Abstract
The first examples of catalyst-controlled stereoselective macrocyclic ring-closing metathesis reactions that generate Z-enoates as well as (E,Z)- or (Z,E)-dienoates are disclosed. Reactions promoted by 3.0–10 mol % of a Mo-based monoaryloxide pyrrolide complex proceed to completion within 2–6 h at room temperature. The desired macrocycles are formed in 79:21 to >98:2 Z/E selectivity; stereoisomerically pure products can be obtained in 43–75% yield after chromatography. Utility is demonstrated by application to a concise formal synthesis of the natural product (+)-aspicilin.
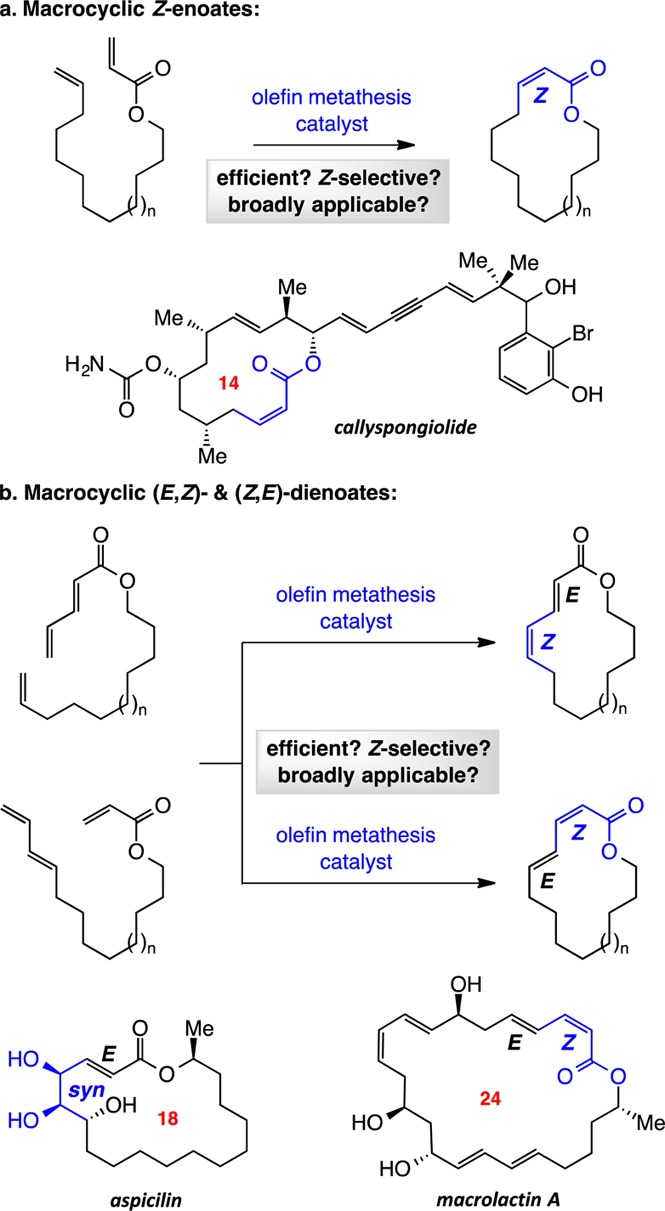
Macrocyclic structures containing a carbonyl unit conjugated to one or more alkenes are present in many biologically active molecules.1 Other than ester bond-forming reactions, Horner–Emmons-type transformations and catalytic ring-closing metathesis (RCM) are two distinct approaches that allow access to large-ring E-enoates. Reactions between an aldehyde and a stabilized phosphonium ylide represent a reliable way of synthesizing macrocycles; RCM, however, obviates the need for protecting group manipulations and/or oxidation state adjustments that often accompany the involvement of a carbonyl group. Macrocyclic Z-enoates can be obtained by reactions performed under modified Horner–Emmons conditions,2 through the use of Still–Gennari-type phosphonate esters3 or by partial hydrogenation of ynoates.4 Catalyst-controlled Z-selective RCM has the potential to give macrocyclic Z-enoates efficiently, facilitating synthesis of molecules such as callyspongiolide5 (Scheme 1a).6 Further, RCM offers an attractive option for preparing macrocyclic (E,Z)-7 or (Z,E)-dienoates; macrolactin A8 is an example of a natural product that contains such a moiety (Scheme 1b). Alternatively, the dienoate unit may be site-selectively and diastereoselectively functionalized;9 the case corresponding to preparation of aspicilin10 is representative (Scheme 1b). Despite the above considerations, macrocyclic RCM processes that deliver large ring enoates and/or dienoates containing a Z-alkene are yet to be disclosed. Here we report catalytic RCM reactions that generate such entities efficiently and stereoselectively.
Scheme 1. Synthesis of Macrocyclic Enoates by Z-Selective RCM.
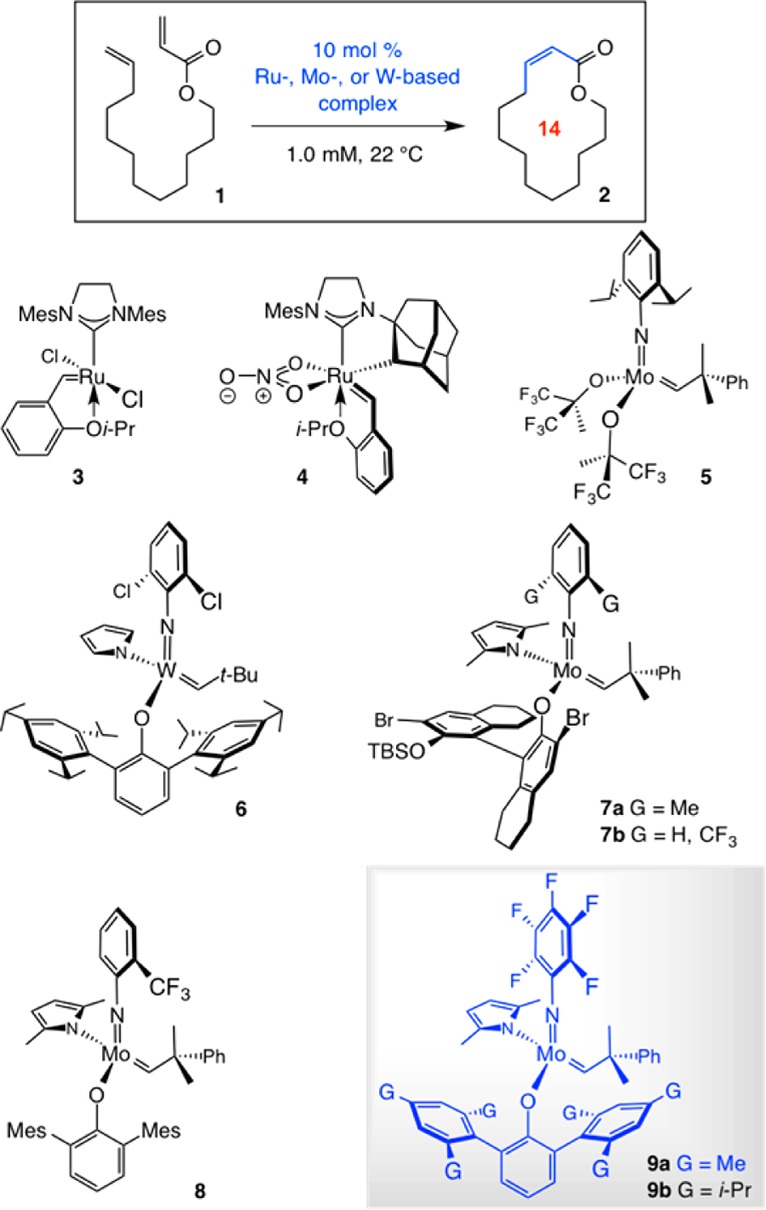
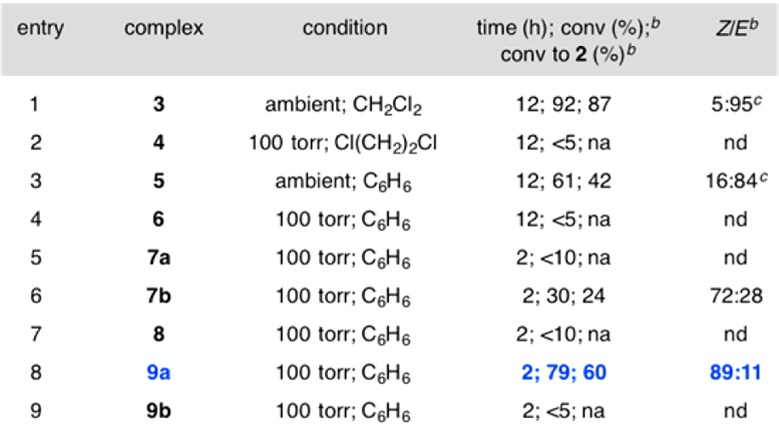
We first examined the ability of Ru, Mo, and W complexes, commonly used and/or shown to be effective in Z-selective macrocyclic RCM,6 to promote the formation of 14-membered-ring Z-enoate 2 (Table 1). Reaction with dichloro-Ru complex 3 was efficient (87% conv, 12 h), generating the lower energy E isomer selectively (5:95 Z/E, entry 1). Under conditions similar to those reported for RCM of unactivated dienes,6c there was no detectable transformation after 12 h in the presence of 10 mol% bidentate Ru complex 4 (entry 2). With bis-alkoxide 5 (entry 3), only 42% conv was observed, presumably due to competitive catalyst deactivation/decomposition; E-2 was again formed preferentially (16:84 Z/E).
Table 1. Screening of Ru, Mo, and W Complexesa.
Reactions were performed under N2 atmosphere.
Conversion (total consumption of 1) and Z:E ratios (±2%) were determined by analysis of 1H NMR spectra of unpurified mixtures.
Product mixture contained ca. 50% of the dimeric macrocycle. See the Supporting Information (SI) for details. Mes = 2,4,6-(Me)3C6H2; na = not applicable; nd = not determined.
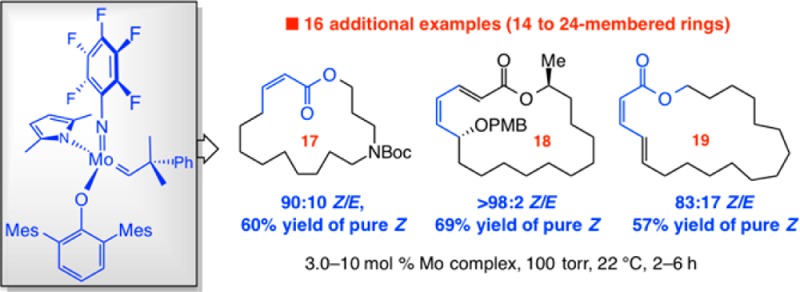
We then turned to examining monoaryloxide pyrrolide (MAP) complexes under conditions that allow for relatively facile reaction (100 Torr, C6H6, 22 °C).6a,6b Use of W or Mo alkylidenes 6 and 7a led to minimal reaction11 (entries 4 and 5, Table 1). Based on computational investigations indicating that a more electron withdrawing imido group can impart electronic stabilization of the metallacyclobutane intermediates to increase efficiency,6d we probed the activity of 2-(trifluoromethyl)phenylimido complex 7b (entry 6). After 2 h, 24% of 2 was formed with 72:28 Z/E selectivity. To enhance selectivity, we opted for a more sizable aryloxide ligand according to our original stereochemical model.12 However, use of MAP complex 8 led to complete loss of activity.
At this point, we chose to prepare and examine MAP alkylidenes 9a,b, complexes that have not been previously utilized. This was based on the hypothesis that electronic activation imposed by the comparatively diminutive pentafluorophenylimido ligand might improve catalyst performance6d while giving rise to elevated Z selectivity by residing opposite to the sterically demanding aryloxides in the metallacyclobutane intermediates. In the event, when 9a was used, 60% conv to 2 was observed along with 89:11 Z/E selectivity (entry 8). When the aryloxide substituents were altered from 2,4,6-trimethylphenyl in 9a to a 2,4,6-tri(isopropyl)phenyl moiety, catalyst activity disappeared entirely (entry 9). Optimization studies indicated that 3.0–5.0 mol% of the in situ-generated MAP complex 9a is sufficient to bring about considerable conversion under 100 Torr of pressure, at ambient temperature and after 2 h (Scheme 2). Longer reaction times did not lead to further transformation.
Scheme 2. Z-Selective Macrocyclic Enoate RCM.
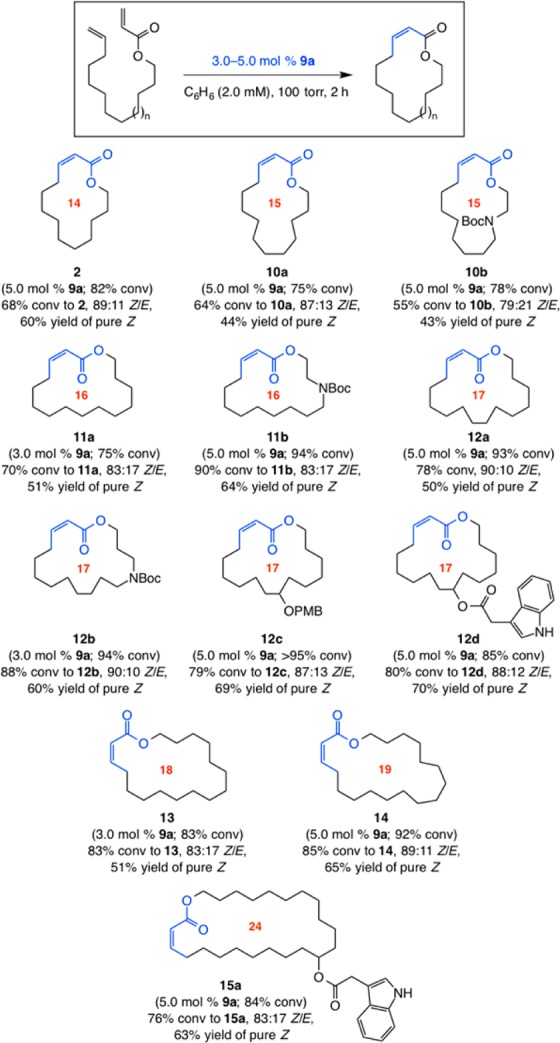
Reactions leading to 2, 10a,b, and 11a performed at 1.0 mM. Conv = formation of desired macrocycle. See the SI for details.
Different macrocyclic Z-enoates, from 14- to 19-membered rings (2, 10–14, Scheme 2), can be synthesized in 79:21–90:10 Z/E selectivity. As the formation of 24-membered-ring 15a indicates, the catalytic protocol may be extended to larger macrocyclic compounds. After silica gel chromatography, pure Z product isomers were isolated in 43–70% yield. Ring closures are often more efficient for ring sizes larger than 15. Consistent with this trend, our efforts to obtain a 12-membered Z-enoate proved unsuccessful (<2% desired product). Depending on the ring size and/or the nature of the substituents, RCM of an acyclic diene precursor that contains additional functionality might result in improved or diminished efficiency.13 Thus, reactions leading to 16- and 17-membered-ring products 11b and 12b proceeded to higher conversion and afforded the Z-enoates in better yield compared to 11a and 12a.
Attempts to promote reactions that produce different α,β-unsaturated amides led to negligible transformation (Scheme 3). The disparity between the reactions of ester and amide substrates might arise from the ability of the latter to form Mo alkylidenes that can be unreactive because of stronger chelation of the amide carbonyl with the transition metal and/or A(1,3)-strain when R = Bn or Boc.14 Moreover, unlike Boc amide substituents or unprotected indole units, the presence of a primary carbamate, such as that in the expected product 15b, leads to substantial activity loss; this might arise from association of the Lewis basic unit with the Mo center and/or the acidity of the said substituent.
Scheme 3.
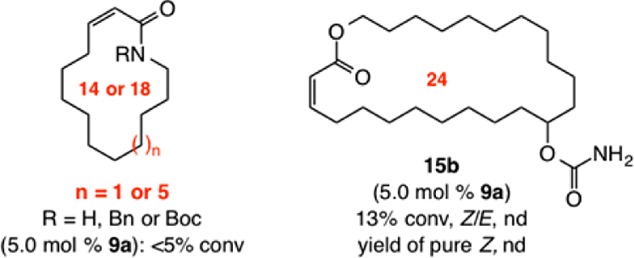
Macrocyclic dienoates of diverse ring sizes can be prepared through the use of 5.0–10 mol% of 9a (Scheme 4). In cases involving E-dienoate as substrates (e.g., 16, Scheme 4a), unlike transformations that afford Z-enoates, cyclizations proceed with complete control of stereoselectivity (>98:2 Z/E) for up to 18-membered rings. In contrast, 20 is formed in 91:9 Z:E selectivity. RCM with Ru complexes represented by 3 and 4 proved inefficient (e.g., <10% conv to 18 with 10 mol% complex after 12 h). With the more active Mo bis-alkoxide 5, 20 mol% loading was required to achieve 50% and 34% conv to 18 and 19, respectively (<10% conv with 10 mol%). In the latter cases, whereas 18 was generated with complete Z selectivity (similar to when 9a was used), there was minimal preference in the case of 19 (65% Z). Catalytic RCM leading to isomeric (Z,E)-dienoates was performed with E-diene substrates (e.g., 21, Scheme 4b).15 Stereoselectivity was lower in these latter systems (cf. 22, 23 vs products in Scheme 4a), but isomerically pure macrocyclic dienoates were obtained after chromatography. Use of Ru dichloride 3 or Mo bis-alkoxide 5 did not yield any desired product. Such disparities underscore the unique ability of Mo MAP complexes to deliver the appropriate balance of longevity and reactivity.
Scheme 4. Z-Selective Formation of Macrocyclic Dienoates by RCM.
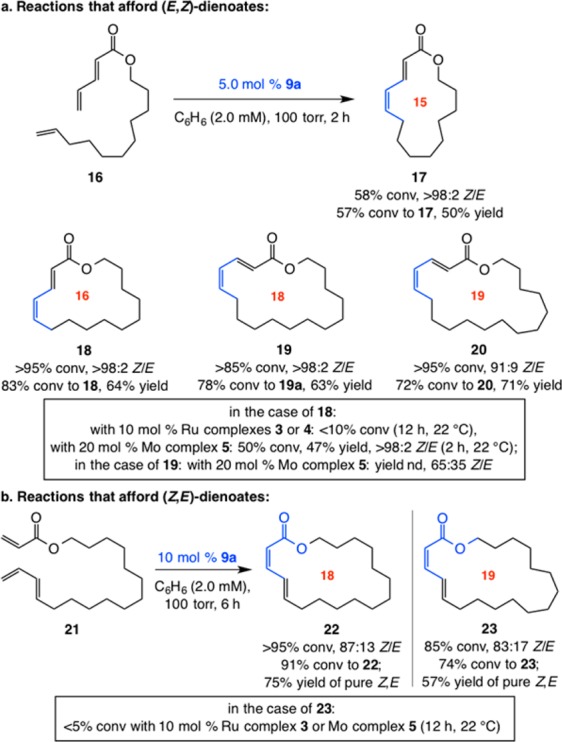
Conv = formation of the desired macrocycle. See the SI for details.
The transformations in Scheme 5 were carried out to demonstrate utility of the approach. Conversion of 24(16) to 25 (80% yield) was followed by RCM with 10 mol% 9a afforded stereoisomerically pure (E,Z)-dienoate 26 in 69% yield. Oxidative removal of the p-methoxybenzyl group generated 27 (91% yield), which has been converted to aspicilin.17
Scheme 5. Application to Formal Synthesis of (+)-Aspicilin.
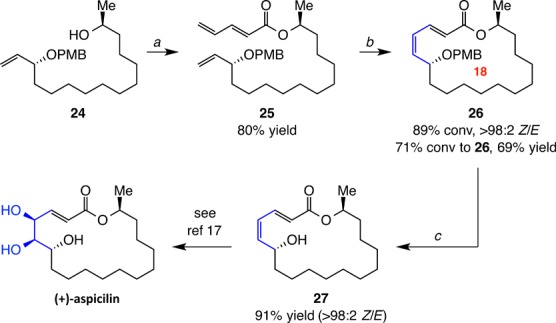
Conditions: (a) pentadienoic acid, pivaloyl chloride, DMAP, Et3N. (b) 10 mol% 9a, C6H6, 22 °C, 100 Torr, 6 h. (c) DDQ, CH2Cl2/H2O, 0 °C, 1 h. Conv = formation of desired macrocycle. See the SI for details.
For cyclic (E,Z)-dienoates (Scheme 4a) that are ≤18-membered, RCM processes are completely Z-selective regardless of the type of Mo alkylidene used (5 or 9a); for 19-membered ring 20, better Z selectivity is obtained with 9a, suggesting considerable catalyst control. This is unlike cyclizations that lead to enoates (Scheme 2) or (Z,E)-dienoates (Scheme 4b). To gain insight regarding the above trends, we calculated (DFT) the thermodynamic stereochemical preferences for unfunctionalized cyclic alkenes (Figure 1, A),6b−6d cyclic enoates (B; cf. Scheme 2); cyclic dienoates with an (E)-α,β-unsaturated carbonyl and a (Z)-γ,δ-alkene (C; cf. Scheme 4a), and those that bear a (Z)-α,β-unsaturated enoate and an (E)-γ,δ-alkene (D; cf. Scheme 4b). With a sufficiently large ring, the thermodynamic preference approaches that of acyclic systems. However, while the energy difference [ΔG(E) – ΔG(Z)] for type A and C rings does not exceed ∼1 kcal/mol (A′ and C′, Figure 1), the E isomer can be significantly more stable in type B and D olefins (ΔΔG ≈ 2 kcal/mol; cf. B′ and D′). For enoates and dienoates, the thermodynamic preference for the E isomer is reached at larger ring sizes compared to the less functionalized variants (A) for which the turning point is at about the 11-membered ring system (13–18-membered rings in B–D). With the less extensive conjugation in a macrocyclic α,β-unsaturated ester, the E alkenes containing more than 13 atoms become favored (B). On the contrary, preference for (E,E)-dienoates is reached when 18–19-membered rings are being generated (C and D, Figure 1). The ΔΔG value in the case of (Z,E)-dienoates (D′) is larger (by ∼1 kcal/mol) for ring sizes above ∼18 atoms [vs (E,Z)-dienoates in C′], consistent with the weaker drive for formation of E alkenes in RCM that furnish (E,Z)-dienoates (C) vs Z,E isomers (D).
Figure 1.
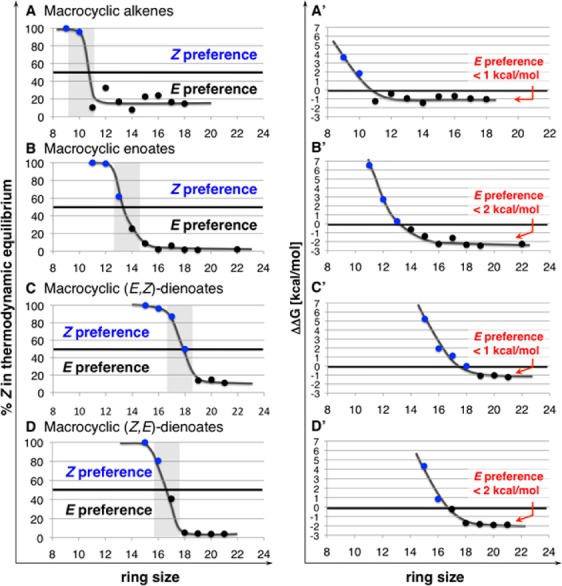
Variations in the thermodynamic preferences of different unsaturated macrocyclic alkenes. See the SI for details of calculations.
These studies expand the applicability of Z-selective olefin metathesis to include a set of transformations that is likely to find substantial utility in chemical synthesis. Development of related cross-metathesis reactions18 and studies on the impact of the resulting Z-enoates and dienoates on the design of multistep routes for preparation of complex biologically active molecules6e,18e are in progrees.
Acknowledgments
Financial support was provided by the NIH (GM-59426). We thank T. J. Mann and A. W. H. Speed for helpful discussions.
Supporting Information Available
Experimental procedures and spectral and analytical data for all products. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Review on biologically active macrocyclic compounds:Yu X.; Sun D. Molecules 2013, 18, 6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Representative reports:; a Williams D. R.; Kiryanov A. A.; Emde U.; Clark M. P.; Berliner M. A.; Reeves J. T. Angew. Chem., Int. Ed. 2003, 42, 1258. [DOI] [PubMed] [Google Scholar]; b Lucas B. S.; Gopalsamuthiram V.; Burke S. D. Angew. Chem., Int. Ed. 2007, 46, 769. [DOI] [PubMed] [Google Scholar]
- Examples:; a Forsyth C. J.; Ahmed F.; Cink R. D.; Lee C. S. J. Am. Chem. Soc. 1998, 120, 5597. [Google Scholar]; b Smith A. B. III; Minbiole K. P.; Verhoest P. R.; Schelhaas M. J. Am. Chem. Soc. 2001, 123, 10942. [DOI] [PubMed] [Google Scholar]; c O’Neil G. W.; Phillips A. J. J. Am. Chem. Soc. 2006, 128, 5340. [DOI] [PubMed] [Google Scholar]
- Selected cases:; a Evans D. A.; Fitch D. M.; Smith T. E.; Cee V. J. J. Am. Chem. Soc. 2000, 122, 10033. [Google Scholar]; b Wender P. A.; Hegde S. G.; Hubbard R. D.; Zhang L. J. Am. Chem. Soc. 2002, 124, 4956. [DOI] [PubMed] [Google Scholar]; c Nelson S. G.; Cheung W. S.; Kassick A. J.; Hilfiker M. A. J. Am. Chem. Soc. 2002, 124, 13654. [DOI] [PubMed] [Google Scholar]
- Pham C.-D.; Hartmann R.; Böhler P.; Stork B.; Wesselborg S.; Lin W.; Lai D.; Proksch P. Org. Lett. 2014, 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reports on Z-selective macrocyclic RCM and applications to natural product synthesis:; a Yu M.; Wang C.; Kyle A. F.; Jakubec P.; Dixon D. J.; Schrock R. R.; Hoveyda A. H. Nature 2011, 479, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang C.; Yu M.; Kyle A. F.; Jakubec P.; Dixon D. J.; Schrock R. R.; Hoveyda A. H. Chem.—Eur. J. 2013, 19, 2726. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rosebrugh L. E.; Herbert M. B.; Marx V. M.; Keitz B. K.; Grubbs R. H. J. Am. Chem. Soc. 2013, 135, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wang C.; Haeffner F.; Schrock R. R.; Hoveyda A. H. Angew. Chem., Int. Ed. 2013, 52, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yu M.; Schrock R. R.; Hoveyda A. H. Angew. Chem., Int. Ed. 2014, 10.1002/anie.201409120. [DOI] [Google Scholar]
- Synthesis of acyclic (E,Z)-dienoates by Still–Gennari conditions:Chemler S. R.; Coffey D. S.; Roush W. R. Tetrahedron Lett. 1999, 40, 1269. [Google Scholar]
- Report on synthesis of macrolactin A (does not involve macrocyclic RCM):; a Smith A. B. III; Ott G. R. J. Am. Chem. Soc. 1996, 118, 13095. [Google Scholar]; For selected other natural products containing the same (Z,E)-enoate unit, see:; b Shin Y.; Fournier J.-H.; Fukui Y.; Brückner A. M.; Curran D. P. Angew. Chem., Int. Ed. 2004, 43, 4634. [DOI] [PubMed] [Google Scholar]; c Bishara A.; Rudi A.; Aknin M.; Neumann D.; Ben-Califa N.; Kashman Y. Tetrahedron Lett. 2008, 49, 4355. [Google Scholar]
- For instance:; a den Hartog T.; Harutyunyan S. R.; Font D.; Minnaard A. J.; Feringa B. L. Angew. Chem., Int. Ed. 2008, 47, 398. [DOI] [PubMed] [Google Scholar]; b Uraguchi D.; Yoshioka K.; Ueki Y.; Ooi T. J. Am. Chem. Soc. 2012, 134, 19370. [DOI] [PubMed] [Google Scholar]; c Lee K.-s.; Wu H.; Haeffner F.; Hoveyda A. H. Organometallics 2012, 31, 7823. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Luo Y.; Roy I. D.; Madec A. G. E.; Lam H. W. Angew. Chem., Int. Ed. 2014, 53, 4186. [DOI] [PubMed] [Google Scholar]
- Selected disclosures on total synthesis of aspicilin (none involve macrocyclic RCM):; a Enders D.; Prokopenko O. F. Liebigs Ann. 1995, 1185. [Google Scholar]; b Kobayashi Y.; Nakano M.; Kumar G. B.; Kishihara K. J. Org. Chem. 1998, 63, 7505. [DOI] [PubMed] [Google Scholar]; c Dixon D. J.; Foster A. C.; Ley S. V. Org. Lett. 2000, 2, 123. [DOI] [PubMed] [Google Scholar]; d Gandi V. R. Tetrahedron 2013, 69, 6507. [Google Scholar]
- The less reactive W MAP complexes typically require longer reaction times than Mo alkylidenes.
- a Ibrahem I.; Yu M.; Schrock R. R.; Hoveyda A. H. J. Am. Chem. Soc. 2009, 131, 3844. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Meek S. J.; O’Brien R. V.; Llaveria J.; Schrock R. R.; Hoveyda A. H. Nature 2011, 471, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An early study regarding the influence of substituents on the facility of a macrocyclic RCM reaction promoted by a Mo alkylidene:Xu Z.; Johannes C. W.; Houri A. F.; La D. S.; Cogan D. A.; Hofilena G. E.; Hoveyda A. H. J. Am. Chem. Soc. 1997, 119, 10302. [Google Scholar]
- Amide complexes derived from Mo complexes and their structure/activity relationships:; a Sattely E. S.; Cortez G. A.; Moebius D. C.; Schrock R. R.; Hoveyda A. H. J. Am. Chem. Soc. 2005, 127, 8526. [DOI] [PubMed] [Google Scholar]; b Townsend E. M.; Kilyanek S. M.; Schrock R. R.; Müller P.; Smith S. J.; Hoveyda A. H. Organometallics 2013, 32, 4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- With 5.0 mol% 9a after 2 h, ∼70% conv was observed, but since 21 and 22 are not easily separable, conditions that lead to higher conv were used.
- See the SI for preparation of alkene 24.
- Quinkert G.; Heim N.; Glenneberg J.; Billhardt U.-M.; Autze V.; Bats J. W.; Dürner G. Angew. Chem., Int. Ed. Engl. 1987, 26, 362. [Google Scholar]
- Representative Z-selective cross-metathesis reactions:; a Ref (12b); b Kiesewetter E. T.; O’Brien R. V.; Yu E. C.; Meek S. J.; Schrock R. R.; Hoveyda A. H. J. Am. Chem. Soc. 2013, 135, 6026. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mann T. J.; Speed A. W. H.; Schrock R. R.; Hoveyda A. H. Angew. Chem., Int. Ed. 2013, 52, 8395. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ref (6e); e Speed A. W. H.; Mann T. J.; O’Brien R. V.; Schrock R. R.; Hoveyda A. H. J. Am. Chem. Soc. 2014, 10.1021/ja509973r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Herbert M. B.; Marx V. M.; Pederson R. L.; Grubbs R. H. Angew. Chem., Int. Ed. 2013, 52, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Quigley B. L.; Grubbs R. H. Chem. Sci. 2014, 5, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


