Abstract
Objective
To determine the extent to which known pre- and perinatal predictors of childhood obesity also predict weight gain in early infancy.
Study design
We studied 690 infants participating in the prospective cohort Project Viva. We measured length and weight at birth and at 6 months. Using multivariable linear regression, we examined relationships of selected maternal and infant factors with change in weight-for-length z-score (WFL-z) from 0 to 6 months.
Results
Mean (SD) change in WFL-z from 0 to 6 months was 0.23 (1.11), which translates to 4500 grams gained from birth to 6 months of life in an infant with average birth weight and length. After adjustment for confounding variables and birth weight-for-gestational age z-score (-0.28 [95% C.I. -0.37, -0.19] per unit), cord blood leptin (-0.40 [95% C.I. -0.61, -0.19] per 10 ng/ml) and gestational diabetes (-0.50 [95% C.I. -0.88, -0.11] versus normal glucose tolerance) were each associated with slower gain in WFL-z from 0 to 6 months.
Conclusion
Higher neonatal leptin and gestational diabetes predicted slower weight gain in the first 6 months of life. The hormonal milieu of the intrauterine environment may determine growth patterns in early infancy and thus later obesity.
Keywords: obesity
The prevalence of obesity among children less than 5 years of age, and even among infants, has increased dramatically in the last 30 years.1,2 Weight gain in infancy predicts later risk for obesity.3,4,5 Consequently, it has become important to identify modifiable risk factors in early life that contribute to the accumulation of excess weight.
Three systematic reviews reported strong associations between rapid weight gain in infancy and obesity later in childhood and adulthood.3,4,5 For example, Baird et al reported that more versus less rapid weight gain in the first year of life was associated with 1.2 to 5.7-fold increased risk of later obesity.5 More recent studies suggest that the strongest associations are with excess weight gain the first 3-6 months of life,6,7 and that these observations apply to gain in weight-for-length, not merely weight, which is highly associated with length.8 Recent studies have also shown that the associations are present not only for the outcome of obesity defined by body mass index, but also for direct measures of adiposity such as skinfold thickness and air-displacement plethysmography, blood pressure and other cardio-metabolic outcomes in childhood and early adulthood.8-11
A robust literature has emerged regarding pre- and perinatal predictors of childhood adiposity,12-18 including pre-pregnancy body mass index,12 excessive gestational weight gain,13,14 gestational glucose tolerance,15 maternal smoking,16 placental production of corticotrophin-releasing hormone, a proxy for fetal glucocorticoid exposure,17 and higher adiponectin and lower leptin levels in umbilical cord blood at delivery.18 However, few studies have examined whether these factors also predict weight gain in early infancy. Investigating these potential determinants of infant growth could lead to intervention strategies to prevent childhood obesity and its consequences.
The objective of this study was to determine the extent to which known pre- and perinatal predictors of childhood obesity also predict weight gain in early infancy. To address this aim, we analyzed longitudinal data from Project Viva, a prospective pre-birth cohort study of pregnant women and their children.
Methods
Study subjects were participants in Project Viva, a prospective, observational cohort study of gestational factors, pregnancy outcomes and offspring health.19 We recruited pregnant women at their initial prenatal visit at Harvard Vanguard Medical Associates, a large multi-specialty group practice in eastern Massachusetts between April 1999 and July 2002. Details of recruitment and retention procedures are available elsewhere.19 The human subjects committees of all participating institutions approved the study protocols and all mothers gave informed consent.
Of the 2128 children born to mothers in the Project Viva, we excluded 45 infants born <34 weeks gestation and 1046 without research measures of newborn length, which were missing primarily because we did not attempt to measure infants born at night or on weekends. Of the 1037 remaining infants, we obtained weight and length measurements among 690 infants at the 6-month visit (67% follow-up). When we compared the mother-infant pairs from our final cohort of 690 to the 347 excluded participants, we found that mothers in our final cohort had similar prevalence of excessive gestational weight gain (58.2% vs. 58.0%) and mean pre-pregnancy BMI (24.6 v. 25.9 kg/m2). Infants had similar mean measures of cord blood adiponectin (28.7 v. 30.4 μg/mL), leptin (9.6 v. 9.1 ng/mL) and birth weight-for-gestational age z-score (0.27 v. 0.18). However, compared with excluded mothers, mothers in our sample had lower rates of gestational diabetes (5.1% v. 7.1%) and smoking during pregnancy (9.5% vs 18.8%), and they had lower mean measures of 2nd trimester corticotrophin-releasing hormone (CRH) (146.9 v. 172.6 pg/mL). More mothers in our sample reported completion of a college degree (72.0% v. 55.9%), household income > $70, 000 (63.6%% vs. 52.8%) and were married or cohabitating (93.5% v. 86.5%). Among the infants, white race was more frequent in our final sample (69.7% v. 57.5%).
We calculated maternal BMI (kg/m2) from maternal self-report of height and weight at the start of pregnancy obtained in structured interviews during the first trimester. We calculated total gestational weight gain as the difference between the last recorded weight before delivery and the self-reported pre-pregnancy weight. We categorized women as having gained inadequate, adequate, or excessive weight according to 2009 Institute of Medicine guidelines for weight gain during pregnancy.20 We previously reported the validity of self-reported pre-pregnancy weight in our cohort.21 As part of routine clinical care, women underwent glycemic screening for gestational diabetes (GDM) between 26-28 weeks gestation with a non-fasting oral glucose challenge test, in which venous blood was sampled 1 hour after a 50-g oral glucose load. Women with a glucose concentration >140 mg/dL after the 50-g oral glucose challenge test then received a fasting, 100-g 3-hour oral glucose tolerance test. Normal results were a blood glucose <95 mg/dL at baseline, <180 mg/dL at 1 hour, <155 mg/dL at 2 hours and <140 mg/dL at 3 hours.22 Based on the results of the glycemic screening tests we formed 4 categories for our analyses:23 a) Normal glucose tolerance, defined as normal results of the 50-g test or test not done because of low-risk status; b) Failed 50-g test with normal results on the 100-g test; c) Impaired glucose tolerance (IGT), defined as failed 50-g test and 0 or 1 cutpoints on the 100-g test; and d) Gestational diabetes (GDM), defined as failed 50-g test and 2 or more cutpoints on 100-g test.
We obtained maternal smoking status by self-report during the first and second trimester.24 We categorized participants into 3 groups: (1) any smoking before pregnancy; (2) any smoking during early pregnancy; and (3) no history of smoking. We measured concentrations of maternal second trimester corticotrophin-releasing hormone (CRH, pg/mL) in plasma samples that were stored in liquid nitrogen.17 Because CRH is highly correlated with gestational age,25,26 in our analyses we corrected CRH level for gestational age at the time we obtained the blood sample. We collected cord blood samples from the umbilical vein after delivery of the infant, refrigerated whole blood for <24 hours, then spun aliquotted samples for storage in liquid nitrogen. We measured concentrations of adiponectin (μg/mL) and leptin (ng/mL) in stored cord blood plasma by radioimmunoassay.18 We abstracted infant sex, birth weight and gestational age from the medical record. We estimated fetal growth as birth weight-for-gestational age z-score from a national US reference.27
Project Viva staff weighed infants at 6 months with a digital scale (Seca Model 881; Seca Corporation, Hamburg, Germany) and measured length at birth and 6 months with a Shorr measuring board (Shorr Productions, Olney, MD). We chose change in weight-for-length z-score (WFL-z) from 0 to 6 months as our main outcome because it is more likely to represent adiposity than weight alone.28 As a secondary outcome measure we used change in weight-for-age z-score (WFA-z) and length-for-age z-score (LFA-z) from 0 to 6 months. We calculated age and sex-specific WFL-z, WFA-z and LFA-z from the Centers for Disease Control and Prevention 2000 growth chart data.29
Mothers reported information about their age, education, household income, marital status, parity, mode of infant feeding, child sex and race/ethnicity and paternal height and weight in structured interviews and questionnaires. We categorized mode of infant feeding at the 6 month visit in 4 categories: a) exclusive breast feeding; b) mixed breast and formula feeding; c) weaned from breast feeding; and d) exclusive formula feeding. From the electronic medical record, we obtained maternal systolic blood pressure during the 3rd trimester of pregnancy.
Statistical Analyses
We first examined bivariate relationships among our main exposures, other covariates, and our outcome. We ranked change in WFL-z from 0 to 6 months into quartiles, separately for boys and girls. To calculate unadjusted trend p values of each exposure across quartiles of change in WFL-z from 0 to 6 months, we used Mantel-Haenszel Chi-Square for categorical characteristics and linear regression for continuous characteristics with quartiles coded as 1 to 4. After testing model assumptions, we used multivariable linear regression models to examine the relationship between pre- and perinatal factors and change in WFL-z from 0 to 6 months. We evaluated separate models for each main exposure, in which we included covariates that were of a priori interest or confounded the main associations. Adjustment for maternal systolic blood pressure, gestational age at delivery and parity did not change effect estimates and were not included in the final models. Model 1 included the main exposure and the child's exact age in months at the 6 month visit. Model 2 also included maternal and paternal BMI, maternal age, marital status, education, household income and child race/ethnicity. To explore the potential mediating effects, in model 3, we additionally adjusted model 2 for mode of infant feeding, and in model 4 we additionally adjusted model 2 for birth weight for gestational age z-score. We performed data analysis with SAS 9.1 (SAS Institute, Cary NC).
Results
Participant characteristics are shown in Table I. The mean (SD) WFA-z at birth and 6 months were 0.18 (0.98) and 0.36 (0.91) units, respectively. Corresponding LFA-z were 0.05 (0.78) and -0.03 (0.82) units. WFL-z was 0.48 (0.79) units at birth and 0.71 (0.96) units at 6 months. Mean change in WFL-z from 0 to 6 months was 0.23 (1.11) units. This 0.23 change in WFL-z translates to 4.5 kg gained by 6 months in an infant of median birth weight and length, which is 345 g more at 6 months than an infant who remained at median weight and length.
Table 1.
Characteristics of 690 Mother-Infant Pairs in Project Viva, According to Quartile of Change in Weight for Length Z-score 0 to 6 months
| Overall | Quartile of Change in Weight for Length Z-score 0-6 months | P for Trend* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| N | 690 | 172 | 173 | 173 | 172 | |
| Mean Z-score ± SD | −1.17 ± 0.62 | −0.10 ± 0.27 | 0.60 ± 0.25 | 1.60 ± 0.60 | <0.0001 | |
| Maternal Characteristics | ||||||
| Maternal age at enrollement, mean ± SD, yrs | 32.7 ± 5.0 | 32.8 ± 5.0 | 33.0 ± 4.8 | 32.5 ± 5.1 | 32.4 ± 5.1 | 0.35 |
| Prepregnancy BMI, mean ± SD, kg/m2 | 24.6 ± 5.1 | 24.6 ± 5.3 | 24.1 ± 4.8 | 24.2 ± 4.5 | 25.6 ± 5.7 | 0.08 |
| Gestational age at delivery, mean ± SD, weeks | 39.8 ± 1.3 | 39.8 ± 1.2 | 39.8 ± 1.3 | 39.8 ± 1.4 | 39.7 ± 1.4 | 0.34 |
| Maternal systolic blood pressure during third trimester, mean ± SD, mmHg | 111.0 ± 8.1 | 110.3 ± 7.5 | 111.1 ± 8.2 | 110.9 ± 7.9 | 111.8 ± 8.7 | 0.13 |
| Placental CRH, mean ± SD, (pg/mL) | 173 ± 140 | 170 ± 145 | 176 ± 107 | 188 ± 198 | 155 ± 88 | 0.64 |
| Gestational weight gain (IOM category), % (n) | 0.51 | |||||
| Excessive | 396 (58) | 101 (60) | 104 (61) | 95 (56) | 96 (56) | |
| Adequate | 190 (28) | 44 (26) | 45 (26) | 50 (29) | 51 (30) | |
| Inadequate | 94 (14) | 24 (14) | 21 (12) | 26 (15) | 23 (14) | |
| Mother smoking status during index pregnancy, % (n) | 0.65 | |||||
| Smoked during pregnancy | 64 (10) | 15 (9) | 18 (11) | 16 (9) | 15 (9) | |
| Smoked prior to pregnancy | 142 (21) | 35 (25) | 42 (25) | 28 (16) | 37 (22) | |
| Never smoked | 466 (69) | 114 (70) | 110 (65) | 126 (74) | 116 (69) | |
| Maternal tolerance status, % (n) | 0.002 | |||||
| Gestational diabetes mellitus (Failed 50-g test, ≥ 2 high values on 100-g test) | 35 (5) | 17 (10) | 7 ( 4) | 5 ( 3) | 6 ( 4) | |
| Impaired glucose tolerance (Failed 50-g test, 1 high value on 100-g test) | 25 (4) | 9 ( 5) | 10 (6) | 6 ( 3) | 0 ( 0) | |
| Failed 50-g test, normal 100-g test | 57 (8) | 12 (7) | 18 (11) | 13 (8) | 14 (8) | |
| Normal glucose tolerance | 564 (83) | 130 (77) | 136 (80) | 148 (86) | 150 (88) | |
| Nulliparous, % (n) | 333 (48) | 74 (43) | 76 (44) | 85 (49) | 98 (57) | 0.01 |
| Household income > $70,000, % (n) | 413 (64) | 99 (62) | 109 (67) | 102 (62) | 103 (64) | 0.98 |
| College graduate or more, % (n) | 496 (72) | 127 (74) | 124 (72) | 123 (71) | 122 (71) | 0.53 |
| Married or cohabitating, % (n) | 644 (94) | 161 (94) | 162 (94) | 160 (93) | 161 (94) | 0.84 |
| Child Characteristics | ||||||
| Birth weight-for-Gestational Age z-score mean ± SD | 0.27 ± 0.95 | 0.52 ± 0.98 | 0.35 ± 0.96 | 0.20 ± 0.92 | 0.00 ± 0.87 | <0.0001 |
| Cord blood leptin, mean ± SD, (ng/mL) | 9.6 ± 6.6 | 12.4 ± 8.0 | 10.0 ± 6.2 | 7.6 ± 5.3 | 7.8 ± 5.3 | <0.0001 |
| Cord blood adiponectin, mean ± SD, (μg/mL) | 28.7 ± 6.0 | 29.9 ± 6.0 | 29.0 ± 6.9 | 27.8 ± 7.1 | 27.9 ± 6.3 | 0.02 |
| Female, % (n) | 348 (50) | 87 (51) | 87 (50) | 87 (50) | 87 (51) | 1.00 |
| White, % (n) | 481 (70) | 112 (65) | 129 (75) | 118 (68) | 122 (71) | 0.85 |
| Mode of infant feeding, % (n) | 0.81 | |||||
| Exclusive breastfeeding | 178 (26) | 42 (24) | 49 (28) | 45 (26) | 42 (24) | |
| Mixed breast and formula | 177 (26) | 46 (27) | 45 (26) | 45 (26) | 41 (24) | |
| Weaned from breast feeding | 266 (39) | 64 (37) | 62 (35) | 69 (40) | 71 (41) | |
| Exclusive formula feeding | 69 (10) | 20 (12) | 17 (10) | 14 (8) | 18 (10) | |
| Age at 6 month visit, mean ± SD, months | 6.5 ± 0.7 | 6.5 ± 0.7 | 6.6 ± 0.7 | 6.5 ± 0.7 | 6.6 ± 0.8 | 0.23 |
| Paternal BMI, mean ± SD | 26.3 ± 3.9 | 25.9 ± 3.6 | 26.7 ± 4.4 | 25.8 ± 3.4 | 26.7 ± 3.8 | 0.23 |
Based on Mantel-Haenszel chi-square test for categorical variables and linear regression for continuous variables
Bivariate analyses showed that infants who had higher birth weight-forgestational age, higher cord blood leptin and adiponectin, who were born to multiparous mothers, and mothers with gestational diabetes had smaller change in WFL-z from 0 to 6 months, representing slower weight gain (Table I). Maternal age, marital status, CRH level, smoking status, and systolic blood pressure, gestational weight gain category, paternal BMI, gestational age at delivery, household income, child race and mode of infant feeding did not vary by quartile of change in WFL-z from 0 to 6 months (Table I).
In multivariable models adjusting for parental BMI and socioeconomic factors (Table II, Model 2), we observed that infants born to mothers with gestational diabetes (-0.60 [95% C.I. -0.99, -0.21]) and impaired glucose tolerance (-0.63 [95% C.I. -1.11, -0.15]) had slower infant weight gain in the first 6 months of life compared with infants born to mothers with normal glucose tolerance. Additional adjustment for mode of infant feeding in categories (Table II, Model 3) or breastfeeding duration as a continuous measure (data not shown) did not materially change in the magnitude of the association of gestational diabetes and impaired glucose tolerance with infant weight gain. To explore the extent to which the association between maternal glucose tolerance and change in WFL-z from 0 to 6 months is mediated by fetal growth, we additionally adjusted birth weight-for-gestational age z-score. In that analysis, the effect estimates for gestational diabetes (-0.60 to -0.50) and impaired glucose tolerance (-0.63 to -0.51) were modestly attenuated (Table II, Model 4).
Table 2.
Associations of Pre- and Perinatal Factors and Change in Weight-for-Length z-score from 0 to 6 months
| N (% of participants) | Model 1: Predictora | Model 2: Model 1 + covariatesb | Model 3: Model 2 + Mode of infant feeding | Model 4: Model 2 + BW for GA-z scorec | |
|---|---|---|---|---|---|
| Pre- and Perinatal Factors | Effect Estimate (95% C.I.) for change in WFL-z from 0 to 6 months | ||||
| Pre-pregnancy BMI (5 kg/m2) | 688 (100%) | 0.04 (−0.04, 0.12) | 0.03 (−0.07, 0.12) | 0.02 (−0.07, 0.12) | 0.05 (−0.04, 0.14) |
| Gestational weight gain IOM category | 680 (99%) | ||||
| Excessive | −0.07 (−0.26, 0.13) | −0.10 (−0.30, 0.11) | −0.10 (−0.30, 0.11) | 0.04 (−0.17, 0.24) | |
| Adequate | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | |
| Inadequate | −0.08 (−0.35, 0.20) | −0.08 (−0.37, 0.21) | −0.08 (−0.37, 0.21) | −0.09 (−0.37, 0.19) | |
| Maternal glucose tolerance | 681 (99%) | ||||
| Gestational diabetes | −0.57 (−0.95, −0.20) | −0.60 (−0.99, −0.21) | −0.60 (−1.00, −0.21) | −0.50 (−0.88, −0.11) | |
| Impaired glucose tolerance | −0.67 (−1.11, −0.23) | −0.63 (−1.11, −0.15) | −0.63 (−1.11, −0.15) | −0.51 (−0.97, −0.04) | |
| Failed 50-g, passed 100-g test | −0.15 (−0.45, 0.15) | −0.20 (−0.51, 0.12) | −0.20 (−0.51, 0.12) | −0.09 (−0.40, 0.22) | |
| Normal glucose tolerance | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | |
| Maternal smoking during pregnancy | 672 (97%) | ||||
| Never smoked | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | |
| Smoked prior to pregnancy | 0.02 (−0.19, 0.22) | 0.02 (−0.20, 0.24) | 0.02 (−0.20, 0.25) | 0.03 (−0.19, 0.24) | |
| Smoked during pregnancy | 0.10 (−0.19, 0.39) | 0.09 (−0.22, 0.40) | 0.07 (−0.25, 0.38) | 0.04 (−0.26, 0.35) | |
| Placental CRH (100 pg/mL) | 357 (52%) | −0.02 (−0.11, 0.06) | −0.03 (−0.12, 0.06) | −0.03 (−0.12, 0.06) | −0.02 (−0.11, 0.06) |
| Cord blood leptin (10 ng/mL) | 321 (47%) | −0.54 (−0.73, −0.36) | −0.56 (−0.76, −0.36) | −0.57 (−0.77, −0.37) | −0.40 (−0.61, −0.19) |
| Cord blood adiponectin (10 μg/mL) | 320 (46%) | −0.20 (−0.40, 0.01) | −0.19 (−0.40, 0.01) | −0.19 (−0.39, 0.02) | −0.12 (−0.32, 0.07) |
| Birth weight-for-gestational age z-score | 690 (100%) | −0.25 (−0.33, −0.16) | −0.28 (−0.37, −0.19) | −0.28 (−0.37, −0.19) | n/a |
Model 1 unadjusted (child age at 6 m only)
Model 2 adjusted for pre-pregnancy BMI, paternal BMI, maternal age, marital status, education, income, child race/ethnicity
Birthweight for gestational age z-score
In analyses examining leptin as the main predictor, we found that for each 10 ng/mL increment of cord blood leptin, change in weight-for-length z-score was lower in the first 6 months of life by 0.56 units (95% C.I. -0.76, -0.36) (Table II, Model 2). We found no material change in this association when we included mode of infant feeding in our analysis either as a categorical (Table II, Model 3) or as a continuous variable (data not shown). After adjusting for birth weight-for-gestational age z-score, which represents fetal growth, the effect was somewhat attenuated (-0.40 [95% C.I. -0.61, -0.19]) (Table II Model 4).
We also found that for each unit increment of birth weight-for-gestational age z-score, weight-for-length gain was 0.28 units lower (95% C.I. -0.37, -0.19) (Table II Model 2), suggesting that infants with greater fetal growth have slower infant weight gain.
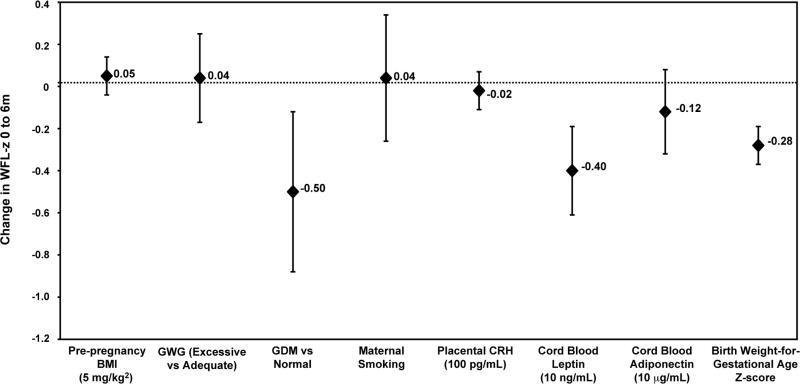
We did not observe direct associations of pre-pregnancy BMI, gestational weight gain, maternal smoking, placental CRH, or cord blood adiponectin and change in WFL-z score from 0 to 6 months (Table II). Results from final regression estimates (Table II, Model 4) of associations of pre-and perinatal factors and change in WFL-z from 0 to 6 months are shown in the Figure.
Figure 1.
Regression estimates of associations of pre- and perinatal factors and change in weight-for-length z-score (WFL-z) from 0 to 6 months, adjusted for pre-pregnancy BMI, paternal BMI, maternal age, marital status, education, income, child age at 6 month visit, child race, and birth weight for gestational age z-score (fetal growth) (Table II, Model 4). BMI = body mass index, GWG = gestational weight gain, GDM = gestational diabetes, CRH = corticotrophin releasing hormone
We found similar results in our secondary outcome of change in WFA-z and LFA-z from 0 to 6 months. In adjusted models we found that infants born to mothers with gestational diabetes (-0.29 [95% C.I. -0.61, -0.02]) and impaired glucose tolerance (-0.41 [95% C.I. -0.79, -0.03]) had slower change in WFA-z in the first 6 months of life compared with infants born to mothers with normal glucose tolerance. We observed that for each 10 ng/mL increment of cord blood leptin, change in WFA-z and LFA-z was lower in the first 6 months of life by 0.31 units (95% C.I. -0.48, -0.13) and -0.13 units (95% C.I. -0.26, 0.00), respectively. In addition, we found an association between excessive gestational weight gain and lower gain in WFA-z and LFA-z from 0 to 6 months (-0.16 [95% C.I. -0.33, 0.00] and -0.19 [95% C.I. -0.32, -0.06], respectively), which was not present in the WFL-z analysis (0.04 [95% C.I. -0.17, 0.24]).
Discussion
In this prospective cohort study, we found that gestational diabetes, a known determinant of later childhood adiposity, predicted slower infant weight gain in the first 6 months of life. This finding was somewhat surprising, given that more rapid infant weight gain predicts greater childhood adiposity. Our result that higher cord blood leptin predicted slower growth, however, is consistent with its association with lower BMI at age 3,18 and raises the possibility that intrauterine leptin exposure may serve as an important factor determining energy metabolism during infancy and thus risk for later obesity.
We confirmed findings from previous cohort studies that infants who are larger at birth have slower weight gain in the first 6 months of life, a phenomenon that may represent postnatal regulation toward genetically driven growth trajectories.30 Our finding of slower weight gain among infants born to mothers with gestational diabetes, even after adjustment for fetal growth, is consistent with an earlier study by Silverman, et al, in which infants born to mothers with gestational diabetes had decreased growth velocity in the first year of life and increased growth thereafter,31 compared with a population reference standard. Gestational diabetes stimulates fetal hyperglycemia, which results in fetal hyperinsulinemia and consequent fetal growth and macrosomia.32 After birth, hypoglycemia may ensue and in turn, changes in blood insulin levels. This sequence of events may lead to slower weight gain in the first several months of life. It is not known why infants of diabetic mothers gain more weight later in childhood.
Our finding that higher cord blood leptin is associated with slower infant weight gain was similarly demonstrated by Ong et al, among infants in the first 2 years of life.33 We previously described an association of higher cord blood leptin and lower 3 year BMI z-score from our same cohort.18 Our results suggest that slower infant growth may serve as an intermediate step between intrauterine leptin exposure and BMI z-score at age 3.
Leptin is an adipocyte-derived hormone that causes decreased appetite and energy intake through actions in the hypothalamus.34 Among obese older children and adults who develop leptin resistance, higher leptin levels predict weight gain, and exogenous leptin administration is not effective in weight loss.35 However, the findings that cord blood leptin is associated with slower infant weight gain (this study) and lower adiposity at age 318 suggests that leptin resistance has not yet taken hold at these earlier ages.
Animal studies support this hypothesis. Vickers et al showed that leptin administration to offspring of undernourished pregnant rat mothers during postnatal day 3 to 13 abolished the otherwise observed increase in energy intake, body weight and fat mass and decreased locomotor activity.36 The physiological effects of leptin during this critical period of development may reflect a direct neurotrophic effect of leptin on the hypothalamus.37 Bouret et al showed that the neuronal projections from the hypothalamus were permanently disrupted in leptin deficient mice.37 Leptin supplementation in neonatal mice, but not adult mice, led to increased neuronal projections.37 These projections relay information to areas of the brain that are important for regulation of feeding and energy balance.37 This early postnatal period in the mouse and rat likely corresponds to the 3rd trimester in the human.
Taken together, these studies support the hypothesis that leptin plays a critical role during a period of developmental plasticity in late gestation. Our finding that elevated cord blood leptin was associated with slower growth in early infancy supports the premise that intrauterine exposure to excess leptin may moderate fat accretion after birth by decreasing appetite and increasing metabolism during infancy and early childhood.
Because many studies show that formula feeding rather than breastfeeding is associated with higher risk of obesity, mode of infant feeding has been a leading explanation for the observed association between rapid weight gain in early infancy and later obesity. However, both observational and experimental evidence suggests that formula feeding is actually associated with less, not more, rapid weight gain in the first few months of life.38 Our findings suggest that the hormonal milieu of the intrauterine environment might be more important than mode of infant feeding in determining how weight gain in infancy raises the risk of later obesity.
Strengths of this study include its prospective study design and evaluation of multiple pre- and perinatal factors that can alter infant growth, as well as a wide range of potential confounders. Although we used weight-for-length measurements, which are more likely to represent adiposity than weight alone, repeated direct measures of adiposity in infancy would be better still. Our main outcome was infant growth from birth to 6 months; we did not evaluate change in weight in shorter time periods within the first six months, nor did we examine increasing weight gain beyond 6 months of age. We included only a subset of the original study cohort due to missing length measures and loss to follow-up from 0 to 6 months. Other limitations include the relatively high socioeconomic status of our participants, which may reduce generalizability. We adjusted for maternal education, household income, child race and mode of infant feeding and did not find material change in our effect estimates. However, we cannot completely exclude the possibility of selection bias. Additional data on the short and long-term effects of intrauterine exposure to leptin and associated hormones are needed to clarify early determinants of obesity and its consequences.
Acknowledgments
Funded by grants from National Institute of Health (HD 034568, HL 064925, HL 068041). The authors declare no conflicts of interest.
Abbreviations
- WFL-z
Weight-for-length z-score
- WFA-z
Weight-for-age z-score
- LFA-z
Length-for-age z-score
- BMI
Body mass index
- CRH
Corticotrophin-releasing hormone
- GDM
Gestational diabetes
- IGT
Impaired glucose tolerance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Peterson KE, Scanlon KS, Fitzmaurice GM, Must A, Oken E, et al. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity (Silver Spring) 2006;14:1107–12. doi: 10.1038/oby.2006.126. [DOI] [PubMed] [Google Scholar]
- 3.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro PO, Victoria CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143–54. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 5.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botton J, Heude B, Maccario J, Ducimetière P, Charles MA. Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr. 2008;87:1760–8. doi: 10.1093/ajcn/87.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Ciruculation. 2005;111:1897–903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 8.Taveres EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth and blood pressure at three years of age. J Pediatr. 2007;151:670–4. doi: 10.1016/j.jpeds.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekelund U, Ong K, Linné Y, Neovius M, Dunger DB, Wareham NJ, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am J Clin Nutr. 2006;83:324–30. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 11.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–42. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, Hauguel de Mouzon S, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. 2009;13:839–46. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 14.Shack-Nielsen L, Mortensen EL, Michaelsen KF, Sorensen T. High maternal pregnancy weight gain is associated with increased risk of obesity in childhood and adulthood independent of maternal BMI. Ped Research. 2005;58:1020. (abstr) [Google Scholar]
- 15.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 16.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to 8 years. Int J Epidemiol. 2006;35:121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW, Rich-Edwards JW, Huh S, Majzoub JA, Oken E, Taveras EM, et al. Maternal corticotrophin-releasing hormone levels during pregnancy and offspring adiposity. Obesity (Silver Spring) 2006;14:1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:1–8. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine . Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 21.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J of Obstet and Gynecol. 2007;196:322, e1–322-e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association Standards of medical care in diabetes- 2008. Diabetes Care. 2008;31:S13–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 23.Herring SJ, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Kleinman KP, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201:61, e1–7. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Huh SY, Taveras EM, Rick-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–8. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97:657–63. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 26.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benn RT. Some mathematical properties of weight-for-height indices used as measures of adiposity. Br J Prev Soc Med. 1971;25:42–50. doi: 10.1136/jech.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics [June 19, 2003];CDC growth charts: United States. 2000 Available at: www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm.
- 30.Karlberg J, Luo ZC. Foetal size to final height. Acta Paediatr. 2000;89:632–6. doi: 10.1080/080352500750043909. [DOI] [PubMed] [Google Scholar]
- 31.Silverman BL, Rizzo T, Green OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40:121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 32.Schartz R, Teramo KA. Effects of diabetic pregnancy on the fetus and newborn. Semin Perinatol. 2000;24:120–35. doi: 10.1053/sp.2000.6363. [DOI] [PubMed] [Google Scholar]
- 33.Ong K, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. Cord Blood Leptin is Associated with Size at Birth and Predicts Infancy Weight Gain in Humans. J Clin Endocrinol Met. 1999;84:1145–8. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- 34.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 35.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 36.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 37.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 38.Kramer MS, Guo T, Platt RW, Shapiro S, Collet JP, Chalmers B, et al. Breastfeeding and infant growth: biology or bias? Pediatrics. 2002;110:343–347. doi: 10.1542/peds.110.2.343. [DOI] [PubMed] [Google Scholar]