Abstract
Objective
Perform gene expression profiling of adult mouse ovary-derived oogonial stem cells (OSCs).
Design
Experimental animal study.
Setting
Research laboratory.
Animal(s)
Adult C57BL/6 female mice.
Intervention(s)
None.
Main outcome measure(s)
Gene expression profiles were compared between freshly isolated and cultured OSCs, as well as between OSCs and embryonic stem cells (ESCs), fetal primordial germ cells (PGCs) and spermatogonial stem cells (SSCs); OSC yield from ovaries versus meiotic gene activation during the estrous cycle was determined.
Result(s)
Freshly isolated OSCs, PGCs and SSCs exhibited distinct gene expression profiles. Cultured OSCs maintained their germline gene expression pattern, but gained expression of pluripotency markers found in PGCs and ESCs. Cultured OSCs also expressed the meiotic marker, stimulated by retinoic acid gene 8 (Stra8). In vivo, OSC yield was higher from luteal versus follicular phase ovaries and this was inversely related to Stra8 expression.
Conclusion(s)
Freshly isolated OSCs exhibit a germline gene expression profile that overlaps with, but is distinct from, that of PGCs and SSCs. After in vitro expansion, OSCs activate expression of pluripotency genes found in freshly isolated PGCs. In vivo, OSC numbers in the ovaries fluctuate during the estrous cycle, with the highest numbers noted during the luteal phase. This is followed by activation of Stra8 expression during the follicular phase, which may signify a wave of neo-oogenesis to partially offset follicular loss through atresia and ovulation in the prior cycle.
Keywords: oogenesis, oocyte, germ cell, oogonial stem cell, Stra8
INTRODUCTION
Since the initial publication in 2004 challenging the decades-old belief that the ovaries of mammals lose replicative germ cells capable of generating new oocytes after birth (1), experimental evidence from many laboratories collectively supports a new paradigm in reproductive biology that accounts for the existence of female germline or oogonial stem cells (OSCs) in postnatal ovaries (reviewed in 2–6). In addition to the successful purification of OSCs by at least three laboratories using different techniques (7–10), transplantation studies analogous to those used for establishing the identity of spermatogonial stem cells (SSCs) in the testes of adult males have demonstrated that OSCs generate competent oocytes in adult ovaries that fertilize to produce viable embryos and offspring (7, 10–12). In addition, methods for the establishment and stable propagation of mouse and human OSCs in culture are now available (7–10, 13). These studies have shown that OSCs undergo spontaneous differentiation in vitro to produce what appear to be oocytes based on morphological criteria, gene expression profiling, meiotic activation and the ability to attract granulosa cells for folliculogenesis (8, 10, 13).
Central to this work is the development and validation of methods to purify OSCs from adult ovarian tissue (13). In 2009, Wu and colleagues reported an immunomagnetic sorting strategy for the isolation of mouse OSCs from dissociated ovarian tissue, relying on an externalized epitope of the germ cell protein, DEAD box polypeptide 4 (Ddx4; also commonly referred to as mouse vasa homolog or Mvh) (14, 15), which had not been identified previously (7, 16, 17). However, the magnetic sorting approach had limitations, as the OSC-containing cell fraction obtained was not homogeneous, and therefore analysis of freshly isolated OSCs could not be performed. This, combined with the relatively long culture period between isolation of the cells and their functional testing by transplantation strategies (7), prompted questions as to whether the cells isolated were truly innately germline at collection or had undergone some type of in vitro transformation event to a stem-like cell prior to transplantation.
To address this, we developed and reported a fluorescence-activated cell sorting (FACS)-based strategy, utilizing an antibody that targets the externalized epitope of Ddx4, for the purification of OSCs free of contaminating oocytes and other cells from ovaries of adult mice (10, 13). Importantly, the same technology was successfully applied to the isolation of OSCs from ovarian cortical tissue of reproductive age women (10, 13). A direct comparison of the immunomagnetic bead strategy reported by Wu and colleagues (7) and our FACS-based approach (10) revealed that, while magnetic sorting results in a mixed population of cells including OSCs and oocytes, comparable fractions obtained by FACS are free of such contamination and can be used for characterization of freshly isolated OSCs. In this regard, OSCs analyzed immediately after FACS-based collection have a gene expression profile fully consistent with primitive germ cells during early specification, and are not the result of some random in-vitro transformation event as erroneously suggested by others (18). Along with the ability to isolate a pure population of OSCs from dissociated ovarian tissue, the use of FACS provides a quantitative assessment of OSC yield. In young adult mouse ovaries, the cells constitute 0.014% ± 0.002% of the total ovarian cell population, and approximately 1.5% ± 0.2% of the viable cell fraction sorted after dissociation (10).
We have also reported that ex-vivo expanded OSCs maintain a primitive germline gene expression profile that is uniform across essentially all cells in culture (10). In males, SSCs are unipotent, having the capability to self-renew and differentiate into spermatozoa (19). Following ex vivo culture, however, isolated SSCs acquire an embryonic stem cell (ESC)-like gene expression profile (20, 21). Whether OSCs propagated in culture possess this same capacity is not known. Accordingly, using our FACS-based methodology for OSC isolation (10, 13), herein we compared gene expression profiles between freshly isolated and cultured mouse OSCs, and between OSCs and other germline and pluripotent stem cell types. We also determined the relationship between OSC numbers and meiotic gene activation in ovaries in vivo during the estrous cycle, based on our observations from the profiling studies that OSCs cultured in vitro activate expression of the meiotic commitment gene, stimulated by retinoic acid gene 8 (Stra8) (22, 23), concomitant with oogenesis.
MATERIALS AND METHODS
Animals
Wild type C57BL/6 mice were obtained from Charles River Laboratories. To generate transgenic mice with Stra8 promoter-driven GFP expression, a 1.4-kb fragment of the mouse Stra8 promoter (–1400 to +11), previously shown to convey premeiotic germ cell-specific expression (24, 25), was amplified from mouse genomic DNA and cloned into the XhoI/EcoRI site of the pEgfp-1 vector (BD Biosciences). The pStra8-Gfp vector was then sent to Genoway to generate pStra8-Gfp transgenic mice using their proprietary Quick Knock-in™ technology involving insertion of the transgene sequence into the neutral Hprt locus (www.genoway.com). This approach eliminates potential confounding effects of random integration of transgenes and variability in copy number commonly associated with conventional pronuclear injection. Mouse lines with germline transmission of the transgene were identified at Genoway, shipped to us and used to establish breeding colonies. All procedures were reviewed and approved by the institutional animal care and use committee of Massachusetts General Hospital.
Cell lines
Mouse ESCs (v6.5) were obtained from Novus Biologicals, expanded as undifferentiated cells in culture under standard conditions (26) and used at passage number 29.
Isolation of OSCs
Adult mouse ovary-derived OSCs were purified by FACS based on cell-surface expression of the C-terminus of Ddx4, and, for some experiments, established as actively dividing germ cell cultures without somatic feeder cells (10, 13). For studies of ex-vivo expanded OSCs, cells at passage number 23 were used.
Isolation of primordial germ cells (PGCs)
For each experimental replicate, 6–12 genital ridges were isolated from embryos at 11.5 days post-coitum (dpc), dissociated and processed by magnetic assisted cell sorting (MiniMACS; Miltenyi Biotec) using a mouse monoclonal antibody to stage specific embryonic antigen-1 (SSEA1; Abcam, ab16285) to isolate PGCs (27). The SSEA1-positive cell fraction enriched for PGCs was snap-frozen for gene expression analysis.
Isolation of SSCs
Spermatogonial stem cells were isolated from dissociated testes of 6-week-old male mice (28), and snap-frozen for gene expression analysis.
Gene expression analysis
Total RNA was extracted using the RNeasy Plus Micro or Mini kit (QIAGEN) and reverse-transcribed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen), according to protocols supplied by the manufacturers. The absence or presence of each mRNA in each sample was assessed by conventional PCR using Platinum Taq polymerase (Invitrogen) and primer pairs specific for amplification of each gene (Supplementary Table 1). All products were sequenced to confirm identity. For quantitative analysis of Stra8 mRNA levels, real-time PCR was performed using a Cepheid Smart Cycler II and primers specific for Stra8 (FAM-labeled D-LUX™ Pre-designed Gene Expression Assays, MLUX3312362; Invitrogen), with levels normalized to β-actin mRNA levels (FAM-labeled certified LUX™ Primer Set for Mouse/Rat β-actin, 101M-01; Invitrogen) in each sample. Expression ratios were then calculated (29).
Immunofluorescence
Ovaries, testes and eviscerated e13.5 fetuses of pStra8-Gfp mice were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned for analysis using antibodies against Stra8 (rabbit polyclonal ab49602, Abcam), Ddx4 (rabbit polyclonal ab13840, Abcam) or GFP (mouse monoclonal sc-9996, Santa Cruz Biotechnology). For immunofluorescence, detection was performed using donkey anti-rabbit Alexa Fluor 546 or donkey anti-mouse Alexa Fluor 488 (Molecular Probes) as secondary antibody. Images were captured using a Nikon E800/BioRad Radiance 2000 confocal microscope or a Nikon ECLIPSE TE2000-S microscope.
Estrous cycle staging
Six-week-old female mice were housed in a controlled environment with food and water ad libitum. Daily vaginal smear cytology was used to monitor estrous cyclicity for each animal for 3–4 weeks prior to ovary collection at the specified stages (30). For each experimental replicate, 2–4 ovaries per stage were prepared for OSC isolation as described above, and OSC yield per dissociated ovary was quantified using FlowJo software (version 9; Tree Star).
Data presentation and statistical analysis
All experiments were independently replicated a minimum of 3 times. Combined data from the replicates, expressed as the mean ± standard error of the mean (SEM), were analyzed by a one-way analysis of variance followed by Student's t-test or Bonferroni post-hoc analysis using the Statistical Package for Social Sciences (SPSS, version 19) to identify significant differences (P < 0.05). Where appropriate, qualitative images representative of outcomes obtained in the replicate experiments are provided.
RESULTS
Gene expression profile comparisons
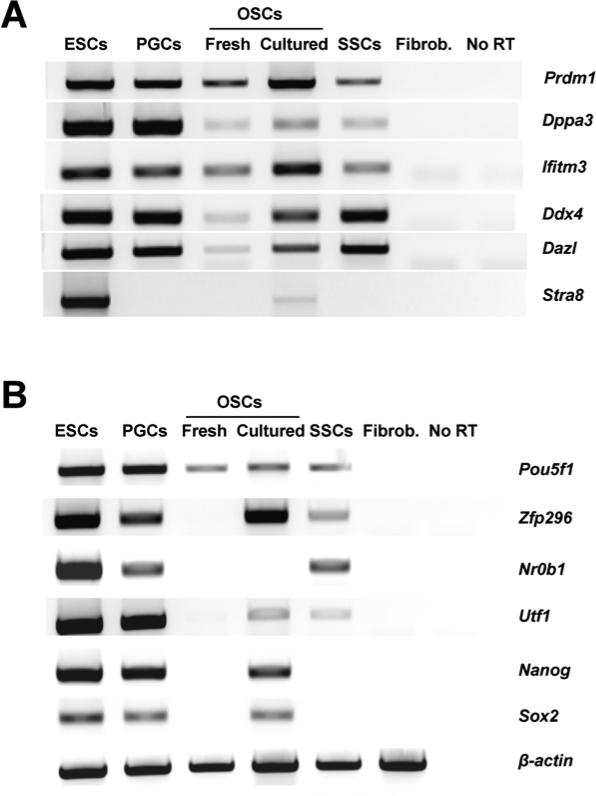
Expression of the germline markers PR domain zinc finger protein 1 (Prdm1; also commonly referred to as Blimp1), developmental pluripotency-associated protein 3 (Dppa3; also commonly referred to as Stella), interferon-induced transmembrane protein 3 (Ifitm3; also commonly referred to as Fragilis), Ddx4 and deleted in azoospermia-like (Dazl) were detected in freshly isolated OSCs, cultured OSCs, ESCs, PGCs and SSCs (Fig. 1A). Notably, of the cell types examined, only ESCs and cultured OSCs expressed detectable levels of Stra8 mRNA (Fig 1A). The pluripotency-associated transcripts POU domain class 5 transcription factor 1 (Pou5f1; also known as octamer binding transcription factor 4 or Oct4), zinc finger protein 296 (Zfp296), dosage-sensitive sex reversal nuclear receptor subfamily 0 group B member 1 (Nr0b1; also commonly referred to as Dax1), undifferentiated embryonic cell transcription factor 1 (Utf1), Nanog and SRY-box containing gene 2 (Sox2) were detectable in ESCs and PGCs, whereas SSCs expressed only Pou5f1, Zfp296, Nr0b1 and Utf1 (Fig. 1B). In striking comparison, of all the pluripotency-associated genes examined, only Pou5f1 was detectable in freshly isolated OSCs; however, ex vivo culture of OSCs resulted in activation of all pluripotency-associated genes with the exception of Nr0b1 (Fig. 1B). As a negative control, adult tail-snip fibroblasts did not show expression of any of the germline or pluripotency-associated genes tested (Fig. 1). A summary of the gene expression analyses, highlighting key comparative differences between the cell populations studied, is presented in Supplementary Table 2.
Figure 1.
Comparative gene expression profiling analysis of germ cell and stem cell markers. (A, B) Representative expression pattern of genes associated with germline development and meiotic commitment (A) or pluripotency (B) in mouse ESCs, embryonic PGCs, adult ovary-derived OSCs (freshly isolated and cultured) and juvenile testis-derived SSCs. Adult tail-snip fibroblasts (Fibrob.) were used as a negative control for specificity of pluripotency and germline gene expression, whereas β-actin mRNA was used as a sample loading control (no RT, PCR analysis of RNA sample without reverse transcription used as a negative control for excluding genomic DNA contamination).
Meiotic gene activation
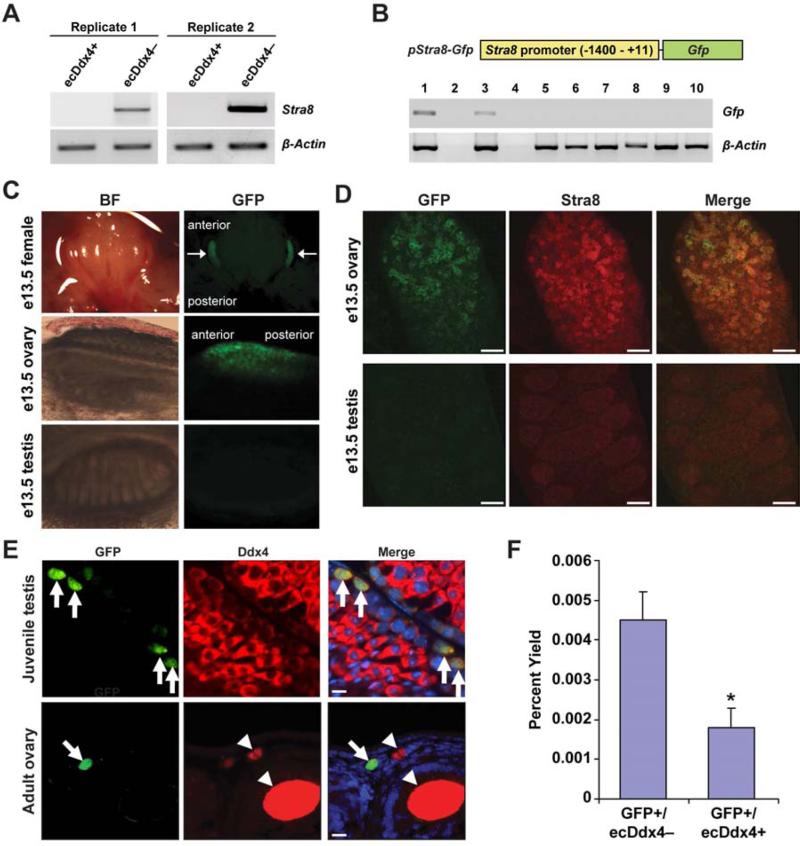
Expression of the meiotic commitment gene, Stra8, was detected only in cultured ESCs and cultured OSCs, which is in keeping with past work showing that both of these cell types can spontaneously generate oocytes in vitro (8, 10, 31). In turn, the absence of Stra8 in freshly isolated OSCs attests to the undifferentiated (pre-meiotic) status of these germ cells prior to ex vivo expansion. To determine if the presence of externalized Ddx4 protein (extracellular-exposed Ddx4 or ecDdx4), which provides the means by which FACS-based isolation of OSCs is accomplished (10, 13), marks only undifferentiated OSCs, we analyzed expression of Stra8 in the ecDdx4-positive and ecDdx4-negative cell fractions obtained from dissociated adult ovaries following FACS. Consistent with results shown earlier (Fig. 1A), ecDdx4-positive cells (OSCs) did not express Stra8 mRNA; however, Stra8 mRNA was detected in the ecDdx4-negative cell fraction (Fig. 2A), which is composed of all other cells including differentiating germ cells as well as small oocytes that express and retain Ddx4 protein entirely in the cytoplasm (10).
Figure 2.
Relationship between externalized Ddx4 protein and meiotic gene activation in OSCs. (A) PCR analysis of Stra8 expression in FACS-purified ovarian cell fractions with externalized Ddx4 protein (ecDdx4-positive or ecDdx4+) or without externalized Ddx4 protein (ecDdx4-negative or ecDdx4–). β-Actin, control gene for sample loading. (B) Schematic of the Stra8 promoter (pStra8)-Gfp construct, and assessment of Gfp and β-actin mRNA levels in tissues from adult pStra8-Gfp transgenic mice: 1, testis; 2, PCR without reverse transcription of testicular RNA; 3, ovary; 4, PCR without reverse transcription of ovarian RNA; 5, heart; 6, kidney; 7, liver; 8, lung; 9, spleen; 10, brain. (C) Brightfield (BF, left panels) and direct fluorescence (GFP, right panels) imaging of female embryos (arrows highlight restricted GFP fluorescence in ovaries), female gonads and male gonads from pStra8-Gfp mice at embryonic day 13.5 (e13.5). (D) Dual immunofluorescence analysis of GFP and Stra8 ovaries and testes of pStra8-Gfp mice at e13.5, showing the presence of both proteins only in fetal ovaries. Scale bars, 10 μm. (E) Dual immunofluorescence analysis of GFP (green) and Ddx4 (red) in juvenile testes of pStra8-Gfp mice after resumption of meiosis (upper panel; arrows indicate GFP-positive germ cells in a randomly selected tubule of a longitudinally sectioned testis) or in ovaries of 2-month-old (adult) pStra8-Gfp mice (lower panel, longitudinally sectioned ovary; arrow indicates a cell positive for both GFP and Ddx4, adjacent to GFP-negative/Ddx4-positive oocytes contained in follicles and highlighted by arrowheads). Merge also shows DAPI-counterstained nuclei (blue); scale bars, 10 μm. (F) Yield of GFP-expressing cells (as a percent of total viable cells sorted) lacking externalized Ddx4 protein (GFP+/ecDdx4–), and of cells positive for both GFP and externalized Ddx4 (GFP+/ecDdx4+), from ovaries of adult pStra8-Gfp mice (mean ± SEM, n = 3; *, P < 0.05).
Analysis of ecDdx4 in ovaries of pStra8-Gfp mice
To more clearly define the relationship between externalized Ddx4 expression and meiotic gene activation, we generated transgenic mice with GFP expression under control of the Stra8 promoter (Fig. 2B). Consistent with the previously reported specificity of this promoter fragment for activation only in germ cells (24, 25), transgene expression in adult pStra8-Gfp mice was restricted to the gonads (Fig. 2B). Moreover, direct fluorescence imaging showed sex- and tissue-specific transgene expression during embryonic development paralleling that of the endogenous Stra8 gene. Specifically, GFP was detected in female gonads at embryonic day 13.5 (e13.5) in an anterior to posterior pattern (Fig. 2C), mirroring that reported for endogenous Stra8 expression associated with female germ cell meiotic commitment at this developmental stage (32). In contrast, GFP was not observed in any other organs of female embryos or in any tissues of male embryos including the testes (Fig. 2C). Dual immunofluorescence staining showed that most GFP co-localized with endogenous Stra8 in e13.5 female gonads (Fig. 2D). A small number of cells were positive for only GFP or Stra8 (Fig. 2D), which may be due to differential kinetics in transcription or protein translation from these two genes. As expected, an absence of Stra8 promoter-driven GFP expression in embryonic testes (Fig. 2C) paralleled an absence of endogenous Stra8 protein (Fig. 2D). However, GFP in male germ cells became detectable concomitant with initiation of meiosis in juvenile testes (Fig. 2E).
We also identified rare cells positive for Stra8 promoter-driven GFP expression in ovaries of adult transgenic females (Fig. 2E). Dual immunofluorescence analysis indicated that these cells co-expressed the germ cell marker Ddx4, whereas adjacent oocytes contained within follicles of pStra8-Gfp mouse ovaries also expressed Ddx4 but lacked GFP (Fig. 2E). The absence of GFP in oocytes of this transgenic line is consistent with the absence of Stra8 in mouse oocytes (33). Like endogenous Stra8-expressing cells in adult mouse ovaries (33, 34), these GFP-positive cells were extremely rare and located near the ovarian surface epithelium. Using FACS, we determined that the majority of cells positive for Stra8 promoter-driven GFP expression were negative for externalized expression of Ddx4 (ecDdx4–; Fig. 2F); however, a small percentage of cells with externalized Ddx4 (ecDdx4+) were identified as also being positive for Stra8 promoter-driven GFP expression (Fig. 2F).
OSC yield versus Stra8 activation during the estrous cycle
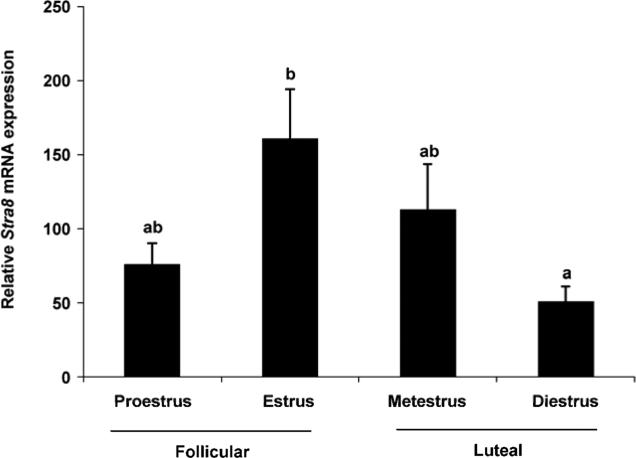
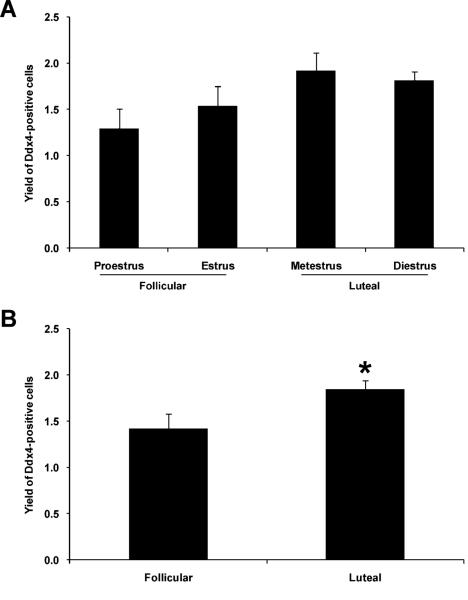
Our observations that OSC differentiation (meiotic gene activation, viz. Stra8 expression) is coupled to a loss of externalized Ddx4 protein (Fig. 2) prompted us to explore the physiological relationship between these events more thoroughly in vivo. Based on past studies, which have reported that the number of oocyte-containing primordial follicles in adult mouse ovaries fluctuates during the estrous cycle (30, 35), we next assessed OSC numbers versus Stra8 activation in ovaries at different stage of the female reproductive cycle. Analysis of the ecDdx4-negative cell fraction by quantitative PCR throughout the estrous cycle identified the highest level of Stra8 expression during estrus, just prior to transition of the females from the follicular phase to the luteal phase of the ovarian cycle (Fig. 3). By comparison, the number of OSCs retrieved by FACS increased as females transitioned to the luteal phase (Fig. 4). Although no significant differences in OSC yield were detected after comparison of each of the four stages of the estrous cycle independently (Fig. 4A), stratification of the data into a comparison of the follicular (proestrus and estrus) versus luteal (metestrus and diestrus) phase revealed the presence of significantly more OSCs in luteal phase ovaries (Fig. 4B).
Figure 3.
Ovarian Stra8 expression patterns during the female reproductive cycle. Quantitative PCR analysis of Stra8 mRNA levels (normalized to β-actin mRNA levels) in adult mouse ovaries at each indicated stage of the estrous cycle (mean ± SEM, n = 3; different letters, P < 0.05).
Figure 4.
Changes in OSC numbers in mouse ovaries relative to stage of the female reproductive cycle. (A, B) Yield of OSCs, as a percent of total viable cells sorted, from ovaries of adult female mice at each indicated stage of the estrous cycle (A) or following stratification of estrous cycle stages into the follicular and luteal phases of the ovarian cycle (B). The data shown represent the mean ± SEM (n = 3; *, P < 0.05).
DISCUSSION
To our knowledge, comparative gene expression profiles between OSCs and other germ cell and stem cell lineages have not been previously reported, despite the fact that some scientists have questioned, without any supporting data, the identity of OSCs as being nothing more than misplaced or ‘laggard’ oogonia (viz. embryonic female PGCs) with no true biological function (36). As shown herein, the numerous and marked differences in gene expression patterns between embryonic PGCs and postnatal ovary-derived OSCs analyzed immediately after isolation provide strong evidence countering such a claim. In fact, in addition to confirming prior observations that freshly isolated OSCs express a primitive germline gene expression profile (7–10), our inclusion of several pluripotency markers in the present study actually demonstrates that PGCs and ESCs exhibit a much greater overlap in gene expression profiles than that of PGCs and OSCs. The most obvious difference is the absence, in OSCs, of the numerous ESC-like pluripotency genes expressed in PGCs. Interestingly, while freshly isolated OSCs do not innately express genes commonly associated with pluripotency, ex vivo expansion of the cells leads to acquisition of most of these factors such that cultured OSCs acquire a closer resemblance to PGCs in terms of gene expression patterns. It is important to point out that our experiments used OSCs at passage 23, which represents approximately 5–6 months of continuous culture. Future studies involving a passage-by-passage analysis of gene expression patterns in cultured OSCs, starting with their initial establishment in vitro (first passage at approximately 2 weeks after plating), will be of interest to determine exactly how much time is required for the gene expression profile of cultured OSCs to change to one more closely resembling that of PGCs. Irrespective, this observation aligns well with that reported for SSCs, which are unipotent in vivo (19) but acquire a multipotent ESC-like cell phenotype following extended culture (20, 21). These latter observations have generated interest in the potential use of SSCs as an alternative to ESCs for patient-specific stem cell therapies (37). Although more studies are needed to determine if cultured OSCs gain multipotency, the finding that OSCs activate genes associated with pluripotency following extended culture at least opens similar possibilities to those considered for multipotent adult germline stem cells derived from cultured SSCs.
Another important observation reported herein is the clear separation of two key processes in OSCs, namely externalized expression of Ddx4 and meiotic commitment. As a point of note to avoid any confusion, we feel it is critical to refer to the viably sorted ovarian cell fractions with and without externalized Ddx4 protein as ‘ecDdx4-positive’ (extracellular Ddx4 epitope exposed) and ‘ecDdx4-negative’ (extracellular Ddx4 epitope not exposed), respectively. Since these cell fractions are sorted based on the immunological presence or absence of the C-terminus of Ddx4 protein on the cell surface, use of the more generic terms, Ddx4-positive and Ddx4-negative, may give the false impression that the latter fraction of cells is defined by the absence of Ddx4 gene expression. This is not the case, a point exemplified most clearly by the fact that oocytes, which contain Ddx4 mRNA as well as Ddx4 protein localized exclusively to the cytoplasm, do not exhibit cell surface expression of Ddx4 (10). Thus, small oocytes would be sorted into the ecDdx4-negative fraction using our FACS protocol (13).
It is currently unclear why OSCs, and not oocytes, exhibit cell surface expression of the C-terminus of Ddx4. One possibility we are now testing is that this difference in protein localization serves to sequester, and thereby silence, the RNA helicase function of Ddx4 in undifferentiated OSCs through membrane insertion of protein at the helicase domain. Once OSCs commit to an oocyte fate, the protein is completely drawn back into the cytoplasm to permit activation of RNA helicase function as a key step in germ cell differentiation. Our finding that mRNA for Stra8, which is one of the earliest known markers of germ cells exiting the mitotic cycle and entering the meiotic cycle (22, 23, 32), is present in germ cells that no longer exhibit externalized expression of Ddx4 [viz. Stra8 mRNA found in only the ecDdx4-negative cell fraction; which remains positive for Ddx4 mRNA (10) and thus still contains germ cells] is consistent with such a model. In turn, the absence of Stra8 mRNA in freshly isolated OSCs, but its detection in pure cultures of OSCs that spontaneously generate oocytes in vitro (8, 10), supports that Stra8-expressing germ cells detected in the ecDdx4-negative ovarian cell fraction likely arise from OSCs undergoing differentiation in vivo. Our FACS-based analysis of ovaries of pStra8-Gfp transgenic mice extends this conclusion by revealing that transcriptional activation of the Stra8 promoter (GFP expression) can be detected in ecDdx4-negative cells as well as in a very small population of cells that still exhibit externalized expression of Ddx4 protein. Since our ability to identify Stra8 promoter activation probably precedes the point at which endogenous Stra8 mRNA would accumulate to levels sufficiently high enough for PCR-based detection in differentiating germ cells, we believe the cells positive for both Stra8 promoter-driven GFP expression and externalized Ddx4 protein represent a rare and transient population of OSC progenitors at the earliest stages of meiotic commitment just prior to complete cytoplasmic internalization of Ddx4.
Outcomes from our additional in vivo experiments agree with this model as well, in that we detected an inverse relationship between OSC numbers and meiotic gene activation in adult mouse ovaries during the estrous cycle. The highest yield of OSCs was obtained from luteal phase ovaries while Stra8 expression peaked during estrus of the follicular phase. If the increase in OSC yield is indicative of proliferation, this finding may indicate that OSCs commit to a wave of mitosis during the luteal phase as a response to follicle loss from ovulation and atresia during the prior cycle. In turn, more OSCs would to be available to activate Stra8 and commit to meiosis during the next follicular phase as a means to at least partially rebuild the ovarian reserve. This sequence of events would help explain prior observations from two different labs that primordial follicle numbers in ovaries of young adult mice also fluctuate during the reproductive cycle, with the highest numbers detected during metestrus into diestrus (30, 35). Although additional work is needed to map the exact relationship between OSC activity and oocyte numbers in vivo, the data presented add to a growing body of evidence that supports not only the presence of OSCs in postnatal mammalian ovaries but also the clear parallels and differences that exist in the genes and pathways that regulate the function of these cells compared to PGCs and SSCs. Studies aimed at identifying the hormones that control self-renewal versus meiotic activation in OSCs (38), as well as defining possible multipotential properties of OSCs after ex vivo expansion, are ongoing and are expected to reveal additional important features of these cells.
Supplementary Material
Capsule.
Oogonial stem cells exhibited a gene expression profile partially overlapping with, but distinct from, other stem cells and germ cells, and showed altered numbers during the reproductive cycle inversely related to meiotic commitment.
Acknowledgments
This work was supported by a Method to Extend Research in Time (MERIT) Award from the National Institute on Aging (NIH R37-AG012279 to J.L.T.), a Ruth L. Kirschstein National Research Service Award (NIH F32-AG034809 to D.C.W.), and a Glenn Foundation Award for Research in the Biological Mechanisms of Aging (J.L.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A.N.I. has nothing to disclose; N.W. has nothing to disclose; Y.T. has nothing to disclose; Y.A.R.W. has nothing to disclose; D.C.W. is a scientific consultant for OvaScience, Inc. (Cambridge, MA); J.L.T. discloses interest in intellectual property described in U.S. Patent 7,195,775, U.S. Patent 7,850,984 and U.S. Patent 7,955,846, and is a co-founder of OvaScience, Inc.
REFERENCES
- 1.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 2.Woods DC, Tilly JL. The next (re)generation of human ovarian biology and female fertility: is current science tomorrow's practice? Fertil Steril. 2012;98:3–10. doi: 10.1016/j.fertnstert.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18:353–4. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- 4.Woods DC, Telfer EE, Tilly JL. Oocyte family trees: old branches or new stems? PLoS Genet. 2012;8:e1002848. doi: 10.1371/journal.pgen.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods DC, White YAR, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci. 2013;20:7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods DC, Tilly JL. An evolutionary perspective on female germline stem cell function from flies to humans. Semin Reprod Med. 2013;31:24–32. doi: 10.1055/s-0032-1331794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 8.Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–70. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–204. doi: 10.1089/scd.2011.0091. [DOI] [PubMed] [Google Scholar]
- 10.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–41. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- 12.Park E-S, Woods DC, White YAR, Tilly JL. Oogonial stem cells isolated from ovaries of adult transgenic female mice generate functional eggs and offspring following intraovarian transplantation. Reprod Sci. 2013;20(Supplement):75A–6A. [Google Scholar]
- 13.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8:966–88. doi: 10.1038/nprot.2013.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci USA. 1994;91:12258–62. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93:139–49. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 16.Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15:393–8. doi: 10.1093/molehr/gap036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abban G, Johnson J. Stem cell support of oogenesis in the human. Hum Reprod. 2009;12:2974–8. doi: 10.1093/humrep/dep281. [DOI] [PubMed] [Google Scholar]
- 18.Oatley JM, Hunt PA. Of mice and (wo)men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod. 2012;86(196):1–2. doi: 10.1095/biolreprod.112.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testis. Physiol Rev. 2012;92:577–95. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2009;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 22.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 23.Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–80. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayernia K, Li M, Jaroszynski L, Khusainov R, Wulf G, Schwandt I, et al. Stem cell based therapeutical approach of male infertility by teratocarcinoma derived germ cells. Hum Mol Genet. 2004;13:1451–60. doi: 10.1093/hmg/ddh166. (2004) [DOI] [PubMed] [Google Scholar]
- 25.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. (2006) [DOI] [PubMed] [Google Scholar]
- 26.Woods DC, White YAR, Niikura Y, Kiatpongsan S, Lee H- J, Tilly JL. Embryonic stem cell-derived granulosa cells participate in follicle formation in vitro and in vivo. Reprod Sci. 2013;20:524–35. doi: 10.1177/1933719113483017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Felici M, Pesce M. Purification of mouse primordial germ cells by MiniMACS magnetic separation system. Dev Biol. 1995;170:722–7. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 28.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–92. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–6. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 32.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–12. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–49. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging. 2009;1:971–8. doi: 10.18632/aging.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen E. Ovogenesis during sexual maturity. Am J Anat. 1923;31:439–70. [Google Scholar]
- 36.Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mardanpour P, Guan K, Nolte J, Lee JH, Hasenfuss G, Engel W, et al. Potency of germ cells and its relevance for regenerative medicine. J Anat. 2008;213:26–9. doi: 10.1111/j.1469-7580.2008.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park E-S, Woods DC, Tilly JL. Bone morphogenetic protein 4 signaling promotes differentiation of adult mouse ovary-derived oogonial stem cells into oocytes in vitro. Reprod Sci. 2013;20(Supplement):189A–90A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.