Abstract
We propose CardioGuard, a brassiere-based reliable electrocardiogram (ECG) monitoring sensor system, for supporting daily smartphone healthcare applications. It is designed to satisfy two key requirements for user-unobtrusive daily ECG monitoring: reliability of ECG sensing and usability of the sensor. The system is validated through extensive evaluations. The evaluation results showed that the CardioGuard sensor reliably measure the ECG during 12 representative daily activities including diverse movement levels; 89.53% of QRS peaks were detected on average. The questionnaire-based user study with 15 participants showed that the CardioGuard sensor was comfortable and unobtrusive. Additionally, the signal-to-noise ratio test and the washing durability test were conducted to show the high-quality sensing of the proposed sensor and its physical durability in practical use, respectively.
Key words: : e-health, home health monitoring, m-health, telehealth, telemedicine
Introduction
Remarkable advances in sensing and communication technology are expanding healthcare services to our everyday lives. Pervasive healthcare applications are increasingly based on daily monitoring of diverse biological signals.1–3 Daily electrocardiogram (ECG) monitoring will open up new opportunities for developing useful healthcare applications because the ECG provides a basic indication of the changes in cardiac activity and in the autonomous nervous system. For example, an application can detect chronic cardiac diseases with intermittent symptoms such as arrhythmia,4,5 which are difficult to detect without daily continuous monitoring. Moreover, heart rate (HR) and HR variability (HRV) enable assessment of the level of stress that users suffer6–8 and can recognize negative emotions in real time.9–11 Thus, an application can provide useful feedback to users and potentially induce behavioral changes.
For the development of a wearable ECG monitoring system, it is essential to accurately measure the signals in a nonintrusive way in everyday situations. However, it is challenging to simultaneously achieve both the usability of wearable sensors and the reliability of measurement. Flexible and conductive materials such as conductive fabrics have been used for wearable sensors because their high flexibility provides a high level of usability. However, such flexibility can severely limit the reliable measurement of ECG signals during diverse daily activities; the deformation of the fabric or the rubbing of the fabric against the skin due to active movement results in serious noise.
In this study, we propose CardioGuard, a nonintrusive, wearable sensor system for daily ECG monitoring for women. The CardioGuard system consists of two major components: (1) a brassiere-based, wearable sensor (bra sensor) and (2) smartphone middleware, designed to be highly usable, reliable, and accessible for ECG-based daily healthcare applications. Specifically, we first designed the bra sensor by leveraging the structure of women's functional underwear. By replacing the existing rigid components of a brassiere (i.e., the brassiere wires) with sensor electrodes, the bra sensor avoids making users feel that they are wearing additional sensors. Additionally, the electrodes can tightly contact the skin of users. As such, the bra sensor minimizes discomfort during the daily activities of wearers while measuring ECG signals accurately, even during movement. In addition, the form factor and the placement of the sensor device are designed to considerably enhance wearability. The sensor device is placed at the cleavage, the free space naturally formed by the structure and shape of a brassiere. The shape and size of the sensor device are designed to entirely fit in the free space while remaining invisible and maintaining the appearance of wearers. The device is light enough that a wearer hardly feels the additional weight.
We also propose smartphone middleware for supporting mobile healthcare applications based on ECG. The middleware allows developers to be free from detailed sensing issues and easily access raw ECG information and its high-level features. It enables developers to build several ECG-based mobile healthcare applications without encountering complicated implementation issues such as issues of reliable monitoring, sensor management, and feature extraction.
Moreover, we present an in-depth study on the robustness of the proposed system against motion artifact caused by daily activities (e.g., when a user is walking, riding a bicycle, or taking a bus). Although there have been many efforts to develop wearable sensing systems targeting daily usage, their evaluation study did not cover diverse daily situations. Furthermore, beyond motion robustness, we study diverse aspects that are important for applying the proposed system to real life; these include sensing quality, usability, and durability.
Related Work
There have been several previous studies on enhancing the usability of wearable biosignal monitoring sensors. Many studies take the approach of using conductive fabrics as the sensor.12–15 Conductive fabrics have enough conductivity to measure electric biosignals and also have high flexibility and softness (similar to common fabrics for clothes) that provide a high level of usability for wearable sensors. However, such flexibility may cause negative effects on measurement accuracy when the sensor is in motion; deformation of fabric or rubbing with the skin due to active movement results in critical noise in the measured signals. This imposes a serious limitation on the reliable measurement of ECG signals during diverse everyday activities. In addition, some types of conductive fabrics are not physically or chemically durable. For example, they may lose conductivity after washing or exposure to sweat. Additionally, some conductive fabrics are not biocompatible. Therefore, it is hard to utilize these fabrics practically as sensors in clothing. Fabric woven from silver-coated conductive yarn overcomes limitations of durability and biocompatibility, but it is expensive.
Vuorela et al.16 proposed a portable system to support long-term ECG recording in daily situations. This system uses an electrode band made of conductive fabric. This provides a high level of wearability. However, its performance in measurement is degraded by the movement of the body. In addition, the user is required to wear an additional body band equipped with electrodes, and this can be a burden. Moreover, the placement of the sensing device hardly considers users' key concerns about wearability and appearance; the device might deform the appearance of clothes when they wear it in daily situations.
Varadan et al.17 developed a daily ECG measurement system applied in a sports bra. They improved the possibility of practical adoption in daily situations by incorporating custom-designed, flexible electrodes into a sports bra. They also built a mobile application to show measured ECG data. However, there are limitations to the application of the system in daily situations because the system is embedded in a sports bra, which is not worn by women for everyday activities. Moreover, the system was not sufficiently evaluated in diverse movement situations (e.g., light running, riding a bus, or riding a bicycle). Thus, its motion robustness was not examined in detail. In addition, although the chemical durability of electrodes was evaluated, other aspects including the system's physical durability and cost were not considered.
The Cardio sports bra18 is a commercial product for sensing HR. However, this product has limitations because it is also a sports bra. In addition, because the product only gives HR updates, it is difficult to develop diverse healthcare applications for it based on ECG-derived information such as HRV. Because detailed technical information about sensor capacity is not provided, it is difficult to judge the reliability of its ECG sensing.
Moreover, previous studies barely considered software to support smartphone healthcare applications. However, the CardioGuard system includes smartphone middleware that takes care of reliable sensing and high-level feature extraction and enables multiple healthcare applications to share derived information. It can save computing resources in the mobile environment by sharing the same processes that are required by multiple applications. Also, it greatly reduces the burden of application development by providing the commonly required functionalities such as sensor management and feature extraction.
Recently, Seeger et al.19 proposed body sensor network middleware to support mobile health monitoring applications. However, they focused on the software architecture of the middleware without providing wearable sensor hardware to enable reliable sensing. The key feature of their middleware is its seamlessness in handling changing sensor configurations between multiple body sensors.
Materials and Methods
System Design and Implementation
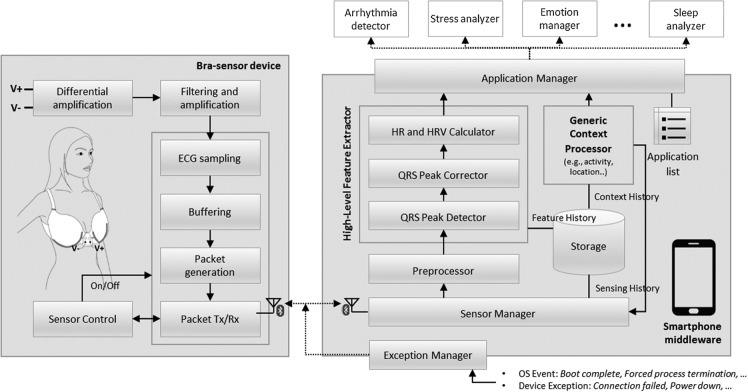
The CardioGuard system is a collaborative system with two major components: the brassiere-based wearable sensor and the smartphone middleware. Figure 1 shows the overall architecture of the CardioGuard system. The bra sensor reliably measures ECG signals using metal-based, dry electrodes. It wirelessly transfers the data to the smartphone middleware. The middleware manages the sensing process and delivers measured signals and extracted high-level features in real time to multiple healthcare applications upon request.
Fig. 1.
The overall architecture of the CardioGuard system. ECG, electrocardiogram; HR, heart rate; HRV, heart rate variability.
Brassiere Sensor
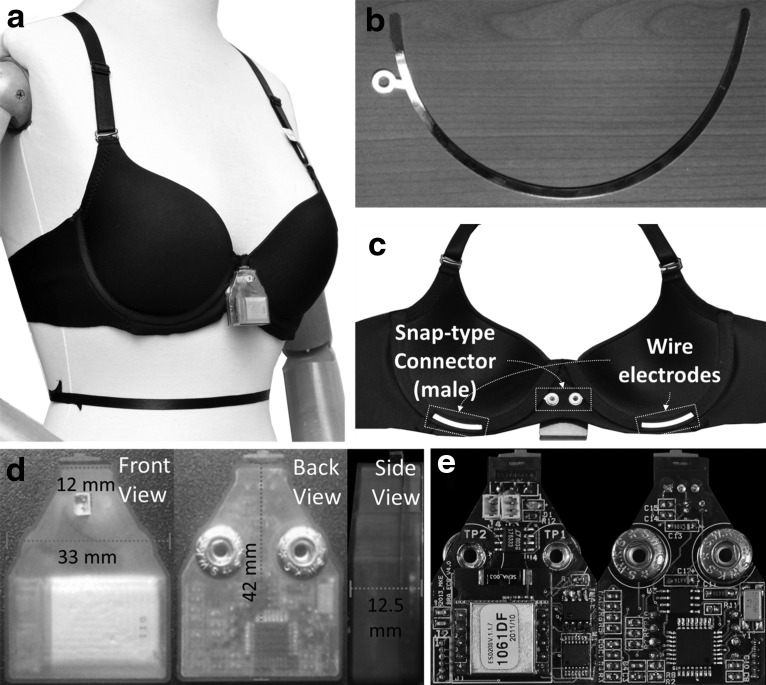
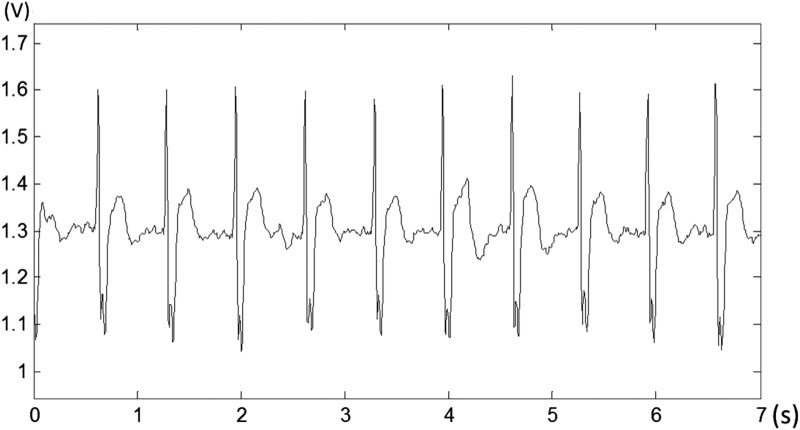
For the design of the bra sensor, we first focused on nonintrusive monitoring of ECG signals to enhance the system's usability in daily life. Figure 2 shows the prototype of the bra sensor and the sensor device. The wire electrodes sense ECG signals, and a tiny computing device preprocesses the signals and transmits the data wirelessly. Figure 3 shows an example of ECG signals measured using the bra sensor and the sensor device. The quality of the signal is good enough to detect ECG features, including the P and T waves and the QRS complex.
Fig. 2.
The prototype of the CardioGuard sensor system. (a) The wearing example. The triangular shape of the sensor device is attached at the cleavage of the bra sensor. (b) Gold-plated wire electrode. (c) The structure of the bra sensor. (d) The front/back/side views of the prototype of the sensor device. The detail size of the device is shown at each view. (e) The hardware and circuit components of the sensor device.
Fig. 3.
An example of the electrocardiogram signal measured by the CardioGuard system.
We used conductive metal for the electrodes to overcome the limitations of conductive fabric in terms of the physical durability, cost, and reliability of sensing during motion. Metal-based, dry electrodes can measure high-quality ECG signals, and their production cost is low. Their ability to withstand deformation from external forces makes them reusable. However, it is challenging to use the metal as a wearable sensor because of its low flexibility. Its rigidity can cause discomfort for the wearer. Corrosion by water and sweat is another disadvantage. We addressed the shortcoming of low flexibility by replacing the rigid wires in the brassiere (which keep the shape of cups) with metal-based electrodes. Figure 2b shows the detailed shape and placement of the electrodes. We carefully determined the thickness of the wire electrodes (0.5 mm) to maintain both durability and flexibility. A small portion of the area of each wire electrode is exposed at the lower center of each cup and directly contacts the skin. These exposed areas usually have close contact with the skin when the brassiere is secured. Such tight contact makes the bra sensor robust against motion artifacts during activities. To protect the skin from metal allergies and prevent corrosion by water and sweat, the wire electrodes are plated with gold. Moreover, the conductivity of gold (4.52×107 S/m) is about seven times greater than that of high carbon steel (6.21×106 S/m), which is the material used for the wire electrodes. Therefore, the gold plating enables the wires to measure a higher quality of signal by increasing their conductivity.
We also carefully designed the sensor device and designated its placement to achieve a high degree of usability and convenience. Figure 2d shows the front and back views of the sensor device. We placed the sensor device at the cleavage that is naturally formed between the two cups. This makes the device invisible when worn (see Fig. 2a). The triangular shape of the sensor device is designed to fully utilize the free space. In addition, the device is small and light enough to wear without discomfort. The detailed size is shown in Figure 2d. Its weight is only 11.4 g. The device uses gold-plated, snap-type metal connectors (shown in Fig. 2e); these are easily attached and detached from the wire electrodes.
The design of the sensor device considers the need for low power consumption. The sensor device buffers the measured signal for a couple of seconds before transmission to save power. The Bluetooth® (Bluetooth SIG, Kirkland, WA) module consumes 25% more battery in transmission mode than in stand-by mode. If the measured data were transmitted instantly after every sample, the Bluetooth module would stay in transmission mode continuously and thus would consume more power. We saved 44% of the power consumption by transmitting the ECG signals every 2 s. At a sampling rate of 128 Hz, the device uses 142.93 mAh with the buffering technique and 205.82 mAh without the buffering technique. The processing and communication modules in the sensor device can be turned on or off by the smartphone middleware.
Smartphone Middleware for Daily ECG-Based Healthcare Applications
Although previous studies did not often consider software support for healthcare applications, we propose an end-to-end monitoring system that includes smartphone middleware to support ECG-based daily healthcare applications. Some studies introduced smartphone healthcare applications based on their own sensing systems,17,20 but these do not include functions to allow other applications to access the sensing data.
The application support software should guarantee continuous monitoring and reliably provide sensing data to healthcare applications. Moreover, it should also enable multiple, concurrent applications to easily share raw ECG data and ECG-derived, high-level features such as HR and HRV. However, the smartphone is not used exclusively for biosignal monitoring. Therefore, the software should not limit the use of other applications and functions on the smartphone. The software also should not cause inconvenience by using too much of the smartphone system's resources. In addition, the software should automatically recover the monitoring process after unexpected termination by the smartphone operating system.
The CardioGuard system includes smartphone middleware that guarantees reliable ECG monitoring and supports healthcare applications to obtain measured ECG signals. The detailed architecture of the smartphone middleware is shown in Figure 1. The middleware wirelessly receives the ECG signal from the sensor device and processes and forwards the data in real time to multiple healthcare applications upon request. The middleware is implemented as a background service to avoid conflicts with the daily usage of the smartphone. For reliable and continuous sensing, the middleware is designed to keep itself alive after unexpected termination by the system or a reboot. It can also automatically recover a broken connection with the sensor device caused by a power-off or range-over.
Moreover, the middleware includes a high-level feature extractor to reduce the burden of healthcare application development. It allows multiple applications to share the extracted features, such as HR and HRV; accordingly, it avoids the unnecessary resource consumption that would result from deriving those features redundantly by individual applications. The feature extractor consists of three processing components: the QRS peak detector, the QRS peak corrector, and the HR and HRV calculator.
In addition, the middleware includes a component to manage the history of measured ECG data and extracted feature data as well as a context processor to obtain the user's context information using sensors embedded in the smartphone (e.g., Global Positioning System and accelerometer). Based on the recognized user condition, the middleware can assess the possibility of reliable sensing and control the monitoring process. For example, when the sensor recognizes that the user is running at a high speed and there might be significant noise, it can stop the ECG sensing and resume it after the user stops running or reduces her speed.
The detailed processing flow of the middleware is as follows. First, the preprocessor clarifies the QRS peaks in the ECG signals and removes power noises once again using a digital band-pass filter (7–35 Hz). Second, a QRS peak detector detects QRS peaks in the filtered signals. We use a custom-developed, fast, and efficient QRS peak detection algorithm that is suitable for a limited computing environment such as a mobile phone. The algorithm saves computational cost by reducing the size of traversal data using its internal prefilter. It efficiently finds the candidate QRS peaks and then quickly detects QRS peaks among such reduced candidates. Note that we do not cover this algorithm in detail in this article. Third, false-positive peaks are removed, and missing peaks are interpolated by a QRS peak corrector. The QRS peak corrector first filters out the peaks whose power is larger or smaller than a certain threshold or whose interval to the previous/next peak is in an abnormal range. Then, it interpolates the missing peaks using a piecewise-cubic Hermite interpolation method, which shows a superior performance.21 Finally, HR and HRV are calculated from the peaks.
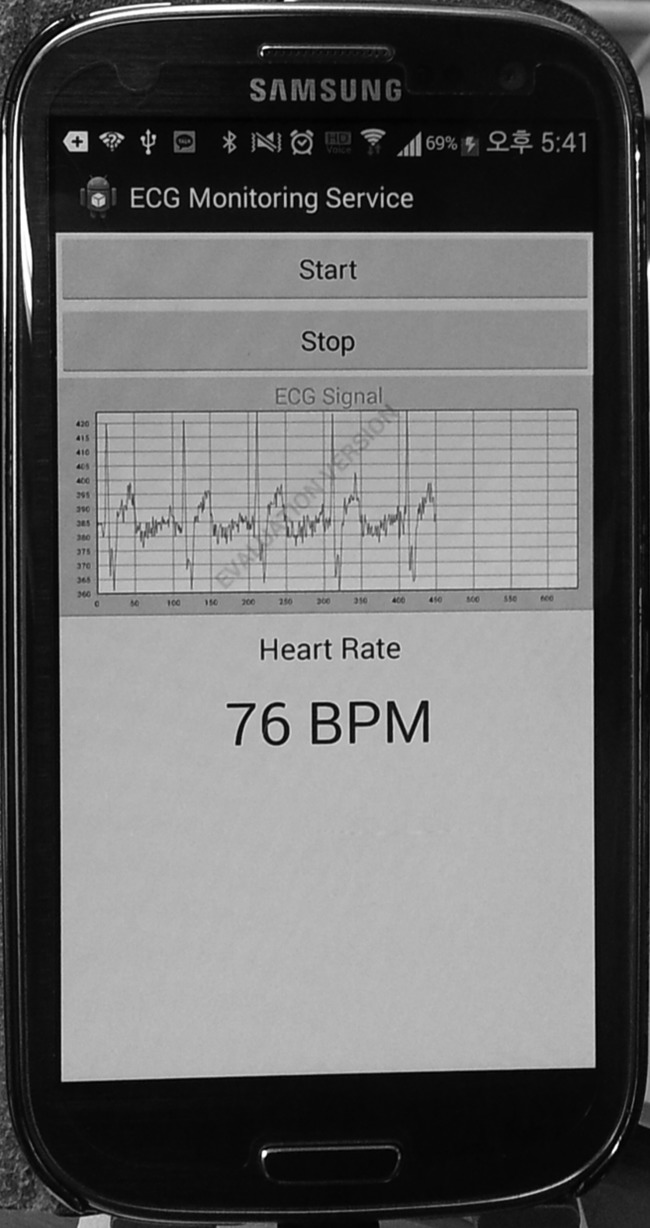
The CardioGuard system provides application programming interfaces (APIs) (Table 1) to support diverse daily healthcare applications. The key primitive is registerDataListener. Once the applications register their callback functions using the APIs, the middleware delivers the requested data to the applications at a requested interval via the callback function. We developed an example healthcare application using the provided APIs. Figure 4 shows the user interface of the application. The example application enables cardiac patients to check their ECG and HR instantly and accurately in daily situations. When the user presses the start button, the application requests the raw data and HR data, using the provided APIs, from the CardioGuard system; the update intervals are 50 ms and 1 s, respectively. Then, the middleware transfers the requested data to the application via the registered callback functions. The callback functions simply update the waveform and HR view upon receiving the data. The application was easily implemented without considering the details of the reliable monitoring and feature extraction, which are fully delegated to the CardioGuard system.
Table 1.
Key Application Programming Interfaces
| registerDataListener(callback(data), data_type, update_interval) |
| * data_type=RAW_DATA | HR | HRV |
| * update_interval |
| - The unit of millisecond (larger than 50 ms) for RAW_DATA, |
| - The unit of second for HR, |
| - 1 | 2 | 5 minutes for HRV |
| class RAW_DATA { long timestamp; double [] raw_data; } |
| class HR { long timestamp; int HR; } |
| class HRV{ long timestamp; float LF; float HF; float LF/HF;…}; |
HR, heart rate; HRV, heart rate variability; HF, high frequency; LF, low frequency.
Fig. 4.
A screenshot of the example healthcare application. The application shows the electrocardiogram (ECG) waveform and heart rate of the user in real time. BPM, beats/min.
Evaluation and Results
We evaluated the proposed sensor system in terms of its reliability and usability in daily environments. To examine the reliability of the sensor measurement, we performed two tests: a signal-to-noise ratio (SNR) test and a QRS peak detection test during daily activities. The SNR test quantitatively examined the quality of the signals measured by the proposed sensor. The QRS peak detection test assessed the reliability of the device in diverse daily situations of typical users. The usability of the proposed system was evaluated through interviews and a questionnaire survey. We also performed a washing durability test to validate the device's feasibility for practical use.
SIGNAL QUALITY
We performed the SNR test to evaluate the device's signal quality. We prepared a simulated ECG measurement environment in which we imitated a woman's body wearing the bra sensor using a mannequin and an ECG simulator, MiniSim.22 We attached copper tape on the left and right breasts of the mannequin and connected the two points with the RA and LA channels of the ECG simulator. Then, the bra sensor was worn on the mannequin so that the wire electrodes contacted the copper tape. The raw signal measured by the sensor device was acquired using a laptop with a Bluetooth interface. The source signal was acquired using a Food and Drug Administration–approved ECG measurement system, the BioPac (Goleta, CA) MP150 ECG module,23 connected directly to the ECG simulator. Both of the signals were measured for 200 s and were time-synchronized.
The results of the SNR test showed that the bra sensor can measure high-quality ECG signals. The SNR of the raw signal was 10.64; the ECG signal power was 12 times stronger than the noise power. When the raw signal was processed using a digital filter (a 5th-order Butterworth low-pass filter with a cutoff frequency of 35 Hz), the SNR increased to 12.28; the ECG signal power was 17 times stronger than the noise.
Reliable Monitoring of Daily Activities
To evaluate the device's sensing reliability, we conducted a QRS peak detection test during diverse daily situations. The test was designed to examine how accurately the bra sensor measured ECG signals during daily activities compared with a reference ECG measurement system. As a metric, we used the QRS peak (R peak) detection ratio (RDR), which is the ratio of the number of QRS peaks detected from the bra sensor signal to the number of QRS peaks detected from the reference signal. The QRS peaks of each signal were detected manually by experienced experts. The ECG signals measured by the bra sensor were collected by the proposed middleware running on a Galaxy S3 smartphone (Samsung, Seoul, Republic of Korea). The signal of the embedded three-axis accelerometer in the smartphone was acquired simultaneously to quantitatively measure the degree of movement. The reference signals were acquired using a portable four-channel ECG Holter monitor, the Shimmer 2R,24 with Ag-AgCl electrodes with standard leads. The reference device sampled the ECG at the same sampling rate as the sensor device and saved the signals in the local SD memory.
Ten female participants from the age group of 19–32 years were recruited for the test. Their body mass indexes ranged from 17.17 to 22.24 kg/m2. Each participant wore a bra sensor fitted to her size (34AA, 34A, and 36AA in U.S. sizes). The reference device and the smartphone were also attached to the left upper arm using a band during the test. The participants were asked to perform predefined activities for specified amounts of time (Table 2). We selected 12 representative daily activities. For the selection, we considered activities with different degrees of movement and categorized them into three groups, as shown in Table 2. Note that two of the activities, lying down and treadmill walking, were subdivided into more detailed activities.
Table 2.
Twelve Predefined Representative Daily Activities and Protocol
| ACTIVITY | DURATION (S) | PROTOCOL |
|---|---|---|
| Stable | ||
| Resting | 300 | Resting on a chair without any movement |
| Eating | 600 | Eating a sandwich and a cup of noodles using chopsticks while sitting on a chair |
| Desk work | 300 | Typing, using a mouse and writing by hand at the desk |
| Lying | Lying with supine, prone, and lateral postures | |
| Supine | 180 | |
| Prone | 180 | |
| Lateral | 180 | |
| Moving activities without walking | ||
| Car driving | 300 | Playing a racing game with a wheel and pedal joystick |
| Riding a bicycle | 180 | Riding a bicycle on flat land |
| Riding a bus | 200–250 | Riding a bus (two stops) |
| Riding a subway | 90–100 | Riding a subway (one stop) |
| Elevator | 30–50 | Using an elevator without any movement |
| Walking activities | ||
| Walking up and down stairs | 60–100 | Walking up and down two flights of stairs |
| Treadmill walking | Walking on a treadmill at 3, 4, 5, and 6 km/h | |
| 3 km/h | 180 | |
| 4 km/h | 180 | |
| 5 km/h | 180 | |
| 6 km/h | 180 | |
| Natural walking | 250–350 | Walking on a sidewalk |
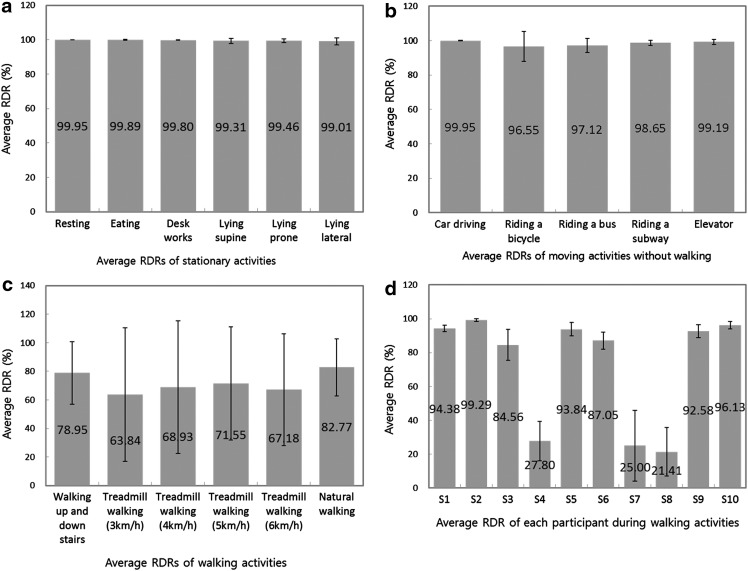
Overall, the test results show the feasibility of the CardioGuard system for reliable ECG sensing during diverse daily activities. The average RDR was 89.53% for all activities. This increased up to 98.29% for all moving activities except walking and to 99.57% for stationary activities such as working at a desk and resting on a chair. In the case of walking activities, the average RDR was relatively low (72.2%). However, there were significant differences between the averages of each individual participant (Fig. 5d). The results of all participants except for three unexpectedly low RDR cases showed the feasibility of the bra sensor for reliable sensing in the walking activities.
Fig. 5.
The average R peak detection ratios (RDRs) and standard deviations for 12 representative activities: (a) stationary activities, (b) movement activities except walking, (c) walking activities, and (d) individual participants for walking activities.
During all stationary and moving activities (except walking), highly reliable ECG data were measured for all participants. Figure 5 shows the detailed results of these tests. All average RDRs for stationary activities were larger than 99%, and their standard deviations (SDs) were very small (from 0.11 to 1.94) (see Fig. 5a). The average RDRs for sitting activities such as resting on a chair, working at a desk, and eating were near 100%. The average RDRs for lying activities were slightly lower; however, the differences were negligible. For moving activities except walking, the average RDRs were also high, ranging from 97.12% to 99.95% (Fig. 5b). Considering that these activities occupy a significant portion of daily activities, we believe that the CardioGuard system exhibits high potential for a reliable ECG monitoring in practical situations.
Figure 5c shows the average RDRs for the group of walking activities. These are relatively low compared with those of the other two groups of activities; they vary from 63.84% to 82.77%. However, they show large SDs of up to 47. This is because there were large variations among participants. In the cases of participants 4, 7, and 8, QRS peaks were rarely detected for walking activities (Fig. 5d). Without these participants, the average RDRs during walking activities were also greater than 92%. From this result, we can see the potential feasibility of the bra sensor for reliable sensing in high movement situations.
To investigate the causes of the low RDR results, we performed three additional examinations. First, we examined the effect of individual differences in body movement during the walking activities on the RDR results. Gait might incur bodily vibration, which results in rubbing or weak contact between the wire electrode and the skin. This might cause severe noise in the ECG sensing. We analyzed the correlation between the strength of bodily vibration and the results of RDRs during the walking activities. For this analysis, we calculated the mean of the root mean squares (RMSs) for acceleration values of each axis. These were collected by a test smartphone during the experiment. We considered high RMS values to represent a strong bodily vibration. We observed that there was no significant difference in the RMS values between the participants whose RDR was low (participants 4, 7, and 8) and the others. Instead, the RMS of the low RDR group was 1.53 (SD=0.15), which is slightly smaller than that of the others (1.66; SD=0.16). The RMS of participant 7 (a member of the low RDR group) was 1.7. This is larger than the average RMS of the high RDR group, but the difference is slight. Moreover, the RMSs of participants 2 and 3 were larger than that of participant 7, but participants 2 and 3 showed high RDRs. From this analysis, we observed that individual difference in potential body vibration did not have a significant effect on RDR results in the walking activities.
Second, we examined the effect of QRS peak power on RDR during the walking activities. In general, it is easier to detect QRS peak correctly if the power of the ECG signal is relatively large. To investigate the influence of QRS peak power on RDR, the mean QRS peak power during the resting activity was calculated and compared among participants. We observed that a large QRS peak power did not guarantee a high RDR. Although participant 4 had the largest QRS peak power (132.07 mV) among all participants, her mean RDR in walking activities was 27.8%. In addition, the QRS peak power of participant 7 was only 3 mV smaller than that of participant 9. However, although the average RDR of participant 9 was 92.58% for walking activities, that of participant 7 was 25%. Thus, we concluded that there is no correlation between QRS peak power and the low RDRs.
Based on these investigations, we speculate that the low RDRs shown in participants 4, 7, and 8 were caused by accidental problems with fitting of their bra sensors. If the banding of the brassiere is relatively loose or if the bra sensor does not fit the user well, the movement of the upper body can result in rubbing or in weak contact between the wire electrode and the skin. This can cause serious noise in the ECG signal measurement. To estimate these fitting problems, we investigated the responses from the questions about the comfort of the bra sensor (Table 3, Q1 and Q2) in the questionnaire-based survey. We considered the fact that participants felt uncomfortable if the bra sensor did not fit them well. The average scores of Q1 and Q2 from the low RDR group (participants 4, 7, and 8) are 3.0 (SD=1) and 3.3 (SD=1.15), respectively. However, those from the relatively high RDR group are 4.71 (SD=0.49) and 4.86 (SD=0.38), respectively. The scores show that the low RDR group felt more uncomfortable than the other group. Accordingly, we conjecture that there were fitting problems that caused severe noise and low RDRs. However, it will be necessary to examine the correlation between fit and detection accuracy in detail in order to answer to this problem more specifically. Additionally, technical solutions to address the problem should be investigated in further studies.
Table 3.
Questions Used in System Usability Evaluation
| ASPECT | QUESTION |
|---|---|
| CardioGuard brassiere | Q1. Do you think wearing the bra sensor is as comfortable as a regular brassiere? |
| Q2. Do you think wire electrodes can fulfill the function of the existing wires in a regular brassiere? | |
| Q3. Do you feel comfortable with the touch of the wire electrodes? | |
| Q4. Do you feel uncomfortable because of the size of the sensor device? | |
| Q5. Do you feel uncomfortable because of the weight of the sensor device? | |
| System usefulness | (After an explanation of some examples of the healthcare applications, including sleep analysis, emotion detection, stress analysis, and the detection of cardiac diseases that are based on heart rate and heart rate variability, that could be possible with the proposed sensor system) |
| Q6. Do you think those healthcare applications with the proposed sensor system are useful for management of your health? | |
| Q7. Are you willing to wear our sensor system in daily life if those applications are provided? |
The current study shows the positive results for the feasibility of the CardioGuard system in daily situations. However, some applications that support clinical decision-making and medical intervention should require severely reliable ECG data. Therefore, it is still necessary to conduct further studies to examine the applicability of the system to these applications.
Usability
To examine the wearability of the bra sensor and the usefulness of the CardioGuard system, we conducted a questionnaire-based survey with 15 female participants. Ten of the participants were the participants from the previous study; the other five participants were additionally recruited. The ages of the new participants ranged from 32 to 48 years, and their bra sizes included 36A, which was not included in the previous reliable monitoring evaluation. To avoid the bias of the response, we recruited all participants who had no prior knowledge on our work.
We carefully designed the questionnaire by customizing a post-study system usability questionnaire,25 which is a conventional tool used to assess users' perceived satisfaction with systems or applications. Table 3 shows detailed questions. Two questions regarding the comfort of the bra sensor (i.e., Q1 and Q2) were designed to ask participants about relative scores compared with the original commercial brassiere used to make our prototype bra sensor. We adopted a 5-point Likert scale for each question.
Before the survey, participants were given enough time to experience and compare both the bra sensor and the commercial brassiere. Additionally, we verbally described several of the potential healthcare applications based on the CardioGuard system mentioned in Table 3 and asked the participants about the usefulness of the applications and their willingness to use the CardioGuard system. In addition, to better understand the participants' opinions, the questionnaires were followed up with individual interviews conducted in person.
Regarding the comfort and function of the bra sensor, most of the participants strongly agreed or agreed with Q1, Q2, and Q3. Mean scores and their SDs were 4.2 (1.03), 4.4 (0.97), and 4.5 (0.53), respectively. They felt that the bra sensor was as comfortable as the regular brassiere and that there was no functional difference compared with the existing wires in the regular brassiere. In addition, they did not feel that the direct contact between the skin and the wire electrode caused discomfort.
Regarding the weight of the sensor device, most of the participants strongly agreed with Q5 (mean score of 4.8 and SD of 0.41). Most of the participants said that they could not feel the weight of the sensor while wearing it. However, in terms of the size of the sensor device, four participants agreed with Q4, and four disagreed with it; the remaining seven participants were neutral. Many of them thought that the size would not matter when they wore loose-fitting clothes. A few participants, however, had concerns that the device might stand out when they wore slim-fitting clothes and would thus spoil the appearance of their clothes. They worried that it might cause other people to regard them as patients who have serious diseases.
Most participants had a positive attitude about the practical uses of the CardioGuard system; most of them agreed with Q6. They answered that the described healthcare applications would be helpful to maintain their health conveniently in daily life. They thought that the applications could provide opportunities to control their body's condition when they felt tired or suffered from a disease. Regarding Q7, most of the participants showed their (strong) willingness to use the CardioGuard system. Ten participants (strongly) agreed with Q7, whereas four were neutral, and one disagreed with it. They thought that the system would be helpful in maintaining their health and that it would not cause discomfort.
A few participants gave additional comments that should be considered in order to improve the CardioGuard system. One participant had concerns about the side effects of wearing an electronic device near her heart for a long time. Some participants commented about the effectiveness of the healthcare applications. One participant thought that the applications should more seriously consider effective ways of showing the health information to users who have no professional medical knowledge. Another participant had concerns about motivating users' behavior for health; she believed that a recommendation of actions would not be enough to encourage behavior changes in users. We found these comments helpful in continuing to develop a practical wearable sensor system and daily healthcare applications.
Washing Durability
To examine the durability of the bra sensor after daily washing, we conducted a washing durability test. As a metric for washing durability, we used SNR values obtained from the same experimental setup for the first SNR test. We washed one bra sensor 20 times using a laundry machine. Before laundering, the sensor device was detached from the bra sensor, and the bra sensor was put in a laundry mesh. Standard detergent (20 g) and tepid water were used for each washing. One laundry cycle included an 8-min washing, one rinse, and a 5-min spin-dry. The SNR values were obtained eight times: before the first wash and after the 1st, 3rd, 5th, 7th, 10th, 15th, and 20th washings.
We did not observe considerable degradation of the signal quality during the 20 washing cycles. The initial SNR was 10.64. After the first wash cycle, the SNR slightly decreased to 10.07. However, it maintained that value as the number of washings increased. Even after 20 wash cycles, the SNR value was 9.96. From this result, we conclude that the functionality of the wire electrodes and the snap-type connectors was not significantly compromised by repeated washings. Considering that a regular brassiere usually falls into disuse after 30 wash cycles, the sensing capacity of the bra sensor is expected to be maintained during its typical lifetime.
Conclusions and Future Work
In this study, we proposed CardioGuard, a brassiere-based, reliable sensor system for practical daily ECG monitoring. CardioGuard achieves the key requirements of daily monitoring, reliability, and usability. Through extensive evaluations, we show that the CardioGuard system achieves a high degree of reliability for daily continuous ECG monitoring. To evaluate the system's reliability, we conducted a QRS peak detection test during 12 representative daily activities with different degrees of movement and with 10 participants. Test results demonstrated that the bra sensor can measure ECG signals reliably. The average QRS peak detection ratio is 89.53% over all activities, and the average increases up to more than 96% except for a few unexpected cases in walking activities. The results showed that the feasibility of the CardioGuard system for reliable ECG monitoring in practical daily situations was high. Through questionnaire-based interviews, we also showed that the CardioGuard system achieves the desired usability from a user's perspective. The users' answers to the questionnaire demonstrated that the comfort of the bra sensor did not differ from the comfort of regular brassieres and that the CardioGuard satisfactorily carried out the function of underwear. Additionally, we conducted an SNR test and a washing durability test to further examine the usability and reliability of CardioGuard system.
In future studies, we will investigate further issues pertaining to the adoption of the CardioGuard system in daily life. One such issue is battery consumption. In addition, we will expand the proposed sensor system to a healthcare application support platform by enhancing the smartphone middleware. First, we will study the methodologies for the generic context processor in the middleware. The processor enables healthcare applications to provide suitable services customized for each user's situation. For example, a stress management application can offer different feedbacks to the user depending on her situation (e.g., when playing a game or when discussing in a business meeting). Second, the performance of feature extraction functions should be evaluated and be enhanced in further studies (e.g., the accuracy of calculating HRV parameters from RR intervals by the CardioGuard system).
Acknowledgments
This work was supported by grant 10041854 from the Research and Development Program of Ministry of Knowledge Economy.
Disclosure Statement
H. Baek is an employee of Samsung Electronics Co., Ltd. S. Kwon, J. Kim, S. Kang, Y. Lee, and K. Park declare no competing financial interests exist.
References
- 1.Korhonen I, Parkka J, Van Gils M. Health monitoring in the home of the future. IEEE Eng Med Biol Mag 2003;22:66–73 [DOI] [PubMed] [Google Scholar]
- 2.Asada HH, Shaltis P, Reisner A, et al. . Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng Med Biol Mag 2003;22:28–40 [DOI] [PubMed] [Google Scholar]
- 3.Lim YG, Hong KH, Kim KK, et al. . Monitoring physiological signals using nonintrusive sensors installed in daily life equipment. Biomed Eng Lett 2011;1:11–20 [Google Scholar]
- 4.Norland CC, Semler HJ. Angina pectoris and arrhythmias documented by cardiac telemetry. JAMA 1964;190:115–118 [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet 2011;378:731–739 [DOI] [PubMed] [Google Scholar]
- 6.Yang H-K, Lee J-W, Lee K-H, et al. . Application for the wearable heart activity monitoring system: analysis of the autonomic function of HRV. Conf Proc IEEE Eng Med Biol Soc 2008;1258–1261 [DOI] [PubMed] [Google Scholar]
- 7.Taelman J, Vandeput S, Spaepen A, Van Huffel S. Influence of mental stress on heart rate and heart rate variability. 4th European Conference of the International Federation for Medical and Biological Engineering New York: Springer, 2009:1366–1369 [Google Scholar]
- 8.Jovanov E, O'Donnell Lords A, Raskovic D, et al. . Stress monitoring using a distributed wireless intelligent sensor system. IEEE Eng Med Biol Mag 2003;22:49–55 [DOI] [PubMed] [Google Scholar]
- 9.Hughes JW, Stoney CM. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosom Med 2000;62:796–803 [DOI] [PubMed] [Google Scholar]
- 10.McCraty R, Atkinson M, Tiller WA, et al. . The effects of emotions on short-term power spectrum analysis of heart rate variability. Am J Cardiol 1995;76:1089–1093 [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Liu G, Hao M. The research on emotion recognition from ECG signal. International Conference on Information Technology and Computer Science, 2009. ITCS 2009.Piscataway, NJ: IEEE, 2009;1:497–500 [Google Scholar]
- 12.Catrysse M, Puers R, Hertleer C, et al. . Fabric sensors for the measurement of physiological parameters. 12th International Conference on Transducers, Solid-State Sensors, Actuators and Microsystems, 2003. Piscataway, NJ: IEEE, 2003;2:1758–1761 [Google Scholar]
- 13.López G, Custodio V, Moreno JI. LOBIN: E-textile and wireless-sensor-network-based platform for healthcare monitoring in future hospital environments. IEEE Trans Inf Technol Biomed 2010;14:1446–1458 [DOI] [PubMed] [Google Scholar]
- 14.Yoon U-j, Noh Y-S, Yoon H-r. Optimization methods for improving the performance of heart rate detection by a wearable ECG system during high-intensity exercise. Biomed Eng Lett 2011;1:143–150 [Google Scholar]
- 15.Pola T, Vanhala J. Textile electrodes in ECG measurement. 3rd International Conference on Intelligent Sensors, Sensor Networks and Information, 2007. ISSNIP 2007.Piscataway, NJ: IEEE, 2007:635–639 [Google Scholar]
- 16.Vuorela T, Seppa V-P, Vanhala J, Hyttinen J. Design and implementation of a portable long-term physiological signal recorder. IEEE Trans Inf Technol Biomed 2010;14:718–725 [DOI] [PubMed] [Google Scholar]
- 17.Varadan VK, Kumar PS, Oh S, et al. . E-bra with nanosensors, smart electronics and smart phone communication network for heart rate monitoring. SPIE Smart Structures and Materials+ Nondestructive Evaluation and Health Monitoring. Bellingham, WA: International Society for Optics and Photonics, 2011;79800S-79800S-79807 [Google Scholar]
- 18.Polar. Cardio sports bra. Available at www.polar.com/us-en/products/earlier_products/CardioSportsBra (last accessed November10, 2014)
- 19.Seeger C, Buchmann A, Van Laerhoven K. An event-based BSN middleware that supports seamless switching between sensor configurations. Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium New York: ACM, 2012:503–512 [Google Scholar]
- 20.Lee Y-G, Jeong WS, Yoon G. Smartphone-based mobile health monitoring. Telemed J E Health 2012;18:585–590 [DOI] [PubMed] [Google Scholar]
- 21.Kim KK, Kim JS, Lim YG, Park KS. The effect of missing RR-interval data on heart rate variability analysis in the frequency domain. Physiol Meas 2009;30:1039. [DOI] [PubMed] [Google Scholar]
- 22.Netech. MiniSim. Available at www.gonetech.com/ (last accessed November10, 2014)
- 23.BioPac. MP150. Available at www.biopac.com/ (last accessed November10, 2014)
- 24.Shimmer Sensing. Shimmer 2R. Available at www.shimmersensing.com/ (last accessed November10, 2014)
- 25.Sauro J, Lewis JR. Quantifying the user experience: Practical statistics for user research. Available at http://store.elsevier.com/product.jsp?isbn=9780123849687&pagename=search (last accessed November10, 2014)