Abstract
Introduction
Staphylococcus aureus bacteremia is associated with significant morbidity and mortality. Given the paucity of recent Canadian data, we estimated the mortality rate associated with S. aureus bacteremia in a tertiary care hospital and identified risk factors associated with mortality.
Methods
We retrospectively reviewed the records of adults with S. aureus bacteremia admitted to a tertiary care centre in southwestern Ontario between 2008 and 2012. Cox regression analysis was used to evaluate associations between predictor variables and all-cause, in-hospital, and 90-day postdischarge mortality.
Results
Of the 925 patients involved in the study, 196 (21.2%) died in hospital and 62 (6.7%) died within 90 days after discharge. Risk factors associated with in-hospital and all-cause mortality included age, sepsis (adjusted hazard ratio [adjusted HR] 1.49, 95% confidence interval [CI] 1.08–2.06, p = 0.02), admission to the intensive care unit (adjusted HR 3.78, 95% CI 2.85–5.02, p < 0.0001), hepatic failure (adjusted HR 3.36, 95% CI 1.91–5.90, p < 0.0001) and metastatic cancer (adjusted HR 2.58, 95% CI 1.77–3.75, p < 0.0001). Methicillin resistance, hepatic failure, cerebrovascular disease, chronic obstructive pulmonary disease and metastatic cancer were associated with postdischarge mortality.
Interpretation
The all-cause mortality rate in our cohort was 27.9%. Identification of predictors of mortality may guide empiric therapy and provide prognostic clarity for patients with S. aureus bacteremia.
Staphylococcus aureus is a versatile and virulent pathogen with the ability to cause many life-threatening illnesses, including necrotizing soft-tissue infections,1 infective endocarditis2,3 and sepsis.4 Staphylococcus aureus is also one of the leading causes of health care associated and hospital-acquired infections,1,5,6 and the rates of infections are increasing steadily, particularly in North America and Europe.5–8
Staphylococcus aureus bacteremia is particularly associated with significant morbidity and mortality,9 and demands rigorous management to prevent further infectious complications, such as infective endocarditis,2,3 vertebral osteomyelitis,10 embolic stroke,9 recurrent infection and metastatic disease. Despite the availability of treatment guidelines11,12 and scoring systems to estimate the likelihood of developing complications,9 mortality from S. aureus bacteremia remains approximately 20%.1,9,12 Many studies from the previous decade6,8,13–16 also demonstrated the increasing prevalence of strains of methicillin-resistant S. aureus (MRSA) within the community and in the hospital, leading to changes in the empiric treatment of S. aureus bacteremia.11
A lack of recent Canadian data on the clinical burden of S. aureus bacteremia may preclude effective prognostication of patients with this illness. Moreover, few studies have examined predictors of mortality after discharge from hospital. This is particularly important for follow-up and management, given the increasing number of patients who are treated for bacteremia and discharged from hospital. The objectives of our current single-centre retrospective cohort study were to estimate the mortality rate associated with S. aureus bacteremia and identify risk factors associated with mortality.
Methods
The present study was approved by the Western University Research Ethics Board (London, Ontario) and the Lawson Health Research Institute (approval No. R-13-350).
Setting
This study was conducted at the London Health Sciences Centre, London, a tertiary care hospital system with 2 academic hospitals in southwestern Ontario that serve a metropolitan population of approximately 435 000.17 In addition to serving the local population, London Health Sciences Centre also receives referrals from 33 rural hospitals in 7 counties, covering a catchment area that spans 21 000 km2 and a population of 2 million.17
Population
All adult (≥ 18 yr) patients with a laboratory-confirmed diagnosis of S. aureus bacteremia between Jan. 1, 2008, and Dec. 31, 2012, were included initially in the study. Staphylococcus aureus bacteremia was defined as occurring in a patient with at least one blood culture result positive for S. aureus. Patients with complicated S aureus bacteremia were defined as having a site of infection remote from the primary focus that was caused by hematogenous seeding (e.g., infective endocarditis or vertebral osteomyelitis) or extension of infection beyond the primary focus (e.g., septic thrombophlebitis or abscess), or recurrent S. aureus bacteremia (defined as a positive culture result obtained from the same case within 12 weeks after the initial culture). All cases of complicated infection were independently defined by radiologic imaging, a positive culture test result for S. aureus from an otherwise normally sterile site or the use of validated diagnostic criteria.9,11,12 Sepsis, severe sepsis, septic shock, soft tissue source and intravascular catheter source were defined according to standard methods.12,18,19 Patients with uncomplicated S. aureus bacteremia exhibited no evidence of complicated or recurrent infection.
Comorbidity was defined as a disease or therapy that could predispose patients to infection, alter defence mechanisms or cause functional impairment, such as coronary artery disease, severe cardiac disease with symptomatic heart failure, peripheral vascular disease, cerebrovascular disease, dementia, severe chronic obstructive pulmonary disease (COPD), connective tissue disease, peptic ulcer disease, liver disease, diabetes, renal disease, active neoplastic disease and HIV infection. Operative intervention was defined as a procedure requiring general anesthesia performed by a surgeon or interventional radiologist in the operating room or angiography suite. Bedside procedures such as upper and lower endoscopy, percutaneous tracheostomy and abscess drains were not considered operations for this study.
Reasons for excluding patients from the study are indicated in Figure 1 and included polymicrobial infection (a positive blood culture result for more than one pathogen), age younger than 18 years, death before the return of a positive culture result (to avoid confounding the analysis for mortality), patients who were not admitted to hospital or left against medical advice, and hospital records with incomplete or missing data.
Figure 1:
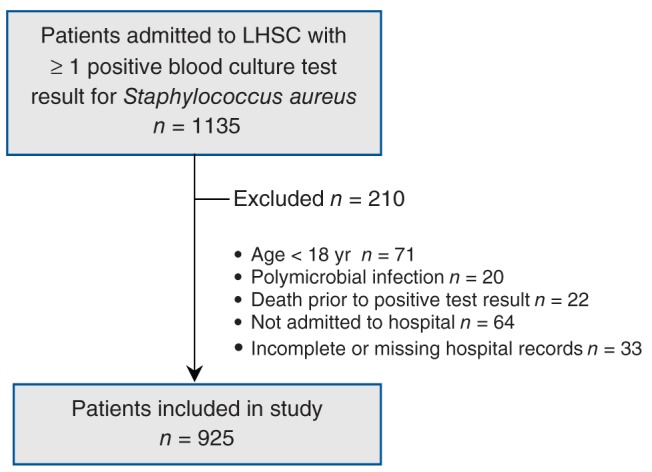
Selection of adult patients admitted to LHSC with S. aureus bacteremia. LHSC = London Health Sciences Centre, London, Ont.
Study design
This was a single-centre retrospective cohort study. Six months after the study period (August 2013), we manually reviewed electronic and paper charts of the patients in the study and recorded data using a standard extraction form. We collected information about demographic characteristics, source of infection, baseline comorbidities, course of illness and outcomes. Chart abstraction was performed separately by 3 reviewers, who each reviewed 40% of the charts: the Cohen’s κ statistic for the coding of categorical and qualitative variables was 0.82, showing excellent interrater reliability. For mortality data, patient information was linked retrospectively to the vital statistics record for Ontario for the period 2008–2012, using the provincial health number or the name and date of birth. All available data were recorded, whereas missing data for key variables were coded as missing and excluded from the analysis; each variable had less than 0.5% of data that was missing.
Outcome measures
Our primary outcome was all-cause mortality, defined as occurring within the hospital or within 90 days after discharge from hospital. Secondary outcomes included attributable in-hospital mortality,9 defined as patients who died with persistent signs or symptoms of systemic infection, positive blood culture results or a persistent focus of infection in the absence of another explanation for death during their hospital admission for S. aureus bacteremia, and postdischarge mortality (0–30 d, 31–90 d, and after 90 d).
Statistical analysis
Descriptive statistics of patient variables were calculated for all patients with S. aureus bacteremia. Continuous data were expressed as median (interquartile range [IQR] 25th–75th percentile), and categorical variables were reported as frequencies and percentages. The significance of trends in MRSA infections, the proportion of people who inject drugs, and in-hospital mortality was assessed with the Cochran–Armitage χ2 test for trend.
Univariable logistic regression was used to evaluate the association between mortality (all-cause, in-hospital and postdischarge) and clinically relevant patient characteristics that were selected based on published studies.18,20–23 For patients with recurrent infections, only the first episode of infection was included in the univariable and multivariable analyses to preserve the assumption of independence of observations and to ensure that each individual contributed only once to analyses. Variables that were significantly associated (p < 0.1) with the primary or secondary outcomes were included in 3 multivariable logistic regression models obtained using stepwise regression. The significance levels for variables entering into and remaining in the models were set at 0.05 and 0.10, respectively. Diagnostic testing for collinearity across the selected variables showed that no variance inflation factor was greater than 5.0. Odds ratios (ORs) and Wald 95% confidence intervals (CIs) were computed. Calibration of each multivariable model was assessed using the Hosmer−Lemeshow goodness-of-fit test24 to evaluate whether significant discrepancies existed between the observed and expected mortality.
A Kaplan−Meier survival curve was generated for unadjusted survival during the hospital stay and for up to 90 days postdischarge using right-censored data. We also performed a multivariable Cox proportional-hazards analysis to evaluate the association between risk factors and survival time, stratified by the patient-level variables that were statistically significant in our analyses. Models were developed for each outcome (all-cause, in-hospital and 90-day postdischarge mortality) in a stepwise fashion. All variables in the models were found to satisfy the proportional hazards assumption. All tests were 2-tailed, and a p value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using SAS, version 9.3 (SAS Institute Inc.).
Results
We identified 925 patients who met our inclusion criteria and were admitted to London Health Sciences Centre for S. aureus bacteremia during the study period (Figure 1); their demographic characteristics are shown in Table 1. Of the 925 patients, 585 (63.2%) were male and 163 (17.6%) were people who inject drugs. Staphylococcus aureus bacteremia caused by MRSA developed in 362 (39.1%) patients, whereas sepsis, severe sepsis or septic shock developed in 642 (69.4%) patients in the study. In addition, 109 patients (11.8%) had pneumonia, whereas 175 patients (18.9%) had a soft-tissue infection, and 53 patients (5.8%) had an intravascular catheter as a source of their infection.
Table 1: Characteristics of patients with Staphylococcus aureus bacteremia admitted to a regional tertiary care centre between 2008 and 2012 (n = 925).
| Characteristic | No. (%) of patients* |
|---|---|
| Median age, yr (range) |
65 (18–102) |
| Male gender |
585 (63.2) |
| Comorbidities |
|
| HIV positive or AIDS |
20 (2.2) |
| Intravenous drug use |
163 (17.6) |
| Diabetes mellitus |
250 (27.0) |
| Cardiac disease (coronary artery disease or chronic heart failure) |
244 (26.3) |
| Peripheral vascular disease |
70 (7.6) |
| Cerebrovascular disease |
137 (14.8) |
| Dementia |
53 (5.7) |
| COPD |
84 (9.1) |
| Liver disease |
172 (18.6) |
| Hepatitis B or C co-infection |
54 (5.8) |
| Cirrhosis |
30 (3.2) |
| Pre-existing renal disease |
175 (18.9) |
| Solid malignancy |
171 (18.5) |
| Leukemia and lymphoma |
31 (3.4) |
| Metastatic malignancy |
77 (8.3) |
| Infection characteristics |
|
| MRSA |
362 (39.1) |
| Sepsis |
642 (69.4) |
| Dominant focus of infection |
|
| Central or peripheral intravascular catheter |
53 (5.8) |
| Pneumonia |
109 (11.8) |
| Skin and soft tissue infection |
175 (18.9) |
| Other† | 10 (1.1) |
Note: COPD = chronic obstructive pulmonary disease, MRSA = methicillin-resistant Staphylococcus aureus. *Unless otherwise indicated. †Includes foci that occur in less than 1% of cases each, such as central nervous system infection (n = 3), lung abscess (n = 3) and urinary tract infection (n = 4).
In this study, 189 patients (20.4%) were admitted with recurrent bacteremia (Table 2), and a complicated infection developed in 248 patients (26.8%) (Table 2). The most common complications were infective endocarditis, vertebral osteomyelitis, deep tissue infections, and epidural and psoas abscesses. During the course of the hospital stay, renal failure requiring dialysis developed in 80 patients (8.6%), prolonged mechanical ventilation lasting more than 21 days was needed by 58 patients (6.3%) and hepatic failure developed in 23 patients (2.5%). Clostridium difficile enterocolitis developed in 22 patients (2.4%) and ischemic colitis developed in 17 patients (1.8%). The median length of stay in hospital was 14 days, and a total of 225 patients (24.3%) were admitted to the intensive care unit (ICU) during their stay in hospital. In our study, 196 (21.1%) patients died in hospital and 62 (6.7%) patients died within 90 days after being discharged from the hospital.
Table 2: Clinical outcomes in patients with Staphylococcus aureus bacteremia admitted to a regional tertiary-care centre between 2008 and 2012 (n = 925).
| Outcome | No. (%) of patients |
|---|---|
| Recurrent S. aureus bacteremia infection |
189 (20.4) |
| One recurrence |
161 (17.4) |
| Two recurrences |
14 (1.5) |
| Three or more recurrences |
14 (1.5) |
| Complicated S. aureus bacteremia |
248 (26.8) |
| Infective endocarditis |
72 (7.8) |
| Septic arthritis |
28 (3.0) |
| Deep tissue abscess |
56 (6.0) |
| Osteomyelitis |
|
| Vertebral |
25 (2.7) |
| Nonvertebral (i.e., hand or foot) |
6 (0.6) |
| Epidural abscess |
22 (2.4) |
| Septic thrombophlebitis |
2 (0.2) |
| Psoas abscess |
12 (1.3) |
| Meningitis |
4 (0.4) |
| Embolic stroke |
8 (0.9) |
| Other complications* |
13 (1.4) |
| Complications during hospital stay |
|
| Operative intervention |
258 (27.9) |
| Renal failure requiring dialysis |
80 (8.6) |
| Hepatic failure |
23 (2.5) |
| Prolonged ventilation (> 21 d) |
58 (6.3) |
|
Clostridium difficile infection |
22 (2.4) |
| Ischemic colitis |
17 (1.8) |
| Myocardial infarction |
10 (1.1) |
| Admission to the intensive care unit |
225 (24.3) |
| All-cause mortality† |
258 (27.9) |
| In-hospital mortality |
196 (21.1) |
| Postdischarge mortality (0–30 d) |
30 (3.2) |
| Postdischarge mortality (31–90 d) |
32 (3.5) |
| Mortality after 90 d postdischarge | 94 (10.2) |
*Includes patients with mycotic aneurysm (n = 7), empyema (n = 4) and pericarditis (n = 2). †Defined as all mortalities that occurred in hospital and up to 90 days postdischarge.
We examined the trends in proportions of MRSA, people who inject drugs and in-hospital mortality throughout the study period. The proportion of MRSA strains isolated from patients with S. aureus bacteremia increased significantly from 40% in 2008 to 51% in 2012 (Figure 2; p = 0.045). The proportion of people who injected drugs in our study population also rose significantly during the study period (14% in 2008 to 22% of patients in 2012, p = 0.0034). In-hospital mortality, however, declined significantly from 29% in 2008 to 11% in 2012 (p < 0.0001; Figure 2).
Figure 2:
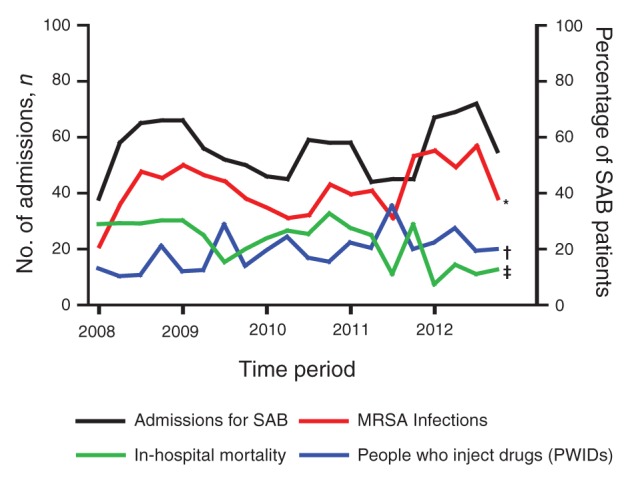
Quarterly admissions for patients with Staphylococcus aureus bacteremia at LHSC (2008−2012). LHSC = London Health Sciences Centre, London, Ont. *p < 0.05, †p < 0.01, ‡p < 0.001 by Cochran−Armitage χ2 test for trend.
When we performed univariable and multivariable logistic regression analyses on all-cause mortality (Appendix 1, available at www.cmajopen.ca/content/X/X/XXX/suppl/CD1, and Table 2, respectively), age, methicillin resistance, diagnosis of sepsis, admission to the ICU, development of hepatic failure and metastatic cancer were all independently associated with increased mortality. Operative intervention was associated with a reduced risk of all-cause mortality. These factors, with the exception of methicillin resistance, were also independently associated with increased in-hospital mortality (Appendix 1). The clinical variables independently associated with increased 90-day postdischarge mortality (Appendix 1) were methicillin resistance, hepatic failure, cerebrovascular disease, COPD and metastatic cancer.
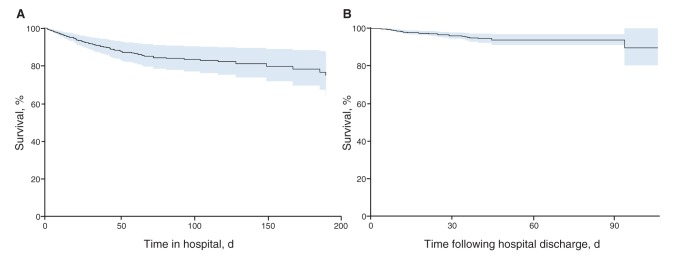
Unadjusted Kaplan–Meier survival curves for patients during their hospital stay and in the 90 days following their hospital discharge are shown in Figure 3. After adjustment in our proportional hazards regression models, age, sepsis, admission to the ICU, development of hepatic failure and metastatic cancer, but not methicillin resistance, were independently associated with reduced overall and in-hospital survival (Tables 3 and 4, respectively). Similar to the logistic regression model, proportional hazards analysis demonstrated that hepatic failure, cerebrovascular disease, COPD, metastatic cancer and methicillin resistance were associated with reduced survival in the 90-day postdischarge period (Table 4).
Figure 3:
Unadjusted Kaplan–Meier survival analysis for patients with Staphylococcus aureus bacteremia during their hospital stay (A) and for 90 days following their discharge from hospital (B). The data shown is right censored, and the shaded areas represent 95% confidence intervals.
Table 3: Factors associated with all-cause mortality.
| Variable | Adjusted HR* (95% CI) | p value |
|---|---|---|
|
Age, yr |
||
| < 60 |
1.00 (ref) |
− |
| 61–70 |
1.54 (0.98–2.41) |
0.06 |
| 71–80 |
3.07 (2.04–4.62) |
< 0.0001 |
| > 80 |
5.18 (3.54–7.57) |
< 0.0001 |
|
Sepsis |
||
| No |
1.00 (ref) |
− |
| Yes |
1.49 (1.08–2.06) |
0.02 |
|
Admission to the intensive care unit |
||
| No |
1.00 (ref) |
− |
| Yes |
3.78 (2.85–5.02) |
< 0.0001 |
|
Hepatic failure |
||
| No |
1.00 (ref) |
− |
| Yes |
3.36 (1.91–5.90) |
< 0.0001 |
|
Operative intervention |
||
| No |
1.00 (ref) |
− |
| Yes |
0.67 (0.49–0.92) |
0.01 |
|
Metastatic cancer |
||
| No |
1.00 (ref) |
− |
| Yes | 2.58 (1.77–3.75) | < 0.0001 |
Note: CI = confidence interval, HR = hazard ratio. *Adjusted for variables listed in the table.
Table 4: Factors associated with in-hospital and 90-day postdischarge mortality.
| Variable | In-hospital mortality |
Postdischarge mortality (90 d) |
||
|---|---|---|---|---|
| Adjusted HR* (95% CI) | p value | Adjusted HR† (95% CI) | p value | |
|
Age, yr |
||||
| < 60 (ref) |
1.00 (ref) |
− |
− |
− |
| 61–70 |
1.24 (0.79–1.95) |
0.4 |
− |
− |
| 71–80 |
2.30 (1.53–3.47) |
< 0.0001 |
− |
− |
| > 80 |
4.22 (2.89–6.17) |
< 0.0001 |
− |
− |
|
Methicillin resistance |
||||
| No |
− |
− |
1.00 (ref) |
− |
| Yes |
− |
− |
1.74 (1.04–2.89) |
0.03 |
|
Sepsis |
||||
| No |
1.00 (ref) |
− |
− |
− |
| Yes |
1.44 (1.04–1.99) |
0.03 |
− |
− |
|
Admission to intensive care unit |
||||
| No |
1.00 (ref) |
− |
− |
− |
| Yes |
2.35 (1.78–3.11) |
< 0.0001 |
− |
− |
|
Hepatic failure |
||||
| No |
1.00 (ref) |
− |
1.00 (ref) |
− |
| Yes |
2.20 (1.25–3.88) |
0.01 |
5.42 (1.60–18.35) |
0.01 |
|
Prolonged ventilation > 21 d |
||||
| No |
− |
− |
− |
− |
| Yes |
− |
− |
− |
− |
|
Cerebrovascular disease |
||||
| No |
− |
− |
1.00 (ref) |
− |
| Yes |
− |
− |
2.16 (1.21–3.86) |
0.01 |
|
COPD |
||||
| No |
− |
− |
1.00 (ref) |
− |
| Yes |
− |
− |
2.43 (1.28–4.61) |
0.01 |
|
Metastatic cancer |
||||
| No |
1.00 (ref) |
− |
1.00 (ref) |
− |
| Yes | 2.79 (1.90–4.10) | < 0.0001 | 5.32 (2.80–10.07) | < 0.0001 |
Note: CI = confidence interval, COPD = chronic obstructive pulmonary disorder, HR = hazard ratio, ref = reference. *Adjusted for age, sepsis, admission to the intensive care unit, hepatic failure and metastatic cancer. †Adjusted for methicillin resistance, hepatic failure, cerebrovascular disease, COPD and metastatic cancer.
Interpretation
Our study describes the morbidity and mortality associated with S. aureus bacteremia and identifies predictors of all-cause, in-hospital and 90-day postdischarge mortality using data from a large retrospective Canadian cohort. All-cause mortality in our study population was 27.9%, although the in-hospital mortality rate declined significantly during the study period. During the same timeframe, the frequency of MRSA identified among patients with bacteremia, and the number of people who inject drugs, also increased significantly. We also determined that increasing age, sepsis, admission to the intensive care unit, hepatic failure and metastatic cancer are independent predictors of all-cause mortality and in-hospital mortality. Additionally, methicillin resistance, hepatic failure, cerebrovascular disease, chronic obstructive pulmonary disease and metastatic cancer were key predictors of mortality in the 90-day postdischarge period.
Factors that were previously shown to be predictive of in-hospital mortality correlated with the variables identified in our study, including age,25 sepsis,25,26 admission to the ICU,27 hepatic failure,25 COPD and metastatic cancer.28,29 The large sample size allowed us to investigate the association between specific patient characteristics and outcomes, rather than aggregating comorbidities using scales such as the Charlson Comorbidity Index. An additional novelty of this study is the identification of risk factors associated with 90-day postdischarge mortality, given that an increasing number of patients with S. aureus bacteremia are surviving beyond the length of their hospital stay.30–32 Based on our survival analysis, our results suggest that patients sustain short- to long-term adverse consequences from S. aureus bacteremia long after they are treated for their acute episode.30
Our study also extends the findings of a Canadian population-based analysis in 2007 by Laupland and colleagues,8who observed MRSA bacteremia in 11% of bacteremic S. aureus infections. The proportion of methicillin resistance among S. aureus bacteremia isolates is significantly higher in our study but is comparable to several American studies.2,13,33–35 Klevens and colleagues5 reported that MRSA strains accounted for 64% of hospital-acquired S. aureus infections isolated from ICUs, whereas Styers and colleagues15 observed that between 47.9% and 59.2% of hospital-acquired S. aureus isolates demonstrated methicillin resistance. Despite the implementation of infection control strategies and empiric treatment coverage for MRSA, the proportion of methicillin-resistant strains among patients with S. aureus bacteremia continued to rise significantly in our study, mimicking the trend seen in North America and Europe.6,8,36,37 Whereas the frequency of MRSA continued to rise, the implementation of protocols to rapidly identify and treat patients with severe infections38 likely helped to reduce in-hospital mortality during the study period. The adoption of similar measures is also felt to have contributed to a comparable observation made by Benfield and colleagues36 in a study in Denmark, where case fatality associated with hospital- and community-acquired S. aureus bacteremia declined by 43% and 23%, respectively, over a 19-year period.
Our observation that methicillin resistance is not an independent predictor of all-cause mortality corroborates the findings of a comparable American study by Pastagia and colleagues,25 although several older studies14,39 reported a significant association. These contradictory results suggest that genetic variations between MRSA strains in different geographic regions may affect disease severity and alter outcomes.16 However, given that methicillin resistance was associated with reduced postdischarge survival, our study affirms the need for increased awareness and rigorous treatment of MRSA infections,8,13,29 as well as continued vigilance in screening and identifying patients with MRSA.6,40
The preponderance of males and older age of our study population supports previous observations that males and elderly individuals are at increased risk for developing S. aureus bacteremia.8 However, some of the patient-specific comorbidities associated with mortality in our study (cerebrovascular disease, COPD and malignancy) are different from the risk factors associated with the acquisition of S. aureus bacteremia (diabetes, heart disease, need for dialysis and hepatitis C virus) as identified by Laupland and colleagues,8 suggesting that the interaction between patient-specific factors and pathogen virulence may play a critical role in determining eventual outcome rather than patient comorbidities alone. Although it was beyond the scope of our current work, prospective studies that correlate the molecular and genetic analyses of S. aureus isolates with clinical outcomes may provide additional insight into the pathophysiology of S. aureus and the susceptibility of specific at-risk populations.
Our study also highlights the impact of S. aureus bacteremia and its complications on health care resource utilization. Our rate of complicated and recurrent S. aureus bacteremia infections (50%) is slightly higher than a prospective observational cohort study by Fowler and colleagues,9 in which a complicated or recurrent infection developed in 311 (42.9%) of 724 patients with S. aureus bacteremia during a 12-week period. In our study, a significant proportion of patients required admission to the ICU, and a smaller number required prolonged mechanical ventilation or dialysis for renal failure. These complications, in addition to the infectious consequences of S. aureus bacteremia such as infective endocarditis and osteomyelitis, significantly increase the morbidity of the illness,1,11,12 prolong hospital stay41 and reduce survival.9,11,12 Therefore, the results of our study may prompt a closer examination of treatment strategies for S. aureus bacteremia to optimize outcomes.
Limitations
Although this study benefits from its large size, it is limited by its retrospective design and single-centre setting. In addition, we may have missed cases of S. aureus bacteremia if patients in the region sought health care from other facilities. We were unable to assess the duration and adequacy of antibiotic therapy for all patients involved in the study, limiting our ability to control for these confounders by regression analysis. Although complications of S. aureus bacteremia can be difficult to identify at the time of the initial positive blood culture test result and may be prone to selection bias, our review of the medical records throughout the patients’ hospital stay is likely to have helped to reduce the bias and improved the identification of complications. Selection bias is also likely to have contributed to our finding that operative intervention was associated with reduced all-cause and postdischarge mortality, given that patients selected for surgery may be healthier than their nonsurgical counterparts. Therefore, further stratification and analysis of the subgroup undergoing surgical intervention and comparison with a matched nonsurgical cohort are necessary to better understand the effect of surgery on survival in S. aureus bacteremia. In addition, we observed that a moderate number of patients died more than 90 days after discharge from the hospital. Because it was difficult to attribute these mortalities to an acute episode of S. aureus bacteremia, our analysis of all-cause and postdischarge mortality was limited to patients who died within 90 days of discharge. A propensity-matched, population-based, retrospective cohort analysis comparing patients with and without the infection may provide valuable insight into whether S. aureus bacteremia increases mortality in the long term.
Conclusion
We presented the results of a large retrospective cohort study involving patients with S. aureus bacteremia, which highlights the significant clinical impact of an important and pervasive disease. In addition to describing the burden of S. aureus bacteremia in a Canadian cohort, we also identified important risk factors associated with all-cause, in-hospital and postdischarge mortality. The results of our study may help with the inpatient management of S. aureus bacteremia, provide prognostic clarity for affected patients and aid in the follow-up of at-risk patient populations after their hospital stay.
Supplementary Material
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998;339:520-32. [DOI] [PubMed] [Google Scholar]
- 2.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005;293:3012-21. [DOI] [PubMed] [Google Scholar]
- 3.Miro JM, Anguera I, Cabell CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005;41:507-14. [DOI] [PubMed] [Google Scholar]
- 4.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997−1999. Clin Infect Dis 2001;32(Suppl 2):S114-32. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis 2006;42:389-91. [DOI] [PubMed] [Google Scholar]
- 6.Simor AE, Ofner-Agostini M, Bryce E, et al. The evolution of methicillin-resistant Staphylococcus aureus in Canadian hospitals: 5 years of national surveillance. CMAJ 2001;165:21-6. [PMC free article] [PubMed] [Google Scholar]
- 7.Tadros M, Williams V, Coleman BL, et al. Epidemiology and outcome of pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) in Canadian hospitals. PLoS ONE 2013;8:e75171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis 2008;198:336-43. [DOI] [PubMed] [Google Scholar]
- 9.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003;163:2066-72. [DOI] [PubMed] [Google Scholar]
- 10.Park KH, Chong YP, Kim SH, et al. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J Infect 2013;67:556-64. [DOI] [PubMed] [Google Scholar]
- 11.Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis 2009;48(Suppl 4):S254-9. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell DH, Howden BP. Diagnosis and management of Staphylococcus aureus bacteraemia. Intern Med J 2005;35(Suppl 2):S17-24. [DOI] [PubMed] [Google Scholar]
- 13.Kuehnert MJ, Hill HA, Kupronis BA, et al. Methicillin-resistant Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis 2005;11:868-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-9. [DOI] [PubMed] [Google Scholar]
- 15.Styers D, Sheehan DJ, Hogan P, et al. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob 2006;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simor AE, Ofner-Agostini M, Bryce E, et al. Laboratory characterization of methicillin-resistant Staphylococcus aureus in Canadian hospitals: results of 5 years of National Surveillance, 1995–1999. J Infect Dis 2002;186:652-60. [DOI] [PubMed] [Google Scholar]
- 17.Census profile. Ottawa: Statistics Canada; 2011. Available: http://www12.statcan.gc.ca/census-recensement/2011/dp-pd/prof/details/page.cfm?Lang=E&Geo1=POPC&Code1=0480&Geo2=PR&Code2=35&Data=Count&SearchText=London&SearchType=Begins&SearchPR=01&B1=All&Custom=&TABID=1 (accessed 2014 Oct. 14).
- 18.Conterno LO, Wey SB, Castelo A. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 1998;19:32-7. [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [DOI] [PubMed] [Google Scholar]
- 20.Mylotte JM, Aeschlimann JR, Rotella DL. Staphylococcus aureus bacteremia: factors predicting hospital mortality. Infect Control Hosp Epidemiol 1996;17:165-8. [DOI] [PubMed] [Google Scholar]
- 21.Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 2000;31:1170-4. [DOI] [PubMed] [Google Scholar]
- 22.Schønheyder HC, Gottschau A, Friland A, et al. Mortality rate and magnitude of Staphylococcus aureus bacteremia as assessed by a semiquantitative blood culture system. Scand J Infect Dis 1995;27:19-21. [DOI] [PubMed] [Google Scholar]
- 23.Jensen AG, Wachmann CH, Espersen F, et al. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med 2002;162:25-32. [DOI] [PubMed] [Google Scholar]
- 24.Hosmer, DW, Lemeshow, S, Sturdivant, RX. Applied logistic regression. 2nd ed. Hoboken (NJ): Wiley; 2004. [Google Scholar]
- 25.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, et al. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis 2012;18:1072-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang CI, Song JH, Chung DR, et al. Bloodstream infections in adult patients with cancer: clinical features and pathogenic significance of Staphylococcus aureus bacteremia. Suppor Care Cancer 2012;20:2371-8. [DOI] [PubMed] [Google Scholar]
- 27.Castillo JS, Leal AL, Cortes JA, et al. Mortality among critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia: a multicenter cohort study in Colombia. Rev Panam Salud Publica 2012;32:343-50. [DOI] [PubMed] [Google Scholar]
- 28.Joo EJ, Peck KR, Ha YE, et al. Impact of acute kidney injury on mortality and medical costs in patients with methicillin-resistant Staphylococcus aureus bacteraemia: a retrospective, multicentre observational study. J Hosp Infect 2013;83:300-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang FD, Chen YY, Chen TL, et al. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control 2008;36:118-22. [DOI] [PubMed] [Google Scholar]
- 30.Fätkenheuer G, Preuss M, Salzberger B, et al. Long-term outcome and quality of care of patients with Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2004;23:157-62. [DOI] [PubMed] [Google Scholar]
- 31.Wolkewitz M, Frank U, Philips G, et al. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother 2011;66:381-6. [DOI] [PubMed] [Google Scholar]
- 32.Wang HE, Szychowski JM, Griffin R, et al. Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 2014;4:e004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strausbaugh LJ, Crossley KB, Nurse BA, et al. Antimicrobial resistance in long-term-care facilities. Infect Control Hosp Epidemiol 1996;17:129-40. [DOI] [PubMed] [Google Scholar]
- 34.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 2007;13:1840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999–2008. Arch Intern Med 2012;172:363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benfield T, Espersen F, Frimodt-Moller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 2007;13:257-63. [DOI] [PubMed] [Google Scholar]
- 37.Frederiksen MS, Espersen F, Frimodt-Moller N, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J 2007;26:398-405. [DOI] [PubMed] [Google Scholar]
- 38.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73. Erratum in Crit Care Med 2004;32:1448 [dosage error in article text] and Crit Care Med 2004;32:2169-70. [DOI] [PubMed]
- 39.Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002;162:2229-35. [DOI] [PubMed] [Google Scholar]
- 40.Loeb MB, Main C, Eady A, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev 2003;CD003340. [DOI] [PubMed] [Google Scholar]
- 41.Chang FY, MacDonald BB, Peacock JE, Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322-32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.