Abstract
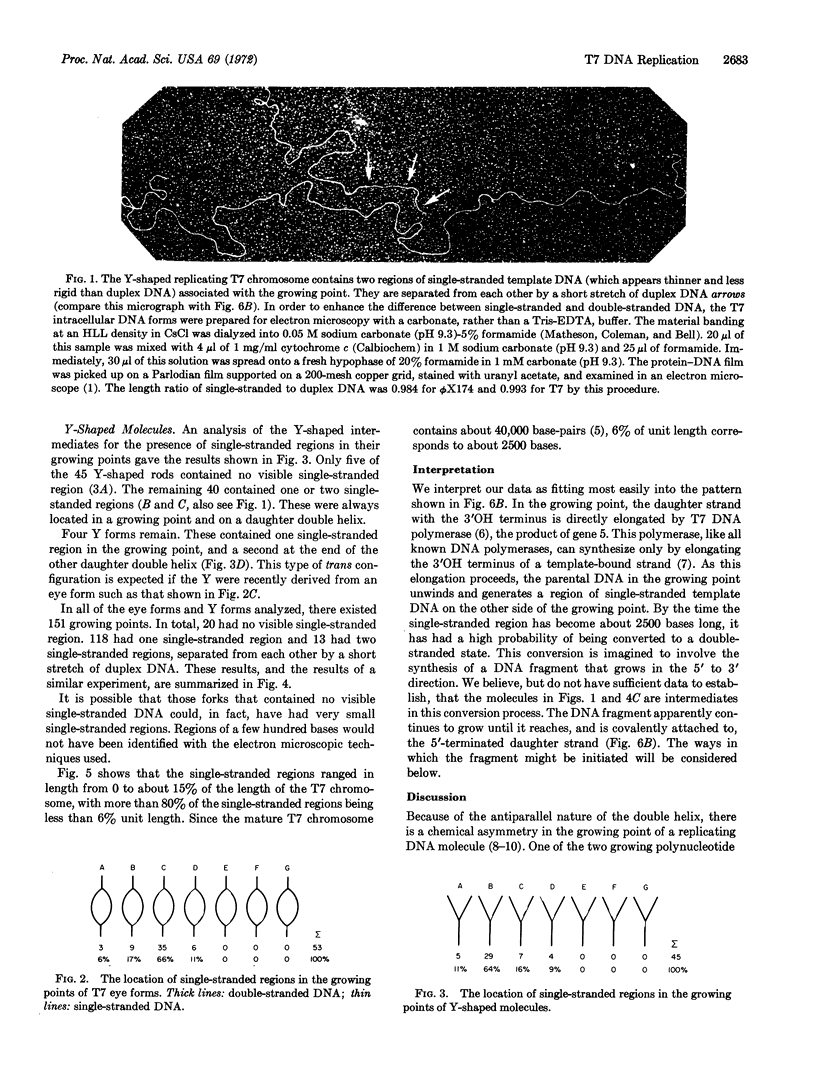
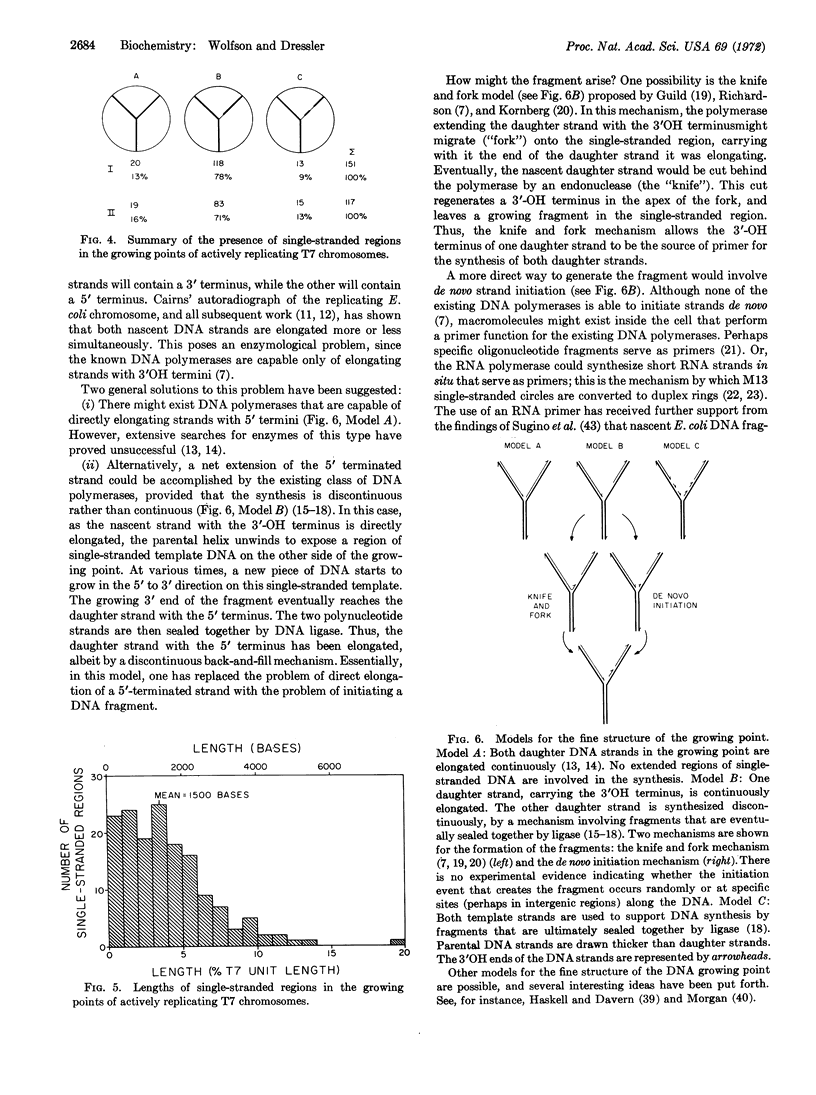
In partially replicated T7 chromosomes, the points where parental strands are separating and new DNA is being synthesized can be seen in the electron microscope to contain regions of single-stranded template DNA. The single-stranded regions are located on only one of the two daughter arms of the replicating chromosome. Inman and Schnös observed such single-stranded regions in 50% of the growing points of replicating lambda DNA, and, as reported in this paper, we find them in about 85% of the growing points of T7 DNA. Both studies support the conclusion that DNA synthesis involves the direct elongation of one daughter strand in the growing point. Evidently, this elongation is accompanied by the unwinding of the parental double helix to expose a region of single-stranded DNA which is then converted to the duplex state by a discontinuous mechanism involving the synthesis of DNA fragments.
Keywords: DNA replication, E. coli, electron microscopy
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai R., Thomas C. A., Jr Spontaneous renaturation of newly-synthesized bacteriophage T7 deoxyribonucleic acid. J Mol Biol. 1970 Jul 14;51(1):145–155. doi: 10.1016/0022-2836(70)90276-7. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulian M. Initiation of the replication of single-stranded DNA by Escherichia coli DNA polymerase. Cold Spring Harb Symp Quant Biol. 1968;33:11–20. doi: 10.1101/sqb.1968.033.01.006. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Ginsberg B., Hurwitz J. Unbiased synthesis of pulse-labeled DNA framents of bacteriophage lambda and T4. J Mol Biol. 1970 Sep 14;52(2):265–280. doi: 10.1016/0022-2836(70)90030-6. [DOI] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Haskell E. H., Daverin C. I. Pre-fork synthesis: a model for DNA replication. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1065–1071. doi: 10.1073/pnas.64.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Structure of branch points in replicating DNA: presence of single-stranded connections in lambda DNA branch points. J Mol Biol. 1971 Mar 14;56(2):319–325. doi: 10.1016/0022-2836(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Lark K. G. DNA replication in Escherichia coli: location of recently incorporated thymidine within molecules of high molecular weight DNA. Proc Natl Acad Sci U S A. 1970 Oct;67(2):629–636. doi: 10.1073/pnas.67.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Veomett G. E. A possible function of DNA polymerase in chromosome replication. Biochem Biophys Res Commun. 1970 Nov 25;41(4):973–980. doi: 10.1016/0006-291x(70)90180-4. [DOI] [PubMed] [Google Scholar]
- Manor H., Deutscher M. P., Littauer U. Z. Rates of DNA chain growth in Escherichia coli. J Mol Biol. 1971 Nov 14;61(3):503–524. doi: 10.1016/0022-2836(71)90062-3. [DOI] [PubMed] [Google Scholar]
- Mitra S., Kornberg A. Enzymatic mechanisms of DNA replication. J Gen Physiol. 1966 Jul;49(6):59–79. doi: 10.1085/jgp.49.6.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R. Model for DNA replication by Kornberg's DNA polymerase. Nature. 1970 Sep 26;227(5265):1310–1313. doi: 10.1038/2271310a0. [DOI] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K. Mechanism of DNA replication possible discontinuity of DNA chain growth. Jpn J Med Sci Biol. 1967 Jun;20(3):255–260. [PubMed] [Google Scholar]
- Okazaki R., Sugimoto K., Okazaki T., Imae Y., Sugino A. DNA chain growth: in vivo and in vitro synthesis in a DNA polymerase-negative mutant of E. coli. Nature. 1970 Oct 17;228(5268):223–226. doi: 10.1038/228223a0. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Okazaki R. Mechanism of DNA chain growth. Vii. Direction and rate of growth of T4 nascent short DNA chains. J Mol Biol. 1972 Feb 28;64(1):61–85. doi: 10.1016/0022-2836(72)90321-x. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. The structure of DNA. Cold Spring Harb Symp Quant Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- WILKINS M. H. Physical studies of the molecular structure of deoxyribose nucleic acid and nucleoprotein. Cold Spring Harb Symp Quant Biol. 1956;21:75–90. doi: 10.1101/sqb.1956.021.01.007. [DOI] [PubMed] [Google Scholar]
- Weiss B. Terminal cross-linking of DNA strands by an enzyme system from Escherichia coli infected with bacteriophage T4. Proc Natl Acad Sci U S A. 1970 Mar;65(3):652–659. doi: 10.1073/pnas.65.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R. Nature of DNA precursors. Nat New Biol. 1971 Sep 22;233(38):99–103. doi: 10.1038/newbio233099a0. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]




