Abstract
Aim: Household ancient remedies reported here are described in the National List of Essential Medicines and have traditionally been used in Thailand to treat infection-related ailments. However, the safety and effectiveness of these remedies have been poorly evaluated. The aim of this study was to evaluate the antibacterial properties of these remedies against seven gram-positive and gram-negative multidrug-resistant bacteria species. Phytochemical constituents and cytotoxicity of these remedies were also determined.
Methods: Seven remedies, consisting of Um-Ma-Luk-Ka-Wa-Tee, Chan-Ta-Lee-La, Kheaw-Hom, Learng-Pid-Sa-Mud, Pra-Sa-Chan-Dang, Dhart-Ban-Chob, and Tree-Hom, were prepared by a licensed traditional medical doctor using a mixture of medicinal plants. Antibacterial activity of ethanol extracts of the remedies was determined by using a broth microdilution method. Qualitative phytochemical screening analysis was carried out to identify the presence of major components. Cytotoxicity activities of the extracts against Vero cells were assessed by green fluorescent protein–based assay.
Results: With the exception of Dhart-Ban-Chob extract, significant minimum inhibitory concentrations (MICs) of <16 to 32 μg/mL were observed for the remedy extracts depending on the bacterial strains. The Um-Ma-Luk-Ka-Wa-Tee extract was noncytotoxic against Vero cells and possessed the highest activity, with MICs of <16 to 31 μg/mL against all methicillin-resistant Staphylococcus aureus isolates.
Conclusions: Remarkable antibacterial activities against multidrug-resistant pathogens, as well as low toxicity on Vero cells, of Um-Ma-Luk-Ka-Wa-Tee support the use of this remedy in traditional medicine. Further investigation on other biological activities related to traditional applications, appropriate biomarkers, and treatment mechanisms of the household remedy are required.
Introduction
Antimicrobial drug resistance is recognized as one of the greatest problems in both developing and developed countries, in hospitals as well as in the community. Many studies have estimated the excess mortality and morbidity, and economic impact of infections caused by antibiotic-resistant pathogens.1,2 Even though enormous efforts have been made to discover new drugs, to implement a more appropriate prescribing antibiotic strategy, and to seek new ways of using older antibiotics, the prevalence of bacterial resistance has been increasing worldwide. In Europe and the United States, the excess hospital costs associated with the infections were as high as $30 billion.1–3 Infections with the multidrug-resistant bacteria are estimated to cause around 25,000–99,000 deaths per year.2,4
Gram-positive cocci have emerged as significant pathogens associated with nosocomial infections in the past two decades. On the basis of results from the SENTRY Antimicrobial Surveillance Program, Staphylococcus aureus and coagulase-negative staphylococci are the microorganisms most frequently isolated from bloodstream infections, and about one third of the infections are caused by these pathogens.5 Methicillin-resistant S. aureus (MRSA) accounts for approximately 30% of S. aureus isolates and is also resistant to clindamycin, ciprofloxacin, and levofloxacin.5
Multidrug-resistant (MDR) gram-negative pathogens are less prevalent than gram-positive bacteria. However, infections due to pan–drug-resistant strains are sometimes untreatable. Escherichia coli is one of the most common causes of bloodstream infections, and data from Europe revealed that 9.3% (13,950 cases) of the isolates were resistant to a third-generation cephalosporin, a regularly used antibiotic.2 The 30-day mortality rate among patients with third-generation cephalosporin–resistant E. coli bloodstream infections was 2.5 times higher than that of the susceptible group.
Acinetobacter baumannii and Pseudomonas aeruginosa can cause a variety of infections and quickly become resistant to commonly prescribed antimicrobials. In a study from Karachi, Pakistan, nearly 50% of babies born with the infections caused by Acinetobacter spp. died, and approximately 70% of isolates were resistant to all antibiotics except polymyxin.6 Despite the introduction of antipseudomonal antibiotics, P. aeruginosa continues to be a serious cause of infection associated with high rates of morbidity and mortality. Most studies have found that mortality rates varied from 30% to 60% among all patients with P. aeruginosa bloodstream infections and have remained high during the past few decades.7,8 In view of all this, the need to discover alternative and effective antibacterial agents is urgent.
Traditional Thai medicine (TTM) is a system of traditional medicine of the Kingdom of Thailand originated during the Sukhothai period (1238–1377) and was formally accepted as a primary health care resource in the late 19th century.9 Thai household ancient remedies have been noted in the National List of Essential Medicines, List of Herbal Medicinal Products D, 2006. Fifty ancient remedies and 21 herbal products were approved by the Ministry of Public Health for their effectiveness in treating ailments and are described in the National List of Essential Medicines, List of Herbal Medicinal Products A.D. 2011.10 However, scientific evidence of the biological effects of Thai household ancient remedies is limited.
Therefore, this study evaluated the in vitro antibacterial potential of seven Thai household ancient remedies that are traditionally used to treat infection-related ailments against seven gram-positive and gram-negative multidrug-resistant bacteria species. Moreover, the phytochemical constituents and cytotoxicity of these remedies were additionally determined. Results obtained from this study provide scientific information to support the use of herbal-based remedies for treatments of infectious diseases, particularly those caused by multidrug-resistant pathogens.
Materials and Methods
Thai household ancient remedies
Information on household ancient remedy uses in this paper was based on the National List of Essential Medicines, List of Herbal Medicinal Products A.D. 2006. The selection of seven Thai household ancient remedies—Um-Ma-Luk-Ka-Wa-Tee, Chan-Ta-Lee-La, Kheaw-Hom, Learng-Pid-Sa-Mud, Pra-Sa-Chan-Dang, Dhart-Ban-Chob, and Tree-Hom—screened in this study was based on their traditional claims for treatment or relief of fever, diarrhea, or sore throat. The list of medicinal components, medical applications, and dosages is summarized in Table 1 from the National List of Essential Medicines, List of Herbal Medicinal Products, 2nd edition, B.E. 2554 (2011).10 The powdered remedies, prepared by a licensed Thai traditional medical doctor, Mr. Somchai Ontong (TM.P. no. 22907), were purchased from Traditional Thai Medicine Hospital, Prince of Songkla University, Hat Yai, Thailand.
Table 1.
Medical Applications and Ingredients of Selected Thai Household Ancient Remedies
| Household remedies (net weight of remedy powder) | Herbal components (parts used) | Weight (g per the net weight) | Ailments/uses | Dosagea |
|---|---|---|---|---|
| Chan-Ta-Lee-La (33 g) | Angelica dahurica Benth. (root) | 4 | For relief of fever | Adults: 3–5 T×3 |
| Atractylodes lancea (Thunb.) DC. (rhizome) | 4 | Children: 1–2 T×3 | ||
| Artemisia annua L. (whole plant) | 4 | |||
| Myristica fragrans Houtt. (mace) | 4 | |||
| Dracaena loureiri Gagnep. (heartwood) | 4 | |||
| Gymnopetalum chinensis (Lour.) Merr. (fruit) | 4 | |||
| Tinospora crispa (L.) Miers ex Hook.f. & Thomson (climber) | 4 | |||
| Eurycoma longifolia Jack. (root) | 4 | |||
| Pogostemon cablin (Blanco) Benth. (leaf ) | 1 | |||
| Pra-Sa-Chan-Dang (64 g) | Symplocos racemosa Roxb. (root) | 4 | For relief of fever and thirst | Adults: 1 tsp×4 |
| Bouea macrophylla Griff (root) | 4 | Children: 0.5 tsp×4 | ||
| Citrus aurantifolia Swingle (root) | 4 | |||
| Kaempferia galanga L. (rhizome) | 4 | |||
| Ligusticum sinense Oliv. (rhizome) | 4 | |||
| Myristica fragrans Houtt. (mace) | 4 | |||
| Caesalpinia sappan Linn. (heartwood) | 4 | |||
| Nelumbo nucifera Gaertn. (stamen) | 1 | |||
| Mesua ferrea L. (pollen) | 1 | |||
| Mammea siamensis (flower) | 1 | |||
| Jasminum sambac (flower) | 1 | |||
| Dracaena loureiri Gagnep. (heartwood) | 32 | |||
| Kheaw-Hom (18 g) | Pogostemon cablin (Blanco) Benth. (leaf ) | 1 | For relief of fever from chickenpox and measles and for thirst relief | Adults: 1–2 tsp×3 |
| Limnophila rugosa (Roth.) Merr (leaf ) | 1 | Children: 0.5 tsp×3 | ||
| Cordyline fruticosa (L.) Goeppert (leaf ) | 1 | |||
| Eupatorium stoechadosmum Hance (leaf ) | 1 | |||
| Vetiveria zizanioides (L.) Nash ex Small. (root) | 1 | |||
| Kaempferia galanga L. (rhizome) | 1 | |||
| Myristica fragrans Houtt. (heartwood) | 1 | |||
| Dracaena loureiri Gagnep. (heartwood) | 1 | |||
| Angiopteris evecta Hoffm. (ND) | 1 | |||
| Globba malaccensis Ridl. (ND) | 1 | |||
| Tacca chantrieri Andre. (ND) | 1 | |||
| Sophora exigua Craib. (ND) | 1 | |||
| Cyathea borneensis Copel.(ND) | 1 | |||
| Aristolochia spp. (root)b | 1 | |||
| Mimusops elengi L. (flower) | 1 | |||
| Mesua ferrea L. (pollen) | 1 | |||
| Mammea siamensis Kosterm. (pollen) | 1 | |||
| Nelumbo nucifera Gaertn. (stamen) | 1 | |||
| Tree-Hom (64 g) | Terminalia sp. (fruit) | 4 | For relief of fever | Children: |
| Terminalia bellirica (Gaertn.) Roxb (fruit) | 4 | Laxatives for children | at 1–2 mo: 1 T×1 | |
| Phyllanthus emblica (fruit) | 4 | at 3–5 mo: 2–3 T×1 | ||
| Coriandrum sativum L. (seed) | 4 | at 6–12 mo: 5–7 T×1 | ||
| Aristolochia spp. (root) | 1 | |||
| Angelica dahurica Benth. (root) | 1 | |||
| Glycyrrhiza glabra (root) | 1 | |||
| Trigonella foenum-graecum L. (seed) | 1 | |||
| Terminalia chebula Retz. (fruitfruit) | 22 | |||
| Rheum palmatum L. (steamed root) | 22 | |||
| Learng-Pid-Sa-Mud (18 g) | Cyperus rotundus L. (rhizome) | 1 | For treatment of diarrhea and dysentery | Adults: 5–7 T×3 |
| Curcuma zedoaria Rose (rhizome) | 1 | Children: | ||
| Oroxylum indicum (L.) Kurz (bark) | 1 | at 1–2 mo: 1 T×3 | ||
| Musa paradisiaca L.; ABB Group (root) | 1 | at 3–5 mo: 2 T×3 | ||
| Allium sativum Linn. (bulk) | 1 | at 6–12 mo: 3–5 T×3 | ||
| Piper retrofractum Vahl (fruit) | 1 | >1 y: 5–7 T×3 | ||
| Learng-Pid-Sa-Mud (Continued) | Dipterocarpus alatus Roxb. Ex G.Don (gum) | 1 | ||
| Tachardia lacca Kerr. (lac) | 1 | |||
| Uncaria gambir (Hunter) Roxb. (leaf and limb) | 1 | |||
| Acacia catechu (L.f.) Willd. (heartwood) | 1 | |||
| Impatiens balsamina L. (leaf ) | 1 | |||
| Punica granatum L. (leaf ) | 1 | |||
| Curcuma longa L. (rhizome) | 6 | |||
| Dhart-Ban-Chob (112 g) | Zingiber officinale Roscoe (rhizome) | 4 | For treatment of diarrhea and bloated stomach | Adults: 3–5 T×3 |
| Atractylodes lancea (Thunb.) DC (rhizome) | 4 | Children: 2–3 T×3 | ||
| Dischidia rafflesiana Wall. (leaf ) | 4 | |||
| Angelica sinensis (Oliv) Diels (root) | 4 | |||
| Angelica dahurica Benth. (root) | 4 | |||
| Abroma augusta (L.) L. f. (seed) | 4 | |||
| Lawsonia inermis L.(seed) | 4 | |||
| Pimpinella anisum L. (seed) | 4 | |||
| Trachyspermum ammi (L.) Sprague (seed) | 4 | |||
| Lepidium sativum Linn. (seed) | 4 | |||
| Diospyros decandra Lour. (fruit) | 4 | |||
| Myristica fragrans Houtt. (mace) | 4 | |||
| Syzygium aromaticum (L.) Merr. & L.M. Perry (flower) | 4 | |||
| Cinnamomum camphora Th Fries (leaf ) | 4 | |||
| Cinnamomum bejolghota (Ham.) Sweet (bark) | 4 | |||
| Amomum krervanh Pierre (fruit) | 4 | |||
| Coriandrum sativum L.(seed) | 4 | |||
| Coriandrum sativum L. (seed) | 4 | |||
| Pogostemon cablin (Blanco) Benth. (leaf ) | 4 | |||
| Aristolochia spp. (root) | 4 | |||
| Piper retrofractum Vahl (fruit) | 4 | |||
| Kaempferia galanga L. (rhizome) | 4 | |||
| Picrorrhiza kurroa Benth. (rhizome) | 8 | |||
| Terminalia chebula Retz. var. chebula (fruit) | 16 | |||
| Um-Ma-Luk | Aristolochia spp. (root) | 7 | Used to relieve cough and reduce phlegm | Adults: 1–2 T×3 |
| Ka-Wa-Tee (85 g) | Dischidia rafflesiana Wall. (leaf ) | 7 | Children: 0.5–1 T×3 | |
| Lawsonia inermis L. (root) | 7 | |||
| Coriandrum sativum L. (root) | 7 | |||
| Phyllanthus emblica (fruit) | 7 | |||
| Terminalia bellirica (Gaertn.) Roxb. (fruit) | 7 | |||
| Glycyrrhiza glabra (root) | 43 |
Dosage: tsp, teaspoon; T, 0.5-g tablet; ×1, once a day; ×2, twice a day; ×3, three times a day; ×4, four times a day.
Aristolochia spp. (root) was removed from Thai traditional herbal recipes in 2011.
ND, no data available.
Remedies extracts
Five hundreds grams of each remedy was mixed with 1,000 mL of 95% ethanol. The mixtures were left for 7 days at 30°C and then filtered through Whatman no. 1 filter paper. The collected filtrates were further concentrated to dryness using a rotary evaporator and kept at 55°C. Yields (%; w/w) of each extracts were calculated as the ratio of the weight of the extract to the weight of the recipe powder (Table 2). The sample were stored at −40°C and further used for antibacterial tests.
Table 2.
Extraction Yields, Cytotoxicity Effects, and Phytochemical Constituents of Selected Thai Household Ancient Remedies
| Phytochemical constituentsb | ||||||
|---|---|---|---|---|---|---|
| Household remedies | Extraction yield (%; w/w) | Cytotoxicitya (IC50; μg/mL) | 1 | 2 | 3 | 4 |
| Chan-Ta-Lee-La | 2.5 | 19.5 | + | − | + | + |
| Pra-Sa-Chan-Dang | 4.2 | >50 | + | + | + | + |
| Kheaw-Hom | 3.7 | >50 | + | − | + | + |
| Tree-Hom | 8.7 | >50 | − | − | + | − |
| Learng-Pid-Sa-Mud | 7.3 | 19.7 | + | + | + | + |
| Dhart-Ban-Chob | 7.9 | >50 | + | + | + | + |
| Um-Ma-Luk-Ka-Wa-Tee | 3.9 | >50 | + | + | + | − |
50% inhibitory concentration of ellipticine (a positive control) was 0.8 μg/mL.
The results showed the presence (+) or absence (−) of phytochemical compounds including alkaloid, triterpenoid, condensed tannin, and hydrolysable tannin.
Stock solutions of the extracts were prepared in advance at a concentration of 80 mg/mL in dimethylsulfoxide (DMSO; Merck, Darmstadt, Germany). Before the bioassay, working solutions were prepared by diluting the stock solutions to two times the maximum desired final testing concentrations in Mueller-Hinton broth (MHB; Difco, Strasbourg, France). The final concentration of DMSO in all assays was 1.25% or less, which is nontoxic for the tested pathogens.
Phytochemical screening tests
Qualitative phytochemical screening analysis of Thai household ancient remedy extracts was carried out to identify the presence of secondary metabolites, including alkaloids, terpenoids, condensed tannins, and hydrolysable tannins using Dragendorff reagent, Liebermann-Burchard reagent, and ferric chloride reagent, respectively.11
Antibacterial properties
Tested pathogens
Microorganisms used to determine antibacterial activities of different extracts were as follows: (1) gram-positive bacteria: S. aureus ATCC 25923, clinically isolated methicillin resistant S. aureus (MRSA NPRC R003-R005), S. epidermidis ATCC 35984, coagulase-negative staphylococci (NPRC 301 and 308), and coagulase-positive staphylococci (NPRC 506–507) isolated from acne lesions; (2) gram-negative bacteria: P. aeruginosa ATCC 10145, clinically isolated MDR P. aeruginosa (2097 and 5351), MDR A. baumannii (NPRC AB002, 004, 005, and 034), E. coli ATCC 25922, MDR E. coli (2746-08 and 2809-08), and E. coli O15:H7 RIMD 05091078. All bacterial strains were obtained from Natural Products Research Center and Department of Microbiology, Faculty of Science, Prince of Songkla University. The strains were maintained on a Trypticase soy agar (Difco) slant at 4°C and activated at 37°C for 24 hours with a Trypticase soy agar plate prior to any antimicrobial tests.
Bacterial cultures were prepared by transferring three to five well-isolated colonies into a tube containing 2 mL of MHB (Difco) and grown overnight at 37°C. The turbidity of the culture was adjusted with sterile MHB to match 0.5 McFarland standard.
Assessment of minimum inhibitory concentration
A modified broth microdilution method using 96-well microplate according to the Clinical and Laboratory Standard Institute12 was used to obtain minimum inhibitory concentrations (MICs) of the crude extracts against the tested pathogens. Each plant extract (80 mg/mL) was serially diluted two-fold to obtain 1 mg/mL final testing concentration in the first well. The range of final testing concentration of each extract was 16–1000 μg/mL. An equal volume of 100 μL fresh bacterial culture concentration corresponding to 106 colony-forming units/mL was added to the wells. The plate was covered with lids and incubated for 24 hours at 37°C. The bacterial growth was measured by recording the absorbance at 620 nm, using a microplate reader (Sunrise, Tecan, Switzerland). The MIC was defined as the lowest concentration of the test agent that had restricted growth to a level less than 0.05 at 620 nm after the incubation period.
A growth control and a blank control were taken using the inoculated broth added into 1.25% of DMSO and fresh MHB added into each concentration of the extract, respectively. The MICs of antibiotics, including vancomycin and rifampicin, for all the reference strains were simultaneously determined. Each experiment was performed at least twice.
As previously proposed by Rios and Recio13 and Kuet,14 extracts were classified as significantly active when MICs were less than 100 μg/mL, moderately active when the values were 100–625 μg/mL, and weakly active when the values were greater than 625 μg/mL.
Kinetic growth inhibition assay
Kinetic growth inhibition assay was used to observe the growth inhibitory effects of effective remedies (Learng-Pid-Sa-Mud, Kheaw-Hom, and Um-Ma-Luk-Ka-Wa-Tee) on representative gram-positive pathogen (MRSA NPRC R003, S. epidermidis ATCC 35984, coagulase-positive staphylococci NPRC 308) and gram-negative pathogens (MDR A. baumannii NPRC AB034, MDR E. coli 2746-08, and MDR P. aeruginosa 5351). Bacterial inoculum prepared as described above (1 mL) was mixed with 1 mL of MHB containing each remedy extract at final concentrations of 16, 32, 64, 125, 250, 500, and 1000 μg/mL. The tubes were incubated at 37°C, and samples (100 μL) were taken at 0, 6, 12, and 18 hours. Bacterial growth of each sample was measured by recording the absorbance at 620 nm.
Cytotoxicity assay
Cytotoxicity activities of the formula extracts against Vero cells were determined by green fluorescent protein–based assay at the National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Pathum Thani, Thailand. Ellipticine used as a positive control exhibited cytotoxicity against Vero cell line with 50% inhibitor concentration (IC50) of 0.8 μg/mL.15
Results
Table 1 provides the ethno-botanical information of the ancient household remedies, including scientific name, plant part used, weight ratio of medicinal components, usages, and dosage. The remedies were prepared using a mixture of plants as follows: Dhart-Ban-Chob, Kheaw-Hom, Learng-Pid-Sa-Mud, Pra-Sa-Chan-Dang, Tree-Hom, Chan-Ta-Lee-La, and Um-Ma-Luk-Ka-Wa-Tee containing 24, 17, 13, 12, 10, 9, and 7 medicinal plants, respectively. In total, this study reports seven plant-based formulas containing 68 species that belonged to 48 families; most of these medicinal plants belong to the Zingiberaceae and Umbelliferae families. Different plant parts, such as root, rhizome, wood, fruit, mace, leaf, and flower, were used to prepare the remedies; of these, root, rhizome, and leaf were the most commonly used plant material. The most frequently mentioned medicinal plants used as household remedies were Myristica fragrans (found in four remedies: Chan-Ta-Lee-La, Pra-Sa-Chan-Dang, Kheaw-Hom, and Dhart-Ban-Chob), Aristolochia pierrei (found in four remedies: Tree-Hom, Um-Ma-Luk-Ka-Wa-Tee, Kheaw-Hom, and Dhart-Ban-Chob), Dracaena loureiri (found in three remedies: Chan-Ta-Lee-La, Pra-Sa-Chan-Dang, and Kheaw-Hom), and Angelica dahurica (found in three remedies: Chan-Ta-Lee-La, Tree-Hom, and Dhart-Ban-Chob). Our preliminary phytochemical test revealed that condensed tannins were common principals found in all tested remedies. Other compounds, including alkaloids, triterpenoids, and hydrolysable tannins, were present in most of the selected remedies (Table 2).
To assess antibacterial activity, the ethanol extracts were tested against five species (20 isolates) of gram-positive and gram-negative pathogens. The evaluated MICs are reported in Table 3. The rationale for using these 16 clinical isolates was that their antibiotic susceptibility profile is fairly representative of most antibiotic-susceptible and antibiotic-resistant isolates. The activity of these remedy extracts was interpreted as inhibition at any MICs of 1000 μg/mL or less. The extracts that did not exhibit inhibition up to the concentration limit of 1000 μg/mL were considered inactive. The antibacterial activity of the remedies was further classified as significant if the MIC was less than 100 μg/mL, moderate if the MIC was greater than 100 and less than or equal to 625 μg/mL, or weak if the MIC was greater than 625 μg/mL. The MICs obtained in this study for all the tested extracts ranged from less than 16 to 1000 μg/mL against gram-positive pathogens, while MICs varied from 125 to greater than 1000 μg/mL against gram-negative bacteria. With the exception of Dhart-Ban-Chob extract, the notable MICs of less than 16 to 32 μg/mL was observed for the remedy extracts depending on the bacterial strains. MRSA isolates used in this investigation were the most susceptible bacterial strain and were found to be susceptible to the extracts of Pra-Sa-Chan-Dang, Kheaw-Hom, Learng-Pid-Sa-Mud, and Um-Ma-Luk-Ka-Wa-Tee.
Table 3.
Minimum Inhibitory Concentration of Ethanol Extracts of Thai Household Ancient Remedies Against Human Pathogens
| MIC of the remedy extractsa,b (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Tested pathogens | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Staphylococcus aureus ATCC 25923 | 250 | 62c | 31c | 250 | 250 | 500 | 125 |
| Methicillin resistant S. aureus (MRSA) NPRC R003 125 | 125 | 31c | 125 | 31c | 250 | 31c | |
| MRSA NPRC R004 | 125 | 31c | 31c | 250 | 1000 | 250 | 31c |
| MRSA NPRC R005 | 250 | 62c | 1000 | 125 | 31c | 500 | <16c |
| S. epidermidis ATCC 35984 | 250 | 125 | 31c | 125 | <16c | 250 | <16c |
| Coagulase-positive staphylococci NPRC 301 | 1000 | 1000 | 125 | 62c | 250 | 1000 | 31c |
| Coagulase-positive staphylococci NPRC 308 125 | 125 | 125 | 125 | 1000 | 500 | 125 | |
| Coagulase-negative staphylococci NPRC 506 | 31c | 250 | 250 | 250 | 125 | 125 | 125 |
| Coagulase-negative staphylococci NPRC 507 62c | 125 | 125 | 125 | 1000 | 125 | 125 | |
| Pseudomonas aeruginosa ATCC 10145 | 500 | 250 | >1000 | 500 | 250 | 250 | 250 |
| MDR P. aeruginosa 2097 | 500 | 250 | 125 | 500 | 125 | 500 | 250 |
| MDR P. aeruginosa 5351 | 1000 | 1000 | 1000 | 250 | 500 | 1000 | >1000 |
| MDR Acinetobacter baumannii NPRC AB002 | >1000 | 1000 | >1000 | 1000 | 1000 | >1000 | >1000 |
| MDR A. baumannii NPRC AB004 | 1000 | 1000 | 1000 | 500 | 500 | 500 | 500 |
| MRD A. baumannii NPRC AB005 | >1000 | 1000 | >1000 | >1000 | 1000 | 500 | 1000 |
| MRD A. baumannii NPRC AB034 | 1000 | >1000 | >1000 | 500 | 1000 | 500 | 500 |
| Escherichia coli ATCC 25922 | >1000 | >1000 | >1000 | >1000 | 1000 | 1000 | >1000 |
| MDR E. coli 2746-08 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| MDR E. coli 2809-08 | >1000 | 1000 | >1000 | >1000 | 1000 | >1000 | >1000 |
| E. coli O15:H7 RIMD 05091078 | 250 | >1000 | >1000 | >1000 | 1000 | 1000 | >1000 |
The tested household remedies were Chan-Ta-Lee-La, Pra-Sa-Chan-Dang, Kheaw-Hom, Tree-Hom, Learng-Pid-Sa-Mud, Dhart-Ban-Chob, and Um-Ma-Luk-Ka-Wa-Tee.
MICs of vancomycin on S. aureus ATCC 25923 and rifampicin on P. aeruginosa ATCC 10145/E. coli ATCC 25922 were 0.5 and 40/5 μg/mL, respectively.
MICs considered as representing noteworthy antimicrobial activity.
MIC, minimum inhibitory concentration; MDR, multidrug-resistant.
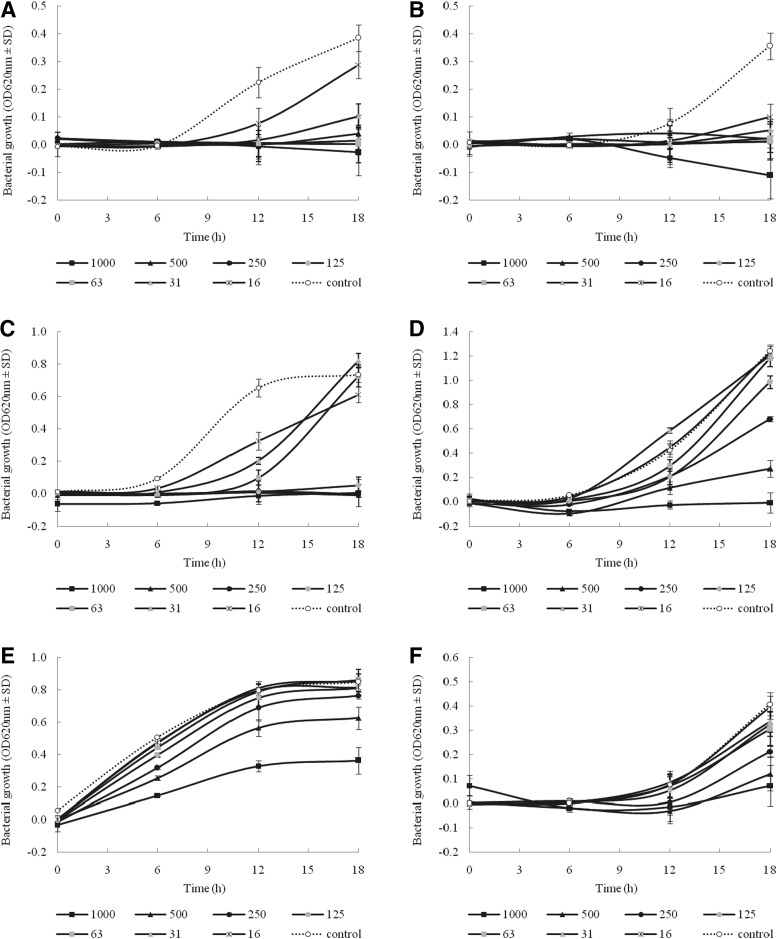
The extract of Um-Ma-Luk-Ka-Wa-Tee possessed the highest and most significant antibacterial activity, with MICs less than 16–31 μg/mL against all MRSA isolates. In addition, this extract exhibited remarkable activity, with MICs of <16 and 31 μg/mL, respectively, against a biofilm-producing isolate, S. epidermidis ATCC 35984 and acne lesion–isolated coagulase-positive staphylococci NPRC 301. The growth studies, as presented in Figure 1A–C, confirmed these results. This remedy showed moderate activity on some isolates of P. aeruginosa and A. baumannii but had weak activity or even total inactivity against E. coli isolates. Although the extract was generally considered inactive against gram-negative pathogens, subinhibitory concentrations of the extract partially inhibited the growth of these bacteria (Figure 1D–F). A certain activity against gram-positive pathogens was also shown by the extract of Kheaw-Hom and Learng-Pid-Sa-Mud, which have an MIC corresponding to 31 μg/mL. On the other hand, these extracts showed no activity with MIC values higher than 1000 μg/mL on tested gram-negative bacteria. Similarly, with Um-Ma-Luk-Ka-Wa-Tee extract, subinhibitory concentration of Kheaw-Hom and Learng-Pid-Sa-Mud affected the growth of E. coli and A. baumannii and A. baumannii and P. aeruginosa, respectively (data not shown).
FIG. 1.
Growth inhibition effects of Um-Ma-Luk-Ka-Wa-Tee ethanol extract on methicillin-resistant Staphylococcus aureus NPRC R003 (A), S. epidermidis ATCC 35984 (B), coagulase-positive staphylococci NPRC 308 (C), multidrug-resistant Acinetobacter baumannii NPRC AB034 (D), multidrug-resistant Escherichia coli 2746-08 (E), and multidrug-resistant Pseudomonas aeruginosa 5351 (F). For all graphs, continuous lines represent time points for test (with different concentrations of the extract) cultures and dashed lines represent control (with 1.25% dimethylsulfoxide) cultures. Each symbol indicates the mean±standard deviation for three independent experiments performed in duplicate. OD, optical density.
Cytotoxicity effects of the remedies were additionally investigated on Vero cells for their potentially safe uses (Table 2). The tested remedies had no cytotoxic effects except for Chan-Ta-Lee-La and Learng-Pid-Sa-Mud, which had IC50 values of 19.5 and 19.7 μg/mL, respectively, on Vero cells. The IC50 value of the positive control ellipticine used in this study was 0.8 μg/mL, which is approximately 24 times higher than those of Chan-Ta-Lee-La and Learng-Pid-Sa-Mud and more than 60 times higher than those of other remedies.
Discussion
Traditional plant-based formulas have been commonly used to treat skin and soft tissue infections, diarrhea, gastritis, fever, and peptic ulcer diseases in Asian countries such as China,16 India,17 Japan,18 Korea,19 and Thailand.20–23 Although previous studies have revealed that folk healers believe that combining more than one plant increases effectiveness of medicines and many diseases were treated using a combination of more than one plant, few pharmacologic studies support this information.24 In the present study, Thai household ancient remedies widely used to treat infection-related ailments were selected from the List of Herbal Medicinal Products A.D. 2006 approved by the Ministry of Public Health of Thailand. On the basis of criteria proposed by Rios and Recio13 and Kuete,14 Um-Ma-Luk-Ka-Wa-Tee (which consists of Aristolochia spp., Dischidia rafflesiana, Lawsonia inermis, Coriandrum sativum, Phyllanthus emblica, Terminalia bellirica, and Glycyrrhiza glabra) possessed significant anti-MRSA activity. The remedy is more effective than the previously reported Thai traditional remedies (Pikutbenjakul,21 Prasaprohyai,22 and Benchalokawichian23), as well as other traditional Thai medicinal plants.
Krai-Krue, a dried root of Aristolochia pierrei Lecomte or Aristolochia tagala Cham, is commonly used in Thai traditional herbal remedies.25 Aristolochic acid and its derivatives, found primarily in Aristolochia spp., are well-documented nephrotoxic and carcinogenic agents.26 According to information from the U.S. Food and Drug Administration, Health Canada, and the Medicine Controls Agency (the United Kingdom), use of botanical products containing the compounds is no longer permitted.27 In 2011, therefore, the National Drug Committee removed Krai-Krue from Thai traditional herbal recipes.20 For this reason, the remedies used in this investigation, including Um-Ma-Luk-Ka-Wa-Tee, did not contain Aristolochia spp. However, neither a Thai traditional preparation containing Krai-Krue, Homnawakod, nor a new version of the formula without this medicinal plants caused any acute nephrotoxicity in vivo. In addition, the quality of the formulas in terms of chemical profile does not differ.20
With the exception of Dischidia rafflesiana, antibacterial activities of L. inermis, C. sativum, P. emblica, T. bellirica, and G. glabra have been documented. Dried powdered Um-Ma-Luk-Ka-Wa-Tee contains approximately 55% (w/w) of G. glabra, traditionally used for treating upper respiratory tract ailments, including cough, hoarseness, sore throat, and bronchitis.28 Various extracts from G. glabra showed antioxidant, anti-inflammatory, and antibacterial activities against human pathogens, such as Bacillus coagulans, E. coli, Salmonella typhimurium, and S. aureus. In previous investigations, MICs (0.3–40 mg/mL) of ethanol and water from root and leaves of this plant against S. aureus, Bacillus subtilis, E. faecalis, Candida albicans, and Mycobacterium tuberculosis were much higher than that of the recipe extract.28–31 Glycyrrhizin generally is considered the major biologically active principal and has been used industrially, but most of the antibacterial activity from this plant is due to glabridin, which was similar to the activities of Um-Ma-Luk-Ka-Wa-Tee (3.9–250 μg/mL).30
Previous studies on L. inermis, traditionally used as antiseptic for burns and wounds in Yemen32 and Nigeria33 have revealed that ethanol, water, and ethyl acetate extracts of the plant exhibited broad-spectrum antibacterial activity on S. aureus, Streptococcus sp., E. faecalis, Proteus mirabilis, Klebsiella pneumoniae, P. aeruginosa, and E. coli at certain concentrations. Lawsone, which was known as the major bioactive constituent, had a wide spectrum of antimicrobial activity, including antiviral, antimycotic, and antiparasitic activities.34 Um-Ma-Luk-Ka-Wa-Tee extract (MIC, 31 to <16 μg/mL) had potent anti-MRSA activity greater than that of L. inermis extract (5–80 mg/mL).33,35 In addition, T. bellerica chloroform extract—as well as the active constituents of this plant, termilignan, thannilignan, and anolignan—show antiviral, antifungal, and antimalarial activities.36 Methanol extract of this plant had inhibitory activities on Bacillus cereus, Listeria monocytogenes, S. aureus, E. coli, and Salmonella anatum, when tested by agar well diffusion method (100 mg/mL; 60 μL/well).37 Air-dried fruits of Phyllanthus emblica have been widely used as a source of natural antioxidants in traditional Chinese and Indian medicine.38 Although anti-MRSA activity of P. emblica has never been reported, extract of P. emblica has been reported to have the ability to prevent the colonization of C. albicans.39
In addition to Aristolochia spp., our results showed that M. fragrans, D. loureiri, and A. dahurica were frequently mentioned medicinal plants used in the household remedies. M. fragrans (seed, seed kernel, and mace), D. loureiri (stem wood), and A. dahurica (fruit and root) are widely used in Asian medicinal ingredients. This finding agrees with values reported in the literature; extracts and chemical constituents of the medicinal plant have been found to possess various biological activities. M. fragrans and its active principals, such as alkyl benzene derivatives (myristicin and elemicin), terpenes, α-pinene, β-pinene, and myristic acid, have been extensively examined for antibacterial, antiviral, antioxidant, anti-inflammatory, anticancer, and anticariogenic activities.40–42 Ethyl acetate, ethanol, and methanol extract possessed significant antibiofilm and antibacterial activities against various oral primary colonizers, such as Streptococcus mutans, S. sanguis, S. sobrinus, S. salivarius, Lactobacillus acidophilus, L. casei, and Actinomyces viscosus.43 D. loureiri (known in Thai as Chan-Dang) has been found to possess antinociceptive, antipyretic,44 antioxidant, and anti-inflammatory activities.45 Flavonoid derivatives of this plant, such as loureirin D and (2S)-pinocembrin, showed moderate antimicrobial activity against S. aureus, B. subtilis, Botrytis cinerea, and Cladosporium herbarum.46 Root of A. dahurica has been stated to have useful properties, including anti-inflammatory, antiasthmatic, antipyretic, and antiacne effects. Studies conducted by Nam47 and Lechner48 and colleagues additionally reported antibacterial activities on S. aureus, MRSA, Mycobacterium fortuitum, and Propionibacterium acnes of extracts from root and its active constituents, falcarindiol.
It would appear that this paper represents the first work exploring in vitro activity against MDR pathogens of the following Thai traditional remedies: Dhart-Ban-Chob, Kheaw-Hom, Learng-Pid-Sa-Mud, Pra-Sa-Chan-Dang, Tree-Hom, Chan-Ta-Lee-La, and Um-Ma-Luk-Ka-Wa-Tee. Additionally, it reports for the first time that the ethanol extract of Um-Ma-Luk-Ka-Wa-Tee possessed significant anti-MRSA activity with low cytotoxic effect. The information obtained from this study suggested that glabridin may possibly be used as a biomarker for antibacterial activity of this household remedy. However, it is necessary to further investigate and understand the relationship between antibacterial activity and glabridin content in the recipe. Further studies on other biological activities related to their traditional application are required to elucidate the appropriate biomarkers as well as to explain the treatment mechanisms of household remedies.
Acknowledgments
This work was supported by grants for a general researcher, the annual income budget of Prince of Songkla University (TTM5406785). The authors are thankful to Miss Stefania Vignotto for editing the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roberts RR, Hota B, Ahmad I, et al. . Hospital and societal costs of antimicrobial- resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009;49:1175–1184 [DOI] [PubMed] [Google Scholar]
- 2.de Kraker ME, Davey PG, Grundmann H. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 2011;8:e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kraker ME, Wolkewitz M, Davey PG, et al. . Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother 2011;66:398–407 [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Richards CL, et al. . Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY Program (2005–2008). Braz J Infect Dis 2009;13:90–98 [DOI] [PubMed] [Google Scholar]
- 6.Saleem AF, Ahmed I, Mir F, Ali SR, Zaidi AK. Pan-resistant Acinetobacter infection in neonates in Karachi, Pakistan. J Infect Dev Ctries 2012;4:30–37 [PubMed] [Google Scholar]
- 7.Tuon FF, Gortz LW, Rocha JL. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis 2012;16:351–356 [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo C, Antoniazzi F, Casari S, et al. . P. aeruginosa bloodstream infections among hematological patients: an old or new question? Ann Hematol 2012;91:1299– 304 [DOI] [PubMed] [Google Scholar]
- 9.Chokevivat V, Chuthaputti A. The role of Thai traditional medicine in health promotion. 6th Global Conference on Health Promotion (6GCHP) Department for the Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, Thailand; 2005, pp. 1–25 [Google Scholar]
- 10.National Essential Drug List Committee. The National List of Essential Drugs: Last update 25th May 2011 (List of Herbal Medicinal Products). Bangkok: War Veterans Administration Printing; 2011 [Google Scholar]
- 11.Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med 2009;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. M07-A8: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard-Eighth Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2009 [Google Scholar]
- 13.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol 2005;100:80–84 [DOI] [PubMed] [Google Scholar]
- 14.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med 2010;76:1479–1491 [DOI] [PubMed] [Google Scholar]
- 15.Chusri S, Settharaksa S, Chokpaisarn J, Limsuwan S, Voravuthikunchai SP. Thai Herbal formulas used for wound treatment: a study of their antibacterial potency, anti-inflammatory, antioxidant, and cytotoxicity effects. J Altern Complement Med 2013;19:671–676 [DOI] [PubMed] [Google Scholar]
- 16.Li S, Zhao J, Liu J, et al. . Prospective randomized controlled study of a Chinese herbal medicine compound Tangzu Yuyang Ointment for chronic diabetic foot ulcers: a preliminary report. J Ethnopharmacol 2011;133:543–550 [DOI] [PubMed] [Google Scholar]
- 17.Shyni GL, Ratheesh M, Sindhu G, Helen A. Anti-inflammatory and antioxidant effects of Jeevaneeya Rasayana: an ayurvedic polyherbal formulation on acute and chronic models of inflammation. Immunopharmacol Immunotoxicol 2010;32:569–575 [DOI] [PubMed] [Google Scholar]
- 18.Ohno T, Inoue M, Ogihara Y. Suppressive effect of Shichimotsu-koka-to (Kampo medicine) on pulmonary metastasis of B16 melanoma cells. Biol Pharm Bull 2002;25:880–884 [DOI] [PubMed] [Google Scholar]
- 19.Hong HT, Kim HJ, Lee TK, et al. . Inhibitory effect of a Korean traditional medicine, Honghwain-Jahage (water extracts of Carthamus tinctorius L. seed and Hominis placenta) on interleukin-1-mediated bone resorption. J Ethnopharmacol 2002;79:143– 148 [DOI] [PubMed] [Google Scholar]
- 20.Tripatara P, Onlamul W, Booranasubkajorn S, et al. . The safety of Homnawakod herbal formula containing Aristolochia tagala Cham in Wistar rats. BMC Complement Altern Med 2012;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo S, Sattaponpan C, Phongpaichit S, Srijan A, Itharat A. Antibacterial activity of Thai medicinal plants Pikutbenjakul. J Med Assoc Thai 2010;93:S131–135 [PubMed] [Google Scholar]
- 22.Sattaponpan C, Kondo S. Antibacterial activity of crude extracts of prasaprohyai formula and its components against pathogenic bacteria. J Med Assoc Thai 2011;94:S153–161 [PubMed] [Google Scholar]
- 23.Nuaeissara S, Kondo S, Itharat A. Antimicrobial activity of the extracts from Benchalokawichian remedy and its components. J Med Assoc Thai 2011;94:S172–177 [PubMed] [Google Scholar]
- 24.Flatie T, Gedif T, Asres K, Gebre-Mariam T. Ethnomedical survey of Berta ethnic group Assosa Zone, Benishangul-Gumuz regional state, mid-west Ethiopia. J Ethnobiol Ethnomed 2009;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sathornviriyapong S, Picheansoonthon C, Tiasakul R, Tiyaworanant S, Reutrakul V. Botanical origin and identification of Krai-Krue herbal plant. Kasetsart J (Nat Sci) 2007;41:420–32 [Google Scholar]
- 26.Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 2002;17:265–77 [DOI] [PubMed] [Google Scholar]
- 27.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008;74:158–169 [DOI] [PubMed] [Google Scholar]
- 28.Nitalikar MM, Munde KC, Dhore BV, Shikalgar SN. Studies of antibacterial activities of Glycyrrhiza glabra root extract. Int J PharmTech Res 2010;2:899–901 [Google Scholar]
- 29.Nirmala P, Selvaraj T. Anti-inflammatory and anti-bacterial activities of Glycyrrhiza glabra L. J Agricult Technol 2011;7:815–823 [Google Scholar]
- 30.Gupta VK, Fatima A, Faridi U, et al. . Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol 2008;116:377–380 [DOI] [PubMed] [Google Scholar]
- 31.Li W, Asada Y, Yoshikawa T. Antimicrobial flavonoids from Glycyrrhiza glabra hairy root cultures. Planta Med 1998;64:746–747 [DOI] [PubMed] [Google Scholar]
- 32.Ali NA, Julich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol 2001;74:173–179 [DOI] [PubMed] [Google Scholar]
- 33.Muhammad HS, Muhammad S. The use of Lawsonia inermis linn. (henna) in the management of burn wound infections. Afr J Biotechnol 2005;4:934–937 [Google Scholar]
- 34.Babu PD, Subhasree RS. Antimicrobial activities of Lawsonia inermis: a review. Academic J Plant Sci 2009;2:231–232 [Google Scholar]
- 35.Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia 2009;80:102–104 [DOI] [PubMed] [Google Scholar]
- 36.Valsaraj R, Pushpangadan P, Smitt UW, et al. . New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod 1997;60:739–742 [DOI] [PubMed] [Google Scholar]
- 37.Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 2007;117:112–119 [DOI] [PubMed] [Google Scholar]
- 38.Liu XL, Cui C, Zhao MM, et al. . Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem 2008;109:909–915 [DOI] [PubMed] [Google Scholar]
- 39.Thaweboon B, Thaweboon S. Effect of Phyllanthus emblica Linn. on candida adhesion to oral epithelium and denture acrylic. Asian Pac J Trop Med 2011;4:41–45 [DOI] [PubMed] [Google Scholar]
- 40.Ozaki Y, Soedigdo S, Wattimena YR, Suganda AG. Anti-inflammatory effect of mace, aril of Myristica fragrans Houtt., and its active principles. Jpn J Pharmacol 1989;49:155–163 [DOI] [PubMed] [Google Scholar]
- 41.Akinboro A, Mohamed KB, Asmawi MZ, Othman AS, Ying TH, Maidin SM. Mutagenic and antimutagenic assessment of methanol leaf extract of Myristica fragrans (Houtt.) using in vitro and in vivo genetic assays. Drug Chem Toxicol 2011;35:412–422 [DOI] [PubMed] [Google Scholar]
- 42.Chirathaworn C, Kongcharoensuntorn W, Dechdoungchan T, Lowanitchapat A, Sa- nguanmoo P, Poovorawan Y. Myristica fragrans Houtt. methanolic extract induces apoptosis in a human leukemia cell line through SIRT1 mRNA downregulation. J Med Assoc Thai 2007;90:2422–2428 [PubMed] [Google Scholar]
- 43.Shafiei Z, Shuhairi NN, Md Fazly Shah Yap N, Harry Sibungkil CA, Latip J. Antibacterial activity of Myristica fragrans against oral pathogens. Evid Based Complement Alternat Med 2012;2012:825362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reanmongkol W, Subhadhirasakul S, Bouking P. Antinociceptive and antipyretic activities of extracts and fractions from Dracaena loureiri in experimental animals. Songklanakarin J Sci Technol 2003;25:467–476 [Google Scholar]
- 45.Likhitwitayawuid K, Sawasdee K, Kirtikara K. Flavonoids and stilbenoids with COX- 1 and COX-2 inhibitory activity from Dracaena loureiri. Planta Med 2002;68:841–843 [DOI] [PubMed] [Google Scholar]
- 46.Ichikawa K, Kitaoka M, Taki M, et al. . Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity. Planta Med 1997;63:540–543 [DOI] [PubMed] [Google Scholar]
- 47.Lechner D, Stavri M, Oluwatuyi M, Pereda-Miranda R, Gibbons S. The anti- staphylococcal activity of Angelica dahurica (Bai Zhi). Phytochemistry 2004;65:331– 335 [DOI] [PubMed] [Google Scholar]
- 48.Nam C, Kim S, Sim Y, Chang I. Anti-acne effects of Oriental herb extracts: a novel screening method to select anti-acne agents. Skin Pharmacol Appl Skin Physiol 2003;16:84–90 [DOI] [PubMed] [Google Scholar]