Abstract
Angiogenesis plays a crucial role in the growth and spread of cancer. New vascularization nourishes cancer cells with oxygen and nutrients, allowing these cells to grow, invade nearby tissue, spread to other parts of the body, and form new colonies of cancer cells. Tumor angiogenesis consists of endothelial cell proliferation, migration, and tube formation into the tumor mass. The study of natural and synthetic angiogenesis inhibitors is a promising area for therapeutics since tumors cannot grow or spread without the formation of new blood vessels. Anti-angiogenic activities have been identified in peptides known as disintegrins. Disintegrins are a family of small proteins (45–84 amino acids in length), many which are found in snake venom that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion. This study reports two recombinant disintegrins (r-mojastin 1 and r-viridistatin 2) inhibiting, with similar effectiveness, distinct steps in angiogenesis such as proliferation, adhesion to fibronectin, migration, and tube formation in vitro and in vivo. Both recombinant disintegrins bind to αvβ3 and αvβ5 receptors that are upregulated in tumor endothelial cells, having a higher binding activity to αvβ3 integrin.
Keywords: Angiogenesis, Endothelial cells, Disintegrins, Integrins, Venoms, Rattlesnakes
Angiogenesis, the formation of new vessels out of the existing vasculature, is involved in numerous processes, such as embryogenesis, wound healing, tissue remodeling, and menstruation. Also, numerous disorders are characterized by either excess or an insufficient number of blood vessels. The best-known disorders are rheumatoid arthritis, psoriasis, restenosis, diabetic retinopathy, and tumor growth (Haubner, 2008). Tumor angiogenesis involves several processes, including endothelial proliferation, migration, invasion, and tube formation all regulated by cell adhesion receptors and specific angiogenesis growth factors produced by tumor cells and the surrounding stroma (Yeh et al., 2001). Integrins of endothelial cells participate in regulating these physiological processes (You et al., 2003).
Tumor cell invasion alone is not sufficient to produce distant metastases; it requires also the transport of malignant cells through blood and/or lymph vessels. Pioneering work by Folkman (1971)showed that avascular tumors could not grow beyond a size of ~ 1 mm in diameter. At this stage, passive diffusion of nutrients and oxygen becomes rate limiting for the tumor nodule, which is then forced to enter a state of so-called “tumor dormancy.” In most cases, tumor vascularization is achieved by sustained angiogenesis, with a significant contribution of bone marrow-derived vascular and hematopoietic progenitor cells. Indeed, tumor angiogenesis is one of the hallmarks of cancer (Geiger and Peeper, 2009). Tumor angiogenesis involves increased endothelial cell proliferation and migration, and tube formation into the tumor mass (Silva et al., 2008).
Because angiogenesis is a key process to tumor growth, developing angiogenesis inhibitors with very few side effects is desirable for cancer treatment. Resistance to anti-angiogenesis drugs is also unlikely to occur, or at least at a much lower rate than seen with traditional cytotoxic chemotherapeutics (Cook and Figg, 2010). The study of tumor angiogenesis, therefore, represents a promising area of research for development of anti-cancer therapeutics (Swenson et al., 2007).
Integrins, a family of noncovalently associated heterodimeric transmembrane glycoprotein adhesion molecules, are a major target of interest, as they are known to play a vital role in pathological angiogenesis (Swenson et al., 2007). They comprise an α-subunit, and a β-subunit, which mediate cell–extracellular matrix (ECM) and cell– cell adhesive interactions. Heterodimer composition confers ligand specificity, with most integrins recognizing several ECM proteins and, in turn, most matrix proteins binding to more than one integrin. Endothelial cells express a subset of mammalian integrins including; the fibronectin receptors, α4β1, α5β1; the collagen receptors, α1β1, α2β1; the laminin receptors, α3β1, α6β1, and α6β4, the osteopontin receptor, α9β1; and the vitronectin receptors, αvβ3 and αvβ5 (Silva et al., 2008).
Snake venoms contain many unique components that affect cell–matrix interaction. Disintegrins represent a family of low molecular weight, cysteine-rich polypeptides that bind specifically to integrins αIIbβ3, α5β1 and αvβ3 expressed on platelets and other cells, including vascular endothelial cells and some tumor cells, leading to inhibition of platelet aggregation, inhibition of cell adhesion, migration and angiogenesis (Calvete, 2013; Huang et al., 2001).
Recombinant disintegrin mojastin 1 (r-mojastin 1) derived from Crotalus scutulatus scutulatus, expressed using a prokaryotic host expression system in Escherichia coli BL21 cells, was shown to be highly active in inhibiting ADP-induced platelet aggregation using platelet-rich plasma and whole blood, platelet ATP release, and platelet adhesion to fibronectin (Sánchez et al., 2010). r-Mojastin 1 was also tested for its ability to inhibit some cellular function such as adhesion to extracellular matrices, migration and invasion in vitro and in vivo, using human urinary bladder carcinoma (T24), skin melanoma (SK-MEL-28), and murine melanoma (B16F10) cells (Lucena et al., 2011). r-Mojastin 1 inhibited SK-MEL-28 cells adhesion to fibronectin and SK-MEL-28 cell migration. The invasion studies in vitro and in vivo shown that r-mojastin 1 inhibited T24 and SK-MEL-28 cells invasion through an artificial basement membrane, and lung tumor metastasis at a dose of 1000 µg/kg, when the r-disintegrin was co-injected with B16F10 cells (Lucena et al., 2011). Recently, we have described the cloning and functional characterization of another recombinant disintegrin derived from Crotalus viridis viridis called r-viridistatin 2, which showed potent anti-metastatic activities against five different human tumoral cell lines. r-Viridistatin 2, efficiently inhibited various functions of the tumoral cells such as adhesion, migration, invasion and lung tumor colonization, with different potency depending of the tumoral cell line used (Lucena et al., 2012).
Considering that angiogenesis contributes to the pathogenesis of many disorders, including cancer, in this study we have described the effects of r-mojastin 1 and r-viridistatin 2 on each distinct step of angiogenesis, including proliferation, adhesion, migration, in vivo angiogenesis, and in vitro tube formation in human umbilical vein endothelium cells (HUVECs).
2. Materials and methods
2.1. Preparation of recombinant disintegrins
Recombinant mojastin 1 and recombinant viridistatin 2 were expressed in E. coli and further purified by two-step chromatography using the method of Sánchez et al. (2010)and Lucena et al. (2012), respectively.
2.2. Cell line and culture conditions
Human umbilical vein endothelium cell (HUVEC) line and endothelial cell growth media were obtained from Lonza (USA). The cells were maintained in endothelial cell basal medium (EMB-2) containing 2% fetal bovine serum (FBS), and supplemented with 0.4% bovine brain extract (BBE), 0.1% human epidermal growth factor (hEGF), 0.1% hydrocortisone, 50 U/mL penicillin, and 50 µg/mL streptomycin in a humidified 5% CO2 air incubator at 37 °C. HUVECs used in all experiments were from passages 2–6.
2.3. Proliferation assay
Two hundred microliters of HUVECs in EMB-2 medium were plated into the wells of 96-well culture plates at 5 × 104 cells/well induplicate andincubatedat 37 °Cin 5%CO2 for 24 h, then the cells were treated with 20 µLof r-mojastin 1 and r-viridistatin 2 at various concentrations for 24 h. Cells were incubated with 10 µL of 3-[4, 5-dimethylthiazol-2-yl] 2,5-diphenltetrazolium bromide (MTT; 5 mg/mL) for 4 h at 37 °C, MTT was aspirated and 100 µL of dimethyl sulfoxide (DMSO) was added to lyse the cells. The absorbance of cell lysate at 570 nm was measured using a Beckman Coulter™ model AD 340 reader. Doxorubicin (4 µL; 2.5 mg/mL), a drug that induced apoptosis in endothelial cells was used as the positive control (Kotamraju et al., 2000). The negative control consisted of cells treated with phosphate buffer saline (PBS), pH 7.4. The percentage of cell proliferation was calculated relative to the negative control, which was defined as 100%. The 50% cytotoxic concentration (CC50) of sample is defined as the venom concentration, which reduced 50% of proliferation. The values of the percentages of cell proliferation inhibition were plotted against disintegrin concentrations, and the CC50 was determined.
2.4. Adhesion assay
Recombinant mojastin 1 and r-viridistatin 2 were used to inhibit the binding of HUVECs on fibronectin coated plate (Juliano et al., 1996). Duplicate wells of a 96-well plate (Falcon® Tissue Culture Plate) were coated with 0.1 mL of fibronectin (10 µg/mL) in phosphate buffer saline (PBS), pH 7.4, and incubated overnight at 4 °C. The plate was blocked by addition of 0.2 mL of PBS in 5% bovine serum albumin (BSA) and incubated at 37 °C for 1 h. Cells were harvested, counted and resuspended in medium containing 1% BSA at 1.3 × 105 cells/mL. Recombinant disintegrins (0.05mL) were added to the cell suspension (0.45 mL) at various concentrations and allowed to incubate at 37 °C for 1 h. The blocking solution was aspirated, and the cell/r-disintegrins fraction suspensions (0.2 mL) were added to the wells coated with fibronectin and incubated at 37 °C for 2 h. The negative control consisted of HUVECs incubated with PBS. The negative control allowed binding of cells to fibronectin. The wells were washed twice with PBS-5% BSA by filling and aspirating. Two hundred microliters of medium in 1% BSA containing 2.5 mg/mL of MTT (5:1 vol/vol) were added to the wells containing cells and incubated at 37 °C for 2 h. The MTT was aspirated and 100 µL of DMSO were added to the wells to lyse the cells. The plate was gently shaken, and the absorbance read at 570 nm using a Beckman Coulter model AD 340 reader. The percent inhibition was calculated by the following formula: [(absorbance of negative control – absorbance of cell/r-disintegrins sample)/absorbance of negative control] × 100.
2.5. Migration assay
HUVEC migration was measured after scraping cells from the bottom of the well as described by Ren et al. (2006). Briefly, HUVECs were plated (1 mL; 9 × 104 cells/mL) on a 48-well plate. After 24 h, the confluent monolayer was scratched with a sterile pipette tip. Detached cells were rinsed away with EMB-2 medium, and then added new medium mixed with recombinant disintegrins. Cells were then incubated in a CO2 chamber and were only removed from the incubator for microscopy images at times 0, 3, 6,12, and 24 h after disintegrins incubation. The concentration of r-viridistatin 2 and r-mojastin 1 used was 6.3 µM. The negative control consisted of HUVECs incubated with PBS, which allowed cell migration to occur. Percent of closure was calculated by the following equation: [(C – E)/C] × 100, where C is the units of distance of cell edge (mm) at zero time for the control, and E is the distance from the cell edge (mm) at the final incubation time for the disintegrin.
2.6. Tube formation assay
Matrigel tube formation assays were performed as described previously by Yeh et al. (2001). Matrigel was diluted to 1 mg/mL in the presence or absence of basic fibroblast growth factor (bFGF) at 30 ng/mL and added to 24-well plates in a total volume of 200 µL in each well to form a gel layer for 30 min at 37 °C. Two hundred microliters of HUVECs (2 × 105 cells/well) were treated with 6.3 µM of r-disintegrins (r-mojastin 1/r-viridistatin 2) for 30 min at 37 °C and plated on diluted matrigel for 24 h. Suramin, a known tube formation inhibitor, was used as a positive control at a concentration of 100 µM (Minea et al., 2012). HUVECs incubated with PBS buffer were used as a negative control. The cells were photographed under a microscope at 10× magnification.
2.7. Matrigel plug angiogenesis assay
Matrigel plug assay was performed as described previously with modification (Yeh et al., 2001). Five hundred microliters of matrigel supplemented with bFGF (500 ng/mL) in the presence of absence of r-viridistatin 2 and rmojastin 1 (22 µM) was injected subcutaneously into the dorsal region of BALB/c mice (weight 18–20 g). After 15 days, matrigel plugs were removed, dissected free from adherent tissue, weighed, and homogenized for hemoglobin quantification.
2.8. Hemoglobin measurement
Hemoglobin was quantified by a colorimetric method as described by Higuchi et al. (2011). Matrigel plug was homogenized in 2 mL Drabkin reagent (Sigma–Aldrich, MO, USA) and centrifuged at 10,000 × g for 15 min. The supernatants were obtained and the hemoglobin in the samples was determined spectrophotometrically at 540 nm. The amount of hemoglobin was calculated from a known amount used as standard assayed in parallel. The results were expressed as µg Hb mg−1 of wet tissue.
2.9. Binding of soluble integrins αvβ3and αvβ5 to immobilized disintegrins
In order to evaluate the interaction of disintegrins (r-mojastin 1 or r-viridistatin 2) with soluble integrins αvβ3 or αvβ5 a modified method of Wang (2010)was used. The microtiter plates (96-well) were coated with 100 µL of rmojastin 1 or r-viridistatin 2 at various concentrations in PBS, pH 7.4 at 4 °C for 18 h. After washing three times with washing buffer (PBS buffer containing 0.05% Tween-20), wells were blocked with 1% (w/v) BSA in PBS containing 0.05% Tween (PBS-T) for 1 h at room temperature. The plates were washed with washing buffer and followed by addition of 100 µL of soluble integrins, αvβ3 or αvβ5 (20 µg/mL), in 0.5% SA in PBS-T. After 2 h incubation, mouse anti-integrin αvβ3 or mouse anti-integrin αvβ5 monoclonal antibody (10 µg/mL) (R&D System, USA)was added and incubated for 1 h at room temperature. After the washing step, 100 µL/well of 1:1500 diluted horseradish peroxidase (HRP)-conjugated goat antimouse IgG (KPL, MD, USA) was added and incubated for 1 h. A final wash was performed and 100 µL/well of TMB substrate solution (0.2 g/L 3,3′,5,5′-tetramethylbenzidine and 0.01%H2O2 in citric acid buffer; KPL)was added. The reaction was stopped with 100 µL/well of TMB stop solution (KPL), and the absorbance was measured in a microplate reader (Beckman Coulter model AD 340) at 450 nm. Commercial echistatin (Sigma–Aldrich,MO, USA), a disintegrin that binds with a high affinity to integrin αvβ3, was used as the positive control. The error bars represent the standard deviations.
2.10. Statistical analyses
Results of proliferation and adhesion were expressed as the mean ± standard deviation (n = 3), and analyzed using the two-tailed t-test, using the software program Graph Pad Prism. A one way-analysis of variance test followed by Newman–Keuls Multiple Comparison Test was used to determine the significance of recombinant disintegrins (r-viridistatin 2 and r-mojastin 1) and the control in inhibiting the migration and angiogenesis in vivo. Differences were statistically significant if p value was less than 0.05. Experiments were performed in triplicate.
3. Results
3.1. HUVECs proliferation studies
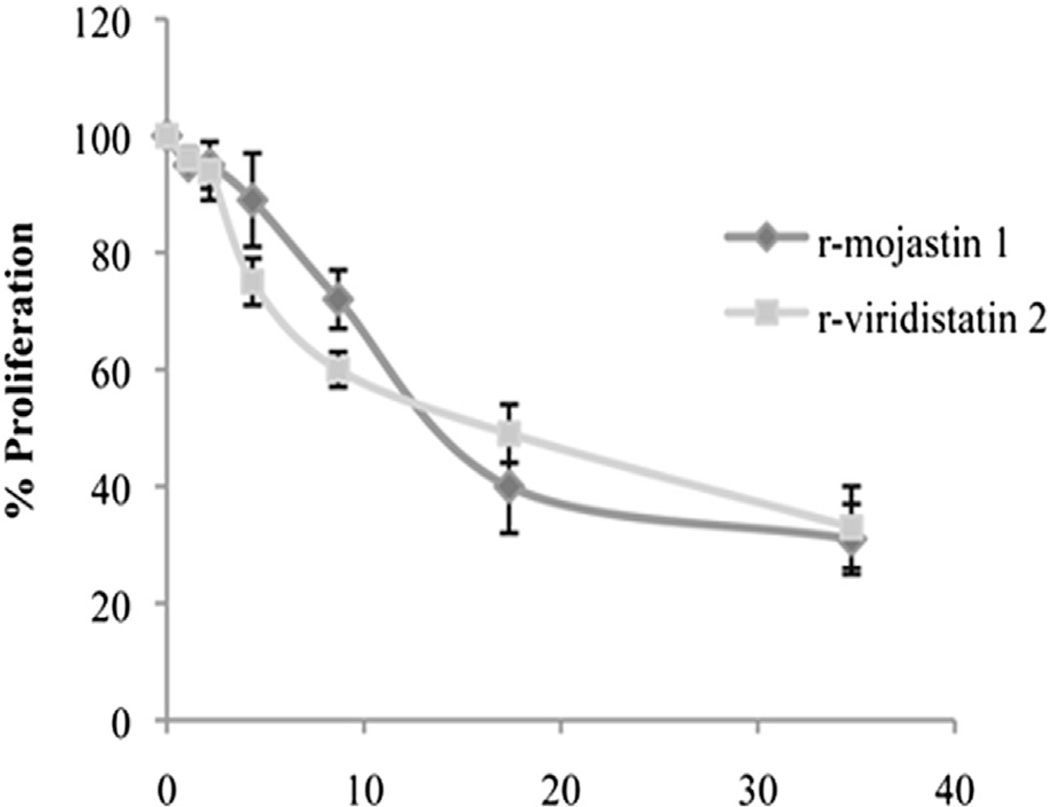
In tumor angiogenesis, endothelial cells proliferate to provide the necessary number of cells for making a new vessel. The results showed that r-viridistatin 2 and r-mojastin 1 dose-dependently inhibited HUVEC cell proliferation with CC50s of 16.2 and 15.6 µM, respectively (Fig. 1). Doxorubicin (85 µM) used as positive control decreased cell proliferation by 55% (data not shown). No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p > 0.05).
Fig. 1.
Proliferation of HUVECs in presence of r-viridistatin 2 and r-mojastin 1. Proliferation was evaluated by incubating 5 × 104 cells with the recombinant disintegrins at various concentrations for 24 h. Cell proliferation was measured using MTT assay. The negative control consisted of cells treated with PBS buffer, pH 7.4. The results are expressed as the percentage of cell proliferation relative to the negative control. The results are expressed as mean ± SD (n = 3).
3.2. Inhibition of cell adhesion in presence of fibronectin
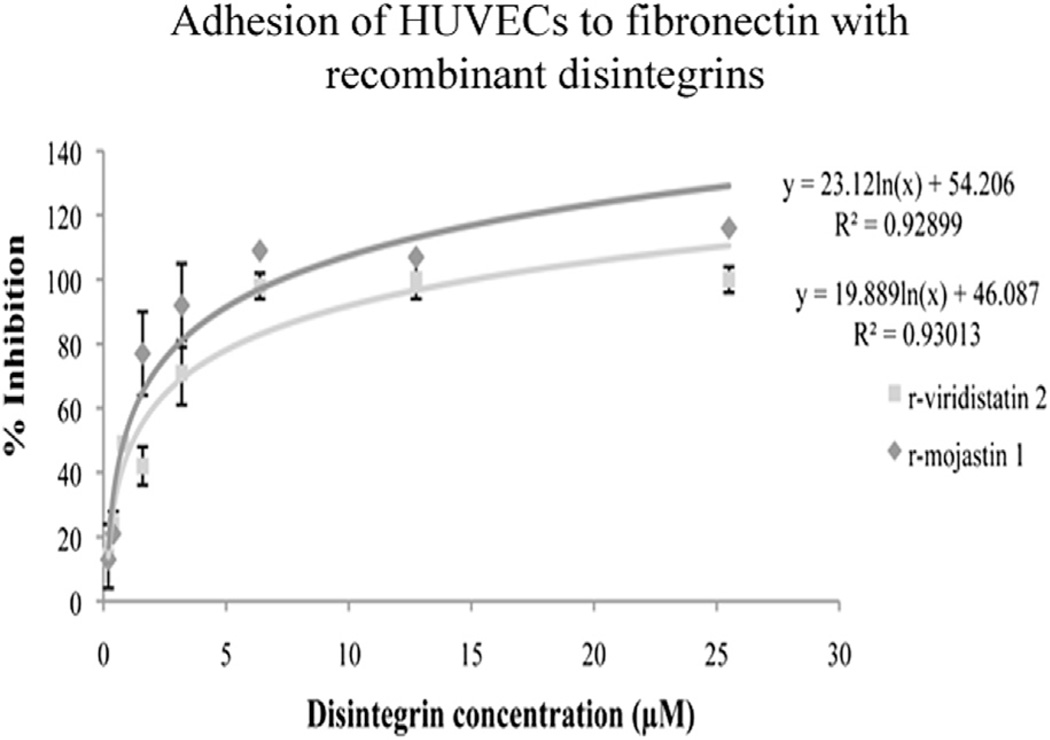
Endothelial cell–matrix contacts play important roles in endothelial cell biology, crucial for the proper functioning of blood vessels, such as endothelial cell growth and survival (Eming and Hubbell, 2011). Recombinant viridistatin 2 and recombinant mojastin 1 inhibited HUVEC adhesion to immobilized fibronectin, in a concentration dependent manner with IC50 values of 1.22 and 0.83 µM, respectively (Fig. 2). No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p > 0.05).
Fig. 2.
Adhesion of HUVECs to fibronectin in presence of r-viridistatin 2 and r-mojastin 1. HUVECs were seeded in 96-well plates in the presence of increasing concentrations of r-viridistatin 2 and r-mojastin 1. Cell adhesion was measured by MTT technique and the results were expressed as % of inhibition. The results are expressed as mean ± SD (n = 3).
3.3. Inhibition of migration
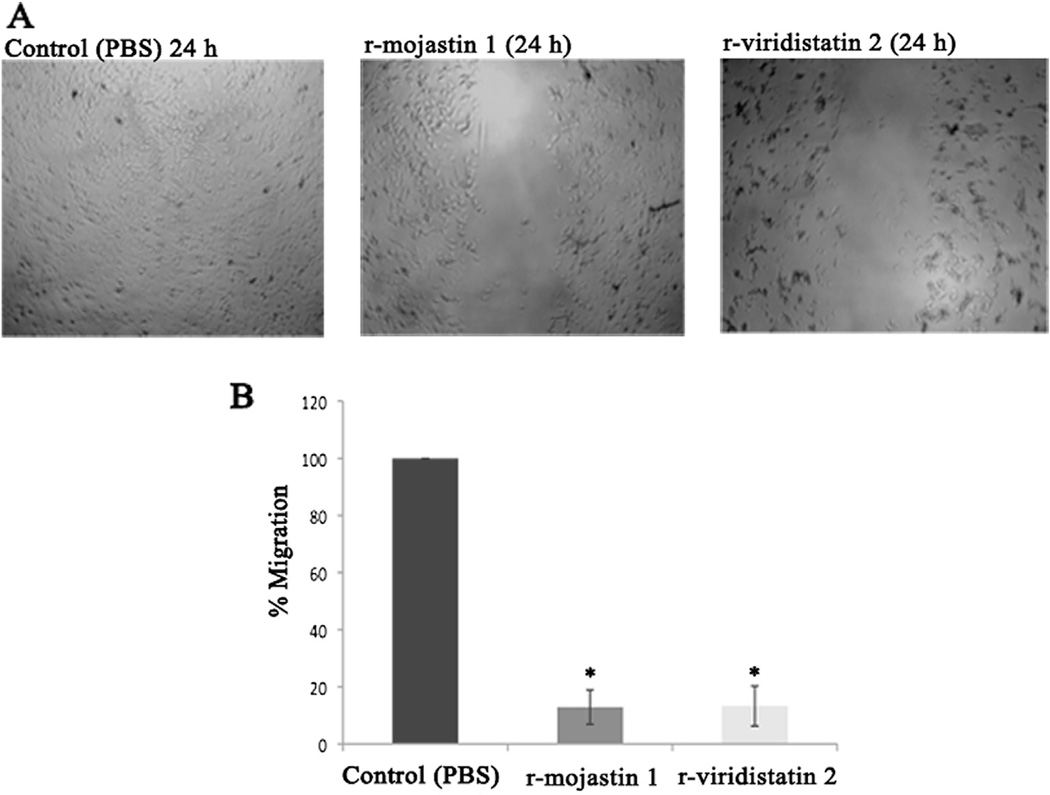
Endothelial cell migration through extracellular matrix is one of the essential responses in angiogenesis (Mahabeleshwar and Byzova, 2008). After 24 h of incubation, r-viridistatin 2 and r-mojastin 1 allowed HUVECs to migrate by 13.3 and 12.9%, respectively, in contrast to PBS used as negative control, which allowed HUVECs tomigrate by 100% (Fig. 3A and B).No statistical difference among r-viridistatin 2 and r-mojastin 1 was observed (p > 0.05).
Fig. 3.
Migration of HUVECs in presence of r-viridistatin 2 and r-mojastin 1 (A & B). HUVECs (9 × 104 cells/mL) were seeded in 48-well plates in the presence of r-viridistatin 2 and r-mojastin 1 at 6.3 µM for 24 h. The negative control consisted of cells treated with PBS buffer, pH 7.4. The results are expressed as the percentage of cell migration relative to the negative control. The results are expressed as mean ± SD (n = 3). *p < 0.05 when compared to control (PBS).
3.4. Inhibition of HUVEC tube formation
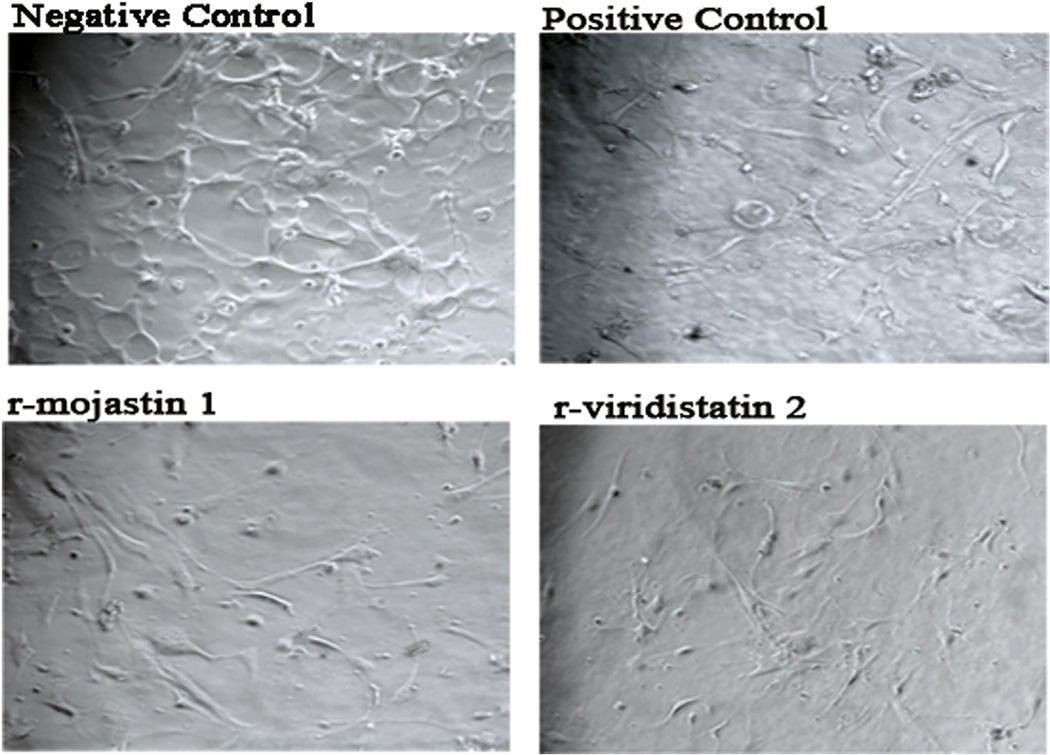
Angiogenesis is modulated overall by the specific interactions of endothelial cells with a variety of adhesion molecules and cytokines. To examine the effect of r-viridistatin 2 and r-mojastin 1 on HUVEC differentiation into vascular structures in vitro, a matrigel-based tube formation assay was performed. As shown in Fig. 4, PBS-treated cells (negative control) displayed a network-like structure after seeding on matrigel. The incubation of HUVECs with 6.3 µM of recombinant disintegrins significantly suppressed the bFGF-stimulated formation of tube-like structures, comparable with the effect produced by suramin at 100 µM (positive control), an inhibitor of tube formation.
Fig. 4.
Effect of r-viridistatin 2 and r-mojastin 1 on bFGF-induced HUVEC tube formation in diluted Matrigel. HUVECs (1 ×106 cells/mL) were seeded on diluted matrigel with bFGF (30 ng/mL) in the presence of r-viridistatin 2 and r-mojastin 1 at 6.3 µM and incubated for 24 h at 37 °C. HUVECs incubated with PBS were used as negative control. Suramin at 100 µM, a known tube formation inhibitor, was used as a positive control. At the end of the incubation time, cells were imaged by confocal microscopy (magnification, ×10).
3.5. In vivo bFGF-induced angiogenesis
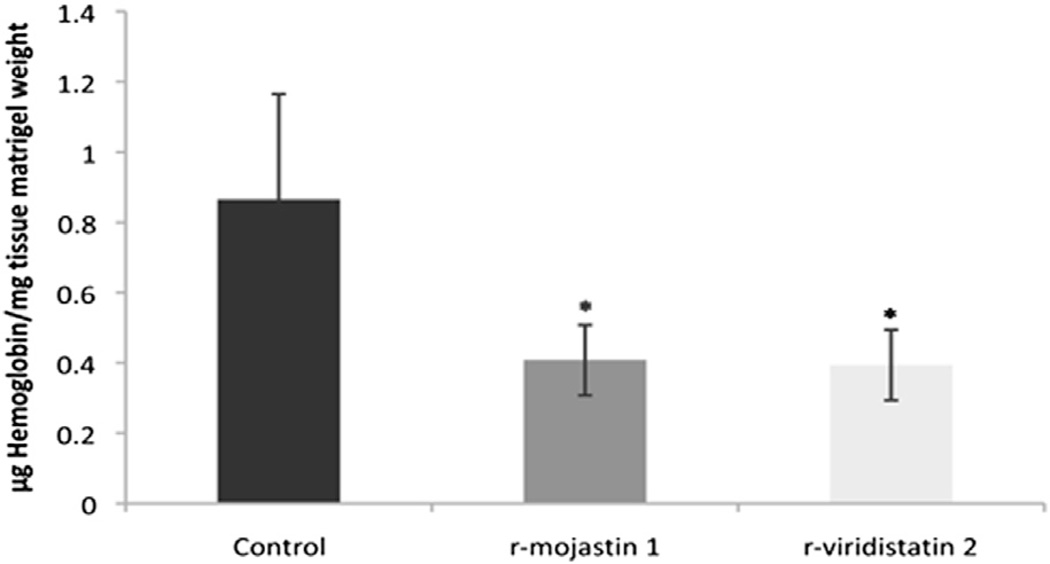
Several in vivo assay systems, including the Matrigel Plug angiogenesis assay, have been developed to permit a more realistic appraisal of the angiogenic response. Since bFGF has been recognized as an important angiogenic factor (Huang et al., 2001), in vivo angiogenic reactions were induced by the subcutaneous injection of matrigel containing bFGF. Effects of r-viridistatin 2 and r-mojastin 1 on angiogenesis were examined by measuring the hemoglobin content in the matrigel. r-Viridistatin 2 and r-mojastin 1 at 22 µM suppress the in vivo bFGF-induced angiogenesis by 55 and 53%, respectively (Fig. 5). No statistical difference among rviridistatin 2 and r-mojastin 1 was observed (p > 0.05).
Fig. 5.
Angiogenesis level in implanted matrigel evaluated in the 15th day after beginning of treatment with PBS (control) or with r-viridistatin 2 and rmojastin 1 at 22 µM. Hemoglobin levels were determined colorimetrically and expressed as microgram per milligram of wet weight of tissue. Results represent the mean ± SEM of five animals for each group. *p < 0.05 when compared to control (PBS).
3.6. Binding of recombinant disintegrins to integrins
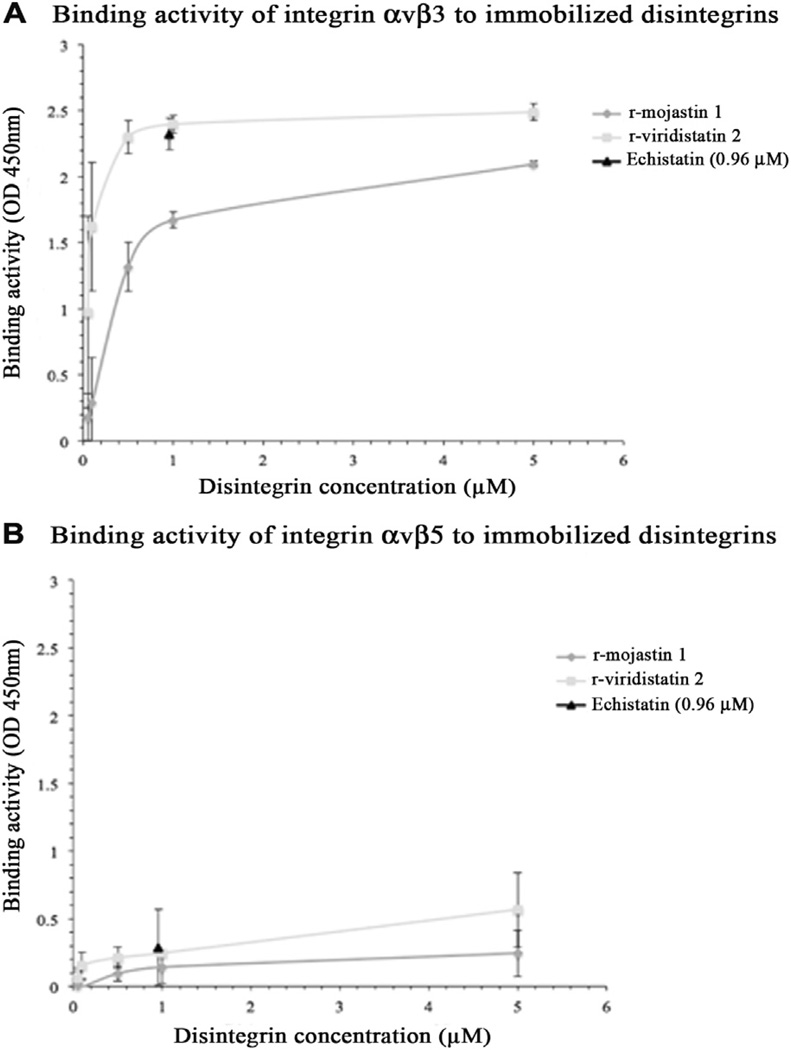
The purified αvβ3 and αvβ5 integrins bound to immobilized r-mojastin 1 and r-viridistatin 2 in a concentration dependent manner, the binding activity was significantly more potent in presence of αvβ3 integrin (Fig. 6). r-Viridistatin 2 showed a greater binding capacity to both integrins αvβ3 and αvβ5 when compare with r-mojastin 1.
Fig. 6.
Interaction of immobilized r-disintegrins with A) integrin αvβ3 and B) integrin αvβ5. A 96-well plate was coated with various concentrations of rmojastin 1 or r-viridistatin 2. After blocking with 1% BSA in PBS-T for 1 h, soluble integrins αvβ3 or αvβ5 (2 µg) were added to each well and the plates were incubated for additional 2 h at room temperature. After washing with PBS-T, anti-αvβ3 or anti-αvβ5 was used as the primary antibody. After 1 h incubation at room temperature and subsequent washing, an HRP conjugated-goat anti-mouse IgG was used as the secondary antibody. The color was developed by TMB substrate solution. Absorbance at 450 nm of the individual well was measured to determine the binding activity. The disintegrin echistatin was used as a positive control and prepared by coating the plate with 0.96 µM echistatin instead of r-mojastin 1 and r-viridistatin 2. The error bars represent the standard deviation from two independent experiments.
4. Discussion
Many physiological and pathological conditions, such as development, tumor growth, inflammation, and tissue repair, induce a neovascularization response in order to cope with the increased oxygen and nutrient demand of the tissue (Van Hinsbergh and Koolwijik, 2008). As previously described, angiogenesis is a multistep process, which offers a variety of targets for therapeutic interventions. Thus, great efforts are being made to develop corresponding drugs. These developments are focused on growth receptor antagonists, metalloproteinase inhibitors, adhesion antagonist, and endothelial cell function antagonists (Haubner, 2008; Verheul and Pinedo, 2007).
Endothelial cell integrins as regulators of tumor angiogenesis are of great importance. Given the central role of integrins in angiogenesis and that growth of solid tumors is dependent on neovascularization, these molecules provide a highly attractive target for antiangiogenic therapy. Of the 30 or more angiogenesis inhibitors in clinical trials for the treatment of cancer, the majority target endothelial cells, with a major subset targeting αvβ3 and αvβ5 integrins, both of which are highly expressed on activated endothelial cells (Silva et al., 2008).
Snake venom disintegrins are present in a variety of species and are functionally divided into three families: RGD, MLD and R/KTS. The RGD family of disintegrins, which bind and inhibit the physiological functions of RGDdependent integrins, constitute the largest and most investigated family (Walsh and Marcinkiewicz, 2011). RGD-reacting integrins are αv integrins (vitronectin receptors), αIIbβ3 (fibrinogen receptor) and α5β1 (fibronectin receptor). Selectivity for RGD-disintegrins to bind αIIbβ3, αvβ3 or α5β1 is determined by the amino acid adjacent to the active motif on the C-terminus. The diversity of the integrinbinding loop, along with other conserved residues among disintegrin molecules, gives rise to different integrin specificities. Therefore, despite the similarities of most disintegrins, they have significant differences in biological activities (Momic et al., 2011; Selistre-de-Araujo et al., 2010). In this study, we reported the activity of r-viridistain 2 and r-mojastin 1 on different steps of angiogenesis, showing that both disintegrins had similar potent inhibitory effect on the pro-angiogenic activities of endothelial cells such as proliferation, adhesion, migration, including in vitro and in vivo angiogenesis.
Unlike in stationary endothelial cells (EC), the αvβ3 integrin is highly expressed by tumor endothelial cells, which helps the binding of new ECs to the provisional matrix components, including vitronectin and fibronectin that are deposited in the tumor microenvironment (Selistre-de-Araujo et al., 2010). Integrin αvβ5 is another receptor found in EC, and known to be dramatically upregulated on endothelial cells during angiogenesis (Eming and Hubbell, 2011). In order to evaluate the possible(s) integrin target(s) for r-mojastin 1 and r-viridistatin 2, binding assays were performed. Our results showed that both recombinant disintegrins bind to αvβ3 integrin, with a higher binding capacity revealed for r-viridistatin 2 (Fig. 6). These data are consistent with previous studies by Scarborough et al. (1993), where they tested the ability of different RGDNP and RGDW peptides to inhibit the binding of vitronectin to αvβ3 integrin; their results showed that RGDNP-containing peptides inhibited the binding of vitronectin to αvβ3 integrin more effectively than the RGDW-containing peptides. r-Viridistatin 2 in our study is a monomeric, medium size disintegrin with 73 amino acids, and an RGDNP-motif region derived from the Prairie rattlesnake (Lucena et al., 2012). r-Mojastin 1 is a monomeric, medium size disintegrin with 71 amino acids, twelve cysteines and an RGDW-motif region derived from the Mohave rattlesnake (Sánchez et al., 2010) (Fig. 7). Both recombinant disintegrins bind weakly to αvβ5 integrin. Considering that r-mojastin 1 and r-viridistatin 2 bind to both receptors, it is probable that r-mojastin 1 and r-viridistatin 2 recognize αvβ3 and αvβ5 integrins blocking EC functions.
Fig. 7.

Comparison of deduced amino acid sequences between r-viridistatin 2 and r-mojastin 1. The gray shaded areas indicate sequence similarities.
These activities of r-viridistatin 2 and r-mojastin 1 were similar to the activities reported for other disintegrins. For instance, triflavin, from Trimeresus flavoridis venom, was one of the first RGD-disintegrins shown to inhibit angiogenesis both in vitro and in vivo. Triflavin strongly inhibited EC migration and TNF-α-induced angiogenesis in the chicken chorioallantoic membrane (CAM) assay (Sheu et al., 1997). Vicrostatin (VCN), a chimeric recombinant disintegrin generated as a genetic fusion between the C-terminal tail of a viperid disintegrin (echistatin) and crotalid disintegrin (contortrostatin), exerts a potent inhibitory effect on EC migration and tube formation in a dose-dependent manner by forcing these cells to undergo significant actin cytoskeleton reorganization and by inducing apoptosis of EC (Minea et al., 2012, 2010). Leucorogin, is another new recombinant disintegrin cloned from Bothrops leucurus, that inhibited in a dose-dependent manner, the vascularization process evaluated by the sponge implant model (Higuchi et al., 2011). Acurhagin-C, an ECD disintegrin from Agkistrodon acutus venom, inhibited EC adhesion and spreading on extracellular matrix proteins, followed by damage to cell functions and finally eliciting anoikis (Wang, 2010). Other examples of disintegrins with anti-angiogenic activities are rhodostomin, agkistin-s, r-adinbitor, r-contortrostatin, accutin and DisBa-01 (Ramos et al., 2008; Ren et al., 2006; Minea et al., 2005; Wang et al., 2004; Yeh et al., 2001, 1998).
Specific inhibitors of angiogenesis have been identified and developed to block tumor growth and metastasis formation using preclinical in vitro and in vivo models (Cook and Figg, 2010). Under normal physiological circumstances, more than 99% of endothelial cells are quiescent. In healthy adults, angiogenesis is only promoted during wound healing and the menstrual cycle (Giuliano and Pagès, 2013; Verheul and Pinedo, 2007). It was proposed that tumor-stimulated endothelial cells have a unique proliferation and migration phenotype compared with quiescent endothelial cells and that targeting this phenotype would be so specific that no major side effects could occur, except for during wound healing and the menstrual cycle. However, recent clinical studies have changed these expectations (Cook and Figg, 2010; Verheul and Pinedo, 2007). Furthermore, treatment with anti-angiogenesis drugs, despite disease stabilization and an increase of progression free survival, gives rise to tumors that often become resistant, and consequently patient relapse occurs (Giuliano and Pagès, 2013).
The toxicities and resistances found in the antiangiogenesis therapies, emphasize the fact that angiogenesis is a very complicated multi-factorial biological process that involves various pathways in the body. In this context, the discovery of new components that can enhance the efficiency of anti-angiogenesis therapy is necessary. The results presented in this work clearly demonstrated that r-viridistatin 2 and r-mojastin 1 can inhibit bFGF-elicited angiogenesis in vivo and in vitro. Because metastasis is highly dependent on angiogenesis, r-viridistatin 2 and r-mojastin 1 may represent novel prototypes in the design of more effective strategies that will lead to improved therapeutics for cancer patients.
Acknowledgments
Funding for this project was provided by NCRR/BMRG, Viper Resource Grant #s 8P40OD01960-10 and 3P40OD01096—10S1 (NNTRC, Texas A&M University- Kingsville, Dr. Sánchez), TAMUK Council for Undergraduate Research (2011–2012; Karen Romo), and the Robert A. Welch Foundation Department, grant # AC-0006 (TAMUK-Department of Chemistry). We would also like to thank Nora Diaz De Leon and Mark Hockmuller (NNTRC serpentarium curator) and all the NNTRC personnel.
Footnotes
Conflict of interests
Authors declare no conflict of interests.
Animal Welfare
All animal experiments were approved by IACUC under protocol # 2012-12-18-A1.
References
- Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Cook KM, Figg WD. Angiogenesis inhibitors – current strategies and future prospects. CA Cancer J. Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Hubbell JA. Extracellular matrix in angiogenesis: dynamic structures with translational potential. Exp. Dermatol. 2011;20:605–613. doi: 10.1111/j.1600-0625.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Geiger TR, Peeper DS. Metastasis mechanisms. Biochim. Biophys. Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Giuliano S, Pagès G. Mechanisms of resistance to antiangiogenesis therapies. Biochimie. 2013;95:1110–1119. doi: 10.1016/j.biochi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Haubner R. Noninvasive tracer techniques to characterize angiogenesis. Handbook Exp. Pharmacol. 2008;185:323–339. doi: 10.1007/978-3-540-77496-9_14. [DOI] [PubMed] [Google Scholar]
- Higuchi DA, Almeida MC, Barros CC, Sanchez EF, Pesquero PR, Lang EAS, Samaan M, Araujo RC, Pesquero JB, Pesquero JL. Leucurogin, a new recombinant disintegrin cloned from Bothrops leucurus (white-tailed-jararaca) with potent activity upon platelet aggregation and tumor growth. Toxicon. 2011;58:123–129. doi: 10.1016/j.toxicon.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Huang TF, Yeh CH, Wu WB. Viper venom components affecting angiogenesis. Haemostasis. 2001;31:192–206. doi: 10.1159/000048063. [DOI] [PubMed] [Google Scholar]
- Juliano D, Wang Y, Marcinkiewicz C, Rosenthal LA, Stewart GJ, Niewiarowski S. Disintegrin interaction with αvβ3 integrin on human umbilical vein endothelial cells: expression of ligand-induced binding site of β3 subunit. Exp. Cell Res. 1996;225:132–142. doi: 10.1006/excr.1996.0164. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. J. Biol. Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Lucena SE, Jing Yia, Soto JG, Parral J, Cantu E, Brannon J, Lardner K, Ramos CJ, Seoane AI, Sánchez EE. Anti-metastatic activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60:31–39. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena S, Sánchez EE, Perez JC. Anti-metastatic activity of the recombinant disintegrin, r-mojastin 1, from the Mohave rattlesnake. Toxicon. 2011;57:794–802. doi: 10.1016/j.toxicon.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Byzova T. Vascular integrin signaling. Methods Enzymol. 2008;443:199–226. doi: 10.1016/S0076-6879(08)02011-9. [DOI] [PubMed] [Google Scholar]
- Minea R, Helchowski C, Rubino B, Brodmann K, Swenson S, Markland F., Jr Development of a chimeric recombinant disintegrin as a cost-effective anti-cancer agent with promising translational potential. Toxicon. 2012;59:472–486. doi: 10.1016/j.toxicon.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minea RO, Helchowski CM, Zidovetzki SJ, Costa FK, Swenson SD, Markland FS. Vicrostatin – an anti-invasive multi-integrin targeting chimeric disintegrin with tumor anti-angiogenic and proapoptotic activities. PloS ONE. 2010;5:e10929. doi: 10.1371/journal.pone.0010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minea R, Swenson S, Costa F, Chen TC, Markland FS. Development of a novel recombinant disintegrin, contortrostatin, as an effective anti-tumor and anti-angiogenic agent. Pathophysiol. Haemost. Thromb. 2005;34:177–183. doi: 10.1159/000092419. [DOI] [PubMed] [Google Scholar]
- Momic T, Arlinghaus FT, Arien-Zakay H, Katzhendler J, Eble JA, Marcinkiewicz C, Lazarovici P. Pharmacological aspects of Vipera xantina palestinae venom. Toxins. 2011;3:1420–1432. doi: 10.3390/toxins3111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos OH, Kauskot A, Cominetti MR, Bechyne I, Salla Pontes CL, Chareyre F, Manent J, Vassy R, Giovannini M, Legrand C, Selistrede-Araujo HS, Crepin M, Bonnefoy A. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin. Exp. Metastasis. 2008;25:53–64. doi: 10.1007/s10585-007-9101-y. [DOI] [PubMed] [Google Scholar]
- Ren A, Wang S, Cai W, Yang G, Zhu Y, Wu X, Zhang Y. Agkistin-s, a disintegrin domain, inhibits angiogenesis and induces BAECs apoptosis. J. Cell. Biol. 2006;99:1517–1523. doi: 10.1002/jcb.20859. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, Guerrero B, Salazar AM, Rodríguez-Acosta A, Galán JA, Tao WA, Pérez JC. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the Mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb. Res. 2010;126:e211–e219. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough RM, Rose JW, Naughton MA, Phillips DR, Nannizzi L, Arfsten A, Campbell AM, Charo IF. Characterization of the integrin specificities of disintegrins isolated form American pit viper venoms. J. Biol. Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- Selistre-de-Araujo HS, Pontes CLS, Montenegro CF, Martin AC. Snake venom disintegrins and cell migration. Toxins. 2010;2:2606–2621. doi: 10.3390/toxins2112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JR, Yen MH, Kan YC, Hung WC, Chang PT, Luk HN. Inhibition of angiogenesis in vitro and in vivo: comparison of the relative activities of triflavin, an Arg-Gly-Asp-containing peptide and anti-alpha(v)beta3 integrin monoclonal antibody. Biochim. Biophys. Acta. 1997;1336:445–454. doi: 10.1016/s0304-4165(97)00057-3. [DOI] [PubMed] [Google Scholar]
- Silva R, D’ Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- Swenson S, Ramu S, Markland FS. Anti-angiogenesis and RGD-containing snake venom disintegrins. Curr. Pharm. Des. 2007;13:2860–2871. doi: 10.2174/138161207782023793. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh VWM, Koolwijik P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc. Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Verheul HMW, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nature. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- Walsh EM, Marcinkiewicz C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon. 2011;58:355–362. doi: 10.1016/j.toxicon.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Wang JH, Wu Y, Ren F, Lu L, Zhao B-C. Cloning and characterization of adinbitor, a novel disintegrin from the snake venom of Agkistrodon halys brevicaudus stejneger. Acta Biochim. Biophys. Sin. 2004;36:425–429. doi: 10.1093/abbs/36.6.425. [DOI] [PubMed] [Google Scholar]
- Wang WJ. Acurhagin-C, an ECD disintegrin, inhibits integrin αvβ3-mediated human endothelial cell functions by inducing apoptosis via caspase-3 activation. Br. J. Pharmacol. 2010;160:1338–1351. doi: 10.1111/j.1476-5381.2010.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Huang TF. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin αvβ3 antagonist and inducing apoptosis. Blood. 1998;92:3268–3276. [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Yang RS, Huang TF. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis elicited by basic fibroblast growth factor and suppresses tumor growth by a selective αvβ3 blockade of endothelial cells. Mol. Pharmacol. 2001;59:1333–1342. doi: 10.1124/mol.59.5.1333. [DOI] [PubMed] [Google Scholar]
- You WK, Seo HJ, Chung KH, Kim DS. A novel metalloprotease from Gloydius halys venom induces endothelial cell apoptosis through its protease and disintegrin-like domains. J. Biochem. 2003;134:739–749. doi: 10.1093/jb/mvg202. [DOI] [PubMed] [Google Scholar]