Abstract
This study meta-analyzed research examining Diffusion Tensor Imaging following pediatric non-penetrating traumatic brain injury to identify the location and extent of white matter changes. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) data from 20 studies were analyzed. FA increased and ADC decreased in most white matter tracts in the short-term (moderate-to-large effects), and FA decreased and ADC increased in the medium- to long-term (moderate-to-very-large effects). Whole brain (short-term), cerebellum and corpus callosum (medium- to long-term) FA values have diagnostic potential, but the impact of age/developmental stage and injury severity on FA/ADC, and the predictive value, is unclear.
Traumatic brain injuries (TBIs) are a leading cause of disability in children and adolescents, and are associated with a variety of cognitive and neurological impairments (Babikian & Asarnow, 2009; Beauchamp et al., 2013; Taylor, 2010). Pediatric TBIs are particularly significant because they are sustained at a time when the brain is still developing and, as such, can alter a child’s developmental trajectory. Consequently, the effects can be more severe and have a greater impact than an equivalent injury sustained during adulthood (Hessen, 2010). Indeed, adults who sustained their TBIs during childhood are at greater risk of experiencing a range of negative outcomes, including social, educational, and employment problems, as well as a lower quality of life (Anderson, Brown, Newitt, & Hoile, 2011; Cattelani, Lombardi, Brianti, & Mazzucchi, 1998). Hence, it is particularly important that we understand the impact of pediatric TBIs in order to ensure that optimal treatment and rehabilitation is provided, with the view to improving life-long outcomes.
Terms relating to the severity of TBI are not used consistently. In this article we use mild TBI (mTBI) to refer to a traumatically induced disruption in brain functioning, as manifested by a Glasgow Coma Scale score of 13–15 and at least one of the following: loss of consciousness that does not exceed 30 minutes, loss of memory for events immediately before or after the accident that results in posttraumatic amnesia not greater than 24 hours, alteration in mental state at the time of the accident (e.g., feeling dazed, disorientated, or confused), and focal neurological deficit(s) that may or may not be transient (Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine, 1993).
Penetrating brain injuries are very rare in children (Koestler & Keshavarz, 2001) and have very different mechanisms of injury and medical outcomes than blunt trauma (Fleisher & Ludwig, 2010); thus the current analysis focuses on non-penetrating TBI. Non-penetrating TBIs, resulting from falls, sporting injuries and traffic accidents, frequently cause both focal and diffuse brain damage (Lee & Newberg, 2005). Diffuse axonal injury is particularly common following TBI due to the traumatic shearing forces that occur when the head is rapidly accelerated or decelerated (Wilde et al., 2010). This damage is thought to be a major contributor to functional outcomes following TBI (Niogi & Mukherjee, 2010). Conventional neuroimaging techniques, such as computerized tomography (CT) and magnetic resonance imaging (MRI), are often used to assess brain damage following a TBI and are highly effective in identifying focal lesions; however these techniques tend to underestimate the amount of white matter damage (Duckworth & Stevens, 2010; Mayer et al., 2010). Thus, standard neuroimaging has failed to adequately detect the full extent of the damage that occurs after a TBI, thereby limiting its usefulness for diagnostic, treatment/management, and prognostic purposes (Niogi & Mukherjee, 2010).
Recent developments in MRI technology have improved our understanding of the impact of TBI on the brain using diffusion tensor imaging (DTI), which assesses changes in the microstructure of white matter and, consequently, is better able to detect diffuse axonal injury following TBI (Caeyenberghs et al., 2010b; Wozniak et al., 2007; Xu, Mukherjee, & Barkovich, 2013). It may therefore also prove to be more useful in predicting functional outcomes.
DTI is a non-invasive MRI technique that measures the three-dimensional diffusion of water molecules within the white matter of the brain (Assaf & Pasternak, 2008; Duckworth & Stevens, 2010) and is based on the principal that this diffusion is normally greater in a direction that is parallel to nerve fibers, than it is perpendicular to them. The diffusion profile of water can be described in terms of its anisotropy; that is, the extent to which there is a uniform direction to the diffusion of the water molecules (Niogi & Mukherjee, 2010). Anisotropy is reduced both by damage that affects the orientation of axonal fibers and damage to the myelin sheaths that surround axons. This damage not only increases the amount of water diffusion, as there are fewer intact structures constraining the water molecules, but it also increases the amount of diffusion that occurs in the direction orthogonal (at right angles) to the white matter tracts because there are fewer longitudinally oriented structures to hinder diffusion. This then leads to a decrease in anisotropy (Mori, 2007; Niogi & Mukherjee, 2010).
When axons are damaged following TBI, the diffusion of water within the nerve fibers is altered, enabling white matter damage to be detected and quantified using a variety of measures that assess these changes (Hanten et al., 2008; Le Bihan et al., 2001; Levin et al., 2008). Several DTI indices have been developed; some focus on anisotropy, the most common of which is fractional anisotropy (FA), and others on diffusivity, including the apparent diffusion coefficient (ADC) and mean diffusivity (MD). FA provides a measure of the degree of uniformity in the direction of the diffusion, with values ranging between 0 and 1. An FA of 0 indicates that there is a complete lack of uniformity in the direction of the movement of water molecules in fibers within a given voxel, and 1 indicates that diffusion occurs in only one direction (parallel to the axon) and is completely restricted in all other directions. ADC, on the other hand, provides a measure of the rate of diffusion of water molecules, which is usually averaged over all directions (Niogi & Mukherjee, 2010). MD is very similar to the ADC and, importantly, ADC and MD values are similar when obtained from the same scanner. Indeed, the terms ADC and MD are often used interchangeably in the literature and were, therefore, necessarily combined for present purposes. Whereas high FA and low ADC or MD values indicate that the cortical white matter tracts are intact and myelinated, low FA and high ADC/MD values indicate that a nerve fiber is injured or poorly developed (Niogi & Mukherjee, 2010). Thus, high FA and low ADC/MD values indicate white matter integrity (Salmond et al., 2006; Tasker et al., 2010; Wu et al., 2010b).
Recent reviews of DTI research examining child, adult, and mixed samples with mild, moderate, and severe TBI have demonstrated its potential to further our understanding of TBI and provide a valuable clinical tool. Specifically, Aoki, Inokuchi, Gunshin, Yahagi, and Suwa, (2012) meta-analyzed research that has examined DTI in mild TBI samples and concluded that it is able to detect changes to white matter microstructure in adults. Similarly, Gardner et al. (2012) conducted a systematic review of DTI findings from adolescents and adults with sports-related mild TBI and concluded that DTI is more sensitive to white matter changes than other imaging techniques. Both studies noted that large white matter tracts—such as the corpus callosum, internal capsule, and longitudinal fasciculus—are most frequently researched because they are often damaged as a result of the shearing forces associated with TBIs (Aoki et al., 2012; Gardner et al., 2012). Moreover, Gardner et al. (2012) proposed that, if DTI can be shown to detect changes in brain structure that is not detected by conventional imaging, it may play a key role in improving the diagnosis and management of TBI, particularly mild TBI.
Many fewer studies have used DTI with pediatric TBI samples. As with adults, much of this research has focused on the corpus callosum, and results have ranged from large increases to FA in the cingulum bundle (Wu et al., 2010b) to large decreases to FA in the same region (McCauley et al., 2011). Other studies have found only minimal changes in some brain regions, for example, Juranek et al. (2012) found minimal decreases to FA in the right hippocampus. Moreover, there is evidence to suggest that DTI findings may vary depending on whether the assessment is conducted in the acute stage after a TBI—when axonal swelling or cytotoxic edema may be associated with an increase in FA and decrease in ADC—or the chronic stage when these effects have reversed and, consequently, FA decreases and ADC increases (Bigler & Bazarian, 2010; Gardner et al., 2012; Wu et al., 2010a). This suggests that it is important to not only consider regional differences, but also time post-injury, when interpreting DTI findings.
AIMS
Thus far, the DTI findings for pediatric TBI samples have yet to be compiled and synthesized, limiting our ability to determine which regions of the brain are most affected and by how much, and the extent to which these changes vary between the acute and post-acute/chronic stages. Existing meta-analyses and systematic reviews have yet to examine pediatric samples in their own right. Given the greater vulnerability of the developing brain to the structural and functional effects of a TBI, it is possible that the findings for pediatric and adult samples may differ.
The current study therefore meta-analyzed existing research that has performed DTI with pediatric TBI samples, together with their healthy peers, in order to provide a clearer understanding of the location and extent of white matter changes, and their evolution over time, following pediatric TBI. In doing so, it provides a basis for assessing whether the measures of damage (FA, MD/ADC) to those regions that are most affected by pediatric TBI accurately predict current and future functioning in order to systematically evaluate their diagnostic and prognostic utility. All levels of injury were examined, including mild, moderate, and severe TBI. The key DTI indices were FA and ADC or MD, and findings for the acute and chronic stages of injury were analyzed separately.
METHODS
Literature Search
A comprehensive search of the literature was conducted in order to identify all research— published before December 2013—that used DTI to examine white matter damage in children and adolescents who had sustained a TBI, compared to that of a non-injured control group. Seven electronic databases (PubMed, PsychINFO, CINAHL, Scopus, Embase, Informit, and Web of Science) were searched using a large number of terms in order to capture all relevant articles. The search strategy included a combination of terms covering brain injury/TBI/brain trauma, child/adolescent/pediatric samples, and DTI/diffusion MRI (see Online Resource A for the logic grids that were used to search each database).
A study was deemed eligible for inclusion in the current meta-analysis if it met the following criteria: (1) participants were children and/or adolescents (mean or median age, or age range <18), (2) it examined a sample that had sustained a non-penetrating TBI caused by an external force, which resulted in at least a brief loss of consciousness, transient amnesia, or confusion (includes mild, moderate, and/or severe TBI, but excludes blows to the head that had no observable consequences), (3) it recruited a non-injured control group, (4) DTI was used to assess white matter damage caused by the TBI, (5) FA and/or ADC/MD data for both the TBI and control samples were reported (e.g., means and SDs, exact p or t values, raw data), and (6) it was an original piece of research (excludes reviews) that was published in English. Case studies were excluded from the present analysis.
These searches identified 1,580 potentially relevant articles. An initial application of the inclusion criteria to the titles and abstracts of these papers, reduced the number to 270 (see Figure 1), 168 of which were duplicates and removed. The full-text versions of the remaining 102 articles were then examined in detail using the same inclusion criteria and a further 70 excluded. Three of the remaining studies did not provide the requisite data (Ewing-Cobbs et al., 2008; Liégois, Tournier, Pigdon, Connelly, & Morgan, 2013; Wilde et al., 2012). The corresponding authors of these studies were therefore contacted by e-mail to request this data: one author replied, but did not have the necessary data, and the other two did not reply. This left a total of 29 studies that were suitable for analysis.
FIGURE 1 .
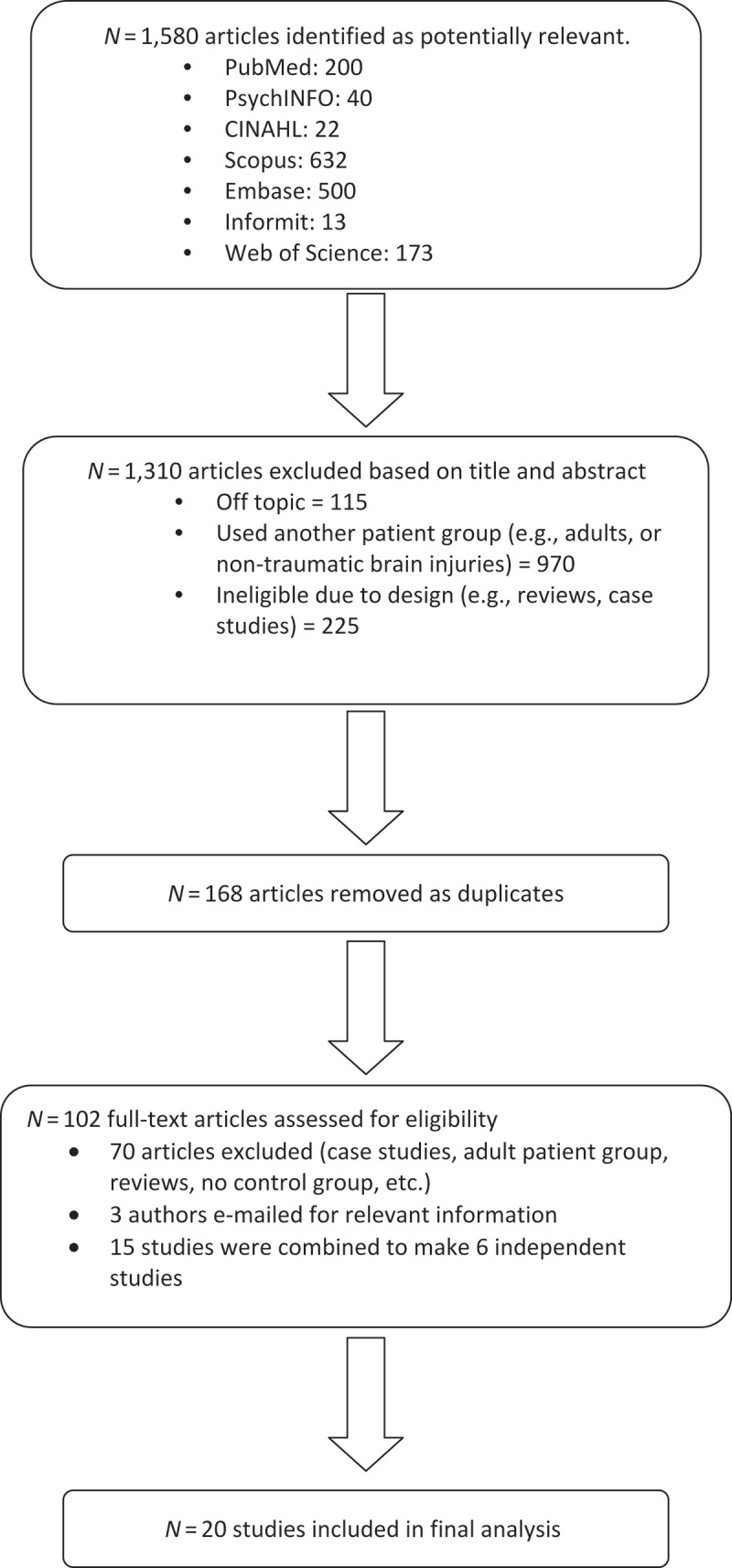
Flow of included studies.
Meta-analytic procedures assume that all samples are independent of one another (Rosenthal, 1995), therefore all studies were checked for independence. Two studies by Borich et al. (Borich, Makan, Boyd, & Virji-Babul, 2013; Virji-Babul et al., 2013), two studies by Yuan et al. (Kurowski et al., 2009; Yuan et al., 2007), three studies by Caeyenberghs et al. (Caeyenberghs et al., 2010a, 2010b, 2011), two sets of two studies by Wilde et al. (Wilde et al., 2006a, 2006b; Oni et al., 2010; Wilde et al., 2010), and another four studies by Wilde et al. (Chu et al., 2010; Wilde et al., 2008; Wu et al., 2010b; Yallampalli et al., 2013) reported data for non-independent samples. The data for the studies within each of these sets were therefore combined and treated as one (i.e., the aforementioned 15 publications equated to 6 independent studies), leaving a total of 20 independent studies in the final analyses.
Data Collection and Preparation
Demographic details (age, gender, handedness), injury details (Glasgow Coma Scale score or category, time post-injury), MRI/DTI information (MRI magnet strength [Tesla: 1.5T or 3T], scanner specifications [brand, model], DTI method of acquisition, DTI metrics [FA, ADC, MD]), region-of-interest (e.g., corpus callosum), and the data required for the calculation of the effect sizes (means and SDs, exact p value, t-statistic or one-way ANOVA, raw data) were extracted from each study. Participants in a study by Wu et al. (2010a) underwent repeated testing at 3 and 18 months in order to evaluate changes in DTI metrics over time. Only the data from the first assessment were used for present purposes because this was more comparable to the intervals used by the other studies. FA data was analyzed separately, and ADC and MD data were analyzed together because the terms were often used interchangeably. All data extraction was undertaken by a trained research assistant working under the close supervision of the first and second authors.
Effect Size Calculation and Interpretation
Cohen’s d (Cohen, 1992), which provides a measure of the standardized mean difference between two groups, was used to assess group differences in the white matter integrity of the TBI and control groups, as measured by FA and ADC/MD. Mean (and SD) FA or ADC/MD measures were recorded for both the TBI and control groups for each region-of-interest that was examined by a study, and used to calculate d. If means and standard deviations were not available, t-tests, F-ratios (one-way) or exact p values were used to calculate d (Lipsey & Wilson, 2001). Effect sizes were calculated even where authors reported an effect size in order to ensure that a consistent formula was used. All effect sizes were calculated using the Comprehensive Meta-analysis program checked by a researcher with expertise in meta-analyses, and all discrepancies were double checked and corrected if necessary.
All Cohen’s d statistics were calculated in such a way that a negative d indicated poorer white matter integrity in the TBI sample, relative to controls; which equates to lower FA values and higher ADC/MD values in the TBI sample. Cohen’s d values of .3, .5 and .8 equate to small, medium, and large effects, respectively (Cohen, 1992).
Mean effect sizes were calculated for each region-of-interest when more than one study examined the same region. Before doing so, the effect sizes from individual studies were weighted by their inverse variance in order to take into account variation in the reliability of an effect size caused by differences in sample sizes (small samples yield less reliable estimates and have greater variance) (Lipsey & Wilson, 2001). A mean weighted effect size was then calculated for each brain region by dividing the sum of the individually weighted effect sizes by the sum of the weights (Lipsey & Wilson, 2001). Four studies performed DTI in the early stages (1-, 2- and 4-weeks post-injury). The remaining studies performed DTI in the medium- to long-term after a TBI; namely 3 or more months post-injury (N = 16; range 3 to 45 months)—these data are reported separately.
A number of additional statistics were calculated to assist in interpreting the results. First, 95% confidence intervals (95% CIs) were calculated to provide a measure of the statistical significance of the effect sizes (Lipsey & Wilson, 2001). CIs that do not span zero indicate that DTI data from the TBI and Control groups differed significantly (p = .05). Second, percentage overlap scores (%OL) were calculated to provide a measure of the extent to which distribution of scores for the TBI and Control groups overlapped (Zakzanis, Leach, & Kaplan, 1999). Third, fail-safe N statistics (Nfs) were calculated to address the potential for publication bias, which refers to the tendency for journals to publish studies that report significant findings (Lipsey & Wilson, 2001). The Nfs statistic provides a hypothetical measure of the number of unpublished studies with non-significant results would need to exist in order to call the current findings into question (Zakzanis et al., 1999). The larger the Nfs, relative to the number of published studies that have examined a particular region, the less likely it is that this number of unpublished studies with null findings would exist. Lastly, a measure of heterogeneity (Q) was calculated in order to determine whether the between-study variability in effect sizes was due to normal sampling error—indicating that a fixed-effects model is appropriate for the effect size calculations—or caused by more systematic differences between studies, potentially due to methodological differences, suggesting that a random-effects model should be used. A significant Q statistic indicates a heterogeneous distribution of effects, which suggests that the variability in the effect sizes is larger than would be expected due to normal sampling error. Notably, however, the Q-test has low statistical power when based on a small number of effect sizes, particularly when the sample sizes are small (Lipsey & Wilson, 2001). A fixed-effects model was therefore used to calculate Cohen’s d, except where the Q statistic was significant.
The inferences made throughout this meta-analysis are based on the combined information from the Cohen’s d statistics, 95% CIs, and Nfs. Specifically, we argue that we can be more confident that FA is lower (i.e., direction of diffusion of water molecules along a nerve fibre is less uniform), and ADC or MD are higher (i.e., the rate of diffusion is higher), in children following a TBI if there are significant (95% CI ≠ 0) moderate to large negative differences in these DTI metrics (Cohen’s d ≤ –.5), together with acceptable Nfs statistics (Nfs > Nstudies).
As the studies included findings from different scanner strengths and brands, we also calculated the mean effect size for studies using 1.5 and 3T strength scanners as well as for studies using Siemens and Phillips machines.
RESULTS
Participant Characteristics
Altogether, the data from 20 independent studies were analyzed, which included a total of 491 children who had sustained a TBI and 465 healthy controls. Summary demographic data are provided in Table 1, where it can be seen that, on average, participants were aged in their early teens (range: 7–17 years). The majority of participants in both groups were right-handed (TBI: 93%, Controls: 88%) boys (TBI: 66%, Controls: 63%). There were no significant differences between the TBI and Control groups in terms of their age (t (38) = 1.16, p = .25), gender (χ2(1, 926) = .19. p = .66) or handedness (χ2(1, 446) = 2.81, p = .09), indicating that they were well matched. Control participants were healthy children recruited from the local community (Nstudies = 7) or children with orthopaedic injuries (Nstudies = 11). An additional two studies reported that control participants were healthy children, but did not report where they were recruited. The TBI group largely sustained moderate (GCS = 9–12) to severe (GCS = 3–8) TBIs (Nstudies = 11) and, on average, were scanned approximately one year after their injury (mean = 14.4 months, SD = 18 months). Five studies examined injuries that ranged from complicated–mild (GCS = 13–15 plus visible brain lesions) to severe, three investigated mild TBIs (GCS = 13–15) (one reported in multiple papers: Borich et al., 2013; Mayer et al,. 2012; Yallampalli et al., 2010) and another examined mild to severe TBI (Wozniak et al., 2007).
TABLE 1 .
Summary Demographic, Injury, and Imaging Information for the TBI and Control Groups
| TBI Group |
Control Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Nstudies | Nparticipants | Mean (SD) | Range | Nstudies | Nparticipants | Mean (SD) | Range | |
| Sample Size | 20 | 491 | 24.6 (14.4) | 6–49 | 20 | 465 | 23.3 (11.7) | 9–46 |
| Age (years) | 20 | 491 | 14.1 (2.1) | 7–17 | 20 | 465 | 13.4 (1.7) | 7–17 |
| Males | 19 | 321 | 19 | 299 | ||||
| Females | 19 | 153 | 18 | 153 | ||||
| Handedness (right) | 9 | 219 (93%) | 8 | 186 (88%) | ||||
| Glasgow Coma Score | 12 | 310 | 9.7 (2.5) | 3–15 | ||||
| Injury category | ||||||||
| Mild | 3 | 40 | ||||||
| Moderate-severe | 11 | 266 | ||||||
| Complicated mild,moderate and severe | 5 | 171 | ||||||
| Mild to moderate | 1 | 14 | ||||||
| Time since injury (years) | 20 | 491 | 1.2 (1.5) | .008–3.9 | ||||
| MRI strength | ||||||||
| 3Tesla | 12 | 196 | 12 | 185 | ||||
| 1.5Tesla | 8 | 295 | 8 | 285 | ||||
Note. GCS = Glasgow Coma Scale; MRI = magnetic resonance imaging; Nstudies = Total number of studies; N participants = total number of participants; SD = standard deviation; TBI = traumatic brain injury.
Four studies performed scans on children with mild TBI at 1-, 2-, or 4-weeks post-TBI and were labeled ‘short-term’. The other 16 studies performed scans 3 or more months post-TBI, and included children with all levels of TBI; these were grouped into a ‘medium to long-term’ category. The majority of studies (n = 12) used a 3T MRI scanner, with the remaining eight using a 1.5T scanner (see Online Resource B for study-specific imaging details, including the scanner strength and brand, image acquisition details, and regions of interest).
Short-Term DTI Changes
FA
As indicated, relatively few studies performed DTI scans in the early stages after an injury (Nstudies = 4) and all examined mild TBI samples. The associated effect sizes for the FA and ADC data (no MD data were reported) are provided in Tables 2 and 3, respectively. FA data was reported for 18 different regions, most of which were only examined by one study; the exceptions being the whole brain (composite white matter score), fornix, and the right and left anterior corona radiata, which were each investigated by two studies (see Table 2). Notably, there were large and significant positive differences in the FA values of 16 regions (89%): composite whole brain, corpus callosum, anterior corona radiata (left and right), cerebral peduncle, fornix, superior corona radiata, left cingulum bundle, anterior thalamic radiation (left and right), right forceps minor, right inferior longitudinal fasciculus, inferior fronto-occipital fasciculus (left and right), subcallosal cortex, right thalamus, and left white matter. Paradoxically, mean FA values were higher than those seen in healthy individuals in almost all regions that have been assessed in the first few weeks after a mild TBI. Although all of these findings had adequate Nfs statistics, they should be treated cautiously as they were based on a maximum of two studies, with a narrow range of injuries (mild TBI), and generally involved small samples. Moreover, the %OL statistics indicate that, with the exception of the composite whole brain score (12%), there was considerable overlap in the distribution of FA scores for the TBI and control groups (≥ 31%).
TABLE 2 .
Cohen’s d Effect Sizes Comparing the FA Values of the TBI and Control Groups in the Early Post-Injury Interval (1–4 Weeks) (Rank-Ordered by Effect Size)
| 95% CIs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Nstudies | NTBI | Ncontrol | Mean dw | dw SE | Lower Limit | Upper Limit | Nfs | %OL | Q | Study References |
| Composite whole brain | 2 | 22 | 20 | 1.61# | 0.36 | 0.89 | 2.33 | 11 | 12 | 3.75NS | Chu et al., 2010; Virji-Babul et al., 2013 |
| Total Corpus Callosum | 1 | 10 | 10 | 1.42# | 0.03 | 2.80 | 6 | 31 | Wilde et al., 2008 | ||
| Left Anterior Corona Radiata | 2 | 28 | 26 | 1.15# | 0.29 | 0.57 | 1.73 | 9 | 39 | 0.44NS | Borich et al., 2013; Mayer et al., 2012 |
| Cerebral Peduncle | 1 | 16 | 16 | 1.13# | 0.08 | 2.19 | 4 | 40 | Mayer et al., 2012 | ||
| Fornix | 2 | 23 | 21 | 1.12# | 0.32 | 0.48 | 1.76 | 9 | 39 | 0.54NS | Borich et al., 2013; Yallampalli et al., 2010 |
| Right Anterior Corona Radiata | 2 | 28 | 26 | 1.04# | 0.29 | 0.47 | 1.61 | 8 | 43 | 0.12NS | Borich et al., 2013; Mayer et al., 2012 |
| Superior Corona Radiata | 1 | 16 | 16 | 1.01# | −0.03 | 2.05 | 4 | 44 | Mayer et al., 2012 | ||
| Left Cingulum Bundle | 1 | 12 | 11 | 1.00# | 0.13 | 1.87 | 4 | 44 | Wu et al., 2010b | ||
| Right Anterior thalamic radiation | 1 | 12 | 10 | .91# | 0.02 | 1.78 | 3 | 48 | Borich et al., 2013 | ||
| Right forceps minor | 1 | 12 | 10 | .90# | 0.02 | 1.78 | 3 | 48 | Borich et al., 2013 | ||
| Left anterior thalamic radiation | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Right Inferior longitudinal fasciculus | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Right Inferior fronto-occipital fasciculus | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Left Inferior fronto-occipital fasciculus | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Subcallosal cortex | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Right thalamus | 1 | 12 | 10 | .89# | 0.01 | 1.77 | 3 | 48 | Borich et al., 2013 | ||
| Left White Matter | 1 | 16 | 16 | .85# | 0.18 | 1.87 | 3 | 50 | Mayer et al., 2012 | ||
| Right Cingulum Bundle | 1 | 12 | 11 | .79 | −0.06 | 1.64 | 3 | 53 | Wu et al., 2010b | ||
Note. #indicates moderate or large and significant effect, with adequate Nfs; Nstudies = Total number of studies included in analysis, NTBI = total number of participants in traumatic brain injury (TBI) group, Ncontrol = total number of Control participants, mean dw = mean weighted effect size, SE = standard error, 95% CIs = 95% confidence intervals, Nfs = Fail safe N,%OL = percentage overlap between the distribution of scores for the TBI and Control groups, NS = nonsignificant. A significant Q indicates a heterogeneous distribution. Where Q statistics were sig, as indicated by *, a random-effects model was used to calculate d. FA = fractional anisotropy.
TABLE 3 .
Cohen’s d Effect Sizes Comparing the ADC Values of the TBI and Control Groups in the Early Post-Injury Interval (1–4 Weeks)(Rank-Ordered by Effect Size)
| 95% CIs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Nstudies | NTBI | Ncontrol | Mean dw | dw SE | Lower Limit | Upper Limit | Nfs | %OL | Q | Study References |
| Total Corpus Callosum | 1 | 10 | 10 | 1.95# | 0.45 | 3.46 | 8 | 19 | Wilde et al., 2008 | ||
| Right Cingulum Bundle | 1 | 12 | 11 | 1.87# | 0.89 | 2.85 | 8 | 21 | Wu et al., 2010b | ||
| Left Cingulum Bundle | 1 | 12 | 11 | 1.24# | 0.35 | 2.14 | 5 | 36 | Wu et al., 2010b | ||
| Composite whole brain White Matter | 2 | 22 | 20 | −0.44 | 0.40 | −1.22 | 0.34 | 26 | 69.6 | 32.58NS | Chu et al., 2010; Virji-Babul et al., 2013 |
Note. #indicates moderate or large and significant effect, with adequate Nfs; Nstudies = Total number of studies included in analysis, NTBI = total number of participants in traumatic brain injury (TBI) group, Ncontrol = total number of Control participants, mean dw = mean weighted effect size, SE = standard error, 95% CIs = 95% confidence intervals, Nfs = Fail safe N,%OL = percentage overlap between the distribution of scores for the TBI and Control groups, NS = nonsignificant. A significant Q indicates a heterogeneous distribution. ADC = apparent diffusion coefficient.
ADC
ADC data on the other hand, was only available for four brain regions (refer to Table 3); only one of which—composite whole brain—was investigated by multiple studies (Nstudies = 2), albeit with a small sample. There were large and significant positive differences, with very good Nfs statistics, in the ADC values of three regions (75%): the total corpus callosum, and the left and right cingulum. These regions were only examined by single studies and the findings indicate that the ADC values were, somewhat surprisingly, lower in the early stages after a mild TBI than they were in healthy controls.
Medium- to Long-Term DTI Changes
FA
FA values were reported for 52 different regions-of-interest; 25 of which were examined by multiple studies. As seen in Table 4 the most commonly explored areas were: the genu of the corpus callosum (Nstudies = 7), followed by the splenium, body and total corpus callosum (Nstudies = 6), the uncinate fasciculus (left and right) (Nstudies = 5), and the anterior and posterior limb of the internal capsule (Nstudies = 4). Fewer studies calculated DTI metrics for the whole brain and other brain regions (Nstudies = 1–3).
TABLE 4 .
Cohen’s d Effect Sizes Comparing the FA Values of the TBI and Control Groups in the Medium to Long Term (Rank-Ordered by Effect Size)
| 95% C.I. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Nstudies | NTBI | Ncontrol | Mean dw | dw SE | Lower Limit | Upper Limit | Nfs | %OL | Q | Study References |
| Whole brain | 2 | 41 | 30 | −3.78 | 3.03 | −9.71 | 2.16 | 36 | 3 | 45.40* | Caeyenberghs et al., 2011; Adamson et al., 2013 |
| Cerebellum | 1 | 24 | 17 | −3.28# | −4.23 | −2.34 | 15 | 5 | Caeyenberghs et al., 2011 | ||
| Total Corpus Callosum | 6 | 118 | 117 | −3.08# | 0.74 | −4.52 | −1.63 | 86 | 7 | 83.40* | Caeyenberghs et al., 2011; Levin et al., 2008; Scheibel et al., 2011; Wilde et al., 2006b; Wozniak et al., 2007; Wu et al., 2010a |
| Left ventromedial | 2 | 41 | 45 | −2.42 | 1.94 | −6.21 | 1.38 | 22 | 13 | 18.59* | Levin et al., 2008; Scheibel et al., 2011 |
| Supracallosal | 1 | 14 | 14 | −1.98# | −3.26 | −0.70 | 9 | 19 | Wozniak et al., 2007 | ||
| Posterior Limb Internal Capsule | 4 | 74 | 74 | −1.95# | 0.63 | −3.18 | −0.72 | 35 | 19 | 25.61* | Caeyenberghs et al., 2011; Kurowski et al., 2009; Levin et al., 2008; Scheibel et al., 2011 |
| Anterior Limb Internal Capsule | 4 | 74 | 74 | −1.86# | 0.58 | −3.00 | −0.73 | 33 | 21 | 22.29* | Caeyenberghs et al., 2011; Kurowski et al., 2009; Levin et al., 2008; Scheibel et al., 2011; Wilde et al., 2011 |
| Anterior corona radiata | 1 | 24 | 17 | −1.59# | −2.29 | −0.88 | 7 | 26 | Caeyenberghs et al., 2011 | ||
| Left dorsolateral | 3 | 90 | 84 | −1.54# | 0.50 | −2.51 | −0.56 | 20 | 29 | 13.28* | Levin et al., 2008; Levin et al., 2011; Scheibel et al., 2011 |
| Body CC | 6 | 138 | 137 | −1.53# | 0.25 | −2.02 | −1.05 | 40 | 29 | 14.22* | Kurowski et al., 2009; Levin et al., 2008; Levin et al., 2011; Scheibel et al., 2011; Wilde et al., 2006b; Wu et al., 2010a |
| Right dorsolateral | 3 | 90 | 84 | −1.51# | 0.50 | −2.48 | −0.53 | 20 | 29 | 13.46* | Levin et al., 2008; Levin et al., 2011; Scheibel et al., 2011 |
| Genu CC | 7 | 179 | 168 | −1.40# | 0.34 | −2.06 | −0.74 | 42 | 32 | 42.72* | Ewing-Cobbs et al., 2008; Kurowski et al., 2009; Levin et al., 2008; Levin et al., 2011; Scheibel et al., 2011; Wilde et al., 2006b; Wu et al., 2010a |
| Right ventromedial | 2 | 41 | 45 | −1.34# | 0.24 | −1.81 | −0.86 | 11 | 35 | 4.42NS | Levin et al., 2008; Scheibel et al., 2011 |
| Right frontal lobe | 3 | 139 | 130 | −1.19# | 0.13 | −1.45 | −0.93 | 14 | 37 | 0.09NS | Levin et al., 2011; Max et al., 2012; Oni et al., 2010 |
| Left frontal lobe | 3 | 139 | 130 | −1.18# | 0.13 | −1.44 | −0.92 | 14 | 38 | 2.12NS | Levin et al., 2011; Max et al., 2012; Oni et al., 2010 |
| Splenium CC | 6 | 130 | 129 | −1.12# | 0.29 | −1.70 | −0.55 | 28 | 41 | 21.91* | Ewing-Cobbs et al., 2008; Kurowski et al., 2009; Levin et al., 2008; Scheibel et al., 2011; Wilde et al., 2006b; Wu et al., 2010a |
| Uncinate fasciculi | 1 | 40 | 37 | −1.08# | −1.56 | −0.60 | 4 | 42 | McCauley et al., 2011 | ||
| Corticospinal tract | 1 | 24 | 17 | −1.08# | −1.74 | −0.42 | 4 | 42 | Caeyenberghs et al., 2011 | ||
| Brainstem | 1 | 24 | 17 | −1.03# | −1.69 | −0.37 | 4 | 43 | Caeyenberghs et al., 2011 | ||
| Left arcuate fasciculus | 1 | 9 | 9 | −1.01# | −1.99 | −0.03 | 4 | 44 | Scheibel et al., 2011 | ||
| Left Inferior Fronto-Occipital fasciculus | 1 | 9 | 9 | −0.97 | −1.95 | 0.005 | 4 | 45 | Scheibel et al., 2011 | ||
| Left uncinate fasciculus | 5 | 162 | 138 | −0.97# | 0.12 | −1.20 | −0.74 | 19 | 45 | 4.23NS | Johnson et al., 2011; Levin et al., 2011; Max et al., 2012; Scheibel et al., 2011; Schmidt et al., 2013; Wilde et al., 2011 |
| Right uncinate fasciculus | 5 | 162 | 138 | −0.93# | 0.12 | −1.16 | −0.70 | 18 | 48 | 3.14NS | Johnson et al., 2011; Levin et al., 2011; Max et al., 2012; Scheibel et al., 2011; Schmidt et al., 2013 |
| Anterior Commissure | 1 | 16 | 16 | −0.88# | −1.61 | −0.16 | 3 | 49 | Wilde et al., 2006a | ||
| Right Inferior Fronto-Occipital fasciculus | 1 | 9 | 9 | −0.88 | −1.85 | 0.08 | 3 | 48 | Scheibel et al., 2011 | ||
| Isthmus | 1 | 41 | 31 | −0.87# | −1.36 | −0.39 | 3 | 49 | Ewing-Cobbs et al., 2008 | ||
| Cerebral peduncle | 1 | 24 | 17 | −0.87# | −1.52 | −0.22 | 3 | 49 | Caeyenberghs et al., 2011 | ||
| Right temporal lobe | 3 | 125 | 119 | −0.86# | 0.13 | −1.12 | −0.59 | 10 | 50 | 3.09NS | Levin et al., 2008; Levin et al., 2011; Max et al., 2012 |
| Cingulum Bundle | 1 | 40 | 37 | −0.85# | −1.31 | −0.38 | 3 | 50 | McCauley et al., 2011 | ||
| Orbito-frontal White Matter | 1 | 40 | 37 | −0.80# | −1.26 | −0.33 | 3 | 53 | McCauley et al., 2011 | ||
| Superior fasciculus | 2 | 23 | 26 | −0.79# | 0.30 | −1.38 | −0.20 | 6 | 53 | 2.73NS | Kurowski et al., 2009; Wozniak et al., 2007 |
| Left Centrum Semiovale | 1 | 44 | 44 | −0.79# | −1.40 | −0.17 | 3 | 55 | Max et al., 2012 | ||
| Right cingulate | 3 | 104 | 91 | −0.77# | 0.35 | −1.46 | −0.08 | 9 | 53 | 8.92* | Levin et al., 2011; Scheibel et al., 2011; Wilde et al., 2010 |
| Left cingulate | 3 | 104 | 91 | −0.77# | 0.15 | −1.06 | −0.59 | 9 | 54 | 3.74NS | Levin et al., 2011; Scheibel et al., 2011; Wilde et al., 2010 |
| Left temporal lobe | 3 | 125 | 119 | −0.76# | 0.13 | −1.02 | −0.49 | 8 | 53 | 3.37NS | Levin et al., 2008; Levin et al., 2011; Max et al., 2012 |
| Right Centrum Semiovale | 1 | 44 | 44 | −0.75# | −1.36 | −0.14 | 2 | 55 | Max et al., 2012 | ||
| Left frontal White Matter | 2 | 58 | 51 | −0.74# | 0.20 | −1.13 | −0.34 | 5 | 55 | 0.07NS | Kurowski et al., 2009; Levin et al., 2011 |
| Superior peduncle | 1 | 24 | 17 | −0.74# | −1.38 | −0.10 | 2 | 57 | Caeyenberghs et al., 2011 | ||
| Left Hippocampus | 1 | 21 | 20 | −0.70# | −1.34 | −0.07 | 2 | 57 | Juranek et al., 2012 | ||
| Thalamus | 1 | 24 | 17 | −0.64# | −1.28 | −0.004 | 2 | 59 | Caeyenberghs et al., 2011 | ||
| Posterior midbody | 1 | 41 | 31 | −0.60# | −1.07 | −0.12 | 2 | 61 | Ewing-Cobbs et al., 2008 | ||
| Right frontal White Matter | 2 | 58 | 51 | −0.58# | 0.19 | −0.96 | −0.20 | 4 | 62 | 0.25NS | Kurowski et al., 2009; Levin et al., 2011 |
| Rostral midbody | 1 | 41 | 31 | −0.57# | −1.05 | −0.09 | 2 | 63 | Ewing-Cobbs et al., 2008 | ||
| Anterior midbody | 1 | 41 | 31 | −0.53# | −0.10 | −0.05 | 1 | 64 | Ewing-Cobbs et al., 2008 | ||
| Left Amygdala | 1 | 21 | 20 | −0.51 | −0.72 | 0.51 | 1 | 66 | Juranek et al., 2012 | ||
| Inferior fasciculus | 2 | 23 | 26 | −0.49 | 0.29 | −1.04 | −0.07 | 3 | 67 | 2.24NS | Kurowski et al., 2009; Wozniak et al., 2007 |
| Right arcuate fasciculus | 1 | 9 | 9 | −0.46 | −1.09 | 0.76 | 0 | 89 | Scheibel et al., 2011 | ||
| Right Amygdala | 1 | 21 | 20 | −0.43 | −1.05 | 0.19 | 1 | 70 | Juranek et al., 2012 | ||
| Inferior peduncle | 1 | 24 | 17 | −0.42 | −1.04 | 0.21 | 1 | 73 | Caeyenberghs et al., 2011 | ||
| Left Inferior Longitudinal Fasciculus | 3 | 103 | 94 | −0.40 | 0.14 | −0.68 | −0.11 | 3 | 72 | 3.51NS | Levin et al., 2011; Scheibel et al., 2011; Schmidt et al., 2013 |
| Right Hippocampus | 1 | 21 | 20 | −0.11 | −1.13 | 0.12 | 0 | 92 | Juranek et al., 2012 | ||
| Right Inferior Longitudinal Fasciculus | 3 | 103 | 94 | −0.05 | 0.14 | −0.33 | 0.23 | 2 | 96 | 7.77NS | Levin et al., 2011; Scheibel et al., 2011; Schmidt et al., 2013 |
Note. #indicates moderate or large and significant effect, with adequate Nfs; CC = corpus callosum, Nstudies = Total number of studies included in analysis, NTBI = total number of participants in traumatic brain injury (TBI) group, Ncontrol = total number of Control participants, mean dw = mean weighted effect size, SE = standard error, 95% CIs = 95% confidence intervals, Nfs = Fail safe N, %OL = percentage overlap between the distribution of scores for the TBI and Control groups, NS = nonsignificant, *p < .05. A significant Q indicates a heterogeneous distribution. Where Q statistics were sig, as indicated by *, a random-effects model was used to calculate d. FA = fractional anisotropy.
Overall, there were moderate to large and significant negative differences between the FA values of the TBI and control groups in 36 of the 52 (69%) brain regions that have been examined (see Table 4), indicating that a large number of regions were negatively impacted by a TBI (anisotropy decreased). Indeed, regardless of statistical significance, all effect sizes were negative, indicating a clear pattern to these changes.
Of the regions that were assessed by multiple studies, there were large to very large and significant decreases in FA values in the: total corpus callosum; splenium, genu and body of the corpus callosum; anterior and posterior limbs of the internal capsule; right temporal lobe; left and right frontal lobes; left and right uncinate fasciculus; and the left and right dorsolateral regions. In addition, moderate and significant decreases in FA values were reported in the left temporal lobe and left and right cingulate. The Nfs statistics for all of these findings exceeded—and often well-exceeded—the number of studies that examined the specific region of interest, suggesting that publication bias is unlikely to impact on these findings. Also of interest is the finding that the FA values for the cerebellum and total corpus callosum are associated with very low %OL statistics (≤7%), indicating that there was minimal overlap between the FA scores of children in the TBI and Control groups.
ADC and MD
ADC or MD data for the medium- to long-term were reported for a total of 30 different regions, 17 of which were examined by multiple studies (see Table 5). Overall, there were large and significant increases (indicating reduced white matter integrity) in ADC/MD in 10 regions (33%), and moderate and significant increases in a further 16 areas (53%). Of the regions that were assessed by multiple studies, there were large and significant increases in ADC/MD values in the: anterior limb of the internal capsule, genu and total corpus callosum, right frontal white matter, and the left inferior longitudinal fasciculus. In addition, there were moderate and significant increases in the body and splenium of the corpus callosum, left frontal white matter, left dorsolateral region, temporal lobe (left and right), and the right inferior longitudinal fasciculus and the uncinate fasciculus (left and right). Once again, the Nfs statistics for these findings indicate that publication bias is unlikely to be a threat to the validity of these findings. Although there were many moderate to large and significant differences between the mean ADC/MD scores of the two groups, none of them were associated with very low %OL statistics (all ≥37%).
TABLE 5 .
Cohen’s d Effect Sizes Comparing the ADC/MD Values of the TBI and Control Groups in the Medium to Long Term (Rank-Ordered by Effect Size)
| 95% C.I.s |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Nstudie s | NTBI | Ncontrol | Mean dw | dwSE | Lower Limit | Upper Limit | Nfs | %OL | Q | Study References |
| Left inferior fronto-occipital fasciculus | 1 | 6 | 11 | −1.58# | −2.71 | −0.46 | 2 | 26 | Wilde et al., 2011 | ||
| Anterior Limb Internal Capsule | 2 | 38 | 47 | −1.19# | 0.24 | −1.66 | −0.73 | 10 | 37 | 0.34NS | Levin et al., 2008; Wilde et al., 2011 |
| Right ventromedial | 1 | 32 | 36 | −1.08# | −1.59 | −0.57 | 4 | 42 | Levin et al., 2008 | ||
| Genu CC | 3 | 104 | 100 | −1.03# | 0.15 | −1.33 | −0.74 | 12 | 43 | 0.45NS | Levin et al., 2008; Levin et al., 2011; Wu et al., 2010a |
| Right Amygdala | 1 | 21 | 20 | −0.97# | −1.62 | −0.33 | 4 | 45 | Juranek et al., 2012 | ||
| Posterior Limb Internal Capsule | 1 | 32 | 36 | −0.95# | −1.45 | −0.45 | 3 | 46 | Levin et al., 2008 | ||
| Right Frontal White Matter | 2 | 55 | 50 | −0.90# | 0.20 | −1.30 | −0.51 | 7 | 48 | −9.61NS | Levin et al., 2011; Wilde et al., 2011 |
| Total Corpus Callosum | 2 | 55 | 61 | −0.88# | 0.19 | −1.26 | −0.50 | 6 | 49 | 0.05NS | Levin et al., 2008; Wu et al., 2010a |
| Left Hippocampus | 1 | 21 | 20 | −0.84# | −1.48 | −0.20 | 3 | 53 | Juranek et al., 2012 | ||
| Left inferior longitudinal fasciculus | 2 | 85 | 82 | −0.83# | 0.16 | −1.14 | −0.53 | 6 | 51 | 0.07NS | Schmidt et al., 2013; Levin et al., 2011 |
| Right Hippocampus | 1 | 21 | 20 | −0.79# | −1.43 | −0.16 | 3 | 52 | Juranek et al., 2012 | ||
| Orbito-frontal White Matter | 1 | 40 | 37 | −0.78# | −1.24 | −0.31 | 3 | 53 | McCauley et al., 2011 | ||
| Left Amygdala | 1 | 21 | 20 | −0.76# | −1.40 | −0.13 | 2 | 54 | Juranek et al., 2012 | ||
| Left ventromedial | 1 | 32 | 36 | −0.75# | −1.25 | −0.26 | 2 | 55 | Levin et al., 2008 | ||
| Splenium CC | 2 | 55 | 61 | −0.73# | 0.19 | −1.12 | −0.36 | 5 | 55 | 0.63NS | Levin et al., 2008; Wu et al., 2010a |
| Left frontal White Matter | 2 | 55 | 50 | −0.72# | 0.20 | −1.13 | −0.32 | 5 | 54 | 2.30NS | Levin et al., 2011; Wilde et al., 2011 |
| Left Frontal lobe | 1 | 49 | 39 | −0.71# | −1.14 | −0.28 | 2 | 56 | Levin et al., 2011 | ||
| Uncinate fasciculi | 1 | 40 | 37 | −0.70# | −1.16 | −0.24 | 2 | 57 | McCauley et al., 2011 | ||
| Right uncinate fasciculus | 3 | 109 | 100 | −0.70# | 0.19 | −0.98 | −0.42 | 8 | 56 | 0.40NS | Levin et al., 2011; Johnson et al., 2011;Schmidt et al., 2013 |
| Body CC | 3 | 104 | 100 | −0.68# | 0.14 | −0.96 | −0.39 | 7 | 58 | 1.04NS | Levin et al., 2008; Levin et al., 2011; Wu et al., 2010a |
| Right Frontal lobe | 1 | 49 | 39 | −0.66# | −1.09 | −0.23 | 2 | 59 | Levin et al., 2011 | ||
| Left dorsolateral | 2 | 81 | 75 | −0.62# | 0.16 | −0.95 | −0.30 | 4 | 62 | 0.002NS | Levin et al., 2008; Levin et al., 2011 |
| Left temporal lobe | 2 | 81 | 75 | −0.60# | 0.16 | −0.92 | −0.28 | 4 | 61 | 0.00NS | Levin et al., 2008; Levin et al., 2011 |
| Right dorsolateral | 2 | 81 | 75 | −0.59 | 0.17 | −1.56 | 0.38 | 4 | 62 | 8.76 * | Levin et al., 2008; Levin et al., 2011 |
| Left uncinate fasciculus | 4 | 115 | 111 | −0.59# | 0.18 | −0.85 | −0.32 | 8 | 62 | 4.00NS | Levin et al., 2011;Johnson et al., 2011; Wilde et al., 2011;Schmidt et al., 2013 |
| Right temporal lobe | 2 | 81 | 75 | −0.53# | 0.16 | −0.85 | −0.21 | 3 | 64 | 0.49NS | Levin et al., 2008; Levin et al., 2011 |
| Right inferior longitudinal fasciculus | 2 | 85 | 82 | −0.50# | 0.15 | −0.80 | −0.20 | 3 | 66 | 0.01NS | Schmidt et al., 2013; Levin et al., 2011 |
| Cingulum Bundle | 1 | 40 | 37 | −0.49 | −0.95 | −0.04 | 1 | 66 | McCauley et al., 2011 | ||
| Left cingulate | 2 | 95 | 82 | −0.45 | 0.15 | −0.75 | −0.15 | 2 | 69 | 0.77NS | Levin et al., 2011; Wilde et al., 2010 |
| Right cingulate | 2 | 95 | 82 | −0.35 | 0.15 | −0.65 | −0.05 | 1 | 75 | 0.45NS | Levin et al., 2011; Wilde et al., 2010 |
Note. #indicates moderate or large and significant effect, with adequate Nfs; CC = corpus callosum, Nstudies = Total number of studies included in analysis, NTBI = total number of participants in traumatic brain injury (TBI) group, Ncontrol = total number of Control participants, mean dw= mean weighted effect size, SE= standard error, 95% CIs = 95% confidence intervals, Nfs = Fail safe N,%OL = percentage overlap between the distribution of scores for the TBI and Control groups, NS = nonsignificant, *p < .05. A significant Q indicates a heterogeneous distribution. Where Q statistics were sig, as indicated by *, a random-effects model was used to calculate d. ADC = apparent diffusion coefficient, MD = mean diffusivity.
Additional analyses of the mean effect size for studies using 1.5T versus 3T strength scanners, as well as for studies using Siemens and Phillips machines, showed broadly similar effect sizes regardless of scanner strength or brand (see Appendices C to J). Thus, the magnet strength and brand did not appear to impact on the difference in DTI metrics between ABI and control groups, suggesting that the findings from these studies could be analyzed together.
DISCUSSION
The current research analysed data from a total of 20 independent studies that have used DTI to investigate changes to the white matter integrity of children who had sustained a TBI. Data was available for 491 participants with TBI (321 males, 153 females) and 465 controls. The mean age for the TBI group was 14.1 years (SD = 2.1) and the injuries ranged in severity from mild to severe, although most were in the moderate to severe range. The average post-injury interval was 1.2 years, with very few studies examining white matter integrity in the early stages after an injury (Nstudies = 4).
As would be expected, most research has focused on the largest white matter tracts, including the corpus callosum, internal capsule, uncinate fasciculus, and inferior longitudinal fasciculus. Although numerous other regions have been investigated, many were only examined by single small-scale studies, therefore limiting the conclusions that can be drawn regarding these other regions.
All studies that conducted DTI in the early post-injury interval (1 to 4 weeks) examined children who had sustained a mild TBI. These studies found large and significant increases in the FA values of the TBI group, relative to controls, in almost all regions that were assessed, namely: the whole brain, corpus callosum, anterior corona radiata (left and right), cerebral peduncle, fornix, superior corona radiata, left cingulum bundle, anterior thalamic radiation (left and right), right forceps minor, right inferior longitudinal fasciculus, inferior fronto-occipital fasciculus (left and right), subcallosal cortex, right thalamus, and left white matter. These findings indicate that there are widespread changes in anisotropy, even after a mild TBI, such that there is greater uniformity in the direction of diffusion. Although all of these changes were sizeable, only the whole brain FA value showed limited overlap in the distribution of scores for the TBI and Control groups (%OL = 12); suggesting that this measure may have the greatest diagnostic potential.
Early post-injury ADC data, on the other hand, was available for many fewer regions, with large and significant positive changes—equating to lower ADC values—in the total corpus callosum and cingulum (left and right) following mild TBI. This paradoxical increase in FA and reduction in ADC has previously been attributed to axonal swelling and cytotoxic edema in the early stages after a TBI (Bigler & Bazarian, 2010). It remains to be determined whether these same changes occur in the early stages after moderate and severe TBIs in children, and exactly how long they persist.
When DTI was performed in the medium to long term (3 to 45 months), there were moderate to very large decreases in the FA values for the majority of white matter regions that have been examined. Of the regions that were assessed by multiple studies, there were large to very large and significant decreases in the FA values of children who sustained a TBI, affecting: the corpus callosum (total region, splenium, genu, and body); the internal capsule (anterior and posterior); right temporal lobe; frontal lobes (left and right); uncinate fasciculus (left and right); and the dorsolateral regions (left and right). In addition, there were moderate and significant decreases to the FA values in the left temporal lobe and cingulate (left and right).
The pattern of findings for ADC/MD data collected in the medium to long term after pediatric TBI was similar to that of the FA data. Specifically, when considering those regions that were assessed by multiple studies, there were large and significant increases in the ADC/MD values of the internal capsule (anterior limb), corpus callosum (genu and total region), right frontal lobe, and the left inferior longitudinal fasciculus following pediatric TBI. Moreover, there were moderate and significant increases to ADC/MD in the corpus callosum (body and splenium), left frontal lobe, left dorsolateral region, temporal lobe (left and right), right inferior longitudinal fasciculus, and uncinate fasciculus (left and right).
Reductions in FA values and increases in ADC/MD values in the medium to long term post-TBI indicate decreased integrity of white matter microstructure (Niogi & Mukherjee, 2010). These findings are consistent with findings from reviews of the sports-related mild TBI literature in older adolescents and young adults, which have also shown these changes in the post-acute period (Gardner et al., 2012). They also confirm the findings from reviews of the adult mild TBI literature (Aoki at al., 2012), suggesting that the changes may be similar in child and adult samples.
DTI measures have also been used to study structural changes occurring with normal development. In a recent review, Mills and Tamnes (2014) noted that both cross-sectional and longitudinal studies have demonstrated greater structural connectivity between childhood and adolescence, evidenced by increases in FA and decreases in MD in most white matter regions. The rate and timing of these changes vary between brain regions, with fronto-temporal white matter tracts developing later and the cingulum having the most prolonged development. This indicates that age may also be an important factor to consider, as Aoki et al. (2012) found a relationship between age and FA in the splenium in their meta-analysis of adult mild TBI (mean age range: 27 to 42). They suggest that, given that FA increases during childhood and adolescence, with a peak value at around age 30 years, an early TBI may accelerate the later decrease in FA during adulthood. Longitudinal research has yet to examine this.
This meta-analysis has demonstrated that there is a decrease in the integrity of white matter microstructure three or more months after mild to severe pediatric TBI and supports suggestions that DTI may provide a useful biomarker of injury (Bigler & Bazarian, 2010). Comparable changes to the corpus callosum, internal capsule and longitudinal fasciculus of adults have also been reported (Aoki et al., 2012; Gardner et al., 2012). However, more regions appear to be affected following pediatric TBI, supporting recent work that has suggested that children’s brains may be particularly vulnerable to TBI (Hessen, 2010).
LIMITATIONS
While the TBI and Control groups were found to be well-matched in terms of age and gender, it was not always clear whether children were matched in other important ways. For example, some studies recruited healthy controls from the local community (n = 7) and others used orthopaedic controls (n = 11). The latter group is thought to better control for pre-morbid functioning and other injury-related variables (e.g., pain, distress), as there is evidence to suggest that children who sustain a TBI have more pre-existing behavioral and learning problems than those from the general community (Asarnow et al., 1995). This also raises the possibility that some children who experience a TBI may have differences in DTI metrics in comparison to normally developing children that pre-date the TBI. Unfortunately, there were not enough studies that examined the same white matter tracts to examine the impact of the type of control group (orthopaedic vs. healthy controls) on the findings, although recent work comparing community and orthopaedic controls, who were recruited as part of a TBI study suggests these groups may be more comparable than previously thought, at least in adult settings (Mathias, Dennington, Bowden, & Bigler, 2013).
The participant numbers in many studies were relatively small and the severity of the TBIs varied, with most studies investigating moderate and severe TBI and some also examining mild and complicated mild TBIs. Once again, there were insufficient data to examine the impact of injury severity on the current findings, but it is highly likely that this variable contributed to the heterogeneity in the findings from different studies. The age range of participants also varied considerably (7 to 17 years). This is particularly important because there are distinct developmental trajectories for each of the major white matter tracts, commencing in childhood and culminating in adulthood (Imperati et al., 2011), which means that the consequences of a TBI may differ according to the age of the child. A finer-grained analysis of the these same DTI measures and regions is now needed, taking into account both the age of the child and the extent to which specific white matter regions have developed at the time of injury.
There was also variation in the MR scanners used, which may also have contributed to heterogeneity, with 12 studies using a 3T MR scanner and eight a 1.5T scanner. While it has generally been assumed that the stronger the MRI magnetic field (tesla units), the higher the quality of the image and the signal to noise ratio, a recent review was unable to find any studies comparing DTI at 1.5 and 3 T to definitively confirm this (Wardlaw et al., 2012). There is also considerable debate regarding the optimum diffusion imaging parameters, namely the number of diffusion encoding directions and strength, and correct image processing pipeline for DTI studies (Jones & Cercignani, 2010). The use of a group-wise experimental design (control versus patient), to some extent, enables comparison of results across the different studies evaluated for this meta-analysis. In addition, there was variation in the software packages used to analyse the DTI data and in the pre-processing of data, which may contribute to the heterogeneity of findings. However, there is currently insufficient data to analyse the impact of these variables on the findings.
Although DTI has enabled a paradigm-shift in our ability to measure microstructural changes to white matter, a key limitation is the inability of the diffusion tensor model to resolve crossing fibers (Jones & Cercignani, 2010). Estimates from the adult brain suggest that ˜ 90% of white matter voxels contain crossing fibres (Jeurissen, Leemans, Tournier, Jones, & Sijbers, 2013). In areas where fibres cross, diffusivity metrics obtained from the diffusion tensor (i.e., FA) become difficult to interpret. For instance, within regions where white matter is highly organized, anisotropy (FA) is low, making it difficult to distinguish possible diffuse axonal injury. Furthermore, if axonal injury occurs in only one fibre orientation within a given voxel, a paradoxical increase in FA can occur, potentially leading to an erroneous conclusion regarding the presence of inflammation or gliosis (Winston, 2012). Such constraints complicate the interpretation of DTI studies, especially in the more acute stages of injury.
CONCLUSION
In conclusion, this meta-analysis revealed substantial increases in FA and decreases in ADC in the short term (1–4 weeks), together with equivalent or greater decreases in FA and increases in ADC/MD in the medium to longer term (3–45 months), in the majority of white matter tracts following pediatric TBI. In particular, the early FA values for composite whole brain white matter and the medium- to long-term FA values for the cerebellum and total corpus callosum, appear to hold the greatest diagnostic potential as they were associated with the least overlap in the distribution of scores for the TBI and control groups (12%, 5%, and 7%, respectively). Notably, there was often significant heterogeneity in the effect sizes reported by different studies, which may arise from the use of small samples that include a wide range of ages (young children to older adolescents) and injuries (mild to severe TBIs). Research is now needed to examine how age/developmental stage and the severity of an injury impacts on these DTI metrics. It should also examine the relationship between these DTI findings and the cognitive and behavioral outcomes of children who have sustained a TBI in order to evaluate the extent to which DTI can contribute to the assessment and management of pediatric TBI patients. Furthermore, for diffusion MRI to be established as a clinical diagnosis tool for TBI, new image analysis methods have to be developed to enable the robust detection of subtle diffuse axonal injury in individual subjects. As outlined above, most published studies have reported differences in diffusivity measures using group-wise analyses, which is not suitable for measuring heterogeneous brain injuries, nor does it aid clinical decision making regarding whether an individual has sustained a TBI. Recent work employing the tensor model has shown that it is possible to detect diffuse axonal injury in individual patients with mTBI using Enhanced Z-score Microstructural Assessment for Pathology (EZ-MAP) (Lipton et al., 2012). Further studies are required for the translation of this promising technology into clinical practice.
APPENDIX: ONLINE RESOURCES
Table A: Terms Used in Database Search
Databases:
| PubMed | Psycinfo |
| Scopus | Embase |
| Web of Science | CINAHL |
| Informit |
Logic Grid for PUBMED
| DTI | TBI | Child |
|---|---|---|
| (Diffusion tensor imaging[mh] OR diffusion tensor imaging[tiab] OR dti[tiab] OR diffusion magnetic resonance imaging[mh] OR diffusion magnetic resonance imaging[tiab] OR diffusion weighted imaging[tiab] OR dwi[tiab]) | (Brain injuries[mh:noexp] OR Brain hemorrhage, traumatic[mh:noexp] OR Craniocerebral trauma[mh:noexp] OR brain injur*[tiab] OR tbi[tiab] OR brain trauma[tiab]) | (Child[mh] OR child*[tw] OR adolescent[mh] OR adolesce*[tw] OR teen*[tiab] OR paediatric[tiab] or pediatric[tiab]) |
Logic Grid for PsycINFO
| DTI | TBI | Child |
|---|---|---|
| TI ‘diffusion tensor imaging’ OR AB ‘diffusion tensor imaging’ OR TI dti OR AB dti OR TI ‘diffusion magnetic resonance imaging’ OR AB ‘diffusion magnetic resonance imaging’ OR TI ‘diffusion weighted imaging’ OR AB ‘diffusion weighted imaging’ OR TI dwi OR AB dwi | DE ‘brain damage’ OR DE ‘traumatic brain injury’ OR TI ‘brain injur*’ OR AB ‘brain injur*’ OR TI tbi OR AB tbiOR TI ‘brain trauma’ OR AB ‘brain trauma’ | SU child OR TI child* OR AB child* OR SU adolescent OR TI adolesce* OR AB adolesce* OR TI teen* OR AB teen* OR TI pediatric*OR AB pediatric* OR TI paediatric* OR AB paediatric* |
Logic Grid for SCOPUS
| DTI | TBI | Child |
|---|---|---|
| TITLE-ABS-KEY(diffusion tensor imaging) OR TITLE-ABS-KEY(dti) OR TITLE-ABS-KEY(diffusion magnetic resonance imaging) OR INDEXTERMS(diffusion tensor imaging) OR INDEXTERMS(diffusion magnetic resonance imaging) OR TITLE-ABS-KEY(diffusion weighted imaging) OR TITLE-ABS-KEY(dwi) | INDEXTERMS(brain injuries) OR TITLE-ABS-KEY(brain injur*) OR TITLE-ABS-KEY(tbi) OR TITLE-ABS-KEY(traumatic brain injury) OR INDEXTERMS(craniocerebral trauma) OR TITLE-ABS-KEY(brain trauma) OR INDEXTERMS(brain hemorrhage, traumatic) | INDEXTERMS(child) OR TITLE-ABS-KEY(child*) OR INDEXTERMS(adolescent) OR TITLE-ABS-KEY(adolesce*) OR TITLE-ABS-KEY(teen*) OR TITLE-ABS-KEY(pediatric*) OR TITLE-ABS-KEY(paediatric*) |
Logic Grid for Embase
| DTI | TBI | Child |
|---|---|---|
| “diffusion tensor imaging”/de OR “diffusion weighted imaging”/de OR “diffusion tensor imaging”:ti,abORdti:ti,ab OR “diffusion weighted imaging”:ti,ab OR “diffusion magnetic resonance imaging”:ti,ab OR dwi:ti,ab | “Brain injury”/de OR “traumatic brain injury”/de OR “traumatic brain injury”:ti,ab OR tbi:ti,abOR “brain injuries”:ti,ab OR “craniocerebral trauma”:ti,ab OR “brain trauma”:ti,ab | Child/de OR child*:ti,ab OR adolescent/de OR adolesce*:ti,ab OR teen*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab |
Logic Grid for Web of Science
| DTI | TBI | Child |
|---|---|---|
| TS=“diffusion tensor imaging” OR TS=dti OR TS=”diffusion magnetic resonance imaging” OR TI=“diffusion tensor imaging” OR TI=dti OR TI=”diffusion magnetic resonance imaging” OR TI=“diffusion weighted imaging” OR TI=dwi OR TS=”diffusion weighted imaging” OR TS=dwi | TS=“brain injur*” OR TS=tbi OR TS=“traumatic brain injury” OR TS=“brain trauma”OR TS=”craniocerebraltrauma” OR TI=“brain injur*” OR TI=tbi OR TI=“traumatic brain injury” OR TI=“brain trauma” | TS=(child OR child*) OR TS=(adolescent OR adolesce*) OR TS=teen* OR TI=(child OR child*) OR TI=(adolescent OR adolesce*) OR TI=teen* OR TS=(pediatric OR paediatric) OR TI=(pediatric OR paediatric) |
Logic Grid for CINAHL
| DTI | TBI | Child |
|---|---|---|
| TI “diffusion tensor imaging” OR AB “diffusion tensor imaging” OR TI dti OR AB dti OR TI ”diffusion magnetic resonance imaging” OR AB “diffusion magnetic resonance imaging” OR TI “diffusion weighted imaging” OR AB “diffusion weighted imaging” OR TI dwi OR AB dwi | MH “Brain injuries” OR TI “brain injur*” OR AB “brain injur*” OR TI tbi OR AB tbiOR TI “traumatic brain injury” OR AB “traumatic brain injury” OR TI “craniocerebral trauma” OR AB “craniocerebral trauma” OR TI “brain trauma” OR AB “brain trauma” | MH child OR TI child* OR AB child* OR TI adolesce* OR AB adolesce* OR TI teen* OR AB teen* OR TI pediatric OR AB pediatric OR TI paediatric OR AB paediatric |
Logic Grid for Informit (Health Databases)
| DTI | TBI | Child |
|---|---|---|
| (SU=Diffusion tensor imaging OR TI,AB=diffusion tensor imaging OR TI,AB=dti OR SU=Diffusion magnetic resonance imaging OR TI,AB=diffusion magnetic resonance imaging) | (SU=acquired brain injuries OR TI,AB=tbi OR TI,AB= brain injur* OR SU=traumatic brain injury) | (SU=Child OR TI,AB=child* OR SU=Adolescent OR TI,AB=adolesce* OR TI,AB=teen*) |
TABLE B Study Characteristics for the Individual DTI Studies Examined in This Meta-Analysis
| Reference | MR Specifications | Acquisition Voxel Size (mm) Reconstruction Voxel Size (mm) | No. Directions | Duration of DTI | Software Package Used for DTI Pre/Post Processing and Analysis | Number of ROIs |
|---|---|---|---|---|---|---|
| Adamson et al. (2013) | 3T Philips Medical Systems scanner | Echoplanar imaging sequence: TR = 8799ms; TE = 88ms; flip angle = 90°; slice thickness = 2mm; 72 contiguous slices; FOV = 180 × 180 mm2; b = 1000s/mm2 | 33 | FMRIB Software Library (TBSS, FNIRT) | Whole brain | |
| Borich et al. (2013)/ Virji-Babul et al. (2013) | 3T Philips Achieva | Single shot echoplanar imaging sequence; TR = 7013ms; TE = 60ms; FOV = 224 × 224mm; 70 slices; voxel dimension = 2.23; b = 700s/mm2 | 60 | 7 mins | ExploreDTI | Left and right anterior corona radiata, left and right thalamic radiation, right forceps minor, right inferior longitudinal fasciculus, right and left inferior fronto-occipital fasciculus, fornix, left subcallosal cortex, right thalamus, genu CC |
| Caeyenberghs et al. (2010/2011) | 3T Phillips Intera with 8-channel head coil | Diffusion weighted single shot spin-echo echoplanar imaging with data acquisition matrix = 112 × 112; FOV = 220 × 220 mm2; TR = 7916 ms, TE = 68 ms, parallel imaging factor 2.5, and 68 contiguous sagittal slices (slice thickness = 2.2 mm; voxel size = 2 × 2 × 2.2 mm3) | 45 | ExploreDTI | Corpus callosum, corticospinal tract, superior cerebellar peduncle, inferior cerebellar peduncle, cerebral peduncle, anterior corona radiate, ALIC, PLIC | |
| Chu et al. (2010)/ Wu, et al. (2010)a Yallampalli et al. (2010)/ Wilde et al. (2008) | 3T Philips Achieva | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 6318 ms; TE = 51 ms; 70 slices, slice thickness = 2 mm, 0 mm gap; b = 0, and 1000 s/mm2 | 30 | 5 mins | Philips PRIDE registration tool and DTIStudio | Right and left cingulumbundle, fornix, total CC, 3 tissue masks: White Matter, Grey Matter, and CerebroSpinal Fluid used in regional analyses of whole brain |
| Ewing-Cobbs et al. (2008) | 3T Philips Intera | Single shot spin-echo diffusion sensitized echoplanar imaging; FOV = 240 × 240 mm2; TR = 6100 ms; TE = 84 ms; b = 1000 s/mm2; slice thickness = 3 mm with 44 axial slices | 7 mins | In-house-developed DTI design and analysis toolbox | Rostrum, renu, rostral midbody, anterior midbody, posterior midbody, isthmus, and splenium of the corpus callosum | |
| Johnson et al. (2011) | 3T Philips scanner with 8-channel head coil | Single shot spin-echo diffusion sensitized echoplanar imaging sequence; FOV = 240 × 240 mm2; TR = 6100 ms; TE = 84 ms; b = 0, and 1000 s/mm2; matrix = 256 x 256; slice thickness = 3 mm | 21 | 7 mins | Freesurfer v4.1.0 software and FMRIB Software Library (FLIRT) | Uncinate fasciculus |
| Juranek et al. (2012) | 3T Philips scanner with 8-channel phase array head coil | Single shot spin-echo diffusion sensitized echoplanar imaging sequence; FOV = 240 × 240 mm2; TR = 6100 ms; TE = 84 ms; b = 0, and 1000 s/mm2; matrix = 256 × 256; slice thickness = 3 mm | 21 | 7 mins | Freesurfer v4.0.5 software and FMRIB Software Library (BET, FLIRT, DTIFIT) | Amygdala, hippocampus |
| Kurowski et al. (2009)/ Yuan et al. (2007) | 3T Siemens Trio | Single shot spin-echo echoplanar imaging sequence; FOV = 25.6 × 25.6 cm2; TR = 6000 ms; TE = 87 ms; b = 1000 s/mm2; matrix = 128 × 128; slice thickness = 2 mm with 46 axial slices | 12 | 5:48 mins | DTIStudio 2.4 from John Hopkins University | Splenium of the corpus callosum, genu of the corpus callosum, body of the corpus callosum, ALIC, PLIC, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, left frontal white matter, right frontal white matter |
| Levin et al. (2008) | 1.5T Philips Integra | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860s/mm2 | 15 | 5:45 mins | Philips diffusion affine registration tool and Philips fiber tracking 4.1V3 Beta 4 software | Corpus callosum, genu CC, body CC, splenium CC, ALIC, PLIC, frontal lobes (ventromedial, dorsolateral), temporal lobes |
| Levin et al. (2011) | 1.5T Philips scanner | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; slice thickness = 2.7 mm; b = 860 s/mm2 | 15 | 5:45 mins | Philips diffusion affine registration tool and Philips fiber tracking 4.1 V3 Beta 4 software | Corpus callosum, prefrontal white matter, uncinate fasciculus, inferior longitudinal fasciculus, cingulum bundle, frontal lobes, temporal lobes |
| Max et al. (2012) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echolpanar imaging sequence; FOV = 256mm; TR = 10150.5ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 2, and 860 s/mm2 | 15 | 6 mins | Philips PRIDE registration tool and Philips fiber tracking 4.1v3 Beta 2 software | Right and left frontal lobe, right and left uncinate fasciculi, right and left centrum semiovale, and right and left temporal lobes |
| Mayer et al. (2012) | 3T Siemens | Gradient echo sequence; FOV = 180x240 mmTR = 28ms; TE = 20ms; flip angle = 15°; slice thickness = 1.5mm, 88 slices; b = 0 and 860 mm230 | 30 | FMRIB Software Library (TBSS, FNIRT) and Automated Functional Neuro-Imaging (AFNI) | Left and right anterior corona radiata, left white matter tracts, left cerebral peduncle, left superior corona radiata | |
| McCauley et al. (2011) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860 s/mm2 | 15 | 5:45 mins | Philips PRIDE registration tool and Philips fiber tracking 4.1v3 Beta 2 software | Cingulum bundle, orbitofrontal white matter, uncinate fasciculus |
| Oni et al. (2010)/ Wilde et al. (2010) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860 s/mm2 | 15 | 5:45 mins | Philips diffusion affine registration tool and Philips fiber tracking 4.1V3 Beta 4 software | Left and right frontal lobes, left and right cingulum bundle |
| Schiebel et al. (2011) | 3T Philips Achieva | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 224 mm; TR = 6161 ms; TE = 51 ms; slice thickness = 2 mm, 0 mm gap; b = 0, and1000 s/mm2 | 32 | 5 mins | Philips diffusion affine registration tool and Philips fiber tracking 4.1V3 Beta 4 software | Ventromedial and dorsolateral frontal regions, arcuate fasciculus, inferior fronto-occipital fasciculus, inferior longlitudinal fasciculus, uncinate fasciculus, corpus callosum, cingulum bundle, ALIC, PLIC |
| Schmidt et al. (2013) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echolpanar imaging sequence; FOV = 256mm; TR = 10150.5ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860 s/mm2 | 15 | Philips PRIDE registration tool and Philips fiber tracking 4.1v3 Beta 2 software | Right and left inferior left longitudinal fasciculus, right and left uncinated fasciculus | |
| Wilde et al. (2005/2006) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860 s/mm2 | 15 | 5:45 mins | Philips PRIDE registration tool and Philips Fiber Tracking 4.1V3 software | Genu CC, body CC, splenium CC, anterior commissure |
| Wilde et al. (2011) | 3T Philips Intera | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 224 mm; TR = 6161 ms; TE = 51 ms; slice thickness = 2 mm, 0 mm gap; b = 0, and 1000 s/mm2 | 30 | 5:45 mins | Philips PRIDE registration tool and Philips fiber tracking 4.1v3 Beta 2 software | Inferiorfronto-occipital fasciculus, inferior longitudinal fasciculus, frontal white matter, uncinate fasciculus, ALIC, PLIC, cingulum bundle |
| Wozniak et al. (2007) | 3T Siemens Trio | Dual spin echo, single shot, pulsed gradient, echoplanar imaging sequence; FOV = 256 mm; TR = 8300 ms; TE = 86 ms; 64 slices; b = 1000 s/mm2 | 12 | FMRIB Software Library (BET, FLIRT, FAST, FUGUE, FDT) | Inferior frontal mask, superior frontal mask, supracallosal mask, genu CC, midbody CC, splenium CC | |
| Wu, Wilde, Bigler, Li, et al. (2010) | 1.5T Philips Intera | Transverse multi-slice spin-echo single shot echoplanar imaging sequence; FOV = 256 mm; TR = 10150.5 ms; TE = 90 ms; 55 slices, slice thickness = 2.7 mm, 0 mm gap; b = 0, and 860 s/mm2 | 15 | 6 mins | Philips PRIDE registration tool and Philips fiber tracking 4.1v3 Beta 2 software | Genu CC, body CC, splenium CC, as well as total CC |
Note. ROI = region of interest; T = tesla; FOV = field of view; TR = repetition time; TE = echo time; mm = millimetre; ms = millisecond; CC = corpus callosum; ALIC = anterior limb of the internal capsule; PLIC = posterior limb of the internal capsule; MR = magnetic resonance; DTI = diffusion tensor imaging.
TABLE C Mean D for Each Study Using 1.5T Scanner in the Medium to Long Term (FA)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 16 | 16 | 0.26 | Philips | Intera | 1.5 | 5 | −1.6788 | 0.67593 |
| 11 | 40 | 37 | 0.34 | Philips | Intera | 1.5 | 3 | −0.91 | 0.14933 |
| 12 | 49 | 39 | 0.31 | Philips | 1.5 | 16 | −0.925 | 0.4719 | |
| 13 | 32 | 36 | 0.25 | Philips | Integra | 1.5 | 12 | −0.98917 | 0.3765 |
| 15 | 46 | 47 | 0.25 | Philips | Intera | 1.5 | 4 | −0.8875 | 0.35056 |
| 16 | 23 | 25 | 0.33 | Philips | Intera | 1.5 | 4 | −1.4375 | 0.47077 |
| 24 | 44 | 44 | 0.25 | Philips | Intera | 1.5 | 8 | −0.83125 | 0.24503 |
| 26 | 45 | 46 | 0.35 | Philips | Intera | 1.5 | 4 | −0.73 | 0.44083 |
| AVERAGE D | −1.04865 |
TABLE D Mean D for Each Study Using 3T Scanner in the Medium to Long Term (FA)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 24 | 17 | 3.862 | Philips | Intera | 3 | 12 | −1.9425 | 1.88846 |
| 21 | 21 | 20 | 0.25 | Philips | 3 | 4 | −0.4375 | 0.24595 | |
| 22 | 9 | 9 | 3.65 | Philips | Achieva | 3 | 20 | −2.4745 | 1.593 |
| 28 | 17 | 13 | 1.94 | Philips | 3 | 6 | −0.88833 | 0.03656 | |
| 3 | 9 | 12 | 1 | Siemens | Trio | 3 | 9 | −0.71333 | 0.43706 |
| 6 | 41 | 31 | 3.25 | Philips | Intera | 3 | 6 | −0.64 | 0.37084 |
| 8 | 15 | 15 | 0.25 | Philips | 3 | 2 | −0.77 | 0.3677 | |
| 10 | 14 | 14 | 0.683 | Siemens | Trio | 3 | 4 | −1.2675 | 0.61619 |
| AVERAGE D | −1.14171 |
TABLE E Mean D for Each Study in the Short Term (All 3T) (FA)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 11 | 11 | 0.016 | Philips | Achieva | 3 | 2 | 1.17 | |
| 20 | 12 | 11 | 0.008 | Philips | Achieva | 3 | 2 | 0.895 | |
| 29 | 16 | 16 | 0.043 | Siemens | 3 | 6 | 1.077167 | ||
| 25 | 12 | 10 | 0.097 | Philips | Achieva | 3 | 13 | 0.912 | |
| AVERAGE D | 1.01354 |
TABLE F Mean D for Each Study Using 1.5T Scanner in the Medium to Long Term (ADC)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 11 | 40 | 37 | 0.34 | Philips | Intera | 1.5 | 3 | 0.66 | 0.14422 |
| 12 | 49 | 39 | 0.31 | Philips | 1.5 | 16 | 0.56375 | 0.20169 | |
| 13 | 32 | 36 | 0.25 | Philips | Integra | 1.5 | 12 | 0.85 | 0.24768 |
| 15 | 46 | 43 | 0.25 | Philips | Intera | 1.5 | 4 | 0.6025 | 0.11177 |
| 16 | 23 | 25 | 0.33 | Philips | Intera | 1.5 | 4 | 0.86 | 0.22015 |
| 26 | 45 | 46 | 0.035 | Philips | Intera | 1.5 | 4 | 0.6825 | 0.17017 |
| AVERAGE D | 0.70313 |
TABLE G Mean D for Each Study Using 3T Scanner in the Medium to Long Term (ADC)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 21 | 21 | 20 | 0.25 | Philips | 3 | 4 | 0.84 | 0.09274 | |
| 14 | 6 | 11 | 0.25 | Philips | Intera | 3 | 5 | 1.5 | 0.27595 |
| 8 | 15 | 15 | 0.25 | Philips | 3 | 2 | 0.455 | 0.0495 | |
| AVERAGE D | 0.93167 |
TABLE H Mean D for Each Study in the Short Term (All 3T) (ADC)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 10 | 10 | 0.007 | Philips | Achieva | 3 | 2 | −1.283 | 4.57781 |
| 20 | 12 | 11 | 0.008 | Philips | Achieva | 3 | 2 | −1.555 | 0.44548 |
| 25 | 12 | 10 | 0.097 | Philips | Achieva | 3 | 1 | 0.933 | |
| AVERAGE D | −0.635 |
TABLE I Mean D of 3T Siemens Trio Scanners (FA)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 9 | 12 | 1 | Siemens | Trio | 3 | 9 | −0.71333 | 0.43706 |
| 10 | 14 | 14 | 0.683 | Siemens | Trio | 3 | 4 | −1.2675 | 0.61619 |
| AVERAGE D | −0.99041 | 0.39186 |
TABLE J Mean D of 3T Philips Scanners (FA)
| Study Number | TBI Participants | Control Participants | Time Since Injury | Scanner Brand | Scanner Model | MRI Strength | Number of Ds | Mean D | SD |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 24 | 17 | 3.862 | Philips | Intera | 3 | 12 | −1.9425 | 1.88846 |
| 21 | 21 | 20 | 0.25 | Philips | 3 | 4 | −0.4375 | 0.24595 | |
| 22 | 9 | 9 | 3.65 | Philips | Achieva | 3 | 20 | −2.4745 | 1.593 |
| 28 | 17 | 13 | 1.94 | Philips | 3 | 6 | −0.88833 | 0.03656 | |
| 6 | 41 | 31 | 3.25 | Philips | Intera | 3 | 6 | −0.64 | 0.37084 |
| 8 | 15 | 15 | 0.25 | Philips | 3 | 2 | −0.77 | 0.3677 | |
| AVERAGE D | −1.19214 | 0.81882 |
REFERENCES
- Note . References marked with an asterisk indicate studies included in the meta-analysis. [Google Scholar]
- *Adamson C., Yuan W. H., Babcock L., Leach J. L., Seal M. L., Holland S. K, & Wade S. L. Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Injury. 2013:454. doi: 10.3109/02699052.2012.750756. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V., Brown S., Newitt H. Hoile H. Long-term outcome from childhood traumatic brain injury: Intellectual ability, personality, and quality of life. Neuropsychology. 2011:176. doi: 10.1037/a0021217. &. –. doi: [DOI] [PubMed] [Google Scholar]
- Aoki Y., Inokuchi R., Gunshin M., Yahagi N. Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2012:870. doi: 10.1136/jnnp-2012-302742. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow R. F., Satz P., Light R., Zaucha K., Lewis R. McCleary C. The UCLA study of mild closed head injury in children and adolescents. In: Broman S., editor; Michel M. E., editor. Traumatic brain injury in children. New York, NY: Oxford University Press; 1995. pp. 117–146. &. &. –. [Google Scholar]
- Assaf Y. Pasternak O. Diffusion Tensor Imaging (DTI)-based white matter mapping in brain research: A review. Journal of Molecular Neuroscience. 2008:51. doi: 10.1007/s12031-007-0029-0. &. –. doi: [DOI] [PubMed] [Google Scholar]
- Babikian T. Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology. 2009:283. doi: 10.1037/a0015268. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M. H., Beare R., Ditchfield M., Coleman L., Babl F. E., Kean M., … Anderson V. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex. 2013 doi: 10.1016/j.cortex.2012.08.015. 49, 591–598. doi: [DOI] [PubMed] [Google Scholar]
- Bigler E. D. Bazarian J. J. Diffusion tensor imaging: A biomarker for mild traumatic brain injury? Neurology. 2010:626. doi: 10.1212/WNL.0b013e3181d3e43a. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Borich M., Makan N., Boyd L. Virji-Babul N. Combining whole-brain voxel-wise analysis with in vivo tractography of diffusion behavior after sports-related concussion in adolescents: A preliminary report. Journal of Neurotrauma. 2013:1243. doi: 10.1089/neu.2012.2818. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Caeyenberghs K., Leemans A., Geurts M., Linden C. V., Smits-Engelsman B. C. M., Sunaert S, & Swinnen S. P. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabilitation & Neural Repair. 2011:492. doi: 10.1177/1545968310394870. –. doi: [DOI] [PubMed] [Google Scholar]
- *Caeyenberghs K., Leemans A., Geurts M., Taymans T., Linden C. V., Smits-Engelsman B. C. M, … Swinnen S. P. Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Human Brain Mapping. 2010a:992. doi: 10.1002/hbm.20911. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Caeyenberghs K., Leemans A., Geurts M., Taymans T., Linden C. V., Smits-Engelsman B. C. M, & Swinnen S. P. Brain-behavior relationships in young traumatic brain injury patients: Fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia. 2010b:1472. doi: 10.1016/j.neuropsychologia.2010.01.017. –. doi: [DOI] [PubMed] [Google Scholar]
- Cattelani R., Lombardi F., Brianti R. Mazzucchi A. Traumatic brain injury in childhood: Intellectual, behavioural and social outcome into adulthood. Brain Injury. 1998:283. doi: 10.1080/026990598122584. &. –. [DOI] [PubMed] [Google Scholar]
- *Chu Z., Wilde E. A., Hunter J. V., McCauley S. R., Bigler E. D., Troyanskaya M, … Levin H. S. Voxel-based analysis of Diffusion Tensor Imaging in mild traumatic brain injury in adolescents. American Journal of Neuroradiology. 2010:340. doi: 10.3174/ajnr.A1806. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992:155. doi: 10.1037//0033-2909.112.1.155. –. [DOI] [PubMed] [Google Scholar]
- Duckworth J. L. Stevens R. D. Imaging brain trauma. Current Opinion in Critical Care. 2010:92. doi: 10.1097/MCC.0b013e3283374900. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Ewing-Cobbs L., Prasad M. R., Swank P., Kramer L., Cox C. S., Jr., Fletcher J. M, … Levin H. S. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: Relation to neurobehavioral outcomes. Neuroimage. 2008:1305. doi: 10.1016/j.neuroimage.2008.06.031. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher G. R., editor; Ludwig S., editor. Textbook of pediatric emergency medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. &. [Google Scholar]
- Gardner A., Kay-Lambkin F., Stanwell P., Donnelly J., Williams W. H., Hiles A., … Jones D. K. A systematic review of diffusion tensor imaging findings in sports-related concussion. Journal of Neurotrauma. 2012:2521. doi: 10.1089/neu.2012.2628. –. doi: [DOI] [PubMed] [Google Scholar]
- Hanten G., Wilde E. A., Menefee D. S., Li X., Lane S., Vasquez C., … Levin H. S. Correlates of social problem solving during the first year after traumatic brain injury in children. Neuropsychology. 2008:357. doi: 10.1037/0894-4105.22.3.357. –. doi: [DOI] [PubMed] [Google Scholar]
- Hessen E. Very long-term neuropsychological and behavioral consequences of mild and complicated mild TBI: Increased impact of pediatric versus adult TBI. In: Anderson V., editor; Yeates K. O., editor. Pediatric traumatic brain injury: New frontiers in clinical and translational research. Cambridge, UK: Cambridge University Press; 2010. pp. 118–144. &. –. [Google Scholar]
- Imperati D., Colcombe S., Kelly C., Di Martino A., Zhou J., Castellanos F. X. Milham M. P. Differential development of human brain white matter tracts. PLoSOne. 2011:e23437. doi: 10.1371/journal.pone.0023437. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J., Jones D. K. Sijbers J. Investigating the prevalence of complex fibre configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping. 2013:2747. doi: 10.1002/hbm.22099. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Johnson C. P., Juranek J., Kramer L. A., Prasad M. R., Swank P. R. Ewing-Cobbs L. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. Journal of the International Neuropsychological Society. 2011:663. doi: 10.1017/s1355617711000464. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. K. Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in Biomedicine. 2010:803. doi: 10.1002/nbm.1543. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Juranek J., Johnson C. P., Prasad M. R., Kramer L. A., Saunders A., Filipek P. A, … Ewing-Cobbs L. Mean diffusivity in the amygdala correlates with anxiety in pediatric TBI. Brain Imaging & Behavior. 2012:36–48. doi: 10.1007/s11682-011-9140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler J. Keshavarz R. Penetrating head injury in children: A case report and review of the literature. Journal of Emergency Medicine. 2001:145. doi: 10.1016/s0736-4679(01)00363-8. &. –. [DOI] [PubMed] [Google Scholar]
- *Kurowski B., Wade S. L., Cecil K. M., Walz N. C., Yuan W., Rajagopal A, & Holland S. K. Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. Journal of Pediatric Rehabilitation Medicine. 2009:273. doi: 10.3233/prm-2009-0093. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J. F., Poupon C., Clark C. A., Pappata S., Molko N., & Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001:534. doi: 10.1002/jmri.1076. –. doi: [DOI] [PubMed] [Google Scholar]
- Lee B. Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005:372. doi: 10.1602/neurorx.2.2.372. &. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Levin H. S., Wilde E. A., Chu Z., Yallampalli R., Hanten G. R., Li X, … Hunter J. V. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation. 2008:197. doi: 10.1097/01.HTR.0000327252.54128.7c. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Levin H. S., Wilde E. A., Hanten G., Li X., Chu Z. D., Vasquez A. C, … Hunter J. V. Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Developmental Neuropsychology. 2011:273. doi: 10.1080/87565641.2010.549885. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois F., Tournier J. D., Pigdon L., Connelly A. Morgan A. T. Corticobulbar tract changes as predictors of dysarthria in childhood brain injury. Neurology. 2013:926. doi: 10.1212/WNL.0b013e3182840c6d. &. –. doi: [DOI] [PubMed] [Google Scholar]
- Lipsey M. W. Wilson D. B. Practical meta-analysis. Thousand Oaks, CA: SAGE Publications; 2001. &. [Google Scholar]
- Lipton M. L., Kim N., Park Y. K., Hulkower M. B., Gardin T. M., Shifteh K. Branch C. A. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: Intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging & Behavior. 2012:329. doi: 10.1007/s11682-012-9175-2. …. –. doi: [DOI] [PubMed] [Google Scholar]
- Mathias J. L., Dennington V., Bowden S. C. Bigler E. D. Community versus orthopaedic controls in traumatic brain injury research: How comparable are they? Brain Injury. 2013:887. doi: 10.3109/02699052.2013.793398. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Max J. E., Wilde E. A., Bigler E. D., Thompson W. K., MacLeod M., Vasquez A. C, … Levin H. S. Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2012:1208. doi: 10.1016/j.jaac.2012.08.026. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. R., Ling J., Mannell M. V., Gasparovic C., Phillips J. P., Doezema D., … Yeo R. A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010:643. doi: 10.1212/WNL.0b013e3181d0ccdd. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Mayer A. R., Ling J. M., Yang Z., Pena A., Yeo R. A. Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. Journal of Neuroscience. 2012:17961. doi: 10.1523/JNEUROSCI.3379-12.2012. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *McCauley S. R., Wilde E. A., Bigler E. D., Chu Z., Yallampalli R., Oni M. B, … Levin H. S. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. Journal of Neurotrauma. 2011:503. doi: 10.1089/neu.2010.1555. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 1993:86. –. [Google Scholar]
- Mills K. L. Tamnes C. K. Methods and considerations for longitudinal structural brain imaging analysis across development. Developmental Cognitive Neuroscience. 2014:172. doi: 10.1016/j.dcn.2014.04.004. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Introduction to Diffusion Tensor Imaging. Amsterdam: the Netherlands: Elsevier; 2007. [Google Scholar]
- Niogi S. N. Mukherjee P. Diffusion Tensor Imaging of mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 2010:241. doi: 10.1097/HTR.0000000000000030. &. –. doi: [DOI] [PubMed] [Google Scholar]
- *Oni M. B., Wilde E. A., Bigler E. D., McCauley S. R., Wu T. C., Yallampalli R, … Levin H. S. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. Journal of Child Neurology. 2010:976. doi: 10.1177/0883073809356034. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Writing meta-analytic reviews. Psychological Bulletin. 1995:183. doi: 10.1037/0033-2909.118.2.183. –. doi: [DOI] [Google Scholar]
- Salmond C. H., Menon D. K., Chatfield D. A., Williams G. B., Pena A., Sahakian B. J., & Pickard J. D. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. Neuroimage. 2006:117. doi: 10.1016/j.neuroimage.2005.07.012. –. doi: [DOI] [PubMed] [Google Scholar]
- *Scheibel R. S., Newsome M. R., Wilde E. A., McClelland M. M., Hanten G., Krawczyk D. C, … Levin H. S. Brain activation during a social attribution task in adolescents with moderate to severe traumatic brain injury. Social Neuroscience. 2011:582. doi: 10.1080/17470919.2011.588844. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schmidt A. T., Hanten G., Li X., Wilde E. A., Ibarra A. P., Chu Z. D, … Levin H. S. Emotional prosody and diffusion tensor imaging in children after traumatic brain injury. Brain Injury. 2013:1528. doi: 10.3109/02699052.2013.828851. –. doi: [DOI] [PubMed] [Google Scholar]
- Tasker R. C., Westland A. G., White D. K. Williams G. B. Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Developmental Neuroscience. 2010:374. doi: 10.1159/000316806. &. –. doi: [DOI] [PubMed] [Google Scholar]
- Taylor H. G. Neurobehavioral outcomes of pediatric traumatic brain injury. In: Anderson V., editor; Yeates K. O., editor. Pediatric traumatic brain injury: New frontiers in clinical and translational research. Cambridge, UK: Cambridge University Press; 2010. pp. 145–168. &. –. [Google Scholar]
- *Virji-Babul N., Borich M. R., Makan N., Moore T., Frew K., Emery C. A, & Boyd L. A. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatric Neurology. 2013:24. doi: 10.1016/j.pediatrneurol.2012.09.005. –. doi: [DOI] [PubMed] [Google Scholar]
- Wardlaw J. M., Brindle W., Casado A. M., Shuler K., Henderson M., Thomas B. A systematic review of the utility of 1.5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research. European Radiology. 2012:2295. doi: 10.1007/s00330-012-2500-8. … SINAPSE Collaborative Group. –. doi: [DOI] [PubMed] [Google Scholar]
- Wilde E. A., Ayoub K. W., Bigler E. D., Chu Z. D., Hunter J. V., Wu T. C., … Levin H. S. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: Changes within an 18 month post-injury interval. Brain Imaging & Behavior. 2012:404. doi: 10.1007/s11682-012-9150-y. –. doi: [DOI] [PubMed] [Google Scholar]
- *Wilde E. A., Bigler E. D., Haider J. M., Chu Z., Levin H. S., Li X, & Hunter J. V. Vulnerability of the anterior commissure in moderate to severe pediatric traumatic brain injury. Journal of Child Neurology. 2006a:769. doi: 10.1177/08830738060210090201. –. doi: [DOI] [PubMed] [Google Scholar]
- *Wilde E. A., Chu Z., Bigler E. D., Hunter J. V., Fearing M. A., Hanten G, … Levin H. S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006b:1412. doi: 10.1089/neu.2006.23.1412. –. doi: [DOI] [PubMed] [Google Scholar]
- *Wilde E. A., McCauley S. R., Hunter J. V., Bigler E. D., Chu Z., Wang Z. J, … Levin H. S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008:948. doi: 10.1212/01.wnl.0000305961.68029.54. –. doi: [DOI] [PubMed] [Google Scholar]
- *Wilde E. A., Newsome M. R., Bigler E. D., Pertab J., Merkley T. L., Hanten G, … Levin H. S. Brain imaging correlates of verbal working memory in children following traumatic brain injury. International Journal of Psychophysiology. 2011:86. doi: 10.1016/j.ijpsycho.2011.04.006. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wilde E. A., Ramos M. A., Yallampalli R., Bigler E. D., McCauley S. R., Chu Z, … Levin H. S. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Developmental Neuropsychology. 2010:333. doi: 10.1080/87565641003696940. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston G. P. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quantitative Imaging in Medicine & Surgery. 2012:254. doi: 10.3978/j.issn.2223-4292.2012.12.05. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wozniak J. R, Krach L., Ward E., Mueller B. A., Muetzel R., Schnoebelen S, … Lim K. O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007:555. doi: 10.1016/j.acn.2007.03.004. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wu T. C, Wilde E. A., Bigler E. D., Li X., Merkley T. L., Yallampalli R, … Levin H. S. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience. 2010a:361. doi: 10.1159/000317058. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wu T. C, Wilde E. A., Bigler E. D., Yallampalli R., McCauley S. R., Troyanskaya M, … Levin H. S. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. Journal of Neurotrauma. 2010b:303. doi: 10.1089/neu.2009.1110. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Mukherjee P. Barkovich A. J. Pediatric brain injury: Can DTI scalars predict functional outcome? Pediatric Radiology. 2013:55. doi: 10.1007/s00247-012-2481-4. &. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallampalli R., Wilde E. A., Bigler E. D., McCauley S. R., Hanten G., Troyanskaya M., … Levin H. S. Acute white matter differences in the fornix following mild traumatic brain Injury using diffusion tensor imaging. Journal of Neuroimaging. 2013:224. doi: 10.1111/j.1552-6569.2010.00537.x. –. doi: [DOI] [PubMed] [Google Scholar]
- *Yuan W, Holland S. K., Schmithorst V. J., Walz N. C., Cecil K. M., Jones B. V, … Wade S. L. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. Americal Journal of Neuroradiology. 2007:1919. doi: 10.3174/ajnr.A0698. –. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis K. K., Leach L. Kaplan E. Neuropsychological differential diagnosis. Lisse: The Netherlands: Swets & Zeitlinger; 1999. &. [Google Scholar]


