Abstract
Objective: Findings of existing functional MRI (fMRI) studies on the neural mechanisms that mediate effects of acupuncture analgesia are inconsistent. This study analyzes the effects of manual acupuncture on pain ratings and brain activation in response to experimental, electrical pain stimuli.
Design: Fourteen healthy volunteers were examined by using a 1.5-T MRI scanner. The intensity of pain stimuli was adjusted to individual pain ratings on a numeric rating scale. Baseline fMRI was performed during electrical pain stimulation in a blocked design. For the second session, manual acupuncture with repeated stimulation was performed on contralateral acupoints—large intestine 4, liver 3, and stomach 36—before imaging. After imaging, subjective pain ratings and ratings of the de qi sensation were assessed.
Results: Compared with baseline, volunteers showed modulated brain activity under pain conditions in the cingulate gyrus, insula, primary somatosensory cortex, and prefrontal areas after the acupuncture session. In accordance with the literature, anterior insular and prefrontal activity seemed to be correlated with acupuncture treatment.
Conclusion: This study supports the existence of analgesic acupuncture effects that outlast the needling period. Pain-associated brain areas were modulated in direct response to a preceding acupuncture treatment.
Introduction
Over the past few decades, manual or electroacupuncture has emerged as a common modality in Western medicine, especially for pain management.1 As early as 1998, the National Institutes of Health considered acupuncture to be an effective treatment with promising results for pain relief.2 Clinical evidence supports the analgesic effect of acupuncture treatments with respect to behavioral data.3,4 One of the oldest dry needling therapies for treating pain in Traditional Chinese Medicine, acupuncture analgesia is considered a complex model.5 It is believed to work through different mechanisms of inhibition, such as gate control or diffuse noxious inhibitory control, on different levels of the central nervous system;6–10 however, unspecific, cortical mechanisms via descending, inhibitory pathways are certainly and equally involved.11,12 The quality and intensity of a painful sensation depend not only on the presence of sensory components but also on affective and cognitive aspects.13,14 At the brain level, important brain regions comprise the thalamus, anterior cingulate cortex, insular cortex and somatosensory areas (primary and secondary somatosensory cortex).15–17 The prefrontal cortex (PFC) is considered to play a role in cognitive distraction or mechanisms of expectation and anticipation of a painful stimulus.18,19 Functional measurements of pain in a cortical pattern include all these areas.20
In different experimental paradigms, acupuncture induced a greater analgesic effect than did sham procedures, such as the Streitberger needle.4,21 In a series of trials with long-lasting electroacupuncture using high-intensity stimulation, the analgesic effect of acupuncture was not only comparable to that induced by meditation22,23 but also to that of conventional pharmaceutical analgesics.24 Previous neuroimaging studies showed that acupuncture changed activations in sensory, affective, cognitive, and inhibitory regions, including neural networks for pain perception and transmission.25 A recent review focused on the differences between verum and sham acupuncture, among various acupuncture methods, between patients and healthy volunteers, and among different acupuncture points with regard to activity changes in specific brain areas.25
Thus, some evidence already shows acupuncture-specific effects on pain, reflected by modulation in certain brain regions responsible for pain processing, but these effects are far from being established.25 Many of the magnetic resonance imaging (MRI) studies on acupuncture so far have focused on the direct effects of the needling procedure of acupuncture, either alone or in an ongoing pain process with a standardized pain stimulus. The current trial studied the effects of acupuncture on subsequent individualized pain stimuli. To investigate acupuncture-specific modulation of pain perception and sensation, activation patterns were evaluated in response to noxious electrical stimulation during rest compared with acupuncture. According to the literature, the study hypothesis was that brain activation patterns in pain-associated areas (somatosensory cortex, insula, thalamus, cingulum, and prefrontal areas) would differ during acupuncture compared with the baseline condition.
Materials and Methods
The ethics committee of the University Hospital Essen, University of Duisburg Essen, Germany, approved the study (no. 07-3499).
Participants
Eighteen healthy age- and sex-matched volunteers age 18–65 years were examined with functional MRI (fMRI) in a within-subject design. Four participants were excluded because of imaging artifacts (e.g., head movement); thus, 14 participants (7 women and 7 men; mean age±standard deviation, 39±10.3 years) were included in the analysis. No participant had any history of neurologic or psychiatric disease. Volunteers with chronic pain syndrome or known vascular disease were excluded from participation. All participants provided informed written consent before imaging.
Scanner and sequences
Image acquisition was performed with a standard clinical 1.5-T scanner (Magnetom Sonata, Siemens Healthcare, Erlangen, Germany) using an 8-channel receive-only head coil. For structural imaging and individual co-registration of the acquired functional images, a three-dimensional magnetization prepared rapid gradient echo (MPRAGE) sequence with the following parameters was used: repetition time, 1800 ms; echo time, 3.9 ms; inversion time, 1000 ms; flip angle, 10°, field of view, 240×240 mm2; matrix, 256×256; resolution, 0.9×0.9×1.0 mm3. Blood oxygenation level–dependent contrast images were acquired using a gradient echo-planar technique with 36 slices: repetition time, 3100 ms; echo time, 40 ms; flip angle, 90°; field of view, 240×240 mm; matrix, 64×64; resolution, 3.75×3.75×3.0 mm3 (noninterpolated), with parallel acquisition technique (Grappa R=2). Three “dummy” scans were eliminated before data analysis. Each participant underwent two subsequent sessions of 5.1 minutes' duration with a 10-minute break in between, during which the acupuncture procedure was carried out.
Experimental design
To determine the individual stimulus intensity required for pain stimulation throughout the experiment, an electrode was placed and fixed proximally to the left lateral malleolus. The stimulus was set at a region with no motoric nerve stimulation. The required electrical impulse, serving as pain stimulus, was transmitted by two grounded coaxial leads (RG 58 UI, impedance 50 Ohm, length 8 m, grounded by linkage to the MRI cage) tried and tested by the Department of Measurement and Control Engineering. Current was generated outside the scanner room by a standard electrical stimulus generator (Nicolet-Viking IVP, Madison, WI) with a 3-Hz frequency.26 Current intensity (mA) and signal configuration were analyzed and documented by a digital external oscilloscope (Infinium; Hewlett Packard, Palo Alto, CA). Good evidence suggests that electrical current stimuli have the potential to evoke valid functional activity in pain-associated brain areas.27,28
Before the functional session, to determine the individual current that was necessary to set the pain threshold, the current intensity was slowly raised and the volunteer was asked to assess the value of the perceived pain stimulus simultaneously on a numeric rating scale from 0 to 10 (0=no sensation, 10=unbearable pain). The pain level rated an 8, being strong but endurable throughout the experiment, was determined and applied again as a single stimulus or adjusted, if necessary.
The MRI experiment started with an anatomic scan. Afterward the first fMRI session was performed and electrical pain stimuli with the intensity of the predetermined pain threshold (pain level 8) was delivered. The stimulus was presented in a block design using five blocks of painful electrical stimulation alternated by five blocks without stimulation, each block lasting 31 seconds. After termination of the first fMRI session (“preacupuncture session” or “baseline session”), the MRI examination was paused for 10 minutes, with the volunteer resting on the MRI table, and the acupuncture procedure was performed. Subsequently, the second fMRI session, identical to the first one, was performed with delivery of painful electrical stimuli (Fig. 1). This session was carried out chronologically after the acupuncture treatment and named the “postacupuncture session.” Upon completion of the whole experimental procedure, the electrode was carefully removed and the skin was examined for any evidence of trauma. No evidence of such trauma or other adverse effects occurred throughout the study.
FIG. 1.
Temporal experimental setting and acupoints used. fMRI, functional magnetic resonance imaging; LI4, large intestine 4; LV3, liver 3; ST36, stomach 36.
Acupuncture procedure
Acupuncture was subsequently carried out as manual acupuncture on acupoints large intestine 4 (LI4), liver 3 (LV3), and stomach 36 (ST36) contralaterally to the painful electrical stimulation. The chosen acupoints, among others, are commonly used for pain treatment in the present literature.25 Needling was performed with 0.25×25-mm stainless steel needles. All needles were inserted perpendicularly, to a depth of about 1–2 cm, at LI4 and LV3 and about 2–3 cm deep at ST36 (Fig. 1). The de qi feeling was caused by rotating the needle clockwise and counterclockwise with a 180°–360° amplitude for each rotation. Needle stimulation stopped as soon as participants indicated feeling a de qi sensation with a hand sign. The total stimulation time was about 5–10 seconds at each point. Participants were told that de qi is a dull, perhaps hot or slightly sore sensation resulting from the needle stimulation. Needle stimulation inducing de qi was repeated twice, each time after 3 minutes. Acupuncture was performed by a licensed acupuncturist who was not involved in data collection or analysis.
Rating of needling sensation (de qi score)
To evaluate the participants' response to acupuncture stimulation, de qi as the self-reported characteristic needling sensation was scored on a numeric rating scale ranging from 0 (no somatosensory sensation at all) to 10 (maximal tolerable de qi) immediately after each session. The de qi feeling was subsumed as an overall mixture of several sensory perceptions, including soreness, heaviness, numbness, and dispersal of the sensations.29–31
Data analysis
SPM 08 software (Wellcome Department of Cognitive Neurology, London, United Kingdom) was used for data analysis. Realignment, using the sinc interpolation and normalization with bilinear interpolation to the standard stereotactic space corresponding to the template from the Montreal Neurological Institute (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html), was done before statistical analysis. MPRAGE images were individually co-registered. Before implementation of a voxel-by-voxel comparison, according to the general linear model to calculate differences in activation between the active and resting conditions, image smoothing was done with an isotropic Gaussian kernel of 6 mm. The model consisted of a box-car function convolved with the hemodynamic response function32 and the corresponding temporal derivative. High-pass filtering with a cutoff of 128 seconds and low-pass filtering with the hemodynamic response function were applied. Significant signal changes for each statistical contrast were assessed by means of t-statistics on a voxel-by-voxel basis.33 The resulting set of voxel values for each contrast constituted a statistical parametric mapping (SPM) of the t-statistic. The effect of interest was defined for each participant with a contrast vector producing a contrast image (con-image) containing the contrast of the parameter estimated at each voxel.34
For group analysis, single-subject con-images were entered into a random effects model. The con-images were fed into a general linear model that implements a one-sample t-test. For within-condition analyses we conducted one-sample t-tests (n=14) for each condition (baseline session and postacupuncture session) as well as paired t-tests for between-condition analysis contrasting baseline session versus postacupuncture session and vice versa.
A priori–defined region of interest (ROI) masks were used for each hemisphere, involving the prefrontal cortex (dorsolateral, ventrolateral, ventromedial, and orbitofrontal portions), primary and secondary somatosensory cortex, rolandic operculum, insula, cingulate cortex (anterior, medial, and posterior), thalamus, and supplementary motor area. The ROI masks were created using maps from the WFU Pickatlas integrated in SPM08.35 Findings of these hypothesis-driven analyses were reported as significant if surviving familywise error correction for multiple comparisons (p<0.05) over the respective volume of interest. The anatomic location of each resulting cluster was determined using the Montreal Neurological Institute coordinates.
Beside these confirmatory analyses, exploratory whole-brain analyses were computed with a threshold of p<0.001 (uncorrected) for between-group second-level analysis. Functional images were superimposed on standardized T1-weighted images. For all functional areas, time-response curves were calculated in SPM 08.
Results
Stimulation intensity and subjective pain rating
The individual current intensity required to provoke a pain stimulus rated as an intensity of 8 (on a numeric rating scale of 0 to 10) varied between 8.6 and 27 mA (mean±standard deviation, 16.0±5.7 mA). The de qi sensation was not affected by painful electrical stimulation (Δ=0.86; 95% confidence interval [CI], −0.23 to 1.94; p=0.111). In addition, paired t-tests revealed no difference between the pain ratings referring to the two experimental scan blocks (baseline: mean, 7.14±2.47; acupuncture: mean, 6.04±2.46) with a mean difference of Δ=1.1 (95% CI, −0.55 to 2.76; p=0.172).
Functional data
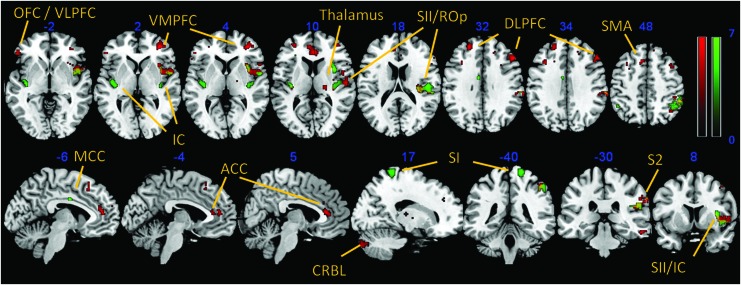
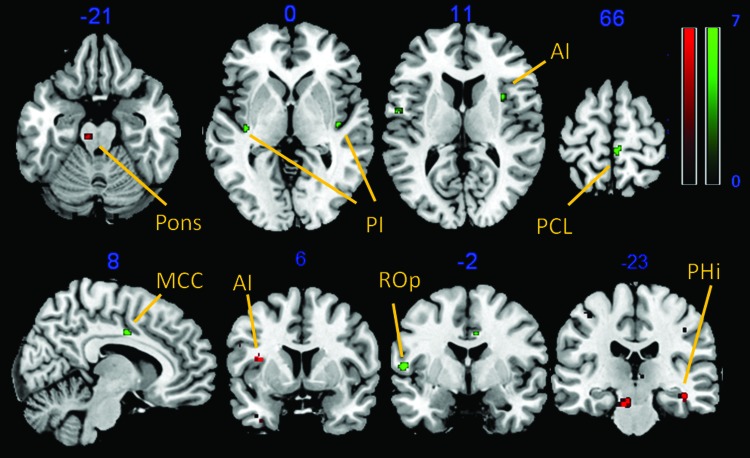
During the first MRI scan, ROI analyses of the baseline session showed a significant cluster in the left and right posterior insula, right anterior insula, right S1 and S2, and left midcingulate cortex (p<0.05, family-wise error corrected). Explorative whole brain analysis revealed additional functional activity in left S2, left cerebellum and right orbitofrontal cortex (OFC) (p<0.001, uncorrected). The second session, following the acupuncture procedure, resulted in significant cluster activation of the bilateral dorsolateral prefrontal cortex (DLPFC), left OFC, right anterior insula, bilateral anterior cingulate cortex, right S2, left supplementary motor area, and right thalamus. Corresponding whole-brain analysis revealed a functional cluster in the right cerebellum, right inferior temporal gyrus, left DLPFC, and right ventromedial prefrontal cortex (Table 1, Fig. 2). For direct comparison of the two functional sessions, a paired t-test analysis regarding the statistical contrast baseline session>post-acupuncture session showed functional cluster in the bilateral, posterior insula, right anterior insula, right midcingulate cortex (MCC), right paracentral lobule, and left rolandic operculum (p<0.001, uncorrected). The opposite contrast post-acupuncture session>baseline session resulted in cluster activation of the left pons, left anterior insula, right parahippocampal gyrus, and left OFC (p<0.001, uncorrected) (Table 2, Fig. 3).
Table 1.
Within-Condition Analyses (One-Sample t-Tests During Baseline and Postacupuncture)
| Baseline | Postacupuncture | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain area | H | x | y | z | t-Value | KE | p-Value | H | x | y | z | t-Value | KE | p-Value |
| Primary somatosensory cortex | R | 20 | −44 | 76 | 5.61 | 3 | 0.021a | |||||||
| Secondary somatosensory cortex | R | 50 | −18 | 16 | 6.58 | 18 | 0.032a | R | 62 | −32 | 36 | 6.76 | 130 | 0.005a |
| Secondary somatosensory cortex | L | −46 | −50 | 46 | 4.47 | 53 | 0.000 | |||||||
| Insula (anterior) | R | 34 | 10 | 12 | 7.55 | 189 | 0.001a | R | 38 | 6 | 2 | 5.72 | 269 | 0.008a |
| Insula (posterior) | L | −38 | −14 | −4 | 6.44 | 211 | 0.003a | |||||||
| R | 32 | −26 | 22 | 5.38 | 6 | 0.036a | ||||||||
| Anterior cingulate cortex | L | −8 | 44 | 16 | 6.81 | 22 | 0.002a | |||||||
| R | 4 | 34 | 12 | 6.85 | 53 | 0.002a | ||||||||
| Midcingulate cortex | L | −6 | −4 | 32 | 6.62 | 24 | 0.013a | |||||||
| Thalamus | R | 22 | −20 | 12 | 5.15 | 25 | 0.018a | |||||||
| Supplementary motor area | L | −8 | 24 | 48 | 6.25 | 39 | 0.014a | |||||||
| Prefrontal cortex (DLPFC) | L | −4 | 30 | 58 | 7.28 | 83 | 0.014a | |||||||
| R | 52 | 28 | 34 | 6.75 | 44 | 0.027a | ||||||||
| Prefrontal cortex (OFC/VLPFC) | L | −52 | 34 | −2 | 7.01 | 269 | 0.031a | |||||||
| L | −26 | 20 | −16 | 5.38 | 11 | 0.033a | ||||||||
| Prefrontal cortex (OFC/VLPFC) | R | 30 | 44 | −6 | 6.21 | 376 | 0.000 | |||||||
| Rolandic operculum | R | 48 | −18 | 18 | 7.35 | 233 | 0.007b | |||||||
| Prefrontal cortex (DLPFC) | L | −36 | 20 | 34 | 5.61 | 42 | 0.001 | |||||||
| Prefrontal cortex (VMPFC) | R | 42 | 46 | 2 | 5.65 | 18 | 0.001 | |||||||
| Inferior temporal gyrus | R | 56 | −30 | −18 | 6.75 | 248 | 0.002b | |||||||
| Cerebellum | R | 20 | −88 | −24 | 4.36 | 64 | 0.000 | R | 22 | −88 | −38 | 7.01 | 561 | 0.000 |
One-sample t-tests (n=14) assessing pain and acupuncture induced neural activation (Montreal Neurological Institute coordinates) separated for each condition (baseline and postacupuncture).
Results that reached significance in region-of-interest analyses (familywise error correction; all p<0.05). For all others not denoted with “a” footnote symbol, results of additional whole-brain analysis (p<0.001 uncorrected).
p<0.05, familywise error correction.
H, hemisphere; R, right; L, left; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
FIG. 2.
Functional clusters (p<0.05, familywise error corrected) superimposed on a T1 template: one-sample t-tests of the baseline condition (green) and the postacupuncture condition (red). Intersections are in yellow. ACC, anterior cingulate cortex; CRBL, cerebellum; DLPFC, dorsolateral prefrontal cortex; MCC, midcingulate cortex; OFC, orbitofrontal cortex; ROp, rolandic operculum; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; SMA, supplementary motor area; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex. Color images available online at www.liebertpub.com/acm
Table 2.
Between-Condition Analysis (Paired Sample t-Test Baseline Versus Postacupuncture Session)
| Baseline > Postacupuncture | Postacupuncture > Baseline | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain area | H | x | y | Z | t-Value | KE | p-Value | H | x | y | z | t-Value | KE | p-Value |
| Insula (anterior) | R | 34 | 10 | 12 | 4.62 | 8 | 0.001 | L | −36 | 8 | 18 | 4.48 | 5 | 0.001 |
| Insula (posterior) | L | −36 | −18 | 2 | 4.66 | 16 | 0.000 | |||||||
| R | 42 | −14 | 0 | 4.48 | 4 | 0.001 | ||||||||
| Midcingulate cortex | R | 8 | −4 | 36 | 4.72 | 9 | 0.000 | |||||||
| Pons | L | −6 | −22 | 20 | 4.64 | 9 | 0.001 | |||||||
| Orbitofrontal cortex | L | −20 | 20 | −12 | 5.66 | 23 | 0.001 | |||||||
| Parahippocampal gyrus | R | 42 | −26 | −12 | 4.35 | 5 | 0.001 | |||||||
| Paracentral lobule (BA6, medial) | R | 4 | −32 | 66 | 4.33 | 7 | 0.000 | |||||||
| Rolandic operculum (BA6, lateral) | L | −54 | −2 | 8 | 5.08 | 20 | 0.000 | |||||||
Paired-sample t-test (n=14) assessing neural activation between the two conditions contrasting baseline > postacupuncture session and vice versa (Montreal Neurological Institute coordinates of local maxima). Results of whole-brain analysis (p < 0.001, uncorrected).
BA6, Brodmann area 6.
FIG. 3.
Paired t-tests (p<0.001, uncorrected) baseline versus acupuncture (green) and vice versa (red). AI, anterior insula; PCL, paracentral lobule; PHi, parahippocampal gyrus. Color images available online at www.liebertpub.com/acm
Discussion
Despite the wide public acceptance and clinical success of acupuncture analgesia,36 a definite scientific explanation of its underlying physiologic and biological mechanisms is still lacking. Recent neuroimaging trials found altered functional brain responses to acupuncture in sensory, affective, cognitive, and inhibitory regions, often including neural networks for pain perception and transmission.37–39 These responses depend on the method, intensity, and frequency of treatment,25 with partly consistent but also conflicting results.40–46 In the current study, functional activation patterns in response to pain processing induced by an electrical, painful stimulus were investigated under a condition with and without the influence of manual acupuncture.
As expected, the experimental stimulus induced a response of the neural networks connected to pain perception and transmission: Functional activity of the bilateral posterior and right anterior insula, right S1 and S2, and left MCC was observed during the baseline session. During the postacupuncture session, significant cluster activation was revealed in regions with a more superordinate function in the modulation of pain perception (left anterior insula, bilateral ACC, right S2, bilateral PFC, left supplementary motor area, and right thalamus). The between-group main analysis resulted in functional activity of the left pons, left anterior insula, right parahippocampal gyrus, and left OFC in the postacupuncture session (postacupuncture>baseline) and revealed activity in the bilateral posterior and right anterior insula, right MCC, right paracentral lobule, and left rolandic operculum during baseline (baseline>postacupuncture). These observations are interpreted to reflect the shortly emerging effects of acupuncture following a pain stimulus of individualized intensity.
During pain perception, activation of S1 is assumed to represent the sensory component of pain. The reduction of electrical pain induced S1 activation in the acupuncture-weighted contrast therefore suggests an acupuncture-induced modulation of the sensory encoding of the painful stimulus in the trial. S2 and the posterior insula are supposed to play a central role within the sensory and discriminative component of pain and thus in its intensity.47 However, Peltz et al. assumed S2 to be connected to the anterior insula and connectivity of the posterior insula to be more related to S1.48 Similar to the acupuncture-induced attenuation of S1 activation, insular activation patterns were changed in the current study by acupuncture because the posterior insula appeared only during the baseline condition. Both effects may reflect an inhibition of the pain induced by the electrical sensory input, possibly at a subcortical level through mechanisms such as diffuse noxious inhibitory control.49
Acupuncture-induced modulation of the anterior insula activation—a region related to transforming processes from pain sensation to cognition21,48,50–52— has been shown before.53 The re-representation between the bilateral anterior and posterior part of the insular cortex may be essential for the subjective component of pain sensation.48,50–52,54 Our results are partly consistent with those of other studies, revealing temporal sustaining activity of the anterior insula and the prefrontal cortex beyond the needling procedure.55,56 We found significant functional cluster in prefrontal brain areas after acupuncture treatment, which supports this theory because there has been evidence that the DLPFC plays a role in the affective component and the control of pain perception by modulating corticosubcortical and corticocortical pathways.57 The activation pattern of ACC, anterior insula, and PFC during acupuncture, also present in this study, may reflect a close connection of these brain regions and the cognitive processing of painful sensations.48 In clinical acupuncture trials,40,58 as well as in imaging studies,40 acupuncture repeatedly leads to significant pain reduction, but several studies show no difference on pain ratings between sham and real acupuncture.59,60
In the current study, subjective pain ratings did not differ significantly between the two experimental sessions; thus, the differences in activation pattern observed may be due to placebo effects. However, retrospective pain ratings of functional sessions are less valid than online ratings. At the same time, the pain ratings might also indicate that participants did not cognitively evaluate the pain as lower. In the discussion of the relevance of the distinction between placebo-mediated and acupuncture-specific effects, the role of expectation must be considered. The analgesic effect of acupuncture increases with the individual grade of expectancy.40,60,61 These responses are assumed to be similar to a placebo response, mediated through the endogenous opiate system.62,63 In this context, the DLPFC may play a central role in mediating placebo-induced analgesia through triggering the midbrain opioid system.64,65 By now most acupuncture studies conclude that the acupuncture-induced decrease in pain perception consists of acupuncture-specific brain activations, as well as cognitively mediated attention-dependent and emotional processes.
One limitation of the current study is the lack of a sham acupuncture condition. Doubts about whether minimal or non–point-specific acupuncture conditions are really physiologically inactive for the treatment of pain have been raised.59,60,66–68 If so, then a comparison of two active treatments is very likely to show no group difference and the conclusion that the investigated treatment is ineffective would be misleading. However, a recent individual meta-analysis based on data from 29 randomized clinical trials with a total of 17,922 patients reported clear differences between real acupuncture and sham procedures for several chronic pain conditions.69 This is a strong argument for incorporating sham procedures as a possible control condition in future studies. Such studies should also consider online pain ratings, which are delivered after each painful stimulus during the fMRI session, instead of retrospective pain ratings. Within a larger sample the sequence order of treatment and baseline should be balanced to control for habituation and other effects of repeated measures.
Conclusion
In line with recent literature, this study showed differences in activation patterns following an acupuncture session, applied before painful electrical stimulation, but also supports the theory that acupuncture and pain mobilize overlapping brain regions and the same intrinsic networks.70,71 The results further support previous findings that the analgesic effect of acupuncture consists of specific brain activation–modulating patterns that outlast the needling period, as well as a component similar to effects known from placebo analgesia.
Author Disclosure Statement
No competing financial relationships exist.
References
- 1.Chen L, Houghton M, Seefeld L, et al. A survey of selected physician views on acupuncture in pain management. Pain Med 2010;11:530–534 [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518–1524 [PubMed] [Google Scholar]
- 3.Schiapparelli P, Allais G, Rolando S, et al. Acupuncture in primary headache treatment. Neurol Sci 2011;32Suppl 1:S15-18 [DOI] [PubMed] [Google Scholar]
- 4.Schliessbach J, van der Klift E, Arendt-Nielsen L, et al. The effect of brief electrical and manual acupuncture stimulation on mechanical experimental pain. Pain Med 2011;12:268–275 [DOI] [PubMed] [Google Scholar]
- 5.Chou LW, Kao MJ, Lin JG. Probable mechanisms of needling therapies for myofascial pain control. Evid Based Complement Altern Med 2012;2012:705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev 2002;40:29–44 [DOI] [PubMed] [Google Scholar]
- 7.Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms of actions. Am J Chin Med 2008;36:635–645 [DOI] [PubMed] [Google Scholar]
- 8.Le Bars D, Cadden SW. What is a wide-dynamic-range cell?. In: Basbaum A, et al., eds. The senses: a comprehensive reference, vol 5: Pain. Vol 5. Amsterdam: Elsevier Churchill Livingstone; 2007:331–338 [Google Scholar]
- 9.Le Bars D, Willer JC. Diffuse Noxious Inhibitory Controls (DNIC). In: Basbaum A, et al., ed. The Senses: A Comprehensive Reference, vol 5: Pain. Amsterdam: Elsevier Churchill Livingstone, 2007:762–773 [Google Scholar]
- 10.Melzack R. From the gate to the neuromatrix. Pain 1999;Suppl 6:S121–126 [DOI] [PubMed] [Google Scholar]
- 11.DeLeo JA. Basic science of pain. J Bone Joint Surg Am 2006;88Suppl 2:58–62 [DOI] [PubMed] [Google Scholar]
- 12.Ohara PT, Vit JP, Jasmin L. Cortical modulation of pain. Cell Mol Life Sci 2005;62:44–52 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Larrea L, Magnin M. [Pathophysiology of neuropathic pain: review of experimental models and proposed mechanisms]. Presse Med 2008;37(2 Pt 2):315–340 [DOI] [PubMed] [Google Scholar]
- 14.Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacol Sin 2009;30:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jänig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. Cambridge: Cambridge University Press, 2006 [Google Scholar]
- 16.Musial F, Michalsen A, Dobos G. Functional chronic pain syndromes and naturopathic treatments: neurobiological foundations. Forsch Komplementmed 2008;15:97–103 [DOI] [PubMed] [Google Scholar]
- 17.Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 2002;22:2748–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amanzio M, Benedetti F, Porro CA, et al. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 2013;34:738–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsenbruch S, Kotsis V, Benson S, et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain 2012;153:382–390 [DOI] [PubMed] [Google Scholar]
- 20.Ploner M, Schnitzler A. [Cortical representation of pain]. Nervenarzt 2004;75:962–969 [DOI] [PubMed] [Google Scholar]
- 21.Kong J, Fufa DT, Gerber AJ, et al. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain 2005;6:55–64 [DOI] [PubMed] [Google Scholar]
- 22.Choi KE, Musial F, Amthor N, et al. Isolated and combined effects of electroacupuncture and meditation in reducing experimentally induced ischemic pain: a pilot study. Evid Based Complement Altern Med 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KE, Rampp T, Saha FJ, et al. Pain modulation by meditation and electroacupuncture in experimental submaximum effort tourniquet technique (SETT). Explore (NY). 2011;7:239–245 [DOI] [PubMed] [Google Scholar]
- 24.Musial F, Choi KE, Gabriel T, et al. The effect of electroacupuncture and tramadol on experimental tourniquet pain. Acupunct Med 2012;30:21–26 [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Pach D, Napadow V, et al. Characterizing acupuncture stimuli using brain imaging with FMRI—a systematic review and meta-analysis of the literature. PLoS One 2012;7:e32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasser TG, Sandalcioglu EI, Wiedemayer H, et al. A novel passive functional MRI paradigm for preoperative identification of the somatosensory cortex. Neurosurg Rev 2004;27:106–112 [DOI] [PubMed] [Google Scholar]
- 27.Naglatzki RP, Schlamann M, Gasser T, et al. Cerebral somatic pain modulation during autogenic training in fMRI. Eur J Pain 2012;16:1293–1301 [DOI] [PubMed] [Google Scholar]
- 28.Yuan W, Ming Z, Rana N, et al. A functional magnetic resonance imaging study of human brain in pain-related areas induced by electrical stimulation with different intensities. Neurol India. 2010;58:922–927 [DOI] [PubMed] [Google Scholar]
- 29.Kong J, Gollub R, Huang T, et al. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med 2007;13:1059–1070 [DOI] [PubMed] [Google Scholar]
- 30.Hui KK, Nixon EE, Vangel MG, et al. Characterization of the “deqi” response in acupuncture. BMC Complement Altern Med 2007;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacPherson H, Asghar A. Acupuncture needle sensations associated with De Qi: a classification based on experts' ratings. J Altern Complement Med 2006;12:633–637 [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage 2000;12:466–477 [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45–53 [DOI] [PubMed] [Google Scholar]
- 34.Penny WD, Holmes AJ. Random-effects analysis. In: Frackowiak RSJ, Friston KJ, Frith C, et al., eds. Human Brain Function, 2nd ed. Vol 2 Waltham, MA: Academic Press, 2003 [Google Scholar]
- 35.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–1239 [DOI] [PubMed] [Google Scholar]
- 36.Lin JG, Chen WL. Review: acupuncture analgesia in clinical trials. Am J Chin Med 2009;37:1–18 [DOI] [PubMed] [Google Scholar]
- 37.Cho SY, Jahng GH, Park SU, et al. fMRI study of effect on brain activity according to stimulation method at LI11, ST36: painful pressure and acupuncture stimulation of same acupoints. J Altern Complement Med 2010;16:489–495 [DOI] [PubMed] [Google Scholar]
- 38.Hui KK, Marina O, Liu J, et al. Acupuncture, the limbic system, and the anticorrelated networks of the brain. Auton Neurosci 2010;157:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo S, Choe IH, van den Noort M, et al. Consecutive acupuncture stimulations lead to significantly decreased neural responses. J Altern Complement Med 2010;16:481–487 [DOI] [PubMed] [Google Scholar]
- 40.Kong J, Kaptchuk TJ, Polich G, et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 2009;45:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla S, Torossian A, Duann JR, Leung A. The analgesic effect of electroacupuncture on acute thermal pain perception—a central neural correlate study with fMRI. Mol Pain 2011;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang J, Jin Z, Wang Y, et al. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum Brain Mapp 2009;30:1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin W, Tian J, Bai L, et al. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol Pain. 2008;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhond RP, Yeh C, Park K, et al. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain 2008;136:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhond RP, Kettner N, Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med 2007;13:603–616 [DOI] [PubMed] [Google Scholar]
- 46.Witt C, Brinkhaus B, Jena S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 2005;366:136–143 [DOI] [PubMed] [Google Scholar]
- 47.Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci 2009;66:375–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peltz E, Seifert F, DeCol R, et al. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage 2011;54:1324–1335 [DOI] [PubMed] [Google Scholar]
- 49.Le Bars D, Willer JC. Pain modulation triggered by high-intensity stimulation: implication for acupuncture analgesia? Int Cong Ser. 2002;1236:11–29 [Google Scholar]
- 50.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–666 [DOI] [PubMed] [Google Scholar]
- 51.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci 2003;26:303–307 [DOI] [PubMed] [Google Scholar]
- 52.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci 2000;3:184–190 [DOI] [PubMed] [Google Scholar]
- 53.Napadow V, Makris N, Liu J, et al. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 2005;24:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 2006;27:715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai L, Qin W, Tian J, et al. Time-varied characteristics of acupuncture effects in fMRI studies. Hum Brain Mapp 2009;30:3445–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bai L, Tian J, Zhong C, et al. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Mol Pain 2010;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126(Pt 5):1079–1091 [DOI] [PubMed] [Google Scholar]
- 58.Brinkhaus B, Witt CM, Jena S, et al. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med 2006;166:450–457 [DOI] [PubMed] [Google Scholar]
- 59.Vas J, Aranda JM, Modesto M, et al. Acupuncture in patients with acute low back pain: a multicentre randomised controlled clinical trial. Pain 2012;153:1883–1889 [DOI] [PubMed] [Google Scholar]
- 60.White P, Bishop FL, Prescott P, et al. Practice, practitioner, or placebo? A multifactorial, mixed-methods randomized controlled trial of acupuncture. Pain 2012;153:455–462 [DOI] [PubMed] [Google Scholar]
- 61.Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain 2007;128:264–271 [DOI] [PubMed] [Google Scholar]
- 62.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 1996;64:535–543 [DOI] [PubMed] [Google Scholar]
- 64.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004;303:1162–1167 [DOI] [PubMed] [Google Scholar]
- 65.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A 2007;104:11056–11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lund I, Naslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist's perspective. Chin Med 2009;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rebhorn C, Breimhorst M, Buniatyan D, et al. The efficacy of acupuncture in human pain models: a randomized, controlled, double-blinded study. Pain 2012;153:1852–1862 [DOI] [PubMed] [Google Scholar]
- 68.Wayne PM, Hammerschlag R, Langevin HM, et al. Resolving paradoxes in acupuncture research: a roundtable discussion. J Altern Complement Med 2009;15:1039–1044 [DOI] [PubMed] [Google Scholar]
- 69.Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med 2012;172:1444–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claunch JD, Chan ST, Nixon EE, et al. Commonality and specificity of acupuncture action at three acupoints as evidenced by FMRI. Am J Chin Med 2012;40:695–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren XJ, Chen HY, Wang BG, et al. Regional homogeneity analysis on acupoint specificity with resting-state functional magnetic resonance imaging. Chin Med J (Engl) 2012;125:1627–1632 [PubMed] [Google Scholar]