Abstract
Objective: Poor descriptions of standard care may compromise interpretation of results in randomised controlled trials (RCTs) of health interventions. We investigated quality of standard care in RCTs of behaviour change interventions for young people with type 1 diabetes and consider implications for evaluating trial outcomes.
Design: We conducted systematic searches for articles published between 1999 and 2012. We extracted standard care descriptions and contacted trial authors to complete a checklist of standard care activities. The relationship between standard care quality and outcomes was examined via subgroup meta-analyses and meta-regression.
Main outcome measures: Standard care descriptions, standard care quality, and relationships between standard care quality with medical and psychological outcomes.
Results: We identified 20 RCTs described across 26 articles. Published descriptions of standard care were limited to service-level features. Author responses indicated standard care provision extended beyond published accounts. Subgroup analyses suggested control groups receiving higher standard care quality showed larger improvements in both medical and psychological outcomes, although standard care quality did not predict outcomes significantly.
Conclusion: The quality of care delivered to control group participants can influence outcomes of RCTs. Inadequate reporting exacerbates this issue by masking variations between trials. We argue for increased clarity in reporting standard care in future trials.
Keywords: type 1 diabetes, control group, reporting, meta-analysis, standard care quality
Introduction
Randomised controlled trials (RCTs) are generally considered to represent the gold standard for evaluating health care interventions (Coates, 2010). By definition, such trials involve the comparison of outcomes between two or more groups (usually an intervention vs. a control), meaning the relative efficacy of the control condition plays an integral part in determining the effect size associated with the intervention under examination (Au, Castro, & Krishnan, 2007). In RCTs of health care interventions, standard care (also known as treatment-as-usual, usual care or routine care) is frequently employed as the control condition to establish if the intervention is a significant improvement over existing practice (Freedland, Mohr, Davidson, & Schwartz, 2011). This research design allows a direct comparison between current and an alternative practice, and thus allows policy-makers to judge the appropriateness of innovations in health care (e.g. through economic evaluations, see Evers, Hiligsmann, & Adarkwah, 2015).
However, the use of standard care control groups has been the subject of much debate; with many pointing out that what constitutes standard care is rarely clear (Burns, 2009; Dawson et al., 2009; Freedland et al., 2011; Mohr et al., 2009; Thompson & Schoenfeld, 2007). Freedland et al. (2011) noted that two trials may include a standard care condition, but this implies few similarities beyond their names. Indeed, standard care has been shown to vary even for the same disease or condition (Burns et al., 2007; de Bruin, Viechtbauer, Hospers, Schaalma, & Kok, 2009). This is perhaps unsurprising as health care provision and spending can differ between countries (Anderson, Reinhardt, Hussey, & Petrosyan, 2003), updated guidelines and technologies are introduced at variable rates (Cappellaro, Fattore, & Torbica, 2009) and what is considered to be best practice evolves with new research findings. Therefore, the composition of standard care may considerably differ across hospitals, countries and over time.
This variation in health care practices raises important issues for the interpretation and generalisability of RCTs of health behaviour change interventions; particularly where standard care is used as the control condition. For example, an intervention compared with highly effective standard care practices could yield a lower effect size than the same intervention compared against less-effective care. Unfortunately, as standard care conditions are often inadequately described (Burns, 2009; Mohr et al., 2009), it is difficult to conclude whether or not a positive trial is the result of an effective intervention or low-efficacy standard care.
Recent research examining interventions to enhance HIV antiretroviral therapy adherence has demonstrated the substantial influence that variation in standard care quality can have on control group outcomes (de Bruin et al., 2009). This, in turn, can distort effect sizes and lead to inaccurate conclusions when synthesising the findings from multiple RCTs (de Bruin et al., 2010). As a result, there are calls for standard care quality to be assessed and reported in future RCTs allowing for control of this in meta-analyses (de Bruin et al., 2009, 2010).
A similar problem in terms of variation in standard care quality potentially affects trials involving young people with type 1 diabetes. Given the complex nature of diabetes self-management and the necessity for a high level of patient involvement in daily care, the composition of standard care is likely to involve a range of behaviour change techniques that may impact on both medical and psychological outcomes. In this paper, we examine recent behaviour change interventions for young people with type 1 diabetes to determine i) the information provided about standard care control conditions in published articles; ii) standard care quality across trials via author completed checklists; and iii) the influence of standard care quality on medical and psychological outcomes in control groups in these interventions.
Method
Literature search and selection procedure
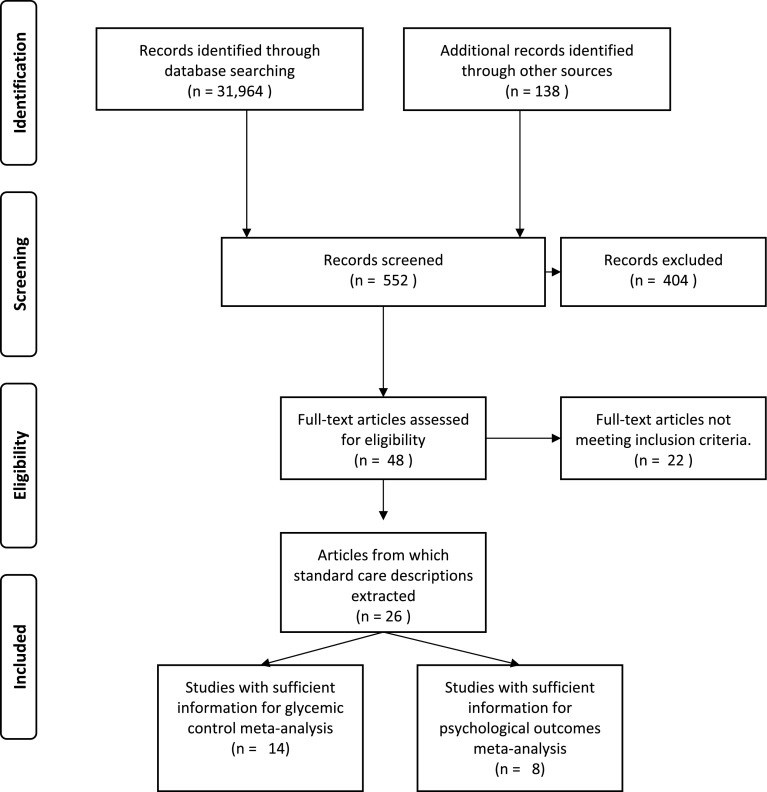
Systematic searches of electronic databases (MEDLINE, Web of Knowledge, PsycINFO, CINHAL, Embase, ProQuest, and Cochrane database of systematic reviews and Conference proceedings citation index) were conducted to identify RCTs of behaviour change interventions, published between July 1999 and November 2012, for young people with type 1 diabetes. Search terms were variants on: type 1 diabetes, psychological interventions and RCTs (see supplementary materials). We included trials that involved young people aged 8–21 years, diagnosed with type 1 diabetes for at least 6 months and measured glycated haemoglobin (HbA1c or GhB) or a validated psychological measure. Non-English language articles and samples with an additional co-morbid chronic illness were excluded. Reference lists and future citations were also examined for additional articles (i.e. the ancestry approach – Johnson & Eagly, 2000). The search strategy yielded a total of 32,102 articles from which non-eligible studies were removed using database filters. This left 552 abstracts to be screened with 48 articles extracted for further review. Overall, there was substantial agreement between reviewers (k = .85, p < .001) with 20 RCTs incorporating a standard care control described across 26 published articles included in the review. The flow of information through the review process can be seen in Figure 1.
Figure 1.
Flow of information through review process.
Published descriptions of standard care
Published manuscripts were examined individually and any details pertaining to standard care practices in each trial were extracted by two researchers (KA, CE). When multiple articles describing one RCT were present (e.g. follow-up reports), details were collated to establish as much detail as possible about standard care. This means that in the trial led by Wysocki, three articles were consulted (Wysocki, Greco, Harris, Bubb, & White, 2001; Wysocki et al., 1999, 2000); in the trial led by Ellis four articles were consulted (Ellis et al., 2005a, 2005b; Ellis, Templin, et al., 2007; Ellis, Yopp, et al., 2007) and in the trial led by de Wit, two articles were consulted (de Wit et al., 2008, 2010).
Assessment of standard care quality
To assess standard care quality, we adapted a checklist developed for use in highly active antiretroviral therapy adherence trials (de Bruin et al., 2009). For example, ‘feedback of CD4 and viral load’ was amended to ‘feedback on most recent HbA1c/A1c’. Other inappropriate items were removed. We derived additional activities from descriptions provided in selected articles and discussions with local Diabetes Specialist Nurses. The final checklist consisted of 19 activities (see Table 1) which were then classified as behaviour change techniques using the taxonomy described by Michie et al. (2011). Activities were coded independently by two researchers (KA, CE) with 100% agreement. Potential checklist responses were ‘Never’, ‘For some patients’, ‘For most patients’, ‘For all patients’ and ‘Don’t know’.
Table 1. Standard care activities assessed and relevant behaviour change techniques.
| Standard care activity | Behaviour change technique (Michie et al., 2011) |
|---|---|
| General information (verbal or written) about diabetes management | Provide information on how to perform the behaviour |
| Information about the consequences of sub-optimal control | Provide information on consequences of the behaviour to the individual |
| Provide contact details for relevant support groups | Plan social support/social change |
| Feedback on most recent HbA1c /A1c | Provide feedback on performance |
| Encouragement of regular monitoring of blood glucose levels | Prompt self-monitoring of behaviour outcome |
| Discuss common barriers and ways to overcome them | Prompt barrier identification/ problem-solving |
| Involve family members in diabetes self-care | Plan social support/social change |
| Set personal goals for diabetes management (behaviour) | Goal setting (behaviour) |
| Set personal targets for optimal HbA1c or Equivalent (outcome) | Goal setting (outcome) |
| Identify individual problems relating to diabetes management and generate solutions | Prompt barrier identification/ problem-solving |
| Use of alarms, cues or reminders to complete diabetes tasks | Teach to use prompts/cues |
| Help plan for holidays, special events and sick days | Relapse Prevention/ Coping Planning |
| Liaison with relevant others (e.g. schools and employers) about diabetes | Plan social support/social change |
| Provide annual review | Provide feedback on performance |
| Adherence to a dietary plan (e.g. carb counting) | Action Planning |
| More frequent contact for those with poor control or most difficulties | Additional: Service Provision |
| Contact with a Dietitian | Additional: Service Provision |
| Contact with a psychologist | Additional: Service Provision |
| Access to 24 h telephone or online support for acute problems | Additional: Service Provision |
We derived a summed score for standard care quality for each trial by scoring ‘Never’ and ‘Don’t know’ responses as 0, ‘For some patients’ as 1, ‘For most patients’ as 2 and ‘For all patients’ as 3. This yielded a theoretical range of 0–57, with higher scores indicating better standard care quality. Additional questions were included to determine the frequency of scheduled appointments, the type of clinic where care was administered (e.g. paediatric, adolescent and adult) and the health professionals involved in care. We contacted primary authors of trials to complete this checklist retrospectively to establish which items were routinely delivered as part of standard care.
Statistical analyses
Effect sizes (d) for glycaemic control (HbA1c or GhB) and psychological outcomes using pre- and post-mean scores, pre- and post-standard deviations and pre/post correlations in the control group were calculated using comprehensive meta-analysis version 2 (Borenstein, Hedges, Higgins, & Rothstein, 2005). As pre/post control group correlations are rarely reported, we calculated effect sizes and assumed positive correlations of .25, .5 and .75 if the actual value was unknown as described by Norris, Lau, Smith, Schmid, and Engelgau (2002). As these approaches yielded essentially identical results, the findings reported in this paper are based on a correlation of .5. To assess the internal reliability of the standard care quality checklist we computed Cronbach’s alpha. Random effects meta-analyses were run using the metan command on Stata 12.1 (StataCorp, 2012; default settings), with heterogeneity assessed via Cochran’s Q test and the I 2 Statistic (Higgins, Thompson, Deeks, & Altman, 2003). Where heterogeneity was significant, subgroup analyses of high vs. low standard care quality (calculated via a median split) were conducted to give an indication of relative efficacy. Standard care quality was then entered as a main predictor in a mixed-effects meta-regression model (metareg and default settings) both individually and in models containing potential confounding trial features (mean age of participants at baseline, gender, attrition, glycaemic control measure and whether only those with poor glycaemic control were selected), using control group effect sizes as the outcome measure.
Results
(1) Information provided about standard care control conditions in published articles
Standard care details provided in published articles for each trial are shown in Table 2. Of the 20 trials, 16 (80%) reported frequency with which patients were seen as part of standard care, which ranged from monthly to every six months. Some details regarding the environment where care was administered were reported in 18 RCTs (90%) with different locations reported across trials. Nine trials (41%) included information about health professionals involved in standard care. There was considerable variety in the make-up of teams involved in standard care, ranging from only paediatricians to large multidisciplinary teams. Additional features of standard care were explicitly reported in a small number of trials. Based on this assessment of published information, we concluded that the information provided about standard care was limited to service-level information, and it was not possible to determine the activities and behaviour change techniques delivered to control group participants.
Table 2. Published details of standard care.
| Trial | Frequency of contact | Details of care environment | Specified health professionals involved in care | Additional details |
|---|---|---|---|---|
| Charron-Prochownik, Ferons-Hannan, Sereika, and Becker (2008) | No Details Provided | Diabetes clinic | No Details Provided | None |
| Cook, Herold, Edidin, and Briars (2002) | Every 3 months | 2 Children’s Hospitals | No Details Provided | None |
| Couper, Taylor, Fotheringham, and Sawyer (1999) | Every 3 months | Women’s and children’s Diabetes Clinic | Paediatric endocrinologist | 24 hour phone support |
| Dietitian | ||||
| Diabetes Educator | ||||
| de Wit et al. (2008, 2010) | Every 3 months | 4 Paediatric Diabetes Clinics | 6 Paediatricians | None |
| Ellis et al. (2004) | Every 3 months | Children’s Hospital | Endocrinologist | Required to test blood sugar 3–4 times per day |
| Endocrinology Clinic | Nurse | Prescribed diet | ||
| Tertiary Care | Dietician | Access to mental health services | ||
| Social Worker | ||||
| Psychologist | ||||
| Ellis et al. (2005a, 2005b), Ellis, Templin, et al. (2007), Ellis, Yopp, et al. (2007) | Every 3 months | Children’s Hospital | Endocrinologist | Access to mental health services |
| Endocrinology Clinic | Nurse | |||
| Tertiary care | Dietician | |||
| Social Worker | ||||
| Psychologist | ||||
| Franklin, Waller, Pagliari, and Greene (2006) | Every 3–4 months | No Details Provided | Multidisciplinary Team (Psychologist specified) | Access to emergency hotline |
| Gay et al. (2006) | Every 3 months | Hospital Outpatient Clinic | Diabetologist | Contact with patient increased by Diabetologist if deemed necessary |
| 5 Paediatric Endocrinologists | ||||
| Graue, Wentzel-Larsen, Hanestad, and Søvik (2005) | Every 3 months | Hospital Paediatric Outpatient Department | Physician | 30 Minute appointments |
| Diabetes Nurse Specialist | Access to Dietitian | |||
| Access to Social worker | ||||
| Access to Clinical Psychologist Discussions of insulin delivery and other issues affecting care | ||||
| Grey, Boland, Davidson, Li, and Tamborlane (2000) | Monthly | Children’s Diabetes Clinic | Physician | Required to test blood sugar ≥ 4 times daily |
| Nurse Practitioners/Certified Diabetes Educators | Between visit telephone contact | |||
| Dietician | ||||
| Social Worker | ||||
| Hains, Davies, Parton, Totka, and Amoroso-Camarata (2000) | Every 3–4 months | Children’s Hospital | No Details Provided | None |
| Diabetes Clinic | ||||
| Laffel et al. (2003) | Every 3–4 months | Paediatric and Adolescent Unit | Multidisciplinary Care (no details specified) | Follow-up visits |
| Diabetes Centre | Contact between visits | |||
| Encouragement around routine diabetes management | ||||
| Received educational materials | ||||
| Lawson, Cohen, Richardson, Orrbine, and Pham (2005) | Every 3 months | Children’s Hospital | Diabetologist | Recommended to test blood sugar ≥ 3 times daily (breakfast, supper and bedtime) with lunchtime tests on weekends and weekdays if possible |
| Diabetes Clinic | Diabetes Nurse Specialist | Between visit contact with Diabetologist or Nurse to adjust insulin as required | ||
| Tertiary care | Diabetes Dietitian | Target preprandial blood sugars of 4–8 mmol/L | ||
| Adherence to diabetes meal plan based on carbohydrate counting or food exchanges | ||||
| Contact with patient increased by Diabetologist if deemed necessary | ||||
| HbA1c tested at each visit | ||||
| Lehmkuhl et al. (2010) | No Details Provided | Paediatric Endocrinology Clinic | No Details Provided | None |
| Mulvaney, Rothman, Wallston, Lybarger and Dietrich (2010) | No Details Provided | Paediatric Diabetes Clinic | No Details Provided | None |
| Murphy et al. (2012) | Every 3 months | 10 Paediatric Diabetes Clinics | No Details Provided | None |
| Olmsted, Daneman, Rydall, Lawson, and Rodin (2002) | Every 3 months | Children’s Hospital | Multidisciplinary Care (no details specified) | Target preprandial blood sugars of 4–10 mmol/L |
| Tertiary care | Adherence to meal plan based on food exchanges or on carbohydrate counting | |||
| Panagiotopoulos, Preston, Stewart, Metzger and Chanoine (2003) | Every 6 months | Children’s Hospital | No Details Provided | Encouraged to regularly test Blood Glucose |
| Diabetes Clinic | Emergency phone contact | |||
| Viklund, Örtqvist, and Wikblad (2007) | No Details Provided | Outpatient Clinic | No Details Provided | None |
| Wysocki et al. (1999, 2000, 2001) | Every 3–4 Months | No Details Provided | Paediatric Endocrinologist | Examination by a physician |
| Physician | GHb tested ≥ 3 times annually; | |||
| Home blood glucose monitoring and recording of test results | ||||
| Diabetes education provided | ||||
| Emphasis on active self-management | ||||
| Prescribed diet | ||||
| Regular physical exercise | ||||
| Annual review for diabetic complications |
(2) Standard care quality across trials via author completed checklists
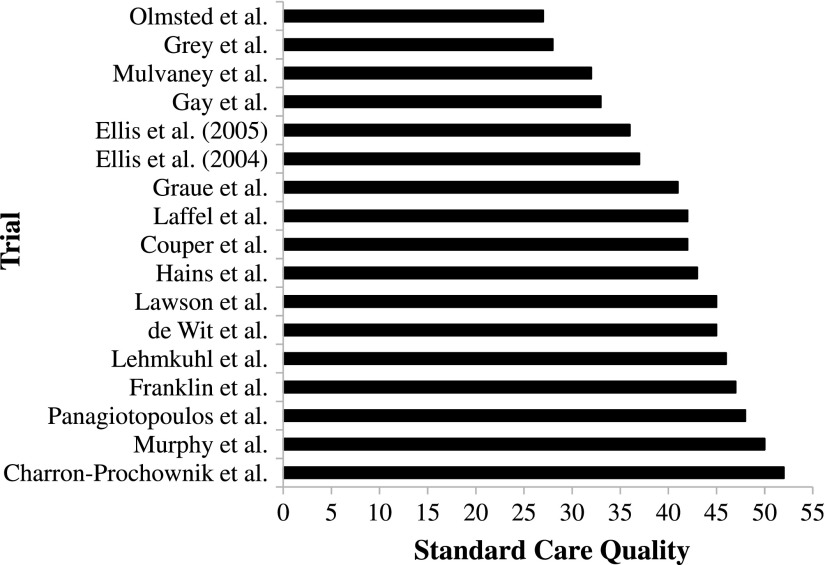
Seventeen authors completed and returned the standard care checklist (85% response rate). Of these, 16 reported scheduling contact with patients every 3 months, with one reporting scheduling contact every 6–8 months. The type of clinic where care was administered was variously described as paediatric (n = 11), paediatric and adolescent (n = 5) and adolescent only (n = 1). The number of professionals involved in standard care ranged from 3 to 7 (M = 4.71 ± 1.15). In terms of checklist responses, total standard care activities delivered to all participants ranged from 2 to 15 (M = 9.94 ± 3.96). The majority of authors were aware of standard care practices with relatively few ‘Don’t Know’ responses (never: n = 12; up to 20%: n = 3; between 20 and 40%: n = 2) 1 . Computed scores for standard care quality ranged from 27 to 52 (M = 40.82 ± 7.50; see Figure 2) and the checklist as a whole was found to be internally consistent (α = .78).
Figure 2.
Variability in standard care quality.
(3) The influence of standard care quality on medical and psychological control group outcomes
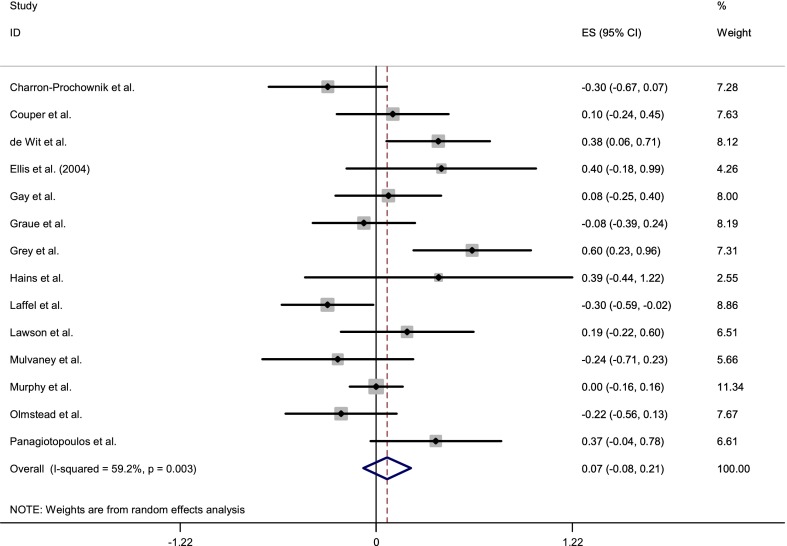
In terms of glycaemic control, 14 trials included sufficient details for inclusion in a meta-analysis. This yielded a total sample of 508 control group participants. Pooling these in a random effects analysis (see Figure 3) produced a non-significant overall control group effect size of .07 (95% CI: −.8 to .22). Cochran’s Q test revealed significant heterogeneity across trials (Q(13) = 31.85, p = .003) with the I 2 Statistic showing moderate variation (59.2%) in effect sizes attributable to heterogeneity.
Figure 3.
Forest plot of standardised effects on glycaemic control.
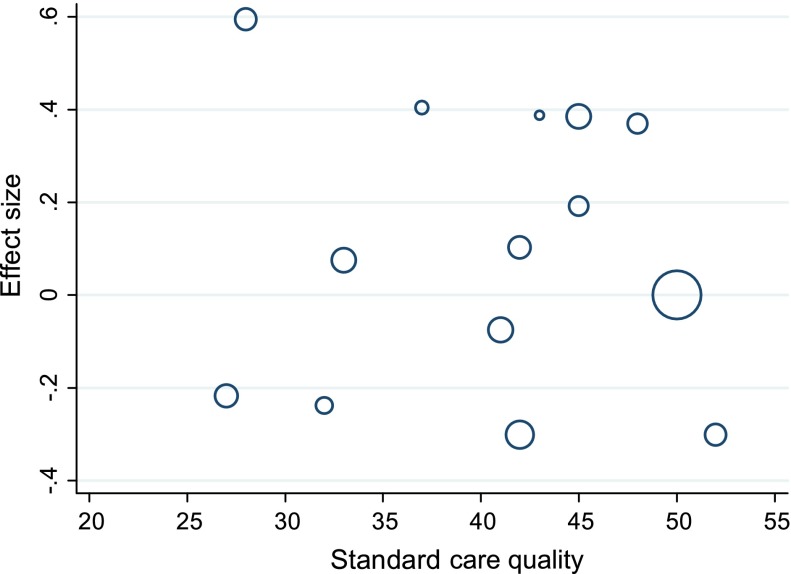
Based on a median split, trials with higher standard care quality scores (>42) had a larger pooled effect size (d = .13, 95% CI: −.09 to .35) than those with lower standard care quality (d = .02, 95% CI: −.19 to .21) (see Figure 4). However, meta-regression analysis revealed standard care quality was not a significant predictor of effect size when considered individually (t = −.31, p > .05) or when considered in models including potentially confounding trial features (mean age of participants at baseline, gender, attrition, glycaemic control measure and whether only those with poor glycaemic control were selected), none of which significantly explained the variation between trials (all p’s > .05).
Figure 4.
Bubble plot of standardised effects on glycaemic control against standard care quality.
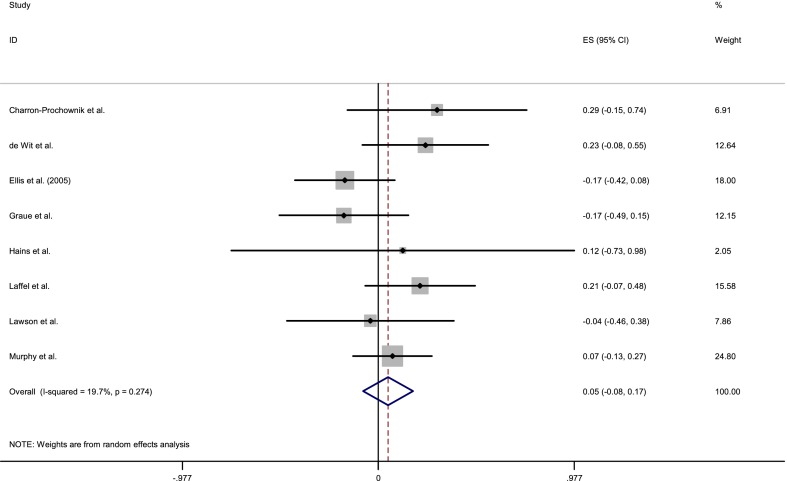
For psychological outcomes, eight trials included sufficient details for inclusion. This yielded a sample of 340 control group participants. A random effects meta-analysis (see Figure 5) revealed an overall effect size of .05 (95% CI: −.08 to .17). Psychological outcomes in the control groups were found to be relatively homogenous (Q(7) = 8.72, p > .05; I 2 = 19.7%). However, as sources of heterogeneity should be explored even when the overall test for heterogeneity is not significant (Thompson & Higgins, 2002), further moderator analyses were conducted.
Figure 5.
Forest plot of standardised effects on psychological outcomes.
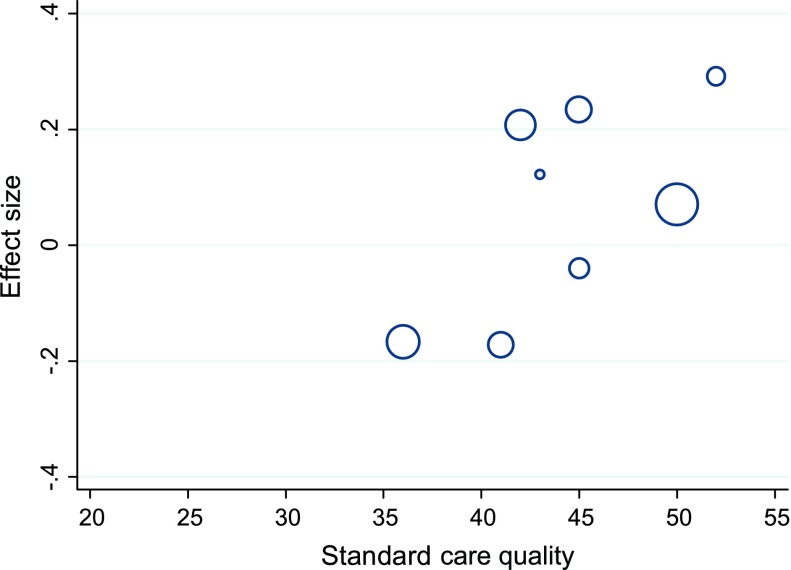
As was the case for glycaemic control, trials with higher standard care quality scores (>42) had a larger pooled effect size on psychological outcomes (d = .12, 95% CI: −.03 to .26) than those with lower standard care quality (d = −.04, 95% CI: −.29 to .21) (see Figure 6). Meta-regression analysis revealed a trend towards standard care quality as a predictor of effect size (t = 1.61, p = .158) when considered individually but this was not apparent when considered in models that included potentially confounding trial features.
Figure 6.
Bubble plot of standardised effects on psychological outcomes against standard care quality.
Discussion
Previous research has illustrated the importance of care quality delivered to control groups in determining effect sizes in RCTs of HIV antiretroviral adherence interventions (de Bruin et al., 2009, 2010). In this study, we aimed to describe standard care reporting and provision in RCTs of behaviour change interventions for young people with type 1 diabetes and the implications for interpreting trial outcomes.
Our results demonstrate that the reporting of standard care in published RCTs of behaviour change interventions for young people with type 1 diabetes is limited, with many articles providing little or no details about what constitutes standard care. Details most frequently reported in published articles were service-level features (e.g. clinic type) rather than information about specific activities designed to change behaviour or improve outcomes. This suggests that little weight is given to describing standard care, in part, perhaps a response to word restrictions in publications, but also perhaps under the assumption that standard care does not differ across health centres. However, even given the limited descriptions in trials we assessed, there was considerable variation in standard care. There were differences in scheduled time intervals of routine appointments, staff involved and activities that were reported as part of standard care.
This variation in standard care was confirmed by authors’ responses to a retrospective checklist of standard care activities. In line with the argument that poor reporting is partly a response to word restrictions in published articles, authors described a greater number of standard care activities than reported in published articles, with previously unreported behaviour change techniques often forming part of care. Standard care quality differed substantially between trials, a finding congruent with previous research (de Bruin et al., 2009), suggesting that variation in standard care may well be the norm in clinical care for chronic conditions.
We also examined the effect of this variation on control group outcomes. Control groups receiving better standard care quality showed larger positive effect sizes than those with lower standard care quality for both glycaemic control and psychological outcomes. This would impact on effect sizes calculated for interventions; with those interventions compared against higher standard care quality appearing less effective than if they were compared against a lower quality standard care. However, when entered as a predictor in meta-regression analyses, standard care quality did not reach statistical significance. In part, this may be due to lack of power, given the relatively small number of interventions we identified for young people with type 1 diabetes. Furthermore, assuming most participants were receiving standard care prior to baseline measures, it is likely the changes captured by our effect sizes across the course of the trial only represent part of the overall influence of standard care.
Despite the inconclusive nature of our findings in terms of impact on control group outcomes, the evidence we present of substantial variation in standard care quality and limited reporting in RCTs is of considerable concern. Previous work has demonstrated the impact of standard care variation on effect sizes and the outcomes of meta-analyses (de Bruin et al., 2009, 2010) an issue which is compounded when reporting of standard care is poor. Despite recommendations that control conditions are described as fully as intervention conditions (Zwarenstein et al., 2008) it seems that in many cases the reporting of control conditions is demonstrably not a priority.
Strengths and limitations
We successfully adapted a checklist previously used to assess standard care quality in HIV antiretroviral adherence trials (de Bruin et al., 2009) and applied it to trials of behaviour change interventions for young people with type 1 diabetes. While the most significant amendment to the checklist involved rewording of items to be relevant for diabetes care, it is important to highlight a difference in terms of response options. In the original checklist, authors were asked to respond ‘yes’, ‘no’ or ‘don’t know’. In our version of the checklist, authors were asked to indicate which activities were routinely delivered using more differentiated response categories (‘never’; ‘for some patients’; ‘for most patients’; ‘for all patients’; and ‘don’t know’). Use of these more differentiated response categories has the potential advantage to capture when an activity is delivered to only some patients. However, given that many authors were necessarily recalling how standard care was delivered many years previously, we cannot be certain about the accuracy with which they were able to report such differences.
A further limitation of this method is that it relies on retrospective accounts of care, at the time of the trial, in some cases over a decade earlier. In addition, trial authors may not themselves have been personally involved in delivering standard care. Due to the limited nature of standard care reporting in trials it was not feasible to derive a standard care quality score from published articles. With improved reporting of standard care, future researchers will be able to assess standard care quality from published articles without needing to contact authors many years post-trial.
This study benefits from systematic searches, pre-determined inclusion criteria that help minimise selection bias and a high response rate from trial authors. We found our checklist for standard care quality to be internally reliable, suggesting the checklist itself captures an underlying factor, which could reasonably be described as the quality of diabetes standard care or adherence counselling. While many of the items were adapted from the checklist developed by de Bruin et al. (2009), additional checklist items were derived in conjunction with informal discussions with consultants and diabetes nurses working in our centre. Further development of the checklist should include consultation with those working in other centres to ensure a comprehensive list of activities that may form part of standard care.
The checklist described in this paper was designed to assess if, and to what proportion of participants, activities are delivered as part of standard care. However, it does not give insight into how well some of these activities are employed (e.g. the quality/extent of activities). Such an examination goes beyond the scope of this paper. However, we would argue that in future reporting, it is essential to include an improved description of standard care and some rationale for why each activity or technique is considered appropriate. Finally, authors should include, where possible, some examples of what each activity involved (e.g. types of goals set and plans made). Only in this way will we be able to assess which behaviour change techniques are being delivered as part of standard care and their role in the outcomes of RCTs.
Concluding remarks
RCTs are generally considered to represent the ‘gold standard’ when testing the efficacy of health behaviour change interventions (Coates, 2010). This special issue of Psychology & Health explores the numerous biases that can potentially undermine or distort outcomes using this methodology and suggests ways to both acknowledge and/or mitigate them (e.g. de Bruin, McCambridge, & Prins, 2015). In this paper, we have explored how poor reporting of standard care limits generalisabilty and potentially introduces bias. There is now ample evidence that standard care for chronic conditions is not uniform across trials. In the case of multi-centre trials, it is important that researchers establish that all participating centres conform to a basic standard care quality at the start of the trial and that any remaining differences between participating centres are adequately described.
We echo the call of de Bruin et al. (2009) that those conducting future RCTs give greater priority to describing what constitutes ‘standard care’ in their trial. At least for trials involving type 1 diabetes, the checklist developed in this study may be a useful tool in this regard. If authors decide against using this checklist in favour of prose-based descriptions, we recommend they at least use language similar to that used in the checklist as this allows for coding of behaviour change techniques. Written descriptions of standard care should extend beyond service-level details and focus on active components of care (e.g. behaviour change techniques). We acknowledge that space is often limited in published articles, yet detailed descriptions could be referenced and made available online. This call shares similarities with the recent focus on the need for improved reporting of intervention conditions in behaviour change interventions (Craig et al., 2008; Michie & Prestwich, 2010). We argue, as have others (Bishop, Fenge-Davies, Kirby, & Geraghty, 2015; Williams, 2010), that it is equally important to consider and describe the nature of the control group since this plays an integral role in evaluation of an RCT.
Acknowledgements
NIHR CLAHRC for South Yorkshire acknowledges funding from the National Institute of Health Research. The views and opinions expressed are those of the authors, and not necessarily those of the NHS, the NIHR or the Department of Health.
CLAHRC SY would also like to acknowledge the participation and resources of our partner organisations. Further details can be found at www.clahrc-sy.nihr.ac.uk.
If you would like access to data, tools, models and materials under CLAHRC SY’s Open Access policy, please contact the lead author to discuss this possibility.
Note
The results presented in the following sections remain unchanged when those who did not know about standard care practice (n = 2,>20%) are removed from the analyses.
References
(* indicates included in the review)
- Anderson G. F., Reinhardt U. E., Hussey P. S., Petrosyan V.2003It’s the prices, stupid: Why the United States is so different from other countries Health Affairs ,89–105. 10.1377/hlthaff.22.3.89 10.1377/hlthaff.22.3.89 [DOI] [PubMed] [Google Scholar]
- Au D. H., Castro M., Krishnan J. A.2007Selection of controls in clinical trials: Introduction and Conference summary Proceedings of the American Thoracic Society ,567–569. 10.1513/pats.200707-099JK 10.1513/pats.200707-099JK [DOI] [PubMed] [Google Scholar]
- Bishop F. L., Fenge-Davies A. L., Kirby S., Geraghty A. W. A.2015Context effects and behaviour change techniques in randomized trials: A systematic review using the example of trials to increase adherence to physical activity in musculoskeletal pain Psychology & Health ,104–121. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L., Higgins J., Rothstein H. Comprehensive meta-analysis version 2. Englewood NJ: Biostat; 2005. [Google Scholar]
- Burns T.2009End of the road for treatment-as-usual studies? The British Journal of Psychiatry ,5–6. 10.1192/bjp.bp.108.062968 [DOI] [PubMed] [Google Scholar]
- Burns T., Catty J., Dash M., Roberts C., Lockwood A., Marshall M.2007Use of intensive case management to reduce time in hospital in people with severe mental illness: Systematic review and meta-regression British Medical Journal (Clinical research ed.) ,336. 10.1136/bmj.39251.599259.55 10.1136/bmj.39251.599259.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellaro G., Fattore G., Torbica A.2009Funding health technologies in decentralized systems: A comparison between Italy and Spain Health Policy ,313–321. 10.1016/j.healthpol.2009.05.004 10.1016/j.healthpol.2009.05.004 [DOI] [PubMed] [Google Scholar]
- *Charron-Prochownik D., Ferons-Hannan M., Sereika S., Becker D.2008Randomized efficacy trial of early preconception counseling for diabetic teens (READY-girls) Diabetes Care ,1327–1330. 10.2337/dc07-1266 10.2337/dc07-1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates V.2010The RCT: A very beautiful technique Practical Diabetes International ,27–31. 10.1002/pdi.1435 [DOI] [Google Scholar]
- *Cook S., Herold K., Edidin D. V., Briars R.2002Increasing problem solving in adolescents with type 1 diabetes: The choices diabetes program The Diabetes Educator ,115–124. 10.1177/014572170202800113 [DOI] [PubMed] [Google Scholar]
- *Couper J. J., Taylor J., Fotheringham M. J., Sawyer M.1999Failure to maintain the benefits of home-based intervention in adolescents with poorly controlled type 1 diabetes Diabetes Care ,1933–1937. 10.2337/diacare.22.12.1933 10.2337/diacare.22.12.1933 [DOI] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M.2008Developing and evaluating complex interventions: the new Medical Research Council guidance British Medical Journal ,a1655.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18824488 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson L., Zarin D. a., Emanuel E. J., Friedman L. M., Chaudhari B., Goodman S. N.2009Considering usual medical care in clinical trial design PLoS Medicine ,e1000111. 10.1371/journal.pmed.1000111 10.1371/journal.pmed.1000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin M., Viechtbauer W., Hospers H. J., Schaalma H. P., Kok G.2009Standard care quality determines treatment outcomes in control groups of HAART-adherence intervention studies: Implications for the interpretation and comparison of intervention effects Health Psychology ,668–674. 10.1037/a0015989 10.1037/a0015989 [DOI] [PubMed] [Google Scholar]
- de Bruin M., Viechtbauer W., Schaalma H. P., Kok G., Abraham C., Hospers H. J.2010Standard care impact on effects of highly active antiretroviral therapy adherence interventions Archives of Internal Medicine ,240–250. 10.1001/archinternmed.2009.536 10.1001/archinternmed.2009.536 [DOI] [PubMed] [Google Scholar]
- de Bruin M., McCambridge J., Prins J. M. Reducing the risk of bias in health behaviour change trials: Improving trial design, reporting, or bias assessment criteria? A review and case study. Psychology & Health. 2015:8–34. doi: 10.1080/08870446.2014.953531. [DOI] [PubMed] [Google Scholar]
- *de Wit M., Delemarre-van de Waal H. A., Bokma J. A., Haasnoot K., Houdijk M. C., Gemke R. J., Snoek F. J.2008Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: A randomized controlled trial Diabetes Care ,1521–1526. 10.2337/dc08-0394 10.2337/dc08-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *de Wit M., Delemarre-van de Waal H. A., Bokma J. A., Haasnoot K., Houdijk M. C., Gemke R. J., Snoek F. J.2010Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: Benefits do not sustain in routine practice Pediatric Diabetes ,175–181. 10.1111/j.1399-5448.2009.00543.x [DOI] [PubMed] [Google Scholar]
- *Ellis D. A., Frey M. A., Naar-King S., Templin T., Cunningham P. B., Cakan N.2005aThe effects of multisystemic therapy on diabetes stress among adolescents with chronically poorly controlled type 1 diabetes: Findings from a randomized Controlled Trial. Pediatrics ,e826–e832. 10.1542/peds.2005-0638 [DOI] [PubMed] [Google Scholar]
- *Ellis D. A., Frey M. A., Naar-King S., Templin T., Cunningham P. B., Cakan N.2005bUse of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: A randomized controlled trial Diabetes Care ,1604–1610. 10.2337/diacare.28.7.1604 [DOI] [PubMed] [Google Scholar]
- *Ellis D. A., Naar-King S., Frey M., Templin T., Rowland M., Greger N.2004Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in poor metabolic control: A pilot investigation Journal of Clinical Psychology in Medical Settings ,315–324. 10.1023/B:JOCS.0000045351.98563.4d [DOI] [Google Scholar]
- *Ellis D. A., Templin T., Naar-King S., Frey M. A., Cunningham P. B., Podolski C.-L., Cakan N.2007Multisystemic therapy for adolescents with poorly controlled type I diabetes: Stability of treatment effects in a randomized controlled trial Journal of Consulting and Clinical Psychology ,168–174. 10.1037/0022-006x.75.1.168 [DOI] [PubMed] [Google Scholar]
- *Ellis D. A., Yopp J., Templin T., Naar-King S., Frey M. A., Cunningham P. B., Niec L. N.2007Family mediators and moderators of treatment outcomes among youths with poorly controlled type 1 diabetes: Results From a randomized controlled trial Journal of Pediatric Psychology ,194–205. 10.1093/jpepsy/jsj116 [DOI] [PubMed] [Google Scholar]
- Evers S. M. A. A., Hiligsmann M., Adarkwah C. C.2015Risk of bias in trial-based economic evaluations: Identification of sources and bias-reducing strategies Psychology & Health ,52–71. [DOI] [PubMed] [Google Scholar]
- *Franklin V. L., Waller A., Pagliari C., Greene S. A.2006A randomized controlled trial of sweet talk, a text-messaging system to support young people with diabetes Diabetic Medicine ,1332–1338. 10.1111/j.1464-5491.2006.01989.x [DOI] [PubMed] [Google Scholar]
- Freedland K., Mohr D., Davidson K., Schwartz J.2011Usual and unusual care: Existing practice control groups in randomized controlled trials of behavioural interventions Psychosomatic Medicine ,323–335. 10.1097/PSY.0b013e318218e1fb.Usual [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gay C. L., Chapuis F., Bendelac N., Tixier F., Treppoz S., Nicolino M.2006Reinforced follow-up for children and adolescents with type 1 diabetes and inadequate glycaemic control: A randomized controlled trial intervention via the local pharmacist and telecare Diabetes & Metabolism ,159–165. 10.1016/s1262-3636(07)70263-x [DOI] [PubMed] [Google Scholar]
- *Graue M., Wentzel-Larsen T., Hanestad B. R., Søvik O.2005Evaluation of a programme of group visits and computer-assisted consultations in the treatment of adolescents with type 1 diabetes Diabetic Medicine ,1522–1529. 10.1111/j.1464-5491.2005.01689.x [DOI] [PubMed] [Google Scholar]
- *Grey M., Boland E. A., Davidson M., Li J., Tamborlane W. V.2000Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life The Journal of Pediatrics ,107–113. 10.1067/mpd.2000.106568 [DOI] [PubMed] [Google Scholar]
- *Hains A. A., Davies W. H., Parton E., Totka J., Amoroso-Camarata J.2000A stress management intervention for adolescents with type i diabetes The Diabetes Educator ,417–424. 10.1177/014572170002600309 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G.2003Measuring inconsistency in meta-analyses British Medical Journal (Clinical research ed.) ,557–560. 10.1136/bmj.327.7414.557 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Eagly A. Quantitative synthesis of social psychological research. In: Reis H., Judd C., editors. Handbook of research methods in social and personality psychology. London: Cambridge University Press; 2000. pp. 496–528.http://digitalcommons.uconn.edu/cgi/viewcontent.cgi?article=1012&context=chip_docs [Google Scholar]
- *Laffel L. M., Vangsness L., Connell A., Goebel-Fabbri A., Butler D., Anderson B. J.2003Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes The Journal of Pediatrics ,409–416.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12712059 10.1067/mpd.2003.138 [DOI] [PubMed] [Google Scholar]
- *Lawson M. L., Cohen N., Richardson C., Orrbine E., Pham B.2005A randomized trial of regular standardized telephone contact by a diabetes nurse educator in adolescents with poor diabetes control Pediatric Diabetes ,32–40. 10.1111/j.1399-543X.2005.00091.x [DOI] [PubMed] [Google Scholar]
- *Lehmkuhl H. D., Storch E. A., Cammarata C., Meyer K., Rahman O., Silverstein J., Geffken G.2010Telehealth behavior therapy for the management of type 1 diabetes in adolescents Journal of Diabetes Science and Technology ,199. 10.1177/193229681000400125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S., Ashford S., Sniehotta F. F., Dombrowski S. U., Bishop A., French D. P.2011A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy Psychology & Health ,1479–1498. 10.1080/08870446.2010.540664 [DOI] [PubMed] [Google Scholar]
- Michie S., Prestwich A.2010Are interventions theory-based? Development of a theory coding scheme Health Psychology ,11–8.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20063930 10.1037/a0016939 [DOI] [PubMed] [Google Scholar]
- Mohr D. C., Spring B., Freedland K. E., Beckner V., Arean P., Hollon S. D., Kaplan R.2009The selection and design of control conditions for randomized controlled trials of psychological interventions Psychotherapy and Psychosomatics ,275–284. 10.1159/000228248 10.1159/000228248 [DOI] [PubMed] [Google Scholar]
- *Mulvaney S. A., Rothman R. L., Wallston K. A., Lybarger C., Dietrich M. S.2010An internet-based program to improve self-management in adolescents with type 1 diabetes Diabetes Care ,602–604. 10.2337/dc09-1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Murphy H. R., Wadham C., Hassler-Hurst J., Rayman G., Skinner T. C., on behalf of Families and Adolescents Communication and Teamwork Study (FACTS) Group 2012Randomized trial of a diabetes self-management education and family teamwork intervention in adolescents with type 1 diabetes Diabetic Medicine ,e249–e254. 10.1111/j.1464-5491.2012.03683.x [DOI] [PubMed] [Google Scholar]
- Norris S. L., Lau J., Smith S. J., Schmid C. H., Engelgau M. M.2002Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control Diabetes Care ,1159–1171.http://www.ncbi.nlm.nih.gov/pubmed/12087014 10.2337/diacare.25.7.1159 [DOI] [PubMed] [Google Scholar]
- *Olmsted M. P., Daneman D., Rydall A. C., Lawson M. L., Rodin G.2002The effects of psychoeducation on disturbed eating attitudes and behavior in young women with type 1 diabetes mellitus International Journal of Eating Disorders ,230–239. 10.1002/eat.10068 [DOI] [PubMed] [Google Scholar]
- *Panagiotopoulos C., Preston J. M., Stewart L. L., Metzger D. L., Chanoine J. P.2003Weekly telephone contact by a diabetes educator in adolescents with type 1 diabetes Canadian Journal of Diabetes ,422–427. [Google Scholar]
- StataCorp . Stata statistical software: Release 12.1. College Station, TX: Author; 2012. [Google Scholar]
- Thompson B. T., Schoenfeld D.2007Usual care as the control group in clinical trials of nonpharmacologic interventions Proceedings of the American Thoracic Society ,577–582. 10.1513/pats.200706-072JK 10.1513/pats.200706-072JK [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. G., Higgins J. P. T.2002How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine ,1559–1573. 10.1002/(ISSN)1097-0258 [DOI] [PubMed] [Google Scholar]
- *Viklund G., Örtqvist E., Wikblad K.2007Assessment of an empowerment education programme. A randomized study in teenagers with diabetes Diabetic Medicine ,550–556. 10.1111/j.1464-5491.2007.02114.x [DOI] [PubMed] [Google Scholar]
- Williams D. M.2010Importance of the nature of comparison conditions for testing theory-based interventions: Comment on Michie and Prestwich (2010) Health Psychology ,467. 10.1037/a0019597 10.1037/a0019597 [DOI] [PubMed] [Google Scholar]
- *Wysocki T., Greco P., Harris M. A., Bubb J., White N. H.2001Behavior therapy for families of adolescents with diabetes: Maintenance of treatment effects Diabetes Care ,441–446. 10.2337/diacare.24.3.441 10.2337/diacare.24.3.441 [DOI] [PubMed] [Google Scholar]
- *Wysocki T., Harris M. A., Greco P., Bubb J., Danda C. E., Harvey L. M., White N. H.2000Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus Journal of Pediatric Psychology ,23–33. 10.1093/jpepsy/25.1.23 10.1093/jpepsy/25.1.23 [DOI] [PubMed] [Google Scholar]
- *Wysocki T., Miller K. M., Greco P., Harris M. A., Harvey L. M., Taylor A., White N. H.1999Behavior therapy for families of adolescents with diabetes: Effects on directly observed family interactions Behavior Therapy ,507–525.http://dx.doi.org/10.1016/S0005-7894(99)80022-7 10.1016/S0005-7894(99)80022-7 [DOI] [Google Scholar]
- Zwarenstein M., Treweek S., Gagnier J. J., Altman D. G., Tunis S., Haynes B., Moher D., for the CONSORT and Pragmatic Trials in Healthcare (Practihc) groups 2008Improving the reporting of pragmatic trials: An extension of the CONSORT statement British Medical Journal ,a2390–a2390. 10.1136/bmj.a2390 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]