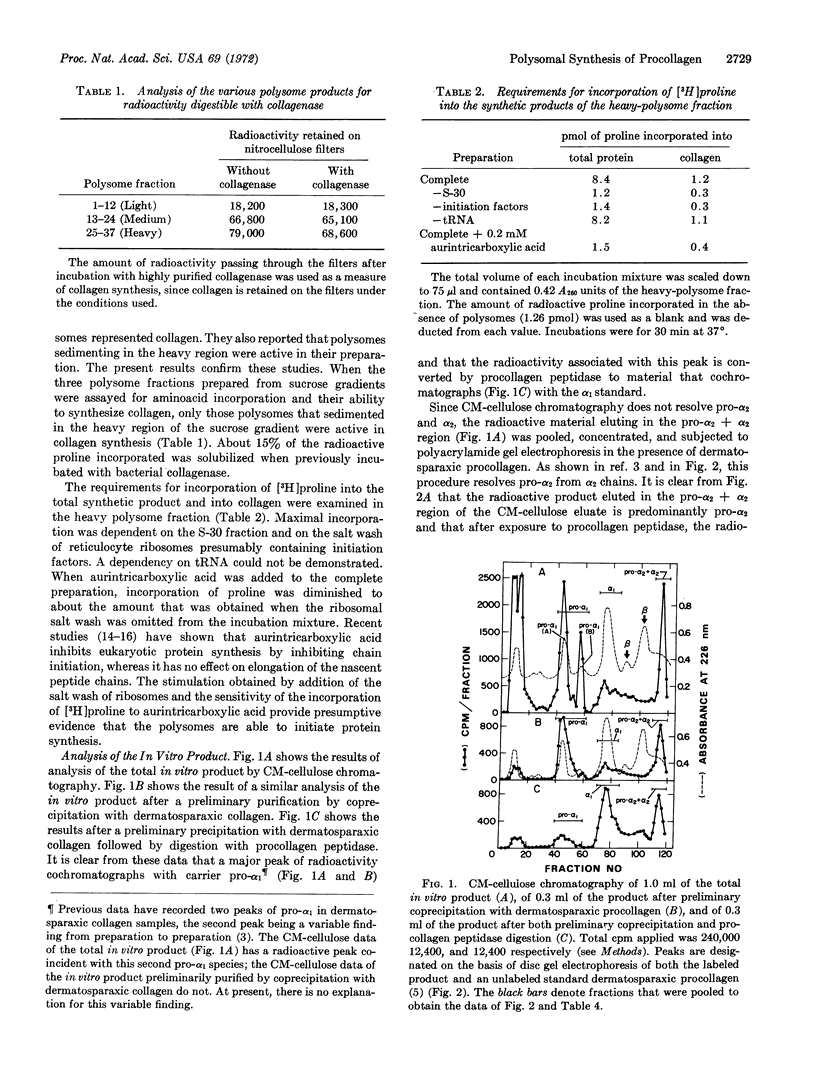
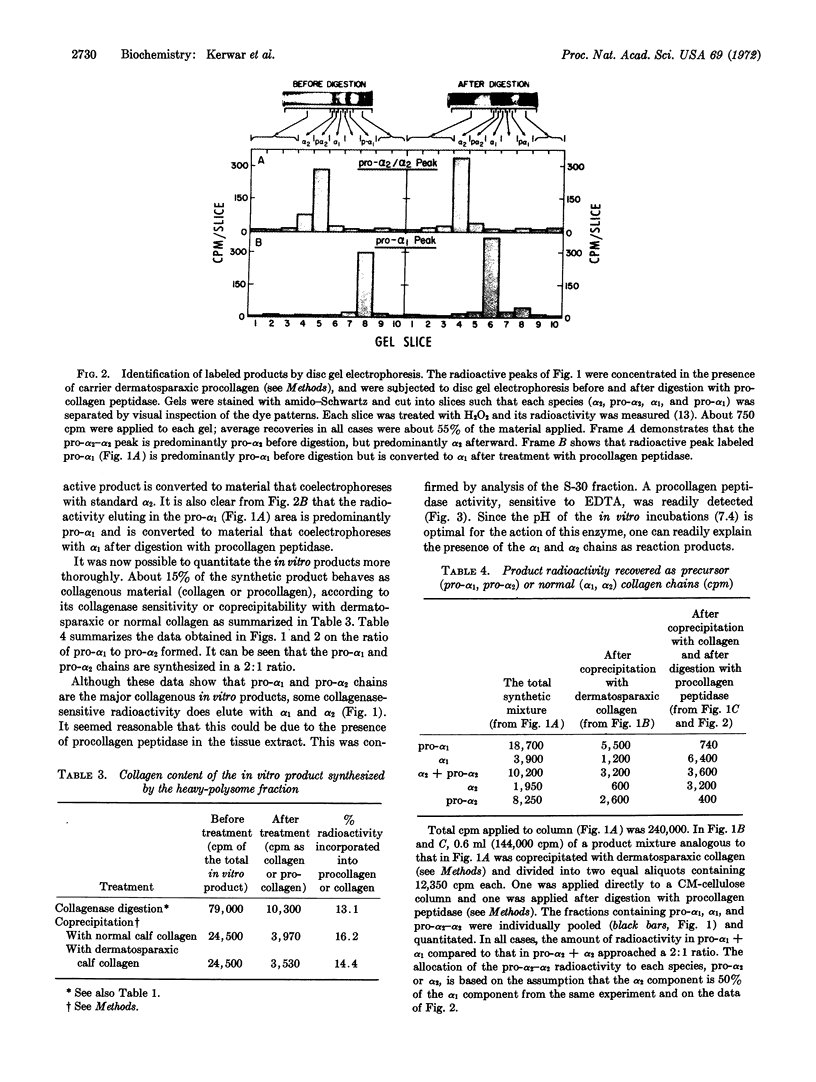
Abstract
The major collagenous products synthesized in a cell-free polysome preparation are pro-α1 and pro-α2, formed in a ratio of 2:1. They are the precursor forms of α1 and α2 chains of normal connective-tissue collagen that are also formed in small amounts. A procollagen peptidase activity has been demonstrated in the supernatant fraction that can account for the formation of α1 and α2 chains from their precursors. The polysomal system is activated by a salt extract of reticulocyte ribosomes and is inhibited by aurintricarboxylic acid, suggesting that the polysomes are able to initiate protein synthesis.
Keywords: collagen, protein synthesis, mRNA, initiation factors
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. L., Pfeiffer S. E., Tanzer M. L. Collagen biosynthesis: synthesis and secretion of a high molecular weight collagen precursor (procollagen). Proc Natl Acad Sci U S A. 1971 Nov;68(11):2638–2642. doi: 10.1073/pnas.68.11.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lukens L. N. Collagen synthesis on polysomes in vivo and in vitro. Nat New Biol. 1971 Jul 14;232(28):37–40. doi: 10.1038/newbio232037a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Marbaix G., Wérenne J., Burny A., Huez G. Effect of aurintricarboxylic acid and of NaF on the binding of globin messenger RNA to reticulocyte 40S ribosomal subunits. Biochem Biophys Res Commun. 1970 Aug 11;40(3):731–739. doi: 10.1016/0006-291x(70)90964-2. [DOI] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Marcus A., Bewley J. D., Weeks D. P. Aurintricarboxylic acid and initiation factors of wheat embryo. Science. 1970 Mar 27;167(3926):1735–1736. doi: 10.1126/science.167.3926.1735. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Schweet R. Isolation of a protein fraction from reticulocyte ribosomes required for de novo synthesis of hemoglobin. Arch Biochem Biophys. 1968 May;125(2):632–646. doi: 10.1016/0003-9861(68)90622-x. [DOI] [PubMed] [Google Scholar]
- Müller P. K., McGoodwin E., Martin G. R. Studies on protocollagen: identification of a precursor of proto alpha 1. Biochem Biophys Res Commun. 1971 Jul 2;44(1):110–117. doi: 10.1016/s0006-291x(71)80165-1. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Sakai T., Gross J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry. 1967 Feb;6(2):518–528. doi: 10.1021/bi00854a021. [DOI] [PubMed] [Google Scholar]
- Stewart M. L., Grollman A. P., Huang M. T. Aurintricarboxylic acid: inhibitor of initiation of protein synthesis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):97–101. doi: 10.1073/pnas.68.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Vuust J., Piez K. A. Biosynthesis of the alpha chains of collagen studied by pulse-labeling in culture. J Biol Chem. 1970 Nov 25;245(22):6201–6207. [PubMed] [Google Scholar]