- Ono H., Yanagihara K., Sakamoto H., et al. (2012). Prostate stem cell antigen gene is expressed in islets of pancreas. Anat. Cell Biol. 45, 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann E., Garin-Chesa P., Heider K.H., et al. (2008). Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 14, 4584–4592 [DOI] [PubMed] [Google Scholar]
- Robbins P.F., Morgan R.A., Feldman S.A., et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki N., Gu J., Yoshida T., and Wu X. (2010). Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin. Cancer Res. 16, 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., and Jemal A. (2012). Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 [DOI] [PubMed] [Google Scholar]
- Tammana S., Huang X., Wong M., et al. (2010). 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum. Gene Ther. 21, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin B.M., O'Reilly E.M., Ko Y.J., et al. (2013). Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Ann. Oncol. 24, 1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang Q.J., Yang S., et al. (2009). A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J. Immunol. 183, 5563–5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Chinnasamy N., and Morgan R.A. (2012). Protein L: a novel reagent for the detection of chimeric antigen receptor (CAR) expression by flow cytometry. J. Transl. Med. 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X.S., Matsushita M., Plotkin J., et al. (2010). Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Li J., Wang Z., et al. (2013). Cellular immunotherapy for carcinoma using genetically modified EGFR-specific T lymphocytes. Neoplasia 15, 544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.