Abstract
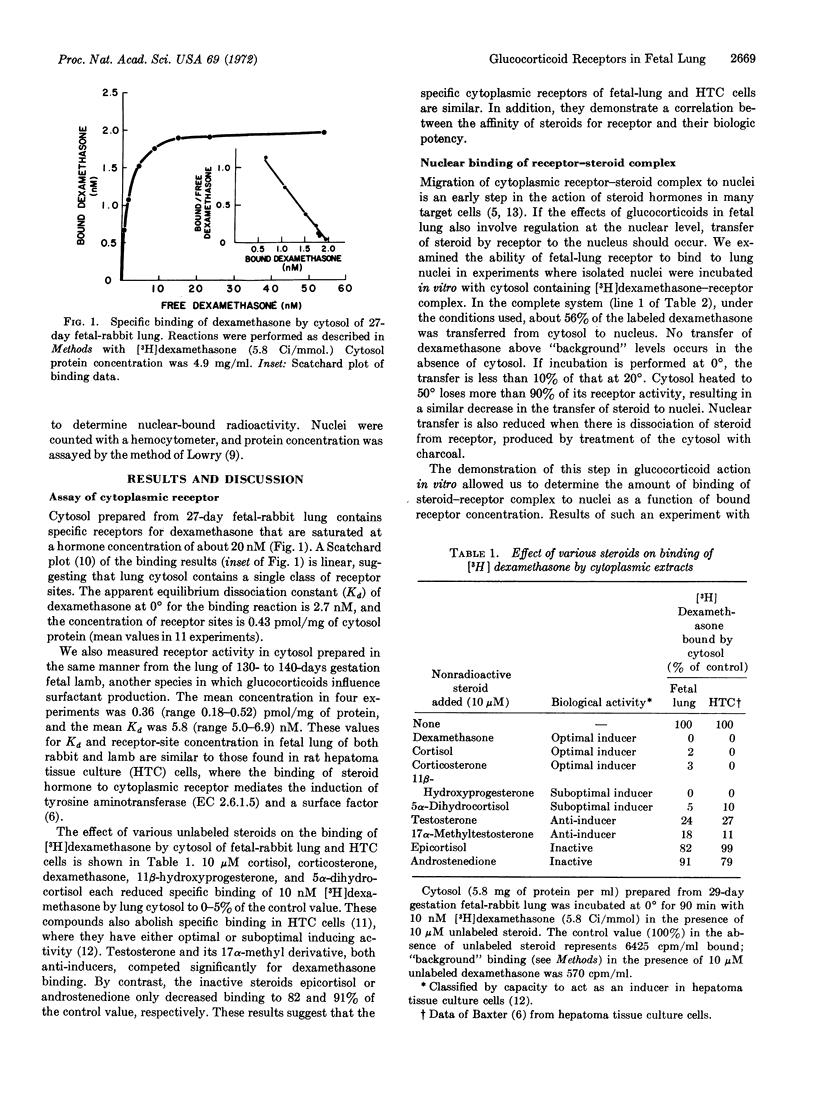
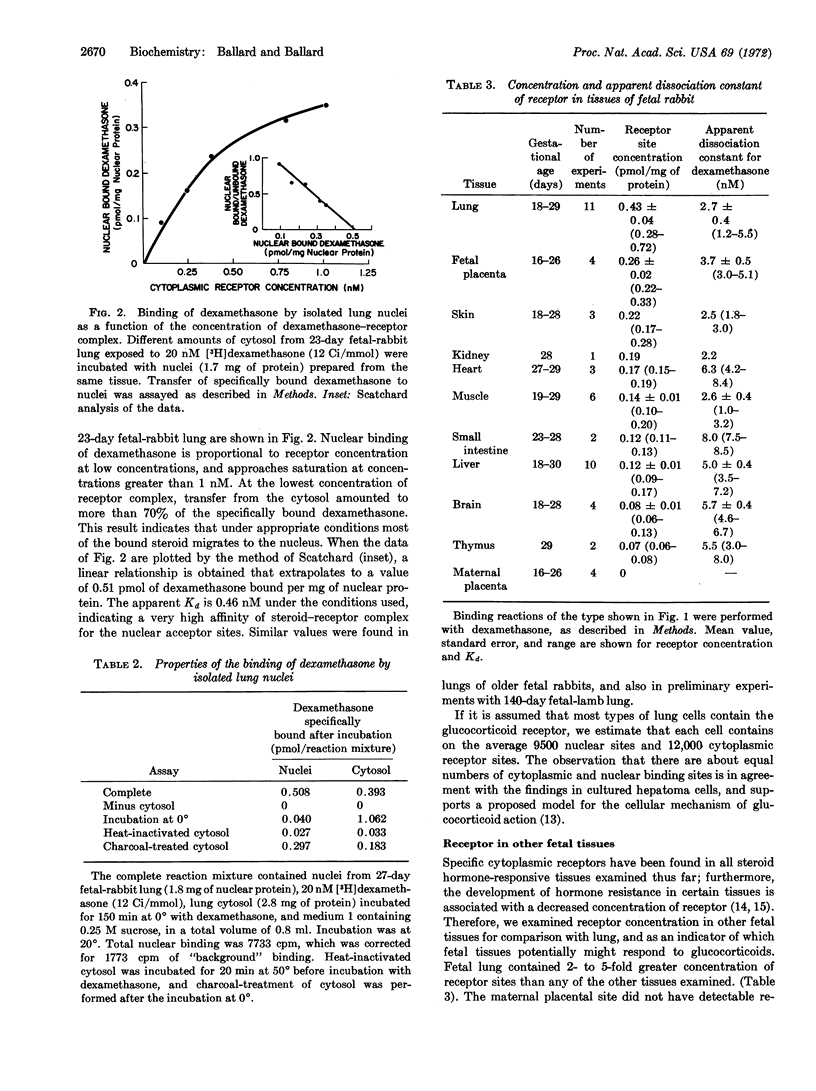
The cellular mechanism of glucocorticoid effects upon fetal lung was examined in studies of specific binding activity for corticosteroids. Cytoplasm of fetal rabbit lung contains receptor sites for [3H]dexamethasone at a concentration of 0.43 ± 0.04 pmol/mg of cytosol protein, and the apparent dissociation constant for the binding reaction is 2.7 ± 0.4 nM. The ability of various steroids to compete with labeled dexamethasone for binding to receptor correlates with their biologic potency. The hormone-receptor complex formed in vitro at 0° binds with high affinity at 20° to isolated lung nuclei. It is estimated that there are 9500 nuclear binding sites and 12,000 cytoplasmic receptor sites per fetal lung cell. During the last 12 days of gestation in a rabbit, the concentration of cytoplasmic receptor in lung is constant and is 2- to 5-times greater than receptor-site concentration in fetal skin, kidney, heart, muscle, gut, liver, brain, thymus, and placenta. These findings demonstrate that the early steps in the mechanism of glucocorticoid action in target tissues are present in lung cells, and suggest that these hormones accelerate fetal lung differentiation and surfactant production in animals by the induction of new protein synthesis mediated by receptor.
Keywords: fetal rabbit, fetal lamb, dexamethasone, nuclear sites
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. The relationship between glucocorticoid binding and tyrosine aminotransferase induction in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):709–715. doi: 10.1073/pnas.65.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. A., Platzker A. C., Tierney D. F., Hobel C. J., Creasy R. K., Margolis A. J., Thibeault D. W., Tooley W. H., Oh W. Assessment of the risk of the respiratory-distress syndrome by a rapid test for surfactant in amniotic fluid. N Engl J Med. 1972 May 18;286(20):1077–1081. doi: 10.1056/NEJM197205182862004. [DOI] [PubMed] [Google Scholar]
- DeLemos R. A., Shermeta D. W., Knelson J. H., Kotas R., Avery M. E. Acceleration of appearance of pulmonary surfactant in the fetal lamb by administration of corticosteroids. Am Rev Respir Dis. 1970 Sep;102(3):459–461. doi: 10.1164/arrd.1970.102.3.459. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M., Ohno S. Effect of the androgen-insensitivity mutation on a cytoplasmic receptor for dihydrotestosterone. Nat New Biol. 1971 Jul 28;232(30):106–107. doi: 10.1038/newbio232106a0. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G., Mulay S., Solomon S. Cortisol receptors in rabbit fetal lung. Biochem Biophys Res Commun. 1972 Apr 28;47(2):411–418. doi: 10.1016/0006-291x(72)90729-2. [DOI] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V., Borer R. C., Jr, Brenner P. H., Anderson G. G., Spellacy W. N. Diagnosis of the respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol. 1971 Feb 1;109(3):440–445. doi: 10.1016/0002-9378(71)90342-5. [DOI] [PubMed] [Google Scholar]
- Hackney J. F., Gross S. R., Aronow L., Pratt W. B. Specific glucocorticoid-binding macromolecules from mouse fibroblasts growing in vitro. A possible steroid receptor for growth inhibition. Mol Pharmacol. 1970 Sep;6(5):500–512. [PubMed] [Google Scholar]
- Jensen E. V., Numata M., Brecher P. I., Desombre E. R. Hormone-receptor interaction as a guide to biochemical mechanism. Biochem Soc Symp. 1971;32:133–159. [PubMed] [Google Scholar]
- Kikkawa Y., Kaibara M., Motoyama E. K., Orzalesi M. M., Cook C. D. Morphologic development of fetal rabbit lung and its acceleration with cortisol. Am J Pathol. 1971 Aug;64(2):423–442. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick A. F., Milholland R. J., Rosen F. Stereospecific glucocorticoid binding to subcellular fractions of the sensitive and resistant lymphosarcoma P1798. Nat New Biol. 1971 Aug;232(33):216–218. doi: 10.1038/newbio232216a0. [DOI] [PubMed] [Google Scholar]
- Kotas R. V., Avery M. E. Accelerated appearance of pulmonary surfactant in the fetal rabbit. J Appl Physiol. 1971 Mar;30(3):358–361. doi: 10.1152/jappl.1971.30.3.358. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McEwen B. S., Magnus C., Wallach G. Soluble corticosterone-binding macromolecules extracted from rat brain. Endocrinology. 1972 Jan;90(1):217–226. doi: 10.1210/endo-90-1-217. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Huff K., Jenning A., Chamness G. C. Mammary carcinoma: a specific biochemical defect in autonomous tumors. Science. 1972 Jan 21;175(4019):335–336. doi: 10.1126/science.175.4019.335. [DOI] [PubMed] [Google Scholar]
- Motoyama E. K., Orzalesi M. M., Kikkawa Y., Kaibara M., Wu B., Zigas C. J., Cook C. D. Effect of cortisol on the maturation of fetal rabbit lungs. Pediatrics. 1971 Oct;48(4):547–555. [PubMed] [Google Scholar]
- Piddington R., Moscona A. A. Precocious induction of retinal glutamine synthetase by hydrocortisone in the embryo and in culture. Age-dependent differences in tissue response. Biochim Biophys Acta. 1967 Jul 25;141(2):429–432. doi: 10.1016/0304-4165(67)90120-1. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Yalovsky U., Zelikson R., Kulka R. G. The effect of hydrocortisone on the accumulation of amylase in embryonic chick pancreas. FEBS Lett. 1969 Mar;2(5):323–326. doi: 10.1016/0014-5793(69)80054-2. [DOI] [PubMed] [Google Scholar]



