Abstract
In July of 2013, samples from a patient with a neurological syndrome were collected from Mantua hospital and sent to the National Reference Laboratory for Arboviruses (National Institute of Health, Rome). On the basis of the symptoms, serological and molecular assays were performed to diagnose either West Nile virus (WNV) or Toscana virus (TOSV) infection. Molecular and serological tests confirmed TOSV infection. Virus isolation was obtained from cerebrospinal fluid. A full genome sequence was determined from this TOSV strain with next-generation sequencing using Ion Torrent technology. Nucleotide and amino acidic sequences grouped phylogenetically with lineage TOSV A and showed a low genome variability.
Key Words: : Phlebovirus, Toscana virus, Sandfly, Isolation, Next-generation sequencing
Introduction
Toscana virus (TOSV; Bunyaviridae, Phlebovirus) is an enveloped, tripartite, single-strand, negative-sense RNA virus, transmitted by phlebotomine sandflies (Phlebotomus perniciosus and P. perfiliewi). It was first isolated from P. perniciosus collected in Italy in 1971. Evidence for its human pathogenicity and neurotropism have been reported since 1983 (Nicoletti et al. 1991, Schwarz et al. 1995, Braito et al. 1998). After a short incubation period, onset of symptoms occurs with nonspecific signs of viral febrile illness associated, or not, with central nervous system (CNS) manifestations, i.e., aseptic meningitis or encephalitis (Jaijakul et al. 2012). Although the vast majority of cases have a favorable outcome, some severe cases of TOSV infections have been described (Baldelli et al. 2004, Vocale et al. 2012).
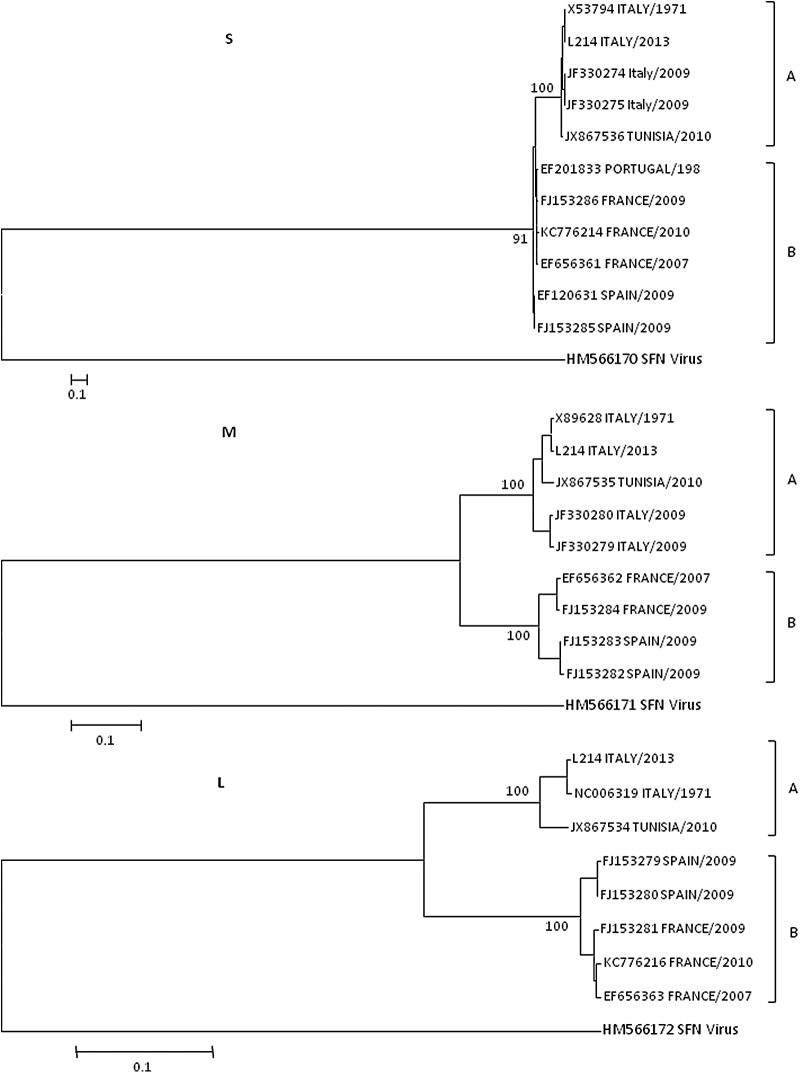
On the basis of the analysis of its three genomic units (S, M, and L), phylogenetic studies have demonstrated that TOSV isolates are categorized into two distinct lineages (Sanbonmatsu-Gamez et al. 2005, Collao et al. 2009). Lineage TOSV A has been identified in Italy, the south of France, and Tunisia, whereas lineage TOSV B is widespread in Portugal and Spain, the south of France, and Morocco. Moreover, the circulation of a third TOSV lineage was recently demonstrated in the Eastern Europe (Punda-Polić et al. 2012). Here we report a TOSV case from the Italian Public Hospital of Mantua, probably imported from Elba Island (central Italy) during the summer of 2013.
Case report
On July 24th, 2013, a 48-year-old Italian man was hospitalized at the Infectious Diseases Department of the Public Hospital of Mantua (Lombardy in northern Italy). He had been suffering from acute headache with vomiting and high-grade fever since July 20th. Physical examination showed neck rigidity and signs of meningoencephalitis. Laboratory results on the cerebrospinal fluid (CSF) showed an elevated white blood cell count (465 H/μL>4, most of which were lymphocytes), with hyperproteinorachia (2.00 H grams/L) and normal glycorrhachia (51 mg/dL).
The PCR assays performed were all negative for herpes simplex, varicella zoster, cytomegalovirus, Epstein–Barr, and enteroviruses. The patient was treated by aciclovir and paracetamol, and on August 8th was discharged as on the mend. Serum, urine, and CSF samples from the patient were sent to the National Reference Laboratory (NRL) for Arboviruses at the Italian National Health Institute (Istituto Superiore di Sanità, Rome) with suspected TOSV or West Nile virus (WNV) infection, and were tested for these neurotropic viruses. The serum sample was analyzed by an immunoglobulin M (IgM)-capture enzyme-linked immunosorbent assay (ELISA) specific for WNV (FOCUS Diagnostics) and for TOSV (homemade ELISA) and by the plaque reduction neutralization test (PRNT).
Total RNA was extracted from serum, CSF, and urine specimens using a QIAamp Viral RNA Mini Kit and RNEasy Mini Kit (Qiagen) according to manufacturer's instructions, and tested by specific real-time reverse transcription PCR for WNV and TOSV (Lanciotti et al. 2000, Pérez-Ruiz et al. 2007). All of the assays were negative for WNV. The patient was positive for the anti-TOSV IgM by ELISA test on serum and by specific TOSV real-time PCR on CSF, whereas urine and serum samples were negative.
Thus, the diagnosis of meningoencephalitis due to TOSV was established. Moreover, CSF was used for viral isolation. After a first inoculation on Vero cells, the supernatant was used for a second inoculation by blind passage and incubated until the appearance of the lytic cytopathic effect. The isolate was identified as TOSV through real-time PCR. Next-generation sequencing using Ion Torrent technology was used to determine the complete genome sequence of this TOSV strain from the isolate virus. Sequences of the S, M, and L segments were submitted in GenBank under accession numbers KM275783, KM507329, and KM507328.
The entire nucleotide sequences of the three genomic segments were aligned and compared with the Italian reference strain (ISS_Phl.3, GenBank accession nos. X53794, X89628, X68414) and with all whole segments of isolates from Italy, Spain, Portugal, France, and Tunisia available in GenBank. P-distance values for both nucleotide and amino acid sequences were calculated. Phylogenetic analysis of L, M, and S segments of TOSV shows that this strain belongs to the lineage TOSV A (Fig. 1). The analysis of each segment group belonging to lineage A (ISS_Phl.3 enclosed) revealed a low degree of genetic variability. The S segment showed a mean nucleotide sequence variation of 2.3%, and the mean amino acid sequence variation was 0.1 % for the nucleoprotein (N) and 2% for the nonstructural protein (NSs). The M segment showed a mean nucleotide sequence variation of 2.7% and a mean amino acid sequence variation of 1.9 % for the polyprotein. The L segment showed a mean nucleotide sequence variation of 2.8%, and the mean amino acid sequence variation was 0.9 %.
FIG. 1.
The selected strains were aligned using BioEdit and analyses were conducted in PhyML (Tamura et al. 2013). The Bayesian information criterion (BIC) was used to determine the model of nucleotide substitution that best fit the data using the selection tool available in MEGA6. The model that best fit the data was the Tamura–Nei (GTR)+G model.
Conclusions
In Mediterranean countries, TOSV is an endemic pathogen and a frequent cause of CNS infection during the warm season. There is no defined surveillance system for TOSV in Italy, and little information is available regarding its epidemiology and pathogenicity. This article describes an acute case of human meningoencephalitis from Mantua. Clinical samples from this case arrived at the NRL for a differential diagnosis between WNV and TOSV neurological diseases. WNV appeared in Italy in 1998 and it has become endemic in some regions, especially Emilia-Romagna, Lombardy, and Veneto, where several cases were reported last year. TOSV cases have been reported in central and southern Italy since the early 1980s in those rural areas where the phlebotomine sandflies competent for TOSV are common. Recently, Vocale et al. demonstrated the presence of autochthonous TOSV in Emilia-Romagna (northern Italy), suggesting an unusual circulation of phlebotomine sandflies in urban areas (Vocale et al. 2012). However, the circulation of TOSV has never been reported in the province of Mantua. The NRL diagnosed TOSV infection by real-time RT-PCR and virus isolation, supported by a specific anti-IgM ELISA assay.
The patient described here traveled in Elba Island (central Italy), an area endemic for TOSV, during the 2 weeks prior to clinical onset, and clinicians hypothesized that the infection could be linked to TOSV. The incubation period of 2 weeks does not allow exclusion of an autochthonous infection, because the presence of Phlebotomus spp. was demonstrated in hilly territories of Lombardy and Emilia-Romagna regions, respectively, north and south of Mantua. However, a dedicated monitoring system failed to detect phlebotomine vectors in the Mantua area, which lies in a lowland considered unsuitable for sand fly breeding (Maroli et al. 2008).
Studies based on sequence comparisons showed that RNA arboviruses are relatively stable in nature, suggesting that a cycle with host alternation (between vertebrate and invertebrate hosts) can interfere with viral evolution by strong conservative sequence selection. This genome stability may result from the requirements to viral replication in two hosts that present competitive niches for replication and adaptation (Strauss and Strauss 1994).
The close relationship between our strain and the ISS_Phl.3 prototype shows that TOSV has held a conserved genome since 1971, when the prototype was isolated. It is probable that this genome stability allowed the virus to maintain its virulence during this time. Further analysis will be needed to explain the biological and ecological reasons for this genome stability, which has been already seen for other phleboviruses (Moutailler et al. 2011).
Acknowledgments
The project participants all contributed significantly to the results of this study. We thank Dr. Luigi Gradoni, Unit of Vector-borne Diseases and International Health at the Italian National Institute of Health (ISS), for valuable comments and suggestions. We also thank Dr. Robert Peter Parker for final linguistic revision.
Author Disclosure Statement
No competing financial interests exist.
References
- Baldelli F, Ciufolini MG, Francisci D, Marchi A, et al. Unusual presentation of life-threatening Toscana virus meningoencephalitis. Clin Infect Dis 2004; 38:515–520 [DOI] [PubMed] [Google Scholar]
- Braito A, Ciufolini MG, Pippi L, Corbisiero R, et al. Phlebotomus-transmitted Toscana virus infections of the central nervous system: A seven-year experience in Tuscany. Scand J Infect Dis 1998; 30:505–508 [DOI] [PubMed] [Google Scholar]
- Collao X, Palacios G, Sanbonmatsu-Gámez S, Pérez-Ruiz M, et al. Genetic diversity of Toscana virus. Emerg Infect Dis 2009; 15:574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaijakul S, Arias CA, Hossain M, Arduino RC, et al. Toscana meningoencephalitis: A comparison to other viral central nervous system infections. J Clin Virol 2012; 55:204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000; 38:4066–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroli M, Rossi L, Baldelli R, Capelli G, et al. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Health 2008; 13:256–264 [DOI] [PubMed] [Google Scholar]
- Moutailler S, Roche B, Thiberge J-M, Caro V, et al. Host alternation is necessary to maintain the genome stability of Rift Valley Fever Virus. PLoS Negl Trop Dis 2011; 5:e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti L, Verani P, Caciolli S, Ciufolini MG, et al. Central nervous system involvement during infection by Phlebovirus Toscana of residents in natural foci in central Italy (1977–1988). Am J Trop Med Hyg 1991; 45:429–434 [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz M, Collao X, Navarro-Marí JM, Tenorio A. Reverse transcription, real-time PCR assay for detection of Toscana virus. J Clin Virol 2007; 39:276–281 [DOI] [PubMed] [Google Scholar]
- Punda-Polić V, Mohar B, Duh D, Bradaric N, et al. Evidence of an autochthonous Toscana virus strain in Croatia. J Clin Virol 2012; 55:4–7 [DOI] [PubMed] [Google Scholar]
- Sanbonmatsu-Gamez S, Perez-Ruiz M, Collao X, Sanchez-Seco MP, et al. Toscana virus in Spain. Emerg Infect Dis 2005; 11:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz TF, Glich S, Jager G. Aseptic meningitis caused by sandfly fever virus, serotype Toscana. Clin Infect Dis 1995; 21:669–671 [DOI] [PubMed] [Google Scholar]
- Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev 1994; 58:491–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, et al. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30:2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocale C, Bartoletti M, Rossini G, Macini P, et al. Toscana virus infections in northern Italy: Laboratory and clinical evaluation. Vector Borne Zoonotic Dis 2012; 12:526–529 [DOI] [PubMed] [Google Scholar]