My Roundabout Entry into the Gene Therapy Field
My father was an electrical engineer, and I loved to tinker with the dismembered parts of electronic equipment that he brought home for me. He liked working on cars, and I learned mechanical skills from him. So it was natural for me to study engineering as an undergraduate, with heavy doses of physics and math. However, during my senior year at Brown University, I took a one-year trip to the Geographic South Pole in Antarctica to study cosmic rays (high-energy particles originating primarily from outside the solar system), and my plans changed. Likely because of the harsh conditions there (10,500-foot altitude equivalent, <1% humidity, outside temperatures dropping below the freezing point of CO2), the long ride there and back on noisy aircraft, or the plain isolation of the site, I recognized the fragility of life and became more interested in biology and medicine.
After finishing my engineering and math degrees and spending a few months on an oceanographic ship, and having spent most of my life on the east coast of the United States, I set sail for Stanford University to learn some biology and hopefully enter medical school. Medical school at Stanford turned out not to be an option, but I was able to find work in a lab in the Stanford Pharmacology Department, under the tutelage of a clever postdoc. Things went well and I was accepted into the PhD program in a lab studying cytochrome P450 regulation, which I hoped would lead me into the field of recombinant DNA and the isolation of the various P450 protein and regulatory genes. The work was very interesting, but my advisor wasn't interested in cloning genes, and so this would have to wait. But while I was there, I took a class in virology run by the Biochemistry Department, and the section on retroviruses got me thinking about their possible use as gene transfer vehicles to treat genetic disease. Interestingly, Richard Mulligan, the other pioneer cited for his work on retroviral vectors, was doing his graduate work on gene transfer in the Biochemistry Department, just down the hall from my lab in pharmacology!
Gene Therapy Beginnings at the Salk Institute
After finishing my PhD in pharmacology, I was extremely fortunate to have Inder Verma accept me into his lab as a postdoctoral fellow starting in 1982. He was doing seminal work on oncogenic retroviruses and was interested in gene therapy. The Salk Institute was also a great place to fly hang gliders, with a soarable cliff right out the back door! However, Inder never did accept my offer to take him flying in my tandem glider. In collaboration with Doug Jolly, a postdoc in Ted Friedmann's lab at the University of California–San Diego, we started our work on retroviruses to treat hypoxanthine phosphoribosyltransferase (HPRT) deficiency. I also worked on the acquisition and activation of oncogenes by retroviruses, primarily by working with a very talented postdoc in Inder's lab, Tom Curran (Curran et al., 1984; Miller et al., 1984a).
Doug Jolly had just cloned the human HPRT cDNA, and genes containing this coding region were ideal for use in our early studies. Cell lines lacking HPRT were available, including cells from Lesch–Nyhan syndrome patients, and techniques to select for conversion of these cells to HPRT+ were well developed. We made a simple murine leukemia virus (MLV)/murine sarcoma virus (MSV) retroviral vector that expressed only the HPRT cDNA, and used replication-competent murine amphotropic retrovirus to rescue the vector and infect fibroblasts and lymphoblasts from Lesch–Nyhan syndrome patients. After selection for HPRT expression, the cells expressed levels of HPRT that were similar to fibroblasts from normal human subjects, confirming that this method of gene transfer was effective in a clinically relevant disease model (Miller et al., 1983). We were also able to transfer and express HPRT in bone marrow cells of mice using this vector (Miller et al., 1984b). Furthermore, we were able to obtain regulated expression of a growth hormone gene inserted into the HPRT vector, despite the strong enhancer/promoters present in the long terminal repeats (LTRs) of the vector (Miller et al., 1984c), showing that proper gene regulation following retroviral gene transfer was possible.
Of course, use of replication-competent (helper) virus to transfer genes into humans would be complicated by immune clearance of infected cells and the possibility of virus-induced cancer. Others had made cell lines that produced helper virus-free vectors (called “packaging” or “helper” cell lines) with limited host ranges that did not include humans (Mann et al., 1983; Watanabe and Temin, 1983). To allow infection of human cells, we made a packaging cell line based on a hybrid virus consisting of a Moloney murine leukemia virus (MoMLV) with its envelope (Env) coding region replaced by that of the 4070A murine amphotropic retrovirus. The packaging cells, called PA12 cells (Miller et al., 1985), were made by transfection of NIH 3T3 cells with virus genome minus the signal needed for packaging of the viral genome into virions, similar in design to the pure MoMLV packaging cells, ψ2 (Mann et al., 1983). The PA12 cells were able to produce a retroviral vector carrying a dihydrofolate reductase gene at titers of over 106 per ml of culture medium, in the absence of helper virus, and could efficiently infect human cells.
On to the Bone Marrow Transplantation Capital of the World
In mid-1984 I started to look for faculty positions, and heard a talk by Don Thomas about the work on bone marrow transplantation pioneered by Don, Rainer Storb, and their colleagues at the Fred Hutchinson Cancer Research Center. Several faculty, including Rainer, were already involved in a fledgling gene therapy effort, and within months, I began work there as an assistant member in a newly created Molecular Medicine program. Investigators at the Fred Hutch had long used an outbred canine model to develop transplantation techniques for humans, and we fairly quickly found that we could indeed transduce canine and human hematopoietic progenitor cells at reasonable rates in culture (Hock and Miller, 1986; Kwok et al., 1986).
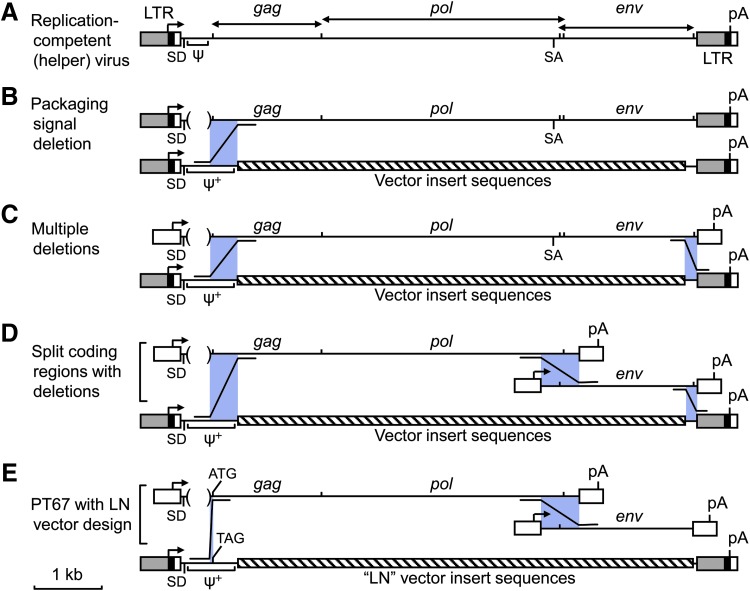
One of the retroviral vectors we used in these studies was the N2 vector from Eli Gilboa's lab (Eglitis et al., 1985), which could be produced at very high titers. Unfortunately, this was often accompanied by the production of helper virus when this vector was introduced into ψ2 mouse-tropic or PA12 amphotropic packaging cells. The N2 vector was indeed produced at higher titer than many other vectors, independent of the production of helper virus. Unlike other vectors that contained a short packaging signal from MoMLV or MoMSV, extending from the LTR to just upstream of the gag coding region, the N2 vector contained sequences from the LTR into the gag coding region. We showed that this larger region promoted better encapsidation of RNAs into virions than did the short, previously defined ψ signal (Fig. 1A), and named this extended packaging signal ψ+ (Bender et al., 1987; Adam and Miller, 1988). It seemed likely that helper virus production from ψ2 and PA12 packaging cells containing the N2 vector was because of recombination between the front end of the N2 vector and the complete helper virus genome downstream of the gag start codon in the packaging cells (Fig. 1B) (Miller et al., 1986).
FIG. 1.
Design of retroviral packaging cell lines to avoid recombination leading to helper virus production. (A) Schematic diagram of the MoMLV replication-competent (helper) retrovirus is shown at top with a scale bar (1 kb) at bottom left. (B–E) Evolving designs for retroviral packaging cell lines. Regions where recombination might occur between packaging cell line components and retroviral vectors to generate helper virus are shown by purple boxes, with Z-shaped lines indicating the direction of recombination. Directional arrows indicate sites of transcription initiation and bidirectional arrows at top indicate the extent of the retroviral protein coding regions. ψ, retroviral RNA packaging signal; ψ+, extended retroviral RNA packaging signal; ATG, gag coding region start codon; LTR, retroviral long terminal repeat; pA, polyadenylation signal; SA, splice acceptor; SD, splice donor; TAG, mutation of the gag start codon to a stop codon to prevent Gag protein translation.
To solve the issue of helper virus production in retrovirus vector stocks, while preserving the high titer afforded by the N2 vector, we generated a new packaging cell line, PA317 (Miller and Buttimore, 1986), and a set of new vectors (“LN” style vectors) (Miller and Rosman, 1989) to prevent recombination leading to helper virus production. The PA317 cells contain a plasmid for virus protein of the type depicted in Fig. 1C, which has multiple deletions designed such that two recombination events are required to produce helper virus. The new vectors we designed have no overlap with the end of the packaging DNA in the PA317 cells such that there is no possibility for homologous recombination in this region. Indeed, we have never see helper virus using these reagents in my lab.
First Gene Therapy Trial
These vector and packaging cell line improvements were critical for the first successful cell marking and gene therapy trials in humans. Steve Rosenberg had been working on a protocol to mark tumor-infiltrating lymphocytes (TIL) with the N2 retroviral vector so that they could be tracked after administration to patients, but were having problems with helper virus in their vector stocks. We provided PA317 cells that made a similar vector, called LNL6 (Miller and Rosman, 1989), that did not produce helper virus, resulting in approval of the human clinical studies and demonstration of effective TIL cell marking (Rosenberg et al., 1990).
Similarly, Mike Blaese and French Anderson were attempting to correct severe immunodeficiency in humans lacking the adenosine deaminase (ADA) enzyme by using a retroviral vector to transfer and express an ADA gene in their lymphocytes, but again were having problems with helper virus contamination of the vector stocks. In this case, my lab had also been working on retroviral vectors to treat ADA deficiency, with expert help from a great collaborator and expert in purine biochemistry from the University of Washington, Bill Osborne (Palmer et al., 1987; Hock et al., 1989; Osborne et al., 1990). We had already tested multiple vector configurations for expression of high levels of ADA, the best of which expressed the ADA cDNA from the retroviral LTR enhancer/promoter. This vector, LASN (LTR-ADA-SV40 promoter-Neo selectable marker) already incorporated features to prevent helper virus production. In collaboration with Ken Culver in Mike's lab, we compared ADA expression from our vector to that of the vector Mike and French were planning to use, the SAX vector [LTR-Neo-SV40 promoter-ADA (Kantoff et al., 1986; Cornetta et al., 1990)]. We found that the LASN vector was the clear winner, and PA317 cells expressing this vector did not generate helper virus. The LASN vector made in PA317 cells was used for the clinical study, which began in 1990 and showed the first evidence for a therapeutic effect from a viral vector (Blaese et al., 1995). Later, Don Kohn used the same vector to transduce umbilical cord hematopoietic cells and return these to ADA-deficient newborns, and showed long-term persistence of transduced cells at low levels (Kohn et al., 1995).
At the end of these early studies, we had developed a robust system for producing helper virus-free retroviral vectors at high titer for transfer and expression of high levels of multiple types of proteins in cells from multiple animal species including humans. We continued to improve the utility of retroviral vectors by switching to a split coding region with multiple deletions design (Fig. 1D) for our packaging cell lines. In particular, our current best amphotropic (actually, 10A1 MLV tropic) packaging cell line is the PT67 line, which generates vectors that can use either the Pit1 or Pit2 phosphate transporters for cell entry (Table 1). Being of a split coding regions with multiple deletions design, three recombinations are required to generate helper virus (Fig. 1D). However, when using the LN-style retroviral vectors (Miller and Rosman, 1989), there is no sequence similarity at the right end to promote recombination, a short region of sequence identity at the left end (59 base pairs) and only 86% sequence identity in the middle region to promote recombination (Fig. 1E) (Miller and Chen, 1996), making such recombinations to generate helper virus extremely unlikely.
Table 1.
Miller Lab Retrovirus Packaging Cell Lines and Cell-Surface Receptors Targeted
| Env protein | Name | Packaging cell line reference | Cell entry receptor | Cell entry receptor references |
|---|---|---|---|---|
| MoMLV ecotropic | PE501 | (Miller and Rosman, 1989) | Cat-1 (SLC7A1) | (Albritton et al., 1989; Kim et al., 1991) |
| 4070A amphotropic | PA317 | (Miller and Buttimore, 1986) | Pit2 (SLC20A2) | (Kavanaugh et al., 1994; Miller et al., 1994) |
| 10A1 MLV | PT67 | (Miller and Chen, 1996) | Pit1 or Pit2 | (Kavanaugh et al., 1994; Miller and Miller, 1994) |
| GALV | PG13 | (Miller et al., 1991) | Pit1 (SLC20A1) | (O'Hara et al., 1990; Kavanaugh et al., 1994) |
| NZB xenotropic | PX325 | — | Xpr1 | (Battini et al., 1999; Tailor et al., 1999; Yang et al., 1999; Vaughan et al., 2012) |
| 98D polytropic | PM571 | (Miller and Miller, 1992) | Xpr1 | |
| MDEV | PD223 | (Wolgamot et al., 1998) | ? | — |
| JSRV | PJ14 | (Rai et al., 2000) | Hyal2 | (Rai et al., 2001) |
| ENTV | PN229 | (Van Hoeven and Miller, 2005) | Hyal2 | (Dirks et al., 2002) |
ENTV, enzootic nasal tumor virus; GALV, gibbon ape leukemia virus; JSRV, jaagsiekte sheep retrovirus; MDEV, Mus dunni endogenous retrovirus; MLV, murine leukemia virus; MoMLV, Moloney murine leukemia virus.
All of these packaging cell lines express the Gag protein and most to all of the Pol protein from MoMLV, and the Env protein from the viruses shown in the first column. The cellular receptors targeted by vectors produced by these packaging lines are listed, when known.
We have also explored the use of additional retroviral Env proteins that target different cell-surface proteins to mediate virus entry (Table 1). Along the way, we identified many of these receptors (for examples, see Table 1), the levels of which help predict which cell types are most susceptible to a particular vector. For example, we found that vectors made using PG13 packaging cells, which express a gibbon ape leukemia virus (GALV) Env, were more effective than vectors made using PA317 amphotropic packaging cells for transducing early hematopoietic cells from baboons, and this result corresponded with higher levels of the GALV receptor Pit1 compared with the amphotropic receptor Pit2 in hematopoietic cells (Kiem et al., 1997).
Definitive Gene Therapy Successes, but with Oncogenic Side Effects in Some Subjects
Further documentation of the clinical utility of retroviral vectors awaited trials in France, Italy, and Great Britain to treat ADA deficiency and another severe combined immunodeficiency (SCID) resulting from defects in the common γ (γc) cytokine receptor subunit. Sustained correction of γc− SCID was first achieved by using a retroviral vector carrying the γc gene (Cavazzana-Calvo et al., 2000; Hacein-Bey-Abina et al., 2002). In this study, a retroviral vector encoding the γc protein and no selectable marker, produced using ψCRIP amphotropic packaging cells (Danos and Mulligan, 1988), was used to transduce autologous CD34+ bone marrow stem cells ex vivo, which were then returned to the patient. Four of five patients treated showed dramatic clinical improvement. Unfortunately, T cell leukemias were later found in four of nine successfully treated patients, which correlated with integration of the gene therapy vector near proto-oncogenes, including LMO2 (Hacein-Bey-Abina et al., 2003, 2008). Three of the affected patients subsequently responded to chemotherapy to ablate the leukemic cells, while one died.
An improved outcome was observed in another study of gene therapy treatment for γc-deficient SCID (Gaspar et al., 2011a). In this study, a retroviral vector encoding only the γc protein, a vector similar to, if not identical to, the vector used by Cavazzana-Calvo et al. but produced using PG13 packaging cells (Table 1), was used to transduce autologous CD34+ bone marrow stem cells ex vivo, which were then returned to the patient. Ten of 10 treated patients showed clinical improvement, but one of these developed an acute T cell leukemia, again associated with vector insertion near the LMO2 proto-oncogene (Howe et al., 2008).
In another example of successful use of retroviral vectors for treatment of human genetic disease, ADA-deficient patients were treated with nonmyeloablative conditioning followed by infusion of autologous CD34+ bone marrow cells transduced with a retroviral vector encoding ADA (Aiuti et al., 2002; Aiuti et al., 2009). In this case, the vector used was the LXSN vector (Miller and Rosman, 1989) into which a human ADA cDNA was inserted, thus similar if not identical to the LASN vector used in earlier ADA− SCID gene therapy trials, and the vector was produced using GP+envAm12 retrovirus packaging cells (Markowitz et al., 1988). All 10 of the treated patients showed clinical benefit with no evidence for abnormal lymphoproliferation.
In a more recent study of gene therapy for ADA− SCID (Gaspar et al., 2011b), ADA-deficient patients again received CD34+ bone marrow cells transduced with a retroviral vector carrying a gene encoding ADA. Here, the vector contained only an ADA gene, and the vector was made by using PG13 retrovirus packaging cells (Table 1). All four patients who showed bone marrow reconstitution, of a total of six treated patients, also showed clinical benefit with no evidence of abnormal lymphoproliferation.
It is interesting to note that none of the 40 ADA− SCID gene therapy subjects treated to date nor those injected with millions of transduced mature T cells have developed leukemia resulting from vector integration (Cavazzana, 2014; Cieri et al., 2014), while those with γc− SCID did. In the case of γc− SCID treatment, it is possible that overexpression of the γc cytokine receptor subunit acted as an oncogene to facilitate leukemia induction by vector insertion near the LMO2 and/or other proto-oncogenes. Much work is underway to better understand the oncogenic mechanisms, and to alter transduction protocols, modify the retroviral vectors used, or utilize lentiviral vectors that show a different genomic target specificity, to avoid oncogenic side effects. Regardless, the therapeutic results of these trials are impressive and substantiate early hopes for the clinical utility of retroviral vectors.
Summary and Future Prospects
Advantages of the retroviral vector system include (1) the ability to generate stable cell lines capable of producing unlimited amounts of vector, unlike other vector systems that rely on repeated transfection and the associated need to remove large amounts of plasmid DNA from vector preparations prior to clinical use; (2) the production of genetically homogeneous vectors from cells carrying a defined, integrated vector copy, as opposed to vectors produced by transfection, which can produce a high proportion of mutated and rearranged vectors; and (3) the key property of retrovirus integration into the genome of target cells, which results in stable vector persistence.
Disadvantages include (1) the inability of retroviral vectors to transduce nondividing cells, including many of the cells in the human body (Miller et al., 1990); (2) the relative frailty of retroviral vectors compared with AAV vectors, for example, making storage at −70°C a necessity to preserve activity; and (3) the transmission to target cells of other retrovirus elements present in packaging cells, such as virus-like 30S (VL30) RNAs (Patience et al., 1998).
Retroviral vectors have positively impacted patient care and have opened the door to a vast array of other gene transfer technologies. My guess is that these vectors based on simple gamma retroviruses will continue to have applications to in vitro modification of patient cells, along with newer lentiviral and foamy viral vectors. Unfortunately, the treatment of rare genetic diseases like SCID will continue to be very expensive procedures that may not justify commercial investment and long-term support. In contrast, other gene therapy approaches such as T-cell modification for treatment of cancer have already generated significant commercial effort. Hopefully, regulatory and treatment costs will come down with time to allow gene therapy to be widely applied.
Acknowledgments
I would like to thank the Human Gene Therapy Pioneer Award committee for the honor of this award. I thank the NIH, and the U.S. citizens whom this institution represents, for the majority of my research funding, and hope that I have provided a reasonable return on this investment. I truly appreciate the great environment for research and outstanding colleagues at the Fred Hutchinson Cancer Research Center and at the University of Washington, and thank the postdocs, students, and technicians in my lab who have contributed greatly to this work and made it fun. And last but not least, I thank my family for their support and tolerance of my long hours in the lab.
Author Disclosure Statement
No competing financial interests exist.
References
- Adam M.A., and Miller A.D. (1988). Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J. Virol. 62, 3802–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A., Slavin S., Aker M., et al. (2002). Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 [DOI] [PubMed] [Google Scholar]
- Aiuti A., Cattaneo F., Galimberti S., et al. (2009). Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 360, 447–458 [DOI] [PubMed] [Google Scholar]
- Albritton L.M., Tseng L., Scadden D., and Cunningham J.M. (1989). A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57, 659–666 [DOI] [PubMed] [Google Scholar]
- Battini J.L., Rasko J.E., and Miller A.D. (1999). A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc. Natl. Acad. Sci. USA 96, 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M.A., Palmer T.D., Gelinas R.E., and Miller A.D. (1987). Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J. Virol. 61, 1639–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaese R.M., Culver K.W., Miller A.D., et al. (1995). T lymphocyte-directed gene therapy for ADA− SCID: initial trial results after 4 years. Science 270, 475–480 [DOI] [PubMed] [Google Scholar]
- Cavazzana M. (2014). Hematopoietic stem cell gene therapy: progress on the clinical front. Hum. Gene Ther. 25, 165–170 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., et al. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672 [DOI] [PubMed] [Google Scholar]
- Cieri N., Mastaglio S., Oliveira G., et al. (2014). Adoptive immunotherapy with genetically modified lymphocytes in allogeneic stem cell transplantation. Immunol. Rev. 257, 165–180 [DOI] [PubMed] [Google Scholar]
- Cornetta K., Moen R.C., Culver K., et al. (1990). Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum. Gene Ther. 1, 15–30 [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A.D., Zokas L., and Verma I.M. (1984). Viral and cellular fos proteins: a comparative analysis. Cell 36, 259–268 [DOI] [PubMed] [Google Scholar]
- Danos O., and Mulligan R.C. (1988). Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA 85, 6460–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks C., Duh F.M., Rai S.K., et al. (2002). Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76, 2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis M.A., Kantoff P., Gilboa E., and Anderson W.F. (1985). Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science 230, 1395–1398 [DOI] [PubMed] [Google Scholar]
- Gaspar H.B., Cooray S., Gilmour K.C., et al. (2011a). Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 3, 97ra79. [DOI] [PubMed] [Google Scholar]
- Gaspar H.B., Cooray S., Gilmour K.C., et al. (2011b). Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 3, 97ra80. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Le Deist F., Carlier F., et al. (2002). Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 346, 1185–1193 [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., et al. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Garrigue A., Wang G.P., et al. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 118, 3132–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R.A., and Miller A.D. (1986). Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature 320, 275–277 [DOI] [PubMed] [Google Scholar]
- Hock R.A., Miller A.D., and Osborne W.R. (1989). Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood 74, 876–881 [PubMed] [Google Scholar]
- Howe S.J., Mansour M.R., Schwarzwaelder K., et al. (2008). Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118, 3143–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P.W., Kohn D.B., Mitsuya H., et al. (1986). Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA 83, 6563–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M.P., Miller D.G., Zhang W., et al. (1994). Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. USA 91, 7071–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiem H.P., Heyward S., Winkler A., et al. (1997). Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood 90, 4638–4645 [PubMed] [Google Scholar]
- Kim J.W., Closs E.I., Albritton L.M., and Cunningham J.M. (1991). Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352, 725–728 [DOI] [PubMed] [Google Scholar]
- Kohn D.B., Weinberg K.I., Nolta J.A., et al. (1995). Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat. Med. 1, 1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok W.W., Schuening F., Stead R.B., and Miller A.D. (1986). Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc. Natl. Acad. Sci. USA 83, 4552–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R.C., and Baltimore D. (1983). Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 33, 153–159 [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., and Bank A. (1988). Construction and use of a safe and efficient amphotropic packaging cell line. Virology 167, 400–406 [PubMed] [Google Scholar]
- Miller A.D., and Buttimore C. (1986). Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol. Cell. Biol. 6, 2895–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., and Chen F. (1996). Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J. Virol. 70, 5564–5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.G., and Miller A.D. (1992). Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J. Virol. 66, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.G., and Miller A.D. (1994). A family of retroviruses that utilize related phosphate transporters for cell entry. J. Virol. 68, 8270–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., and Rosman G.J. (1989). Improved retroviral vectors for gene transfer and expression. BioTechniques 7, 980–990 [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., Jolly D.J., Friedmann T., and Verma I.M. (1983). A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc. Natl. Acad. Sci. USA 80, 4709–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., Curran T., and Verma I.M. (1984a). c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell 36, 51–60 [DOI] [PubMed] [Google Scholar]
- Miller A.D., Eckner R.J., Jolly D.J., et al. (1984b). Expression of a retrovirus encoding human HPRT in mice. Science 225, 630–632 [DOI] [PubMed] [Google Scholar]
- Miller A.D., Ong E.S., Rosenfeld M.G., et al. (1984c). Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science 225, 993–998 [DOI] [PubMed] [Google Scholar]
- Miller A.D., Law M.F., and Verma I.M. (1985). Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol. Cell. Biol. 5, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., Trauber D.R., and Buttimore C. (1986). Factors involved in production of helper virus-free retrovirus vectors. Somat. Cell Mol. Genet. 12, 175–183 [DOI] [PubMed] [Google Scholar]
- Miller D.G., Adam M.A., and Miller A.D. (1990). Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10, 4239–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.D., Garcia J.V., von Suhr N., et al. (1991). Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 65, 2220–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.G., Edwards R.H., and Miller A.D. (1994). Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc. Natl. Acad. Sci. USA 91, 78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara B., Johann S.V., Klinger H.P., et al. (1990). Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1, 119–127 [PubMed] [Google Scholar]
- Osborne W.R., Hock R.A., Kaleko M., and Miller A.D. (1990). Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum. Gene Ther. 1, 31–41 [DOI] [PubMed] [Google Scholar]
- Palmer T.D., Hock R.A., Osborne W.R., and Miller A.D. (1987). Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc. Natl. Acad. Sci. USA 84, 1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience C., Takeuchi Y., Cosset F.L., and Weiss R.A. (1998). Packaging of endogenous retroviral sequences in retroviral vectors produced by murine and human packaging cells. J. Virol. 72, 2671–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., DeMartini J.C., and Miller A.D. (2000). Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 74, 4698–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., Duh F.M., Vigdorovich V., et al. (2001). Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98, 4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Aebersold P., Cornetta K., et al. (1990). Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 323, 570–578 [DOI] [PubMed] [Google Scholar]
- Tailor C.S., Nouri A., Lee C.G., et al. (1999). Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA 96, 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeven N.S., and Miller A.D. (2005). Improved enzootic nasal tumor virus pseudotype packaging cell lines reveal virus entry requirements in addition to the primary receptor Hyal2. J. Virol. 79, 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.E., Mendoza R., Aranda R., et al. (2012). Xpr1 is an atypical G-protein-coupled receptor that mediates xenotropic and polytropic murine retrovirus neurotoxicity. J. Virol. 86, 1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., and Temin H.M. (1983). Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol. Cell. Biol. 3, 2241–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgamot G., Rasko J.E., and Miller A.D. (1998). Retrovirus packaging cells expressing the Mus dunni endogenous virus envelope facilitate transduction of CHO and primary hematopoietic cells. J. Virol. 72, 10242–10245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.L., Guo L., Xu S., et al. (1999). Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat. Genet. 21, 216–219 [DOI] [PubMed] [Google Scholar]