Abstract
Stem cell-based strategies for ovarian regeneration and oocyte production have been proposed as future clinical therapies for treating infertility in women. However, utilization of embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) to produce oocytes has had limited success in vitro. A recent report of the isolation and characterization of endogenous oocyte-producing or oogonial stem cells (OSCs) from ovaries of reproductive-age women describes the first stable and pure human female germ cell culture model in which a subset of cells appear to initiate and complete meiosis. In addition, purified human OSCs introduced into adult human ovarian cortical tissue generate oocytes that arrest at the diplotene stage of meiosis and successfully recruit granulosa cells to form new primordial follicles. This overview examines the current landscape of in vitro and in vivo gametogenesis from stem cells, with emphasis on generation of human oocytes. Future research objectives for this area of work, as well as potential clinical applications involving the use of human OSCs, are discussed.
Keywords: Infertility, IVF, stem cells, germline stem cells, oocyte, ovary, aging
INTRODUCTION
Some improvements in clinical outcomes for women with fertility problems associated with primary ovarian insufficiency (POI), premature ovarian failure (POF) and age-associated ovarian dysfunction have been made over the past decade or so through expansion of assisted reproductive technologies (ART) (1, 2). However, the often prohibitive number of in-vitro fertilization (IVF) cycles required for success and the low numbers of live births, particularly for older women, remain major hurdles. Both the clinical management of problems associated with ovarian insufficiency and failure, as well as basic research into new therapies, have largely been restricted by a decades-old belief that women are born with a pool of oocytes that is not amenable to replacement or renewal (3). In 2004, this traditional thinking was challenged by a study in mice suggesting that ovarian oocyte and follicle formation is not restricted to the fetal or perinatal period, but rather extends well into adulthood (4). The stark contrast of these findings and conclusions to previously held views in the field resulted in considerable skepticism by some members of the scientific and medical communities (5–8; reviewed in 9, 10).
Nonetheless, a rapidly growing number of highly-compelling independent studies have subsequently confirmed that ovaries of adult mice contain a rare population of mitotically-active germ cells that can be isolated and propagated in culture for months, and that give rise to oocytes in vitro and upon transplantation into ovaries of recipient mice in vivo (11–16). While the role of these newly-discovered cells in adult ovarian function and fertility under normal physiological conditions is yet to be been determined, prior studies of adult ovaries in other animal models, such as Drosophila (17–21) and the teleost medaka (22), show that OSCs are active contributors to the adult oocyte pool used for reproduction. In mice, 5-bromo-2'-deoxyuridine (BrdU) incorporation into DNA in combination with the germ cell marker, Ddx4 (DEAD-box polypeptide 4, also referred to as mouse vasa homolog or Mvh), has been used to demonstrate the presence of actively dividing germ cells within the ovary (4, 12). Although interesting from a basic science perspective, these findings have little, if any, clinical implication unless reproductive-age women possess a comparable population of germline stem cells capable of generating new oocytes. To this end, we recently reported that ovaries of women in their twenties and early thirties contain a rare population of OSCs which generate oocytes in defined cultures in vitro and following introduction into human ovarian tissue in xenografts in vivo (16). The use of stem cell-based strategies as fertility treatment options are often viewed as being clinically applicable only in the distant future; however, these new findings in the area of human ovarian biology bring stem cell-based approaches for treating infertility in women one significant step closer to reality.
ISOLATION OF HUMAN OSCs
The procedure used for the isolation of human OSCs from adult ovary tissue was adapted from a protocol carefully validated using adult mouse ovaries as a model (16). A previous report had demonstrated that mouse OSCs can be isolated using an immunomagnetic bead-based isolation approach that targets a cell surface-expressed domain of the germ cell-specific protein, Ddx4 (12). After attempting for months to repeat this work, our laboratory was able to finally achieve isolation of OSCs from adult mouse ovaries using immunomagnetic beads; however, the resultant cell preparation was contaminated with small oocytes (16). We therefore re-developed and refined this protocol for use with fluorescence-activated cell sorting (FACS), which offers four major advantages over the magnetic bead-based approach used previously. First, OSCs isolated from adult mouse ovaries by FACS are free of contaminating oocytes. Second, FACS permits inclusion of a fluorescent cell viability dye, such as propidium iodide or 4',6-diamidino-2-phenylindole (DAPI), which allows exclusion of dead or dying (membrane compromised) cells from the final viable cell pool obtained. Third, FACS allows one to verify that a relatively uniform population of cells, based on size, is isolated. Lastly, multiple markers can be simultaneously explored as a means to confirm specificity of the approach used to isolate OSCs.
Freshly-isolated mouse OSCs purified by FACS possess a gene expression signature fully consistent with that of primitive germ cells and these cells can be expanded in vitro for months without loss of these identifying markers (16). During culture, a subset of mouse OSCs regularly form what appear to be oocytes based on morphology, size, and expression of classic oocyte markers, including Nobox (newborn ovary homeobox), Ybx2 (Y-box protein 2, also referred to as Msy2), Lhx8 (LIM homeobox protein 8) and Gdf9 (growth differentiation factor 9), as well as Zp1, Zp2 and Zp3 (zona pellucida glycoproteins 1, 2 and 3). In addition, using viral transduction to generate mouse OSCs with stable expression of green fluorescent protein (GFP) for cell tracking, such cells returned to ovaries of adult female mice by direct injection differentiate in vivo into GFP-positive oocytes that orchestrate de-novo follicle formation. Importantly, exogenous gonadotropin hormone stimulation of female mice receiving intraovarian transplantation of GFP-expressing mouse OSCs results in ovulation of fully mature GFP-positive eggs that fertilize to produce viable blastocysts (16).
This rigorous validation using mouse ovaries provided a strong impetus to then test this protocol for isolation of OSCs from ovaries of reproductive age women. Using de-identified cortical tissue obtained from ovaries surgically removed from women in their twenties and thirties as part of sex reassignment with written informed consent, human OSCs were successfully purified by FACS (16). This same FACS protocol has since been applied to routinely isolate OSCs from de-identified ovarian tissue samples obtained from women, with written informed consent, in their forties and fifties undergoing gynecologic surgeries for various benign reasons (D.C. Woods and J.L. Tilly, unpublished data). The cells obtained are indistinguishable from those reported just recently which were purified from ovaries of younger women (16). As is the case for mouse OSCs, freshly-isolated human OSCs express a primitive germline gene expression profile that is maintained during ex-vivo expansion of the cells in culture. In addition, human OSCs maintained in vitro spontaneously generate oocytes with all of the same attributes identified for oocytes produced by mouse OSCs cultured in parallel under identical conditions (16). To evaluate the ability of human OSCs to participate in oogenesis and follicle formation, GFP-expressing human OSCs were first aggregated with dissociated adult human ovarian cortex and monitored for the onset of follicle formation following in vitro culture for up to 72 hours. As early as 24 hours after aggregation and culture, large ovoid GFP-positive cells surrounded by a tightly compact layer of somatic cells closely resembling small follicles can be detected. This rapid onset to follicle formation observed in vitro was then used to set the time frame for testing the ability of OSCs to participate in follicle formation in adult human ovary tissue in vivo (16).
Due to the obvious limitations of studying human organ function in vivo, a xenograft model was employed in which human GFP-expressing OSCs were directly injected into adult human ovarian cortical tissue pieces. These tissues were then transplanted subcutaneously into immunocompromised adult female mice as hosts. Within 1–2 weeks after transplantation, GFP-positive oocytes can be found in the xenografted tissue, surrounded by flattened somatic cells enclosed by a clearly visible basement membrane in histologically normal primordial follicles (16). The oocytes contained within these newly formed follicles express both LHX8, an oocyte-specific transcription factor in the ovary (23), and more importantly YBX2, which marks oocytes at the diplotene stage of meiosis (24, 25). As expected, both of these markers are present in the same spatial localization pattern in oocytes of existing follicles present in the ovarian tissue prior to human OSC injection (16). This significance of these findings is two-fold. The first is that human OSCs spontaneously give rise to what appear to be normal diplotene-stage oocytes in adult human ovarian cortex within only a week or two after their introduction back into the tissue. Although xenografting of human ovary tissue is not equivalent to a physiologically normal environment per se, this approach has been used previously by us and others to study human oocyte and follicle development in an in-vivo setting (26–28). The second point of significance is that human OSCs were directly injected into ovarian cortical tissue from reproductive-age women, and de-novo follicle formation spontaneously occurred in a fairly rapid time-frame (16). This indicates that human ovaries are fully capable of supporting folliculogenesis during adulthood, similar to what has been reported for animal models (3, 12, 16). Accordingly, it is now imperative to more clearly define the contribution of these cells to adult ovarian function under physiological conditions, and how the existing mechanistic framework for age-related ovarian failure may need to be adjusted to account for the existence of OSCs and their potential support of oogenesis during adulthood as variables.
OOGENESIS: A COMPARISON OF HUMAN OSCs VERSUS HUMAN ESCs
The prospect of using human OSCs to produce developmentally-competent oocytes in vitro has enormous clinical potential. For many infertile women, oocyte or embryo donation is the only viable option to achieve a successful pregnancy, although the child is not biologically her own. As such, much effort has been put forth to generate oocytes in vitro from cellular sources other than those normally used to produce oocytes, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), with the goal of the latter being to generate patient-specific oocytes for IVF procedures (29–31). However, despite nearly a decade of work since the first description of mouse ESC-derived oocytes was published (32), progress has been limited and there has not been a single report of a fertilizable oocyte produced from ESCs or iPSCs of any species.
In mouse models of in vitro oogenesis, ESCs are specified into germ cells in essentially undefined conditions amidst a milieu of differentiating cell types and their respective secreted factors (29–32). The heterogeneity of differentiating ESC cultures has made the study of in-vitro primordial germ cell (PGC) specification and oogenesis challenging. As one example of the hurdles faced, scientists in this field rely heavily on reporter assays to trace these cells, since ESC-derived germ cells are not readily distinguishable based on morphology and differential surface marker expression until they reach the oocyte-like stage, which occurs approximately 20 days into the differentiation process (31, 33). Using lineage-specific reporter assays, early germ cell specification from ESCs occurs within the first 5 days of the initiation of differentiation following removal of leukemia inhibitory factor and feeder cells (usually mitotically-inactive mouse embryonic fibroblasts or MEFs), in either adherent monolayer or embryoid body (EB) cultures (34–36). While both methodologies can lead to the formation of oocyte-like cells and follicle-like structures in vitro, it has been postulated that differentiating EBs actually favor production of male germ cells (36, 37). Subsequent commitment to meiosis occurs between days 5–10 of culture (31, 33, 38), and oocyte-like cells can be distinguished from more primitive germ cell precursors based on morphology and genetic markers between days 16–40, depending on culture conditions (32, 39–41). Thus, a prolonged culture time is required to actually identify and isolate oocyte precursor cells or oocyte-like cells from differentiating ESCs (31–33). During this time, however, key signaling events required for the proper differentiation of oocytes from PGCs go astray, resulting in an `in-vitro block' of normal meiotic progression (33).
The extended time frame for germ cell specification from ESCs presents an additional problem, that being a high propensity for the PGCs that form to acquire characteristics similar to ESCs as they transform into embryonic germ cells (EGCs). This artifact of a prolonged differentiation process not only reduces the number of germ cells available for commitment to meiosis but also, and more importantly, represents a significant clinical risk for transplantation strategies as EGCs, like ESCs, give rise to teratomas after transplantation (33, 42, 43). Additionally, as mentioned earlier ESC-derived oocyte-like cells are meiotically defective, and completion of meiosis in germ cells derived from ESCs in vitro has yet to be demonstrated. For example, Novak et al. (44) reported that oocyte-like cells derived from mouse ESCs in vitro actually lack a full complement of meiotic proteins and fail to progress through meiotic prophase I. While the meiotic marker Sycp3 (synaptonemal complex protein 3) is detectable in mouse ESC-derived oocyte-like cells, these cells exhibit aberrant nuclear morphology and chromosomal alignment (44). The inability of ESC-derived oocyte-like cells to progress through meiosis has also been confirmed by Nicholas et al. (33) as well as by Tedesco et al. (45), who reported abnormal nuclear distribution of Sycp3 and the related meiotic protein Sycp1.
Far less is known of the derivation of germ cells and oocytes from human ESCs and iPSCs. This may be due, at least in part, to differences in the innate properties of mouse and human ESCs to differentiate into germ cells. Despite unique global gene expression profiles, many of the same markers expressed by early-stage mouse PGCs are also expressed in mouse ESCs, leading some to believe that these properties reflect a common origin from germ cell precursors (46, 47). Undifferentiated human ESCs, on the other hand, express a limited number of germ cell markers, and may be more closely related to the epiblast (31, 48). As such, human ESCs may have a very different inherent germ cell differentiation potential than their mouse counterparts. In any case, spontaneous and induced specification of PGCs has been documented in human ESC cultures (49–55).
The use of OSCs could circumvent many of the issues encountered by researchers studying human in-vitro oogenesis (Fig. 1). For example, unlike differentiating ESCs, the OSCs obtained following FACS purification represent a pure germ cell population, and these cells continue to maintain their germ cell identity and homogeneity following prolonged culture (16). Additionally, whereas ESC-derived oocyte-like cells fail to progress through meiosis, OSC-derived oocytes unenclosed by follicular cells or lacking as-yet undefined endogenous factors appear to complete meiosis in vitro, as evidenced by the formation of haploid cells in both mouse and human OSC cultures (16). While intraovarian transplantation studies using mouse ESC-derived germ cells have shown that the ovarian somatic microenvironment can help direct the differentiation of such germ cells into oocytes contained within immature follicles (33; D.C. Woods and J.L. Tilly, unpublished observations), there has not been any evidence to date describing maturation, ovulation or fertilization of these oocytes. In fact, follicles containing mouse ESC-derived oocytes fail to progress past the primary to secondary stage of development in vivo, and instead undergo atretic degeneration (33; D.C. Woods and J.L. Tilly, unpublished observations).
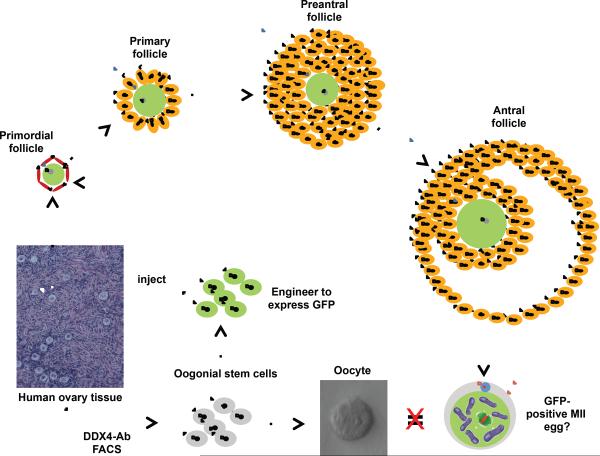
FIGURE 1.
A representative oocyte derived in vitro from human OSCs. Adult human ovary-derived OSCs, engineered to express GFP for cell tracking, spontaneously generate large GFP-positive oocytes during in vitro culture (see ref. 16 for more details).
In stark contrast, mouse OSC-derived oocytes formed following intraovarian transplantation of purified OSCs not only orchestrate proper folliculogenesis but also mature within follicles to the point of producing a metaphase-II egg after ovulation. In addition, through either natural mating trials or IVF protocols, the developmental competency of mouse OSC-derived eggs to fertilize and generate viable embryos and healthy offspring has been independently confirmed (12, 15, 16). While oocytes differentiated from OSCs maintained in vitro have not yet been reported to mature to a fertilizable state, it is clear that oocytes formed from ESCs versus OSCs, at least in vivo, possess a completely different developmental potential to make an egg. With the latter in mind, further investigation into ex-vivo maturation of OSC-derived oocytes is warranted.
Before closing this section, it should be mentioned that the approach employed to track the fate of transplanted mouse ESC-derived germ cells (33) and mouse OSCs (12, 16) is the same, that being the use of green fluorescent protein (GFP) expression in the cells to be transplanted. For Reijo-Pera and colleagues in studies of mouse ESCs, their conclusion that transplantation of “ESC-derived oocytes into an ovarian niche to direct their functional maturation and, thereby, present rigorous evidence of oocyte physiologic relevance and a potential therapeutic strategy for infertility” is based entirely on monitoring the fate of GFP-expressing cells in ovaries. Given this, it is surprising that Reijo-Pera then criticized recent findings of in-vivo oocyte formation from GFP-expressing mouse and human OSCs based on the approach utilized by White et al. (16), stating that “even the green oocytes [derived from transplanted GFP-expressing OSCs] should be viewed with caution, as GFP-tagged cells can fuse with unrelated cells” (56). If such experimental approaches “present rigorous evidence of oocyte physiologic relevance” in the context of mapping ESC-derived germ cell fate in vivo (33), it is unclear why Reijo-Pera would subsequently infer that a different standard be placed on interpretation of comparable outcomes obtained by others using OSCs isolated from adult ovaries as the cells for intraovarian transplantation studies (16).
EGG FORMATION FROM HUMAN OSCS EX VIVO: FACT OR FICTION
Although progress towards development of competent eggs from human ESCs or human iPSCs over the past decade has been disappointing, the recent isolation and characterization of OSCs from ovaries of reproductive age women open the prospects that such a goal may indeed be achievable if cells that serve as a natural precursor for oogenesis are used as starting material (16). Several pieces of evidence already in hand support this proposal. At a very basic level, one can simply draw a parallel between human OSCs and their adult mouse ovary-derived counterparts, the latter of which yield fully competent eggs if the oocytes formed are enclosed by granulosa cells as follicles (16). This finding, when considered with observations that every other aspect of OSC biology, including the spontaneous formation of haploid cells ex vivo in pure germ cell cultures, is conserved between mouse and human OSCs, supports that the primary if not exclusive function of these cells is to produce oocytes (16). This is entirely consistent with what is known of the role of OSCs in maintaining egg production in adult ovaries of less evolved species, such as Drosophila and medaka (17–22).
A second key piece of evidence comes from studies of how human OSCs respond once recombined with adult human ovarian somatic cells either in vitro or in vivo. Specifically, aggregation of human OSCs with dissociated adult human ovarian cortical tissue leads to formation of what appear to be immature follicles containing human OSC-derived oocytes within 24 hours in vitro. These data, along with findings that direct injection of human OSCs into adult human ovarian cortex followed by xenografting results in formation of follicles containing OSC-derived oocytes that express the meiotic diplotene-stage marker YBX2 (16), demonstrate that human OSCs have a strong propensity for oogenesis and folliculogenesis within adult human ovary tissue. A more technologically-advanced strategy will, however, be required to test the complete developmental competency of human OSC-derived oocytes. While in-vitro maturation of primordial and early primary follicles, and their enclosed oocytes, is now a relatively straightforward approach in mice (57, 58), culture technologies that support the advancement of human primordial follicles to mature antral stages have only recently been established (59, 60), with in-vitro development of human metaphase II oocytes not yet reported.
If achievable, such an outcome would have clear clinical applicability as a potential new source of developmentally competent oocytes for ART. To date, the most convincing and promising strategy for in-vitro maturation of human ovarian follicles is a two-step process in which micro-thin ovarian cortical strips containing primordial follicles are cultured under serum-free conditions (59, 60). Following primordial follicle activation and growth, preantral follicles are dissected out of the cortical strips and cultured individually in the presence of activin-A, which supports a further increase in follicle growth and development of an antral cavity. Bearing in mind that human in-vitro follicle growth in this particular system is initiated at the primordial stage, this is a striking achievement (60). Should this emerging technological advance in the field become a reality for the generation of functional metaphase II eggs, not only would it open new dimensions in the treatment of human infertility through ART, but it would also lay the framework needed to test the developmental potential of human OSCs and the oocytes these cells produce. Importantly, the latter goal is closer than some may think, in that GFP-expressing human OSCs have already been shown to generate oocytes that participate in the formation of primordial follicles in vitro and in vivo (16). Since human primordial follicles represent the starting point for the protocol used by Telfer and colleagues to develop antral follicles in vitro (60), a simple marriage of the two procedures at this common juncture point would be all that is required (Fig. 2). Given the ability of mouse OSC-derived oocytes, when contained inside follicles, to mature successfully into fully functional eggs (16), it is certainly reasonable to envision that ovarian follicles containing human OSC-derived oocytes could one day be developmentally advanced in vitro.
FIGURE 2.
Proposed steps for testing development of fully mature metaphase II (MII) eggs from human OSCs ex vivo. Oogonial stem cells isolated from ovarian cortex differentiate into oocytes in vitro; however, the oocytes formed do not arrest at MII, likely due to a lack of normal interaction with follicular somatic cells. This can be overcome by introducing OSCs into adult human ovarian cortical tissue ex vivo, which leads to the formation of new oocytes contained within primordial follicles (see ref. 16 for more details). After incubation of these micro-thin human ovarian cortical strips in vitro, primordial follicles within the strips activate and progress to primary and preantral stages. Preantral follicles are dissected out and further matured the antral stage, at which time oocytes are isolated for in-vitro maturation to the MII phase (see ref. 60 for more details). To distinguish OSC-derived oocytes from those present in the host tissue, human OSCs are transduced to express GFP prior to injection into the cortical tissue strips. This permits tracking of OSC-derived oocyte formation, primordial follicle assembly and activation, and growth maturation in vitro. If successful, resultant GFP-positive MII eggs derived from human OSCs could then be studied in more detail, including spindle appearance, chromosomal integrity and developmental competency.
It should also be mentioned that while cultured mouse and human OSCs appear to be largely homogeneous with respect to germline markers, only a few cells at a time undergo what appears to be a random and spontaneous differentiation process that triggers the formation of an oocyte in vitro (16). This event is restricted to only a subset of cells despite the fact that all of the cells in culture are continually exposed to essentially identical conditions. Why neighboring OSCs exposed to the same conditions fail to activate differentiation, whereas others in close proximity exit the mitotic cell cycle and enter meiosis, remains to be determined. It is also unclear how the fate of such cells, once differentiation and oocyte formation has occurred, is affected by local (microenvironmental) cues within the cultures, and whether or not this fate determination can be modified to more closely mimic an in-vivo environment. Identification of the factors contributing to the initiation or repression of this differentiation event is critical to further defining the parallelism between de-novo oogenesis occurring in vivo and in vitro. The use of human OSCs could therefore provide be a valuable screening approach for discovery and evaluation of factors that influence human oocyte yield and quality in vivo.
HUMAN OSCS: MORE TO THE STORY THAN JUST EGG FORMATION
Finally, although much is still to be learned about human OSCs from both basic science and clinical perspectives, the possible generation of eggs from human OSCs in vitro represents only one of several technologies of relevance to the clinical management of the ovarian reserve and fertility in women (Fig. 3). For example, with protocols now established to purify and culture human OSCs, and to evaluate their oogenic potential in vitro (16), experiments can be designed under defined conditions ex vivo to systematically uncover the critical signaling pathways that regulate the ability of human OSCs to generate oocytes. Given the unique function of OSCs in the body, it is highly likely that these cells will utilize hormones and factors that preferentially, or perhaps even exclusively, target these cells. In turn, the identification of such factors may then facilitate development of new therapies to enhance oogenic activity of OSCs in adult human ovaries in vivo as a means to maintain or rebuild the oocyte reserve, with minimal if any off-target effects. In addition to targeting OSCs in vivo, there is the obvious possibility of isolating human OSCs from a small ovarian biopsy for ex-vivo expansion and subsequent return to the ovaries to generate new oocytes and follicles. While proof-of-principle for this already exists in the recent report of human oocyte formation from OSCs in xenografted adult human ovary tissue (16), we echo the recent views of Telfer and Albertini that any clinical application of ex-vivo egg formation from human OSCs will take years of additional investigation (61). Nonetheless, the work required will proceed at a much faster pace if the “tone of future discourse” on the existence and function of mammalian OSCs by the scientific community can indeed move from one historically rooted in skepticism toward one of “measured enthusiasm” (61).
FIGURE 3.
Proposed technologies for future clinical testing and development based on human OSCs. 1: Once isolated, human OSCs could be cryopreserved and stored for future use. This could be done directly following isolation (fresh cells) or after expansion in vitro to establish a much larger cell pool. 2a: Through the AUGMENT procedure, mitochondria from a woman's OSCs could be isolated and injected into that same woman's oocytes at the time of ICSI to restore bioenergetic potential and enhance IVF success. 2b: As an alternative strategy to overcome mitochondrial or ATP deficits in eggs, human OSCs could serve as a unique screening model for identification of novel bioenergetic activators for female germline cells, which could then be tested for improvements in egg quality or embryo competency. 3: Because of their ex-vivo proliferative potential, human OSCs could serve as an essentially unlimited source of oocytes for generation of mature eggs using step-wise in-vitro organ and follicle culture systems. 4: Human OSCs, following storage without or with ex-vivo expansion, could be autologously returned to the ovaries as a means to increase the size of the ovarian reserve in vivo. 5: High-throughput screening of human OSCs ex vivo for factors, both man-made and natural, that enhance the oocyte-forming activity of these cells could lead to identification of new therapeutics designed to target these cells in vivo as an alternative means to increase the size of the ovarian reserve.
Finally, human OSCs may hold yet another value for clinical development in the form of cellular energy production. For many years, a common belief in the IVF field has been that the decline in egg quality with advancing maternal age is due, at least in part, to a progressively impaired energetic capacity in oocytes (62–64). This energy deficit leads to reduced fertilization potential and poor embryonic developmental competency, if the eggs are successfully fertilized; however, the resultant embryos are still compromised, resulting in much higher rates of implantation failure. In the late 1990s, it was reported that transfer of a small amount of ooplasm from donor eggs of young women into eggs of women with a history of repeat IVF failure resulted in a remarkably high rate of IVF success, with live birth rates nearing those normally observed in much younger women undergoing IVF for the first time (65–68). Despite the apparent clinical success, heterologous ooplasmic transfer was quickly stopped by the U.S. Food and Drug Administration since mitochondria, each one of which in humans contains DNA encoding 37 genes, were present in the donor ooplasm that was transferred. These `foreign' mitochondria, being maternally derived from eggs, underwent normal replication during embryogenesis and fetal development, leading to the birth of children containing mitochondria from two different sources (heteroplasmy) (67, 68). Accordingly, the presence of a `foreign' source of genetic material in these children, arising from the introduction of heterologous oocyte mitochondria at fertilization, represents an example of genetic manipulation of human germ cells for the purpose of reproduction. In other words, the most probable source of the `rejuvenating' factor in the transferred donor egg ooplasm was also the source of the reason why the procedure was prohibited from further use in human IVF protocols in 2002.
What if, however, the apparent clinical success of heterologous ooplasmic transfer for improving IVF outcomes could be reproduced in such a way that removes the `foreign' aspect of the female germline mitochondrial source that rejuvenates failing eggs? Human OSCs may indeed offer such an advantage. Being natural precursor cells for oocytes in adult mammalian ovaries (4, 12, 15, 16), a new strategy we term AUGMENT (autologous germline mitochondrial energy transfer) has been devised, which seeks to use the natural energy-producing potential of a woman's own OSCs as a means to reinvigorate that same woman's eggs for improved IVF success. Such delivery of autologous OSC-derived mitochondria into human oocytes during intracytoplasmic sperm injection (ICSI) may provide the energy boost needed to overcome existing mitochondrial deficits due to aging or other factors that negatively affect either fertilization outcome or post-fertilization embryonic competency. Considering the clinical success already reported from transfer of heterologous female germ cell mitochondria into eggs of women with a history of IVF failure (65–68), along with related studies using animal models showing improved embryonic developmental potential of fertilized eggs after mitochondrial transfer (69, 70), AUGMENT may hold considerable promise for clinical development and testing in the near-term.
Acknowledgements
Work conducted by the authors discussed herein was supported by a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279 to J.L.T.), a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809 to D.C.W.), the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, and Vincent Memorial Hospital Research Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: D.C.W. declares interest as a scientific consultant for OvaScience, Inc. (Cambridge, MA). J.L.T. declares interest in intellectual property described in U.S. Patent 7,955,846 and is a co-founder of OvaScience, Inc.
REFERENCES
- 1.Centers for Disease Control and Prevention, Society for Assisted Reproductive Technology . 2006 assisted reproductive technology success rates: national summary and fertility clinic reports. U.S: Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2008. [Google Scholar]
- 2.Palermo GD, Neri QV, Monahan D, Kocent J, Rosenwaks Z. Development and current applications of assisted fertilization. Fertil Steril. 2012;97:248–259. doi: 10.1016/j.fertnstert.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Zuckerman S. The number of oocytes in the mature ovary. Rec Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 4.Johnson J, Canning T, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 5.Gosden RG. Germline stem cells in the postnatal ovary: is the ovary more like a testis? Hum Reprod Update. 2004;10:193–195. doi: 10.1093/humupd/dmh023. [DOI] [PubMed] [Google Scholar]
- 6.Telfer EE. Germline stem cells in the postnatal mammalian ovary: a phenomenon of prosimian primates and mice? Reprod Biol Endocrinol. 2004;18:24. doi: 10.1186/1477-7827-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfeld C, Flaws JA. Renewed debate over postnatal oogenesis in the mammalian ovary. BioEssays. 2004;26:829–832. doi: 10.1002/bies.20094. [DOI] [PubMed] [Google Scholar]
- 8.Albertini DF. Micromanagement of the ovarian follicle reserve – do stem cells play into the ledger? Reproduction. 2004;127:513–514. doi: 10.1530/rep.1.00247. [DOI] [PubMed] [Google Scholar]
- 9.Powell K. Going against the grain. PLoS Biol. 2007;5:e338. doi: 10.1371/journal.pbio.0050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80:2–12. doi: 10.1095/biolreprod.108.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr JB, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 12.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–349. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- 16.White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong MD, Jin Z, Xie T. Molecular mechanisms of germline stem cell regulation. Annu Rev Genet. 2005;39:173–195. doi: 10.1146/annurev.genet.39.073003.105855. [DOI] [PubMed] [Google Scholar]
- 18.Waskar M, Li Y, Tower J. Stem cell aging in the Drosophila ovary. AGE. 2005;27:201–212. doi: 10.1007/s11357-005-2914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7:344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 23.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA. 2006;103:8090–8095. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu W, Tekur S, Reinbold R, Eppig JJ, Choi YC, Zheng JZ, Murray MT, Hecht NB. Mammalian male and female germ cells express a germ cell-specific Y-Box protein, MSY2. Biol Reprod. 1998;59:1266–1274. doi: 10.1095/biolreprod59.5.1266. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, Schultz RM, Hecht NB. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci USA. 2005;102:5755–5760. doi: 10.1073/pnas.0408718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60:1462–1467. doi: 10.1095/biolreprod60.6.1462. [DOI] [PubMed] [Google Scholar]
- 27.Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril. 2000;73:599–603. doi: 10.1016/s0015-0282(99)00548-8. [DOI] [PubMed] [Google Scholar]
- 28.Matikainen T, Perez GI, Jurisicova A, Schlezinger JJ, Ryu H-Y, Pru JK, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 29.Ko K, Schöler HR. Embryonic stem cells as a potential source of gametes. Semin Reprod Med. 2006;24:322–329. doi: 10.1055/s-2006-952157. [DOI] [PubMed] [Google Scholar]
- 30.Nagano MC. In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod. 2007;76:546–551. doi: 10.1095/biolreprod.106.058271. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas CR, Chavez SL, Baker VL, Reijo Pera RA. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr Rev. 2009;30:264–283. doi: 10.1210/er.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, Boiani M, Schöler HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 33.Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet. 2009;22:4376–4389. doi: 10.1093/hmg/ddp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saiti D, Lacham-Kaplan O. Density gradients for the isolation of germ cells from embryoid bodies. Reprod Biomed Online. 2008;16:730–740. doi: 10.1016/s1472-6483(10)60489-0. [DOI] [PubMed] [Google Scholar]
- 35.Young JC, Dias VL, Loveland KL. Defining the window of germline genesis in vitro from murine embryonic stem cells. Biol Reprod. 2010;82:390–401. doi: 10.1095/biolreprod.109.078493. [DOI] [PubMed] [Google Scholar]
- 36.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 38.Young JC, Dias VL, Loveland KL. Defining the window of germline genesis in vitro from murine embryonic stem cells. Biol Reprod. 2010;82:390–401. doi: 10.1095/biolreprod.109.078493. [DOI] [PubMed] [Google Scholar]
- 39.Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- 40.Qing T, Shi Y, Qin H, Ye X, Wei W, Liu H, Ding M, Deng H. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation. 2007;75:902–911. doi: 10.1111/j.1432-0436.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R, Kerkis I. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells. 2007;9:535–548. doi: 10.1089/clo.2007.0031. [DOI] [PubMed] [Google Scholar]
- 42.Enseñat-Waser R, Santana A, Vicente-Salar N, Cigudosa JC, Roche E, Soria B, Reig JA. Isolation and characterization of residual undifferentiated mouse embryonic stem cells from embryoid body cultures by fluorescence tracking. In Vitro Cell Dev Biol Anim. 2006;42:115–123. doi: 10.1290/0509063.1. [DOI] [PubMed] [Google Scholar]
- 43.Cao F, van der Bogt KE, Sadrzadeh A, Xie X, Sheikh AY, Wang H, Connolly AJ, Robbins RC, Wu JC. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev. 2007;16:883–891. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- 44.Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24:1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- 45.Tedesco M, Farini D, Felici M. Impaired meiotic competence in putative primordial germ cells produced from mouse embryonic stem cells. Int J Dev Biol. 2011;55:215–222. doi: 10.1387/ijdb.103108mt. [DOI] [PubMed] [Google Scholar]
- 46.Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 47.Mise N, Fuchikami T, Sugimoto M, Kobayakawa S, Ike F, Ogawa T, Tada T, Kanaya S, Noce T, Abe K. Differences and similarities in the developmental status of embryo-derived stem cells and primordial germ cells revealed by global expression profiling. Genes Cells. 2008;13:863–877. doi: 10.1111/j.1365-2443.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;270:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells. 2009;27:68–77. doi: 10.1634/stemcells.2007-1018. [DOI] [PubMed] [Google Scholar]
- 51.Chen HF, Kuo HC, Chien CL, Shun CT, Yao YL, Ip PL, Chuang CY, Wang CC, Yang YS, Ho HN. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod. 2007;22:567–577. doi: 10.1093/humrep/del412. [DOI] [PubMed] [Google Scholar]
- 52.Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE, Plath K, Clark AT. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;4:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- 54.Tilgner K, Atkinson SP, Yung S, Golebiewska A, Stojkovic M, Moreno R, Lako M, Armstrong L. Expression of GFP under the control of the RNA helicase VASA permits fluorescence-activated cell sorting isolation of human primordial germ cells. Stem Cells. 2010;28:84–92. doi: 10.1002/stem.263. [DOI] [PubMed] [Google Scholar]
- 55.West FD, Machacek DW, Boyd NL, Pandiyan K, Robbins KR, Stice SL. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells. 2008;26:2768–2776. doi: 10.1634/stemcells.2008-0124. [DOI] [PubMed] [Google Scholar]
- 56.Vogel G. Potential egg stem cells reignite debate. Science. 2012;335:1029–1030. doi: 10.1126/science.335.6072.1029. [DOI] [PubMed] [Google Scholar]
- 57.Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2:94–104. doi: 10.1530/ror.0.0020094. [DOI] [PubMed] [Google Scholar]
- 58.Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- 59.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 60.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 61.Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18:353–354. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- 62.Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ. Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod. 2000;15(Suppl. 2):160–172. doi: 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- 63.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- 64.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of an infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, Wu L, Brenner CA, Warner C, Willadsen S. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 67.Brenner CA, Barritt JA, Willadsen S, Cohen J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril. 2000;74:573–578. doi: 10.1016/s0015-0282(00)00681-6. [DOI] [PubMed] [Google Scholar]
- 68.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 69.El Shourbagy SH, Spikings EC, Freitas M, St. John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 70.Yi Y-C, Chen M-J, Ho JY-P, Guu H-F, Ho E-S. Mitochondrial transfer can enhance the murine embryo development. J Assist Reprod Genet. 2007;24:445–449. doi: 10.1007/s10815-007-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]