Abstract
Objective
To evaluate the efficacy of Taselisib, a selective inhibitor of PIK3CA, against primary uterine serous carcinomas (USC) harboring PIK3CA mutations and HER2/neu gene amplification.
Methods
Sensitivity to taselisib was evaluated by flow-cytometry viability assays in vitro against nine primary USC cell lines. Cell cycle distribution and downstream signaling were assessed by measuring the DNA content of cells and by phosphorylation of the S6 protein by flow-cytometry. Preclinical efficacy of taselisib was also evaluated in vivo in a mouse model.
Results
Four USC cell lines harbored HER2/neu gene amplification by FISH and two of them harbored oncogenic PIK3CA mutations. Taselisib caused a strong differential growth inhibition in both HER2/neu FISH positive and HER2/neu FISH positive/PIK3CA mutated USC cell lines when compared to lines that were FISH negative and PIK3CA wild type (taselisib IC50 mean±SEM= 0.042 ± 0.006 µM in FISH+ versus 0.38 ± 0.06 µM in FISH- tumors, P <0.0001). Taselisib growth-inhibition was associated with a significant and dose-dependent increase in the percentage of cells in the G0/G1 phase of the cell cycle and dose-dependent decline in the phosphorylation of S6. Taselisib was highly active at reducing tumor growth in vivo in USC mouse xenografts harboring PIK3CA mutation and overexpressing HER2/neu (P=0.007). Mice treated with taselisib had significantly longer survival when compared to control mice (P<0.0001).
Conclusions
Taselisib represents a novel therapeutic option in patients harboring PIK3CA mutations and/or HER2/neu gene amplification.
Keywords: Uterine serous tumors, HER2/neu, PIK3CA mutation, GDC-0032, taselisib, PIK3CA-mutant mouse
Introduction
Endometrial cancer is the most common gynecologic malignancy in women in the United States with about 50,000 new cases diagnosed in 2013 [1]. Two main clinicopathological types of endometrial cancers have been described. Type-1 tumors are estrogen-dependent and associated with early stage, low grade and high overall survival. In contrast, Type-2 carcinomas are estrogen-independent neoplasms, characterized by advanced stage at diagnosis, high grade and aggressive clinical course [2]. Uterine serous carcinoma (USC) is the most biologically aggressive subtype of type-2 endometrial cancer [3]. Although this tumor constitutes less than 10% of all endometrial cancers, it accounts for a disproportionately high number of relapses and deaths (40–50%) [4]. Overall 5-year survival rates for USC are between 35–81% for stages I and II, and 0–31% for stages III and IV [5, 6]. This poor prognosis is related to the fact that a significant proportion of women with USC present with advanced stage disease and up to 50% of patients with no myometrial invasion will have extrauterine involvement or metastatic disease detected at the time of comprehensive surgical staging [7] [8]. These challenges in USC patients have led to an extensive search for more effective treatment modalities.
The PI3K/AKT pathway plays a central role in multiple cellular functions including proliferation, survival, metabolism and growth. Several isoforms of the PI3K family are implicated in pathologic processes and diseases and class 1A PI3Ks are often mutated or amplified in multiple human tumors [9]. In agreement with these data, recent molecular profiling analyses have shown that increased signaling in this pathway is associated with aggressive disease and poor prognosis in both Type I and Type II endometrial cancers [10] [11] [12] [13] [14] [15]. Furthermore, amplification of HER2/neu, a member of the ErbB receptor tyrosine kinase (TK) family located upstream to the PI3K/AKT pathway, have been also reported in about 35% of biologically aggressive endometrial cancer such as USC [16] [17, 18]. Consequently, PIK3CA and HER2/neu may represent potential targets for novel drugs against USC. Consistent with this view, multiple research groups, including ours, have provided evidence that HER2/neu/PI3K/AKT inhibitors are indeed endowed with significant anti-cancer activity against PIK3CA mutated or HER2/neu amplified human cancer cell lines [19, 20].
Taselisib, GDC-0032 (Genentech, South San Francisco, CA), is a novel, oral, selective inhibitor of PIK3CA currently in clinical trials. Unlike other PIK3CA inhibitors previously studied in USC by our group (i.e., GDC-0980) [20], taselisib binds the ATP-binding pocket of PI3K with selective preference for the mutated form of PIK3CA [21]. This selectivity profile has been shown to allow for greater sensitivity in vivo at the maximum tolerated dose relative to a pan inhibitor in representative PIK3CA-mutant xenografts [21] [22]. On the basis of this evidence the aims of this study were: 1) to evaluate the in vitro sensitivity of a panel of 9 well characterized primary USC cell lines to taselisib; 2) to compare the activity of taselisib in USC harboring amplification of HER2/neu and PIK3CA mutations versus control USC cell lines harboring wild type PIK3CA or lacking amplification of HER2/neu; 3) to evaluate the effect of taselisib on cell-cycle distribution; 4) to assess changes in phosphoprotein S6 (pS6) as downstream cellular response to taselisib exposure in vitro; 5) to evaluate the in vivo effect of taselisib in a preclinical mouse model harboring a USC xenograft with HER2/neu amplification and PIK3CA mutation.
Materials and Methods
USC cell lines
Nine primary uterine serous carcinoma cell lines were evaluated in our study. Specimens were obtained from fresh tumor biopsies collected at the time of surgery, under approval of the institutional review board. USC cell lines were established as previously described [20]. Source-patient characteristics of the USC cell lines are described in Table 1.
Table 1.
Patient characteristics, HER2/neu amplification and PIK3CA mutations in primary USC cell lines
| CELL LINES ID |
AGE | RACE | STAGE | PIK3CA MUTATIONS |
HER2/neu FISH |
HER2 IHC |
PTEN MUTATIONS |
KRAS MUTATIONS |
RAF MUTATIONS |
AKT MUTATIONS |
|---|---|---|---|---|---|---|---|---|---|---|
| USPC-ARK-1 | 62 | B | IV | 542/1068 | A | 3+ | ND | ND | ND | ND |
| USPC-ARK-2 | 63 | B | IV | ND | A | 3+ | ND | ND | ND | ND |
| USPC-ARK-4 | 73 | W | IV | ND | NA | 0 | ND | ND | ND | ND |
| USPC-ARK-6 | 62 | W | IB | ND | NA | 1+ | ND | ND | ND | ND |
| USPC-ARK-7 | 75 | W | IIC | ND | NA | 2+ | ND | ND | ND | ND |
| USPC-ARK-8 | 88 | W | IIIA | ND | NA | 1+ | ND | ND | ND | ND |
| USPC-ARK-19 | 65 | W | IA | ND | NA | 1+ | ND | ND | ND | ND |
| USPC-ARK-20 | 42 | W | II | 1047/1068 | A | 3+ | ND | ND | ND | ND |
| USPC-ARK-21 | 70 | W | IA | ND | A | 3+ | ND | ND | ND | ND |
FIGO, International Federation of Gynecology and Obstetrics stage 1988; B= black; W= white;
ND= not detected; A= amplified; NA= not amplified.
For comprehensive whole exome genomic results of these cell lines see reference #18
Tumor HER2/neu gene amplification was evaluated by fluorescence in situ hybridization (FISH) while PIK3CA gene mutations were evaluated by PCR and DNA sequencing in all cell lines as previously described [20].
Drug
Taselisib was provided by Genentech Inc, South San Francisco, CA. In all experiments taselisib was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) as a 10 mM stock solution and diluted in culture medium immediately before use.
Drug-response assay
The effect of taselisib on the viability and IC50 of cells was determined by flow-cytometry assays. Tumor cells derived from 9 primary USC cell lines, established as long term cultures in vitro, were plated in six-well tissue culture plates and treated with taselisib at concentrations of 0.05, 0.1, 0.5, 1.0, 2.0 µM at least 24 hrs after plating. After 72 hours of additional incubation, well contents were harvested in their entirety, centrifuged and then stained with propidium iodide (2 µL of a 500 µg/mL stock solution in PBS) for flow cytometric counts. Viable cells were then quantified using flow-cytometry (FACSCalibur, Beckton-Dickinson, San Jose, CA) as percentage of viable cells (mean ± SEM) after exposure to different concentrations of taselisib relative to vehicle-treated cells (i.e., 100% viable). A minimum of 3 independent experiments per USC cell line were performed.
Flow-cytometry analysis of cell cycle in primary USC cell lines
USC cells were seeded in six-well tissue culture plates and 24 hrs later were treated with taselisib. After 24 hrs exposure to 50 nM, 100 nM and 500 nM of drug, treated cells and control cells were permeabilized with ice-cold 70% ethanol and fixed for 30 min at 4° C. After spinning at 2000 rpm for 5 min and discarding supernatant, cells were resuspended in 1 ml of PBS. After additional spinning at 2000 rpm for 5 min, 100 µl ribonuclease (100 µg/ml, DNase free, Sigma) was added for 5 min incubation at room temperature, before exposure to 400 µl of propidium iodide (50 µg/ml in PBS). Taselisib treated and untreated control cells were acquired with FACSCalibur, using Cell Quest software (BD Biosciences, San Jose, CA) and were analyzed using FlowJo software (Ashland, OR).
Flow-cytometry analysis of phosphorylated S6 intracellular levels in primary USC cell lines
Next we evaluated pS6 expression levels in representative HER2/neu amplified USC cell lines harboring PIK3CA mutation (i.e., USPC-ARK-1) before and after exposure to taselisib by flowcytometry. After 4 hrs exposure to 25 nM of taselisib, cells were fixed in 4% formaldehyde and permeabilized with ice-cold 90% methanol. Taselisib treated and untreated control cells were incubated with primary rabbit monoclonal antibody against pS6 (Cell Signaling Technology, Inc., Danvers, MA) following the protocol provided by the manufacturers and stained with a fluorescein isothiocyanate-conjugated goat anti-rabbit F(ab’)2 immunoglobulin as a secondary reagent (Chemicon International, Temecula, CA). Cells (i.e., 10,000 events per sample) were analyzed on FACSCalibur, using Cell Quest software.
In vivo assay of drug effect
To determine the in vivo activity of taselisib, a representative PIK3CA-mutated/FISH+ cell line (USPC-ARK-1) was injected into the subcutaneous region of ten 5–8 week old SCID mice (Harlan Laboratories, Indianapolis, IN). After implantation of cells, tumors were monitored until they reached a tumor volume of 0.1 cm3 prior to initiating the treatment. Mice were then randomized into 2 treatment groups namely, control and taselisib, keeping average tumor volume similar between groups. Each group consisted of 5 mice. The control group was treated with vehicle (0.5% methylcellulose-0.2% Tween 80) while the taselisib experimental group was treated with 11.25 mg/kg of taselisib. Treatments were given orally once a day, 5 days a week. Tumor volume was calculated by the formula V= length × (width)2 × 0.5 [23]. Tumor sizes and body weights were recorded three times per week. The mice in both treatment groups were treated for 1 month with taselisib or placebo after which they were observed for overall survival (OS). When tumor reached 1 cm3 or became necrotic the animals were removed from the study and euthanized according to the rules and regulations set forth by the Institutional Animal Care and Use Committee (IACUC).
Statistical Analysis
Dose response curves of uterine serous carcinoma cell lines after exposure to taselisib have been created using GraphPad Prism5 version 6 Software (GraphPad Software, Inc., San Diego, CA). For each independent experiment of taselisib on a given cell line, the measures of growth under different dose levels were normalized to the mean of the control group receiving no drug, so that all data were expressed as a proportion of the control. Normalized data were then fit via nonlinear regression to a normalized logistic response curve against the base-10 logarithms of dose in M, and the resulting parameter estimates were used to calculate the value of the IC50 (in log10 units) for that experiment. For the flow-cytometry experiments, changes in the phosphorylated S6 protein levels were analyzed comparing the mean intensity of fluorecence (MFI) before and after the exposure to taselisib. Unpaired t-test was used to assess pS6 changes and cell cycle changes in the HER2/neu amplified PIK3CA mutated cell line (USPC-ARK-1). Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered significant at P values < 0.05.
Results
Molecular results
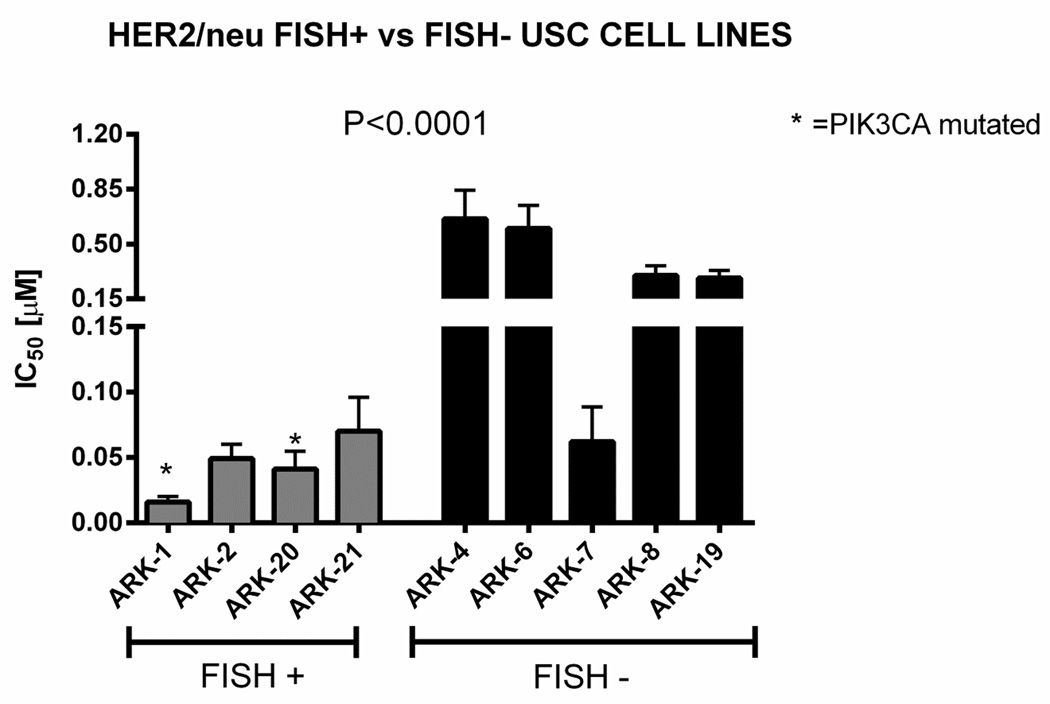
Comprehensive whole exome genomic results of the cell lines studied in our report have been recently reported [18]. Four USC cell lines (i.e., USPC-ARK-1, 2, 20 and 21) were found to harbor HER2/neu gene amplification by FISH while two out of nine (i.e., USPC-ARK-1 and 20) were found to have oncogenic PIK3CA mutations. The missense mutations were located in exon 9 (E542K, c.1624 G>A, USPC-ARK-1) and in exon 20 (H1047R, c.3140 A>G, USPC-ARK-20) (Table 1). Both PIK3CA mutated cell lines were found to harbor HER2/neu gene amplification. No PIK3R1, PTEN, KRAS, RAF and AKT mutations were found in any of the cell lines.
Taselisib activity in vitro
Next, we evaluated the antitumor activity of taselisib in all nine primary cell lines established as long term in vitro cultures (i.e., 4 cell lines harboring HER2/neu gene amplification versus 5 HER2/neu negative cell lines). As shown in Figure 1, using scalar concentrations of taselisib, we found strong growth inhibition in all PIK3CA mutated cell line and/or HER2/neu amplified cell lines when compared to HER2/neu non-amplified cell lines (taselisib IC50 mean±SEM= 0.042 ± 0.006 µM in PIK3CA mutated cell line/FISH+ versus 0.38 ± 0.06 µM in PIK3CA wild type/FISH- tumors, P <0.0001). The USPC-ARK-1 cell line was the most sensitive to taselisib, with a mean inhibitory concentration (IC50) ± standard error (SEM) of 0.014 ± 0.002 µM. The USPC-ARK-4 cell line was found to be the least sensitive, with a mean IC50 of 0.66 ± 0.1 µM. Representative dose response curves are shown in Figure 2A. Dose curves response of the remaining seven cell lines are shown in Figure S1. We further compared the IC50 values of PIK3CA mutated/FISH+ cell lines and PIK3CA wild type/FISH+ cell lines. HER2/neu amplified USC harboring PIK3CA mutations (i.e., USPC-ARK-1, USPC-ARK-20) were significantly more sensitive to taselisib (i.e., mean inhibitory concentration (IC50) ± standard error (SEM) =0.029 ± 0.006 µM) when compared to the PIK3CA wild type HER2/neu amplified cell lines USPC-ARK-2 and USPC-ARK-21 (i.e., mean IC50 values of 0.058 ± 0.007 µM, P =0.01) (Figure 2B).
Figure 1.
Graph showing IC50 values for all 9 USC cell lines after exposure to taselisib. Inhibition of USC cell proliferation was determined by flow-cytometry. The 4 HER2/neu FISH positive cell lines are located to the left of the graph while the 5 HER2/neu FISH negative USC cell lines are located to the right of the graph. The asterisk denotes the 2 USPC cell lines harboring PIK3CA mutation. Cells were treated with taselisib and incubated for 72 hours. Each column on the graph represents the mean of 3 experiments in each group. Mean IC50 values of PIK3CA mutated and/or HER2/neu FISH positive cells compared to HER2/neu FISH negative cell lines are significantly different (P<0.0001).
Figure 2.
Dose response curves of uterine serous carcinoma cell lines after exposure to taselisib. 2A: representative dose response curves with IC50 of a PIK3CA mutated/FISH+ cell line (USPC-ARK-1) vs a PIK3CA wild-type/FISH- cell line (USPC-ARK-4). 2B: scatter plot showing IC50 (mean±SEM) for PIK3CA mutated HER2/neu amplified cell lines compared to the IC50 (mean±SEM) for PIK3CA wild-type HER2/neu amplified cell lines. The PIK3CA mutated cell lines are significantly more sensitive than PIK3CA wild-type cell lines to taselisib (P=0.01).
Effect of taselisib on the cell cycle
To evaluate the potential antiproliferative effects of taselisib in PIK3CA mutated/FISH+ cell line (USPC-ARK-1), the percentage of cell in the G0/G1 phase of the cell cycle was evaluated by flow-cytometry after the exposure to the drug. As shown in Figure 3, treatment with 50 nM, 100 nM and 500 nM of taselisib for 24 hrs was able to significantly increase the fraction of cells in the G0/G1 cell cycle phase in the USC cell lines when compared with the untreated controls (P = 0.0002, P=0.0005, P<0.00001, respectively).
Figure 3.
Graph representing mean of percentage of cells in G0/G1, S, and G2/M phase after exposure to increasing concentration of taselisib in a representative USC cell line (USPC-ARK-1). A statistically significant dose-dependent increase in the percentage of cells in G0/G1-phase was seen at all concentration tested. Treatment with 50 nM, 100 nM and 500 nM of taselisib for 24 h was able to significantly increase the fraction of cells in the G0/G1 cell cycle phase in the USC cell line when compared with the untreated controls (*P = 0.0002, ** P=0.0005, ***P<0.00001).
Taselisib affects pS6 expression levels in USC cell lines
We next evaluated pS6 expression levels in a representative HER2/neu amplified USC cell line harboring PIK3CA mutation (USPC-ARK-1) before and after exposure to taselisib. Flow-cytometry revealed that taselisib was able to significantly reduce S6 phosphorylation after 4 hrs of treatment at 25 nM (Figure 4). In the FISH+/PIK3CA mutated cell lines, MFI for pS6 before treatment ranged from 99.6 ± 4.1 (mean ± SEM) while after taselisib treatment ranged from 32.3 ± 4.6 (P=0.0004).
Figure 4.
Effect on pS6 in uterine serous carcinoma cells lines after exposure to taselisib. Representative graph showing a statistically significant difference in the reduction in mean fluorescence intensity (MFI) of pS6 in PIK3CA mutated/FISH+ cell line (USPC-ARK-1) after 4 hrs exposure to taselisib (P=0.0004).
Taselisib activity in vivo
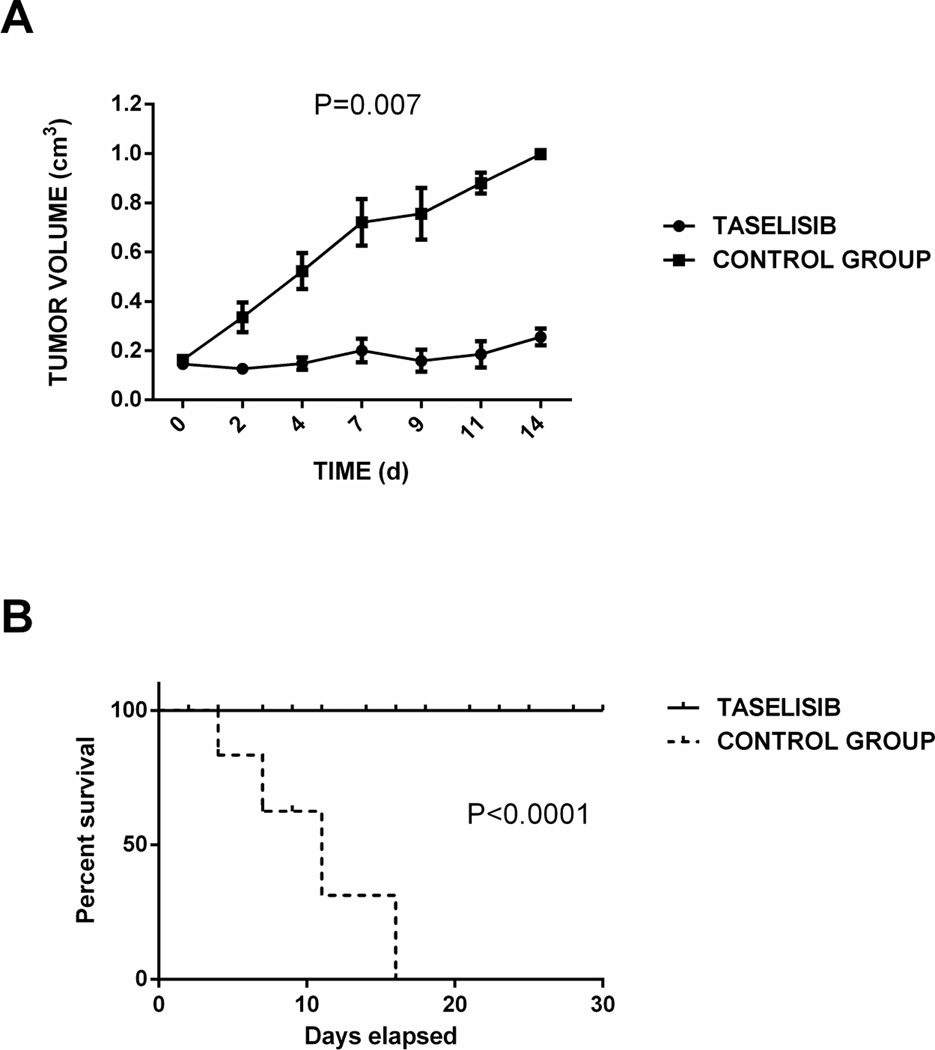
We further evaluated the effect of taselisib in vivo using a xenograft USC model. Ten mice were randomized in two groups, control and taselisib. No signs of general toxicity were seen in any of the 2 treatment groups harboring the USC-xenograft and no animal died during the experiments or had to be prematurely sacrificed due to signs of systemic drug toxicity. One mouse in the control group had to be sacrificed after 7 days because it reached 1 cm3 in tumor volume while the remaining control animals had to be sacrificed within 2 weeks secondary to their tumor volume. Taselisib group showed a significant tumor growth inhibition after 14 days of treatment (P=0.007; Figure 5 panel A) and significantly improved OS when compared to the control group (P<0.0001; Figure 5 panel B). The mean survival of the taselisib-treated mice was 45 days.
Figure 5.
Administration of taselisib results in significant tumor growth inhibition and longer overall survival in established USC xenografs. Panel A: Graph showing a statistically significant difference in tumor growth between the control group treated with vehicle (0.5% methylcellulose-0.2% Tween 80) and the taselisib group at 11.25 mg/Kg/daily (P=0.007). Panel B: Graph showing a statistically significant difference in overall survival between the control group and the taselisib group (P<0.0001).
Discussion
Regardless to the use of surgery, radiation and chemotherapy, most of the patients diagnosed with advanced or recurrent USC experience an extremely poor prognosis with less than 30% surviving 5 years from the time of diagnosis [5, 6]. The development of novel, more effective therapies against advanced/recurrent USC remains a high priority.
In the last few years, in an attempt to better understand the molecular profile of these rare tumors, our group and others have reported the mutational landscape of USC in comprehensive genetic studies [18] [24] [25] [26]. These landmark reports, confirm and extend molecular results from previous studies [28] [29] [30] [31] [32] [33] [34] [26] [25] [35], which have identified the HER2/neu/PI3K/AKT/mTOR pathway as an important target for the treatment of this lethal subset of gynecologic tumors.
In this regard, the HER2/neu/PI3K/AKT/mTOR pathway is well known to regulate a wide variety of essential cellular functions such as cellular growth, glucose metabolism, translational regulation of protein synthesis, cell proliferation and apoptosis, and has recently been implicated in endometrial cancer pathogenesis [9] [13] [16]. Increased PI3K/AKT signaling is associated with aggressive disease and poor prognosis, regardless of endometrial cancer tumor type [13]. Importantly, 80% of PIK3CA somatic mutations identified in human cancer have been shown to cluster in exon 9 (E542K and E545K) and exon 20 (H1047R) and are known to elevate the PIK3CA oncogenic activities via the Akt signaling pathway in a variety of human cancer types [9], [36] [37]. Consistent with this data, our group has recently reported that 61% of PIK3CA mutations occur in exon 9 and 20 in USC [18]. These results are in agreement with earlier results of others demonstrating about 50% of the PIK3CA mutations found in endometrial endometrioid carcinoma to take place in exon 9 and 20 [25] [26] [33] [34] [35].
On the basis of this knowledge, in the last few years several potent and selective dual PIK3CA/mTOR inhibitors have been generated and carried forward into clinical trials. Unfortunately, severe rash, diarrhea and sometimes difficult to control hyperglycemia have been reported in both mice and human studies [38] [39]. Taselisib is a novel, oral, selective inhibitor of PIK3CA, which spares inhibition of PIK3-beta. Isoform-specific PIK3 inhibitors are expected to produce greater target inhibition with fewer adverse events [40]. Clinically, the benefit of targeting a specific isoform of PIK3 has led to more complete target inhibition at lower doses resulting in less adverse effects [41]. In preclinical studies, taselisib demonstrated antitumor activity, inducing tumor growth arrest and regression in PIK3CA mutated xenograft models [21]. Thanks to promising in vitro and in vivo results, taselisib is currently undergoing Phase I/II clinical trials in patients with advanced solid breast cancer (NCT01296555).
In this study, we have evaluated the sensitivity of a series of primary, highly purified and characterized USC cell lines harboring PIK3CA mutations and HER2/neu gene amplification to taselisib and compared our results to control USC cell lines harboring PIK3CA wild type or without HER2/neu gene amplification. Our data consistently demonstrate that taselisib has a stronger growth inhibitory effect on HER2 amplified/PIK3CA mutated USC cell lines in vitro as well in vivo. Importantly, taselisib showed a significantly higher growth inhibitory effect on HER2/neu amplified and PIK3CA mutated USC cell lines when compared with HER2/neu amplified PIK3CA wild type cell lines. These data suggest that USC harboring both genetic anomalies may be even more addicted to the HER2/neu/PI3K/AKT/mTOR pathway than tumors harboring only HER2/neu gene amplification. At this time point it is not completely understood why ARK7 (i.e., a USC cell line harboring a wild type PIK3CA gene) behaves as an outlier in our assays. Our experimental data however (Table 1) allow the speculation that ARK7 harbors a hyperactive HER2/neu/PI3kinase/mTOR pathway due to the overexpression of HER2/neu protein at level 2 + by IHC.
Since in previous studies the PI3K/AKT pathway has been shown to play a key role in regulate G1-S cell cycle transition [42], we next evaluated cell cycle distribution in a representative USC cell line after exposure to taselisib. Our results are consistent with a significant increase in the number of cells blocked in G0/G1 phase of the cell cycle even at the lowest (50 nM) dose of taselisib tested. These results suggest that taselisib treatment impose a blockade of G1-S-phase transition, thereby causing a G1-phase arrest of the cell cycle. It is reasonable to speculate that this cell cycle arrest is an irreversible process that ultimately results in apoptotic cell death. We also evaluated the ability of taselisib to induce a downstream cellular response by measuring phosphorylation of the S6 protein by flow-cytometry. Our results demonstrate that taselisib is able to inhibit the PI3K pathway in a concentration-dependent manner through reduced phosphorylation of S6, a critical downstream target in USC (P=0.0004). These findings are consistent with a recent study reported by our group using another PIK3CA inhibitor (i.e., GDC-0980) in USC [20]. Importantly, our current results using taselisib demonstrates this novel PI3Kalpha inhibitor, which spares inhibition of PI3K-beta, to be more potent (i.e., IC50s 8–10 fold lower) when compared to GDC-0980 [15].
Finally, we evaluated in vivo the effect of taselisib in mice harboring xenografts of a PIK3CA mutated HER2/neu amplified cell line (USPC-ARK-1). Mice treated with vehicle had faster tumor growth and had to be sacrificed at an earlier time point. Taselisib treatment resulted in a dramatically slower rate of tumor growth and a significant improvement in OS (P=0.007 and P<0.0001 respectively). These in vivo results are consistent with our in vitro experimental results with taselisib against multiple primary USC cell lines harboring oncogenic PIK3CA mutations (i.e., E542K and H1047R) and HER2/neu gene amplification. Taken together these findings suggest that taselisib given by oral gavage daily may be a potentially effective therapy for aggressive PIK3CA mutated/HER2 amplified USC.
Single-agent treatment of unselected endometrial cancer patients with PI3K/mTOR inhibitors have so far shown limited clinical efficacy [43] [44] [45]. It has been speculated that PI3K pathway inhibitors may have limited clinical activity if used as single agents, due to the complex network of negative feedback loops involving receptor tyrosine kinases (RTK) and the activation of several survival signaling pathways (i.e., secondary target mutations, activation of parallel pro-survival signaling pathways, and amplification of downstream lesions in the same pathway) [43] [44] [46]. The experimental results reported in this manuscript using both HER2/neu gene amplified and PIK3CA mutated/HER2/neu gene amplified USC cell lines (i.e., molecular features more common in USC when compared to Type I endometrioid adenocarcinoma) [17], strongly suggest that the presence of these markers may potentially allow the identification of USC patients most likely to respond to PI3K pathway inhibitors such as taselisib in vivo.
In conclusion, our study shows the first preclinical demonstration that taselisib, a new PIK3CA inhibitor, may represent a novel, potentially effective therapeutic strategy against PIK3CA mutated and HER2/neu gene amplified USC. Clinical trials of taselisib in USC are warranted.
Supplementary Material
Dose curves response of the remaining seven cell lines after exposure to taselisib.
HIGHLIGHTS.
This is the first research article to evaluate the efficacy of Taselisib in USC.
Taselisib may represent a novel therapeutic agent for advanced/recurrent USC.
PIK3CA mutation and HER2/neu amplification determine sensitivity of USC to Taselisib
Acknowledgments
We wish to thank Genentech for providing taselisib for our experiments. This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation and the Guido Berlucchi Foundation to ADS. This investigation was also supported by NIH Research Grant CA-16359 from the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors report no conflicts of interest
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic oncology. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. The American journal of surgical pathology. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. British journal of cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boruta DM, 2nd, Gehrig PA, Groben PA, Bae-Jump V, Boggess JF, Fowler WC, Jr, et al. Uterine serous and grade 3 endometrioid carcinomas: is there a survival difference? Cancer. 2004;101:2214–2221. doi: 10.1002/cncr.20645. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Asrari F, Trimble EL, Montz FJ. Extended surgical staging for uterine papillary serous carcinoma: survival outcome of locoregional (Stage I-III) disease. Gynecologic oncology. 2001;81:279–286. doi: 10.1006/gyno.2001.6159. [DOI] [PubMed] [Google Scholar]
- 7.Cirisano FD, Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecologic oncology. 1999;74:385–394. doi: 10.1006/gyno.1999.5505. [DOI] [PubMed] [Google Scholar]
- 8.Nicklin JL, Copeland LJ. Endometrial papillary serous carcinoma: patterns of spread and treatment. Clinical obstetrics and gynecology. 1996;39:686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori Y, Kigawa J, Itamochi H, Shimada M, Takahashi M, Kamazawa S, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:892–895. [PubMed] [Google Scholar]
- 11.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer research. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 12.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nature reviews Clinical oncology. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 13.Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 15.Myers AP. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway--the devil is in the details. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5264–5274. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- 16.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Molecular diagnosis & therapy. 2013;17:85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1605–1612. doi: 10.1038/modpathol.2013.113. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PloS one. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English DP, Bellone S, Cocco E, Bortolomai I, Pecorelli S, Lopez S, et al. Oncogenic PIK3CA gene mutations and HER2/neu gene amplifications determine the sensitivity of uterine serous carcinoma cell lines to GDC-0980, a selective inhibitor of Class I PI3 kinase and mTOR kinase (TORC1/2) American journal of obstetrics and gynecology. 2013;209:465, e1–e9. doi: 10.1016/j.ajog.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. Journal of medicinal chemistry. 2013;56:4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Molecular cancer therapeutics. 2014;13:1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 23.Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer discovery. 2013;3:308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Cancer Genome Atlas Research N. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gallo M, O'Hara AJ, Rudd ML, Urick ME, Hansen NF, O'Neil NJ, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatinremodeling and ubiquitin ligase complex genes. Nature genetics. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang Y, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. Journal of the National Cancer Institute. 2012;104:1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 28.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1271–1279. [PubMed] [Google Scholar]
- 29.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 30.Santin AD, Bellone S, Van Stedum S, Bushen W, De Las Casas LE, Korourian S, et al. Determination of HER2/neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecologic oncology. 2005;98:24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 32.Diaz-Montes TP, Ji H, Smith Sehdev AE, Zahurak ML, Kurman RJ, Armstrong DK, et al. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecologic oncology. 2006;100:139–144. doi: 10.1016/j.ygyno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Hayes MP, Douglas W, Ellenson LH. Molecular alterations of EGFR and PIK3CA in uterine serous carcinoma. Gynecologic oncology. 2009;113:370–373. doi: 10.1016/j.ygyno.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. The Journal of pathology. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer research. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 38.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nature reviews Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 39.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nature reviews Clinical oncology. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 41.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottschalk AR, Basila D, Wong M, Dean NM, Brandts CH, Stokoe D, et al. p27Kip1 is required for PTEN-induced G1 growth arrest. Cancer research. 2001;61:2105–2111. [PubMed] [Google Scholar]
- 43.Pavlidou A, Vlahos NF. Molecular alterations of PI3K/Akt/mTOR pathway: a therapeutic target in endometrial cancer. TheScientificWorld Journal. 2014;2014:709–736. doi: 10.1155/2014/709736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer? Oncotarget. 2011;2:1314–1321. doi: 10.18632/oncotarget.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan J, Yu Q. Molecular mechanisms of tumor resistance to PI3K-mTOR-targeted therapy. Chinese journal of cancer. 2013;32:376–379. doi: 10.5732/cjc.012.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose curves response of the remaining seven cell lines after exposure to taselisib.