Abstract
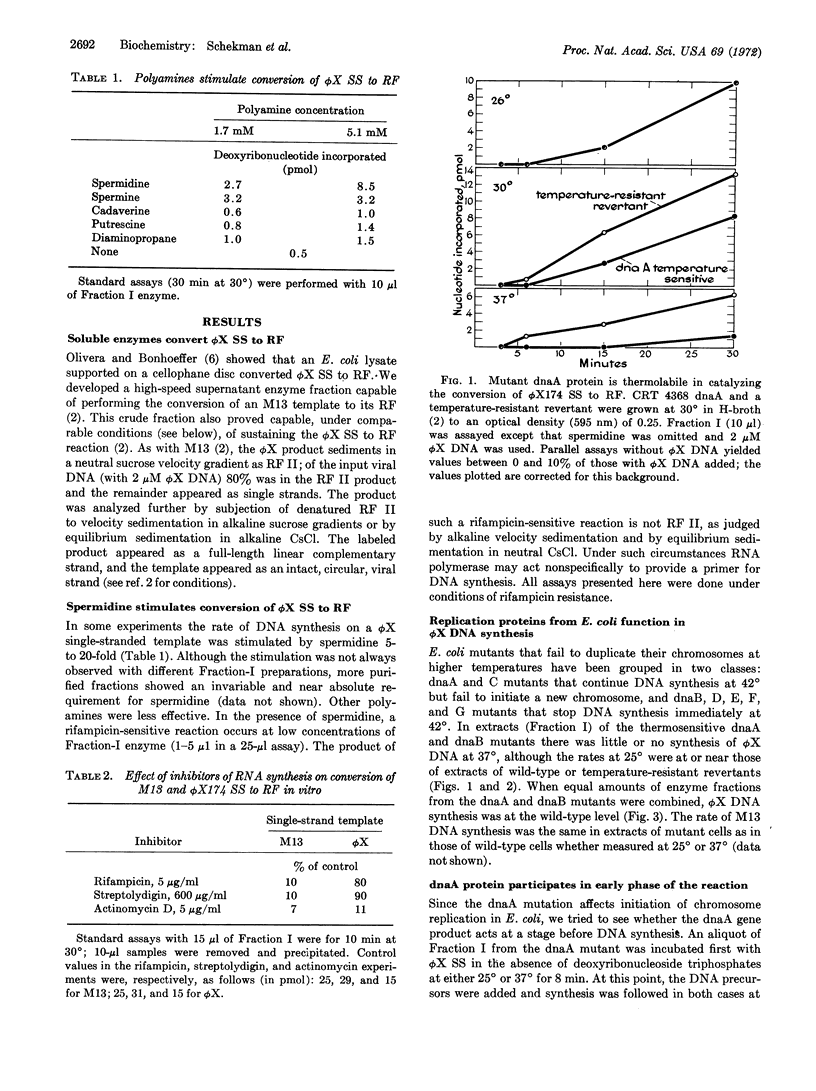
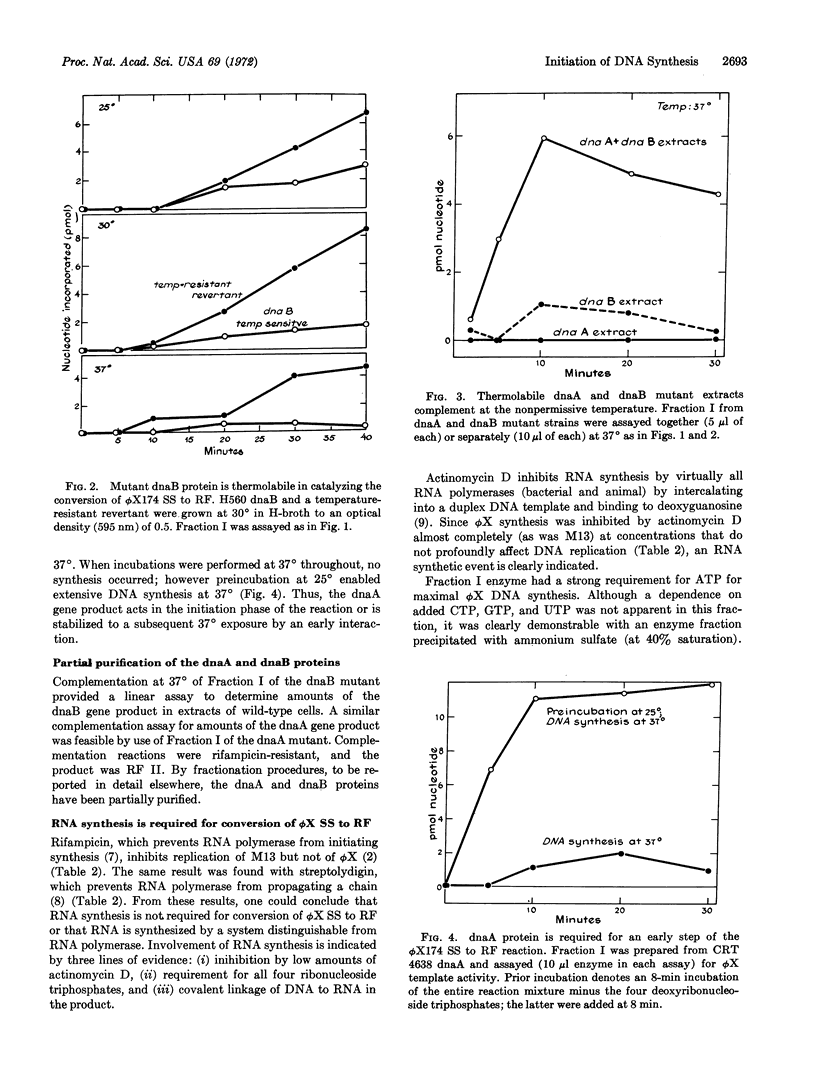
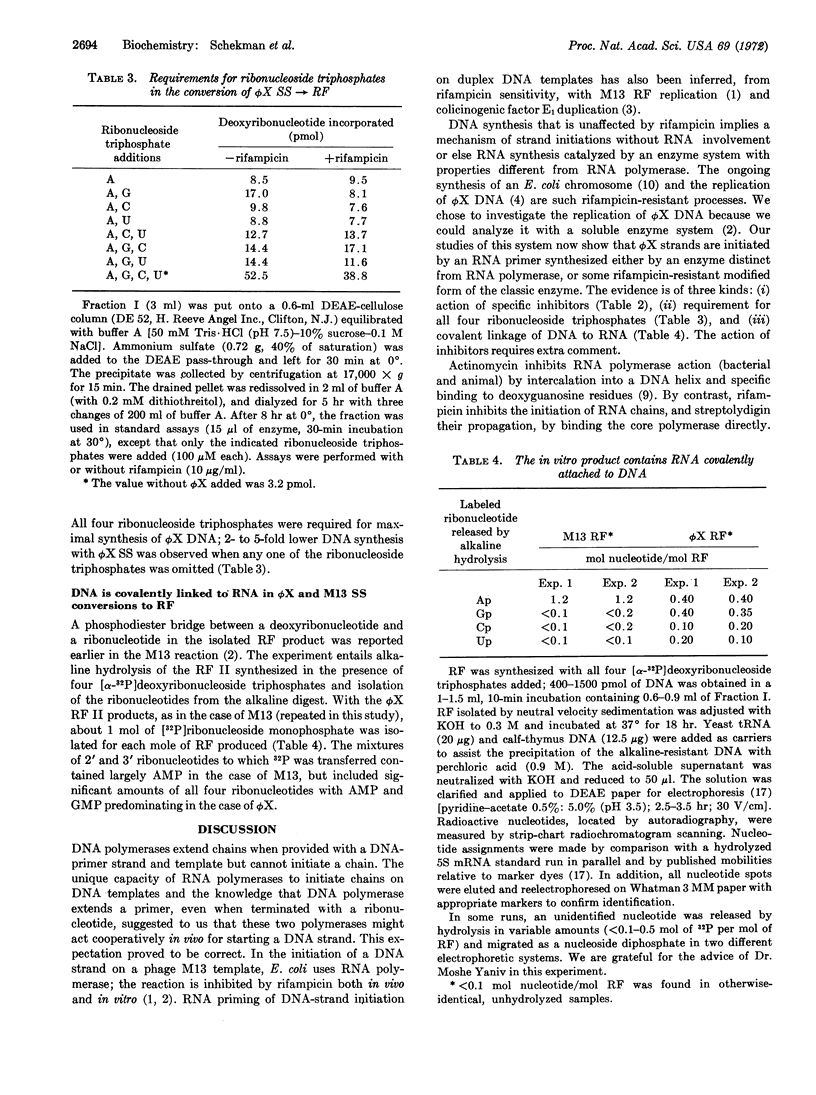
Conversion of single-stranded DNA of phage ϕX174 to the double-stranded replicative form in Escherichia coli uses enzymes essential for initiation and replication of the host chromosome. These enzymes can now be purified by the assay that this phage system provides. The ϕX174 conversion is distinct from that of M13. The reaction requires different host enzymes and is resistant to rifampicin and streptolydigin, inhibitors of RNA polymerase. However, RNA synthesis is essential for ϕX174 DNA synthesis: the reaction is inhibited by low concentrations of actinomycin D, all four ribonucleoside triphosphates are required, and an average of one phosphodiester bond links DNA to RNA in the isolated double-stranded circles. Thus, we presume that, as in the case of M13, synthesis of a short RNA chain primes the synthesis of a replicative form by DNA polymerase. Initiation of DNA synthesis by RNA priming is a mechanism of wide significance.
Keywords: dnaA gene product, dnaB gene product, M13 DNA, spermidine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B., Cranston J. W. Direct inhibition of Col E 1 plasmid DNA replication in Escherichia coli by rifampicin. Nat New Biol. 1972 May 3;237(70):29–31. doi: 10.1038/newbio237029a0. [DOI] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S. Conformations of the single-stranded DNA of bacteriophage M13. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1534–1541. doi: 10.1073/pnas.67.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancini G., Pallanza R., Silvestri L. G. Relationships between bactericidal effect and inhibition of ribonucleic acid nucleotidyltransferase by rifampicin in Escherichia coli K-12. J Bacteriol. 1969 Feb;97(2):761–768. doi: 10.1128/jb.97.2.761-768.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Replication of Phi-X174 DNA by Escherichia coli polA- in vitro (Phi-X174 DNA-DNA replication-E. coli polA-). Proc Natl Acad Sci U S A. 1972 Jan;69(1):25–29. doi: 10.1073/pnas.69.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Voss H., Gucker S. Structure of the DNA of bacteriophage fd. II. Isolation and characterization of a DNA fraction with double strand-like properties. J Mol Biol. 1969 Sep 28;44(3):445–458. doi: 10.1016/0022-2836(69)90372-6. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S., Billen D. Transcription: role in the initiation and replication of DNA synthesis in Escherichia coli and phiX174. Biochim Biophys Acta. 1971 Oct;247(3):383–390. doi: 10.1016/0005-2787(71)90023-2. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C., Sakore T. D., Nordman C. E. Stereochemistry of actinomycin--DNA binding. Nat New Biol. 1971 Jun 16;231(24):200–205. doi: 10.1038/newbio231200a0. [DOI] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Preparation of [alpha-32P]nucleoside and deoxynucleoside 5'-triphosphates from 32Pi and protected and unprotected nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):548–550. doi: 10.1016/0005-2787(69)90105-1. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]



