Abstract
This study aimed to evaluate the ability of BOLD signals at high MRI field (7 Tesla) to map fine-scale single-digit activations in subdivisions (areas 3b and 1) of the human primary somatosensory cortex (SI) in individual subjects. We acquired BOLD-fMRI data from cortical areas around the central suclus in six healthy human subjects while stimulating individual finger pads with 2 Hz air puffs. Discrete, single-digit responses were identified in an area along the posterior bank of the central sulcus corresponding to area 3b and in an area along the crest of the postcentral gyrus corresponding to area 1. In single subjects, activations of digits 1 to 4 in both areas 3b and 1 were organized in a somatotopic manner. The separation of digit representations was measured for adjacent digits, and was approximately 1.6 times greater in area 3b than in area 1. Within individual subjects, the cortical responses to single-digit stimulations and the magnitude of the BOLD signals were reproducible across imaging runs and were comparable across subjects. Our findings demonstrate that BOLD-fMRI at 7 T is capable of revealing the somatotopic organization of single-digit activations with good within-subject reliability and reproducibility, and activation maps can be acquired within a reasonably short time window, which are essential characteristics for several neurological applications within patient populations.
Keywords: fMRI, high MR field, high-resolution imaging, human digit, primary somatosensory cortex, somatotopy
Introduction
Functional magnetic resonance imaging (fMRI) based on measuring changes in blood oxygen level dependent (BOLD) signals has been used extensively to study the functional architecture of somatosensory and other cortices in humans (Fox, 2009; Kayser et al., 2005; Moore et al., 2000; Nelson and Chen, 2008; Sasaki et al., 2005; Schweizer et al., 2008; Silver and Kastner, 2009; Tootell et al., 2008). Such studies provide important insights into not only the normal organization of sensory cortices but also potentially into how neural systems are affected by disease or damage, and how they may change over time during remodeling and with interventions (Borsook et al., 1998; Fox, 2009; Schaechter et al., 2006). FMRI is uniquely suited to address such questions because it is non-invasive and relatively easy to implement, but for ultimate success it is essential that the spatial resolution and sensitivity of fMRI data are adequate for recording fine details in single subjects with adequate within-subject reliability and reproducibility in reasonably short acquisition times (Harel et al., 2006). To date these criteria have not been satisfied, where previous studies typically have reported findings with insufficient spatial resolution, or have used average maps from multiple subjects, reflecting the practical current limits on sensitivity and image contrast to noise ratio (Francis et al., 2000; Gelnar et al., 1998; Krause et al., 2001; Kurth et al., 2000; Maldjian et al., 1999; Nelson and Chen, 2008). Theoretically, the use of ultra-high fields (7 Telsa or above) for fMRI promises to provide greater sensitivity for detecting functional activations and to permit the use of higher spatial resolution acquisitions (Bandettini, 2009). Thus, the advent of commercial ultra-high field MRI systems operating at 7 Tesla (T) provides an opportunity to push the limits of performance of fMRI to examine anew whether single-subject high-resolution functional maps can be acquired in reasonable times in order to be useful for a variety of applications.
In general, throughout the history of MRI, each substantive increase in field strength has in time led to dramatic improvements in the quality of images obtainable, but each major increase in field has also introduced new technical challenges and problems (Bandettini, 2009). Image quality in MRI is always limited by the relative strengths of the available signal and the “noise” - those random fluctuations in images that are unavoidable but which obscure details and make the detection of small signal differences more difficult. In principle the strength of MRI signals increase quadratically with field strength but in practice other factors are also important and affect the achieved signal to noise ratio (SNR). However, experiences to date have demonstrated that SNR increases when moving from 1.5 T or 3 T to 7 T, and the higher SNR can be used to make images with finer spatial resolution, or in less time, or in which small signal differences are easier to detect (Haacke et al., 1999; Stark and Bradley, 1999). In addition, differences in signal induced by functional activation via the BOLD effect will also be magnified at higher fields and therefore should be detected with greater sensitivity (Dula et al., 2010; Gati et al., 1997; Ogawa et al., 1993; van der Zwaag et al., 2009). To date, however, there have been few convincing demonstrations that fMRI at 7T offers significant advantages over lower fields and few illustrations of where the higher spatial resolution provides additional information.
In 1937, Penfield and colleagues, using invasive electrophysiological techniques, successfully mapped body surface representations in the human primary somatosensory cortex, establishing the existence of a somatotopic map or homunculus of the body surface in the human brain (Penfield and Boldrey, 1937). In the subsequent sixty years, with the lack of noninvasive imaging techniques little advancement was made in the understanding of somatosensory organization in human cortex. Following the first development of BOLD fMRI (Ogawa et al., 1990), human somatotopy has been studied extensively at varying field strengths (1.5, 3, and 4 T) (Blankenburg et al., 2003; Eickhoff et al., 2006; Francis et al., 2000; Gelnar et al., 1998; Hinkley et al., 2007; Kurth et al., 2000; Kurth et al., 1998; Maldjian et al., 1999; Overduin and Servos, 2004; Ruben et al., 2006; Schweizer et al., 2008; Weibull et al., 2008). Studies have revealed a topographical organization of digits in area 3b (Gelnar et al., 1998; Jack et al., 1994; Maldjian et al., 1999; Schweizer et al., 2008). In a major step forward, Nelson and Chen (Nelson and Chen, 2008) elegantly demonstrated the existences of topographically organized digit maps in both area 3b and area 1, findings that complement those found by other techniques in non-human primates (Chen et al., 2005; Chen et al., 2007; Friedman et al., 2008; Nelson and Chen, 2008; Sur et al., 1982). Taken together, the existing knowledge on the digit representation within the primary somatosensory cortex provides an excellent model for the evaluation of the performance of BOLD at ultra-high field (7 T). Specifically, in this study we assess 1) whether fMRI at 7 T can reliably resolve the separation of adjacent single digits, 2) the across-run reproducibility of single-digit activation, and 3) the across-subject variation of digit separation in subregions of SI (areas 3b and 1). We demonstrate that fMRI at 7 T can provide millimeter-scale depictions of the fine-detail organization of SI in individual subjects reliably and with good reproducibility of activation and signal magnitude. The robustness of single-trial BOLD activation significantly reduces imaging acquisition duration, an advantage that is desirable for fine-scale mapping studies particularly in patients when only limited scan time is possible. Additionally, the fine-scale mapping capability of 7 T appears to be well suited for studies of differences between subjects and across time.
Methods
Stimulation Protocol
Six healthy human subjects (5 men, 1 woman) gave informed consented for this study in accordance with a protocol approved by the Vanderbilt University Institutional Review Board. A hand and finger cast was connected to an air pressure generator via plastic tubing. Stretchable fabric was fitted across each opening in the cast, allowing for restricted skin displacement in response to an air puff. Air puffs were delivered to the glabrous skin of selected distal finger pads (Figure 1B). The digits (D) were stimulated at 2 Hz in a block design of 24 s on and 24 s off (Figure 1C). The “on” blocks alternated between digits. Each run consisted of 2 or 3 blocks for each digit being studied. Each subject performed multiple runs so as to acquire 6 to 12 stimulation “on” blocks per individual digit. To map the digit somatotopy and evaluate the reproducibility of single-digit activation, we mapped digit responses by stimulating nonadjacent digits (D2 and D4 in Subjects 1 and 2), adjacent digits (D2 and D3 in Subjects 3 and 4), and four digits (D1, D2, D3, and D4 in Subjects 5 and 6).
Figure 1. Experimental setup.
(A) The yellow lines over the sagittal structural image indicate the slice prescription for the functional images. The imaging volume covers SI, SII, posterior insula, and the thalamus.
(B) A hand cast was constructed to deliver air puffs to individual digits through plastic tubing.
(C) Two digits were stimulated separately in a 24 s on and 24 s off blocks design. A single run lasted 288 s (4 min 48 s).
MRI Data Acquisition
Subjects were scanned on a 7 T Philips Achieva magnet with a 16-channel NOVA head coil. Structural images were collected using a three-dimensional fast field echo (3D-FFE) sequence, TR = 3.7 ms, TE = 1.8 ms, and TI = 1300 ms, with a 1 × 1 × 1 mm3 voxel resolution. Functional images were acquired using a gradient-echo echo-planar imaging (GE-EPI) sequence, TR = 2000 ms, TE = 25 ms, flip angle = 80°, and a 192 × 192 field of view (FOV). The voxel resolution was 1 × 1 × 2 mm3. Sixteen oblique coronal slices (0 mm gap thickness) were acquired covering cortical regions around the central sulcus – the primary somatosensory cortex (SI) region, posterior part of the lateral sulcus, and thalamus (Figure 1A). A SENSE acceleration factor of 3, and volume selective 2nd or 3rd order shimming using a pencil-beam method were employed. All EPI images were distortion corrected using a B0 field-map obtained at the start of scanning using two gradient echo scans (Jezzard and Balaban, 1995). All preprocessing and imaging analysis was implemented in BrainVoyager QX following a standard pipeline. Preprocessing included slice scan time correction using a cubic spline, motion correction in three dimensions, and slice alignment using an intra-session registration.
fMRI Data Analysis
BOLD signals were temporally filtered with a 0.007 Hz high-pass filter, including a linear trend removal. The functional images were interpolated into 1 × 1 × 1 mm3 voxels for visualization. To preserve the high spatial resolution, warping into stereotactic space was not performed and spatial smoothing of the functional images was not applied. A general linear model (GLM) was fitted to the data and t-maps were created. Single condition activity maps (DA – Rest) and contrast maps (DA – DB) were examined. Individual subject maps were thresholded at q (FDR) < 0.001 with 4 voxels minimum per cluster. To reveal single-run activation, maps were thresholded at q (FDR) < 0.05 with 4 voxels minimum per cluster. Single condition activity maps were used to define regions of interest (ROIs). Composite maps of cortical digit representation were created to visualize digit topography in SI (area 3b and area 1). Surface volume renderings of the single-digit ROI clusters were also created to visualize the somatotopy. For all subjects the functional maps are presented in their native space with native coordinates reported. These coordinates do not correlate with Talariach nor MNI space. We did not create and do not report any group activation maps.
Identification of Cortical Activations in Areas 3b and 1
The primary somatosensory cortex (SI) lies directly posterior to the central sulcus (CS), and can be indentified by the omega-shaped folding in the axial plane (Moore et al., 2000; White et al., 1997). SI cortex was divided into three cytoarchitecturally distinct subregions by Brodmann in 1909 (Brodmann, 1909), and further subdivided into four subregions a decade later (Vogt and Vogt, 1919). The subregion boundaries can be objectively determined through histological methods (Geyer et al., 1999; Geyer et al., 2000), but no clear anatomical landmarks exist, leading to challenges in defining the boundaries with typical structural MRI techniques. Additionally, the intersubject variability in neural geometry adds to the difficultly in accurately indentifying the location of subregions (Geyer et al., 2001). We therefore use the guidelines put forth by Geyer at al to delineate the boundaries between subregions within SI (Geyer et al., 2000). Area 3a is located within the fundus of the CS, area 3b is located along the posterior bank of the CS, area 1 is located at the crest of the postcentral gyrus, and area 2 is located on the anterior bank of the postcentral sulcus (medial) (Geyer et al., 2000; Krause et al., 2001; Kurth et al., 2000; Nelson and Chen, 2008). These operational definitions are applied throughout the paper when areas 3b and 1 are referenced.
Measure of Interdigit Distance
When measuring interdigit distances in three dimensions, two approaches were taken. We denote the two methods as “Surface” and “Euclidian.” From each single condition activity map (DA – Rest; thresholded at q (FDR) < 0.001) we defined ROIs for each of the digit clusters in both areas 3b and 1. We then determined the three-dimensional coordinates for the peak (highest t-value) voxel within each ROI. In the Surface approach to determine interdigit distance, we traced the three-dimensional path, voxel by voxel, between the peak voxels of neighboring digit responses along the folds of the postcentral gyrus (e.g., 3D distance between D2 and D3 in area 3b). We then calculated the length of the path based on the voxel dimension, 1 × 1 × 1 mm3. For the Euclidian approach we measured the distance between peak voxels according to the Euclidian formula: .
Cortical Response Displacement Across Runs
We tested the spatial reproducibility of cortical responses to finger pad stimulation by calculating peak voxel and center of mass (COM) displacement. We defined ROIs from single condition activity maps (DA – Rest; thresholded at q (FDR) < 0.05) for each individual run. We determined the peak voxel and calculated the COM for each of these digit-specific ROIs and recorded the 3D coordinates. Additionally we defined ROIs from the mean single condition activity maps (DA – Rest; thresholded at q (FDR) < 0.001) across all runs. We recorded the peak voxel and the COM coordinates for each digit-specific ROI. To measure the ROI displacement for each run, we calculated the distance between the mean multi-run peak voxel and the individual run peak voxel; we made the same displacement calculations for the ROI COM measurements. The displacement between ROI coordinates (peak voxel and COM) was calculated using the Euclidian formula.
Magnitude of BOLD Signal
We tested the temporal reproducibility of the BOLD signal magnitude by comparing the area under the curve from single run BOLD signal time courses. The area under the curve encompasses several metrics such as the maximum change in BOLD, time to onset, time to offset, and shape of the curve. We created single condition activity maps (DA – Rest; thresholded at q (FDR) < 0.05) for each run of a single subject and defined ROIs for each of the digit clusters in both areas 3b and 1. The BOLD signal time courses from the five peak voxels in each ROI were averaged for each single condition block (DA) and across blocks, resulting in one mean BOLD signal time course per run. Based on inspection of the time courses and prior knowledge of the hemodynamic response function, we used a trapezoidal approximation to calculate the area under the curve from 4 s after the stimulus onset to 2 s after the stimulus ended. An ANOVA was performed on the BOLD signal magnitude to test whether the variance across runs was greater than the variance across ROIs.
Results
In individual subjects (n = 6), we detected single-digit activations in subregions (areas 3b and 1) of the primary somatosensory cortex (SI). Single-digit activation was robust in single runs, and the activation maps were reproducible across runs. Digit activations were organized topographically in areas 3b and 1 with apparent intersubject variation.
Digit Somatotopy in Area 3b and Area 1 in Individual Subjects
Digit separation within area 3b
We first examined the cortical responses to tactile stimulation of nonadjacent distal finger pads. In digit contrast maps (digit A condition minus digit B condition, Figure 2A) of Subject 1, we observed distinct and focal cortical activations along the posterior bank of the central sulcus (CS), corresponding to area 3b. Where nonadjacent digits 2 and 4 were stimulated separately in subject 1, two separate activation clusters (thresholded at q (FDR) < 0.001; D2 (blue/green) and D4 (orange/yellow)) were observed in three sequential lateral (x = 44) to medial (x = 46) sagittal slices (insets in Figure 2A). The pink box over the large whole head sagittal image indicates the zoomed-in region around CS as displayed in the left most inset (x = 44). In this particular case, due to the great sinuosity of the CS, when viewing in the sagittal plane, the CS of Subject 1 extended from the anterior to the posterior part of the inset window, thus we used two arrows to indicate the location of the CS. D2 activation was located anterior and inferior to D4 activation in area 3b. In 3D space, the spatial extent (in the×medial to lateral direction) of D2 activation also extended both laterally and medially to D4 activation. In this case, we observed visibly discrete separation between cortical responses to nonadjacent neighboring digits D2 and D4.
Figure 2. Contrast maps from two subjects.
(A) A contrast map (D4 - D2) from Subject 1 is overlaid on the subject’s structural image in the sagittal plane and in radiological orientation, thresholded q (FDR) < 0.001. The pink box indicates the area displayed in the left most increased field of view inset. The inset windows are arranged lateral (x = 44) to medial (x = 46). The x-coordinate is in the subject’s native space. There is a clear separation of digit responses along the posterior bank of the central sulcus (area 3b), with D4 response (orange/yellow) located posterior and superior to D2 response (blue/green) in the contralateral cortex. The white arrows indicate the location of the central sulcus (CS), and the white line indicates the area 3b/area 1 boundary.
(B) A contrast map (D2 - D3) from Subject 3 is overlaid on the subject’s structural image in the sagittal plane and in radiological orientation, thresholded q (FDR) < 0.001. The pink box indicates the area displayed in the center increased field of view inset. The inset windows are arranged lateral (x = 43) to medial (x = 45). The x-coordinates are in the subject’s native space. There is a clear separation of digit responses along the posterior bank of the central sulcus (area 3b), with D3 response (blue/green) located superior to D2 response (orange/yellow) in the contralateral cortex. The white arrows indicate the location of the CS, and the white line indicates the area 3b/area 1 boundary.
We next examined whether we could detect activation separations in adjacent digits. Figure 2B shows cortical responses to D2 (orange/yellow) and D3 (blue/green) stimulation in Subject 3 (thresholded at q (FDR) < 0.001). Similar to the observation in Subject 1, D2 activation is located anterior, inferior, and lateral to D3 activation along the posterior bank of the central sulcus in area 3b. There is a sizeable spatial separation between D2 and D3 clusters, which is emphasized in the zoomed-in insets centered over the CS and arranged lateral (x = 43) to medial (x = 45). While the separation of D2 and D3 responses is modest in the×and y planes, there is considerable separation along the z plane, even greater than the separation of nonadjacent digits observed in Subject 1. In general, the cortical responses to single-digit stimulation are unique for each individual subject. Together, the discrete cortical responses to nonadjacent digit stimulation in Subject 1 and adjacent digit stimulation in Subject 3 illustrate that individual digit representations in area 3b are distinct and separable, which suggest minimal overlap across adjacent digits.
Digit topography
We further examined whether the spatially distinct cortical BOLD activations in response to stimulating individual finger pads were organized in a topographic manner in both areas 3b and 1. The finger pad somatotopies of two subjects (Subjects 5 and 6) as displayed in Figure 3 provided evidence for two separate digit maps. The surface volume renderings of Subject 5 (Figure 3A & B) and Subject 6 (Figure 3C & D) revealed subject-specific organization of D1 (red), D2 (blue), D3 (yellow), and D4 (green) in three orientations (axial plane along the blue axis, coronal plane along the green axis, and sagittal plane along the red axis). Two separate digit maps were established. The zoomed-in images (Figure 3B and 3D) illustrate that digit activations in area 3b (rostral clusters) were located anterior and medial to area 1 (caudal clusters), and that the spatial expanse of digit responses are greater in area 3b than in area 1. A similar activation pattern was observed in Subject 6 (Figure 3C and 3D) and the majority of the cases. Within these maps (areas 3b and 1), the digit-specific cortical activations were topographically organized from D1 to D4 in a lateral to medial, anterior to posterior, and inferior to superior pattern.
Figure 3. Surface composite maps of digit topography in two subjects.
(A) Subject 5 and (C) Subject 6 are depicted in surface renderings in the neurological orientation. Part of the facial surface is cut away to expose the T1-weighted structural images of each subject in three orientations, axial (blue axis), coronal (green axis), and sagittal (red axis). The white boxes indicate the volumes displayed in the increased field of view windows. The cortical digit representations in the contralateral cortex are displayed in 3D for (B) Subject 5 and (D) Subject 6. The CS is highlighted by the white arrows. Digit representations are organized topographically in two distinct maps, one along the posterior bank of the CS (area 3b), and along the crest of the postcentral gyrus (area 1). Cortical response to D1 stimulation is illustrated in red, D2 blue, D3 yellow, and D4 green.
Intersubject variability was apparent, and a topographical exception was observed in Subject 5 where a reversal of digit organization in the axial plane in area 1 was present. Single-digit cortical responses from D1 to D4 followed the standard lateral to medial and anterior to posterior pattern, yet an anomalous superior to inferior pattern (Figure 3B). In contrast, digit activations in area 3b followed a typical organization. By virtue of the unique geometry of an individual brain, there were significant variations in the localization of digit representations across subjects. However, each subject revealed a clear topographical organization of the finger pad representations. In sum, while a standard digit topograhy was observed in the majority of subjects, subtle differences in digit somatotopy were apparent, especially in area 1 in one subject.
Differential Spatial Separation of Finger Pads in Area 3b and Area 1
Due to the observed differences in digit organization, we further quantified the separation between digit representations in areas 3b and 1. Of primary interest was the quantifiable separation between adjacent digits (D2 and D3), leading us to exclude the two subjects that were tested with nonadjacent digits from this analysis. Within area 3b, D2 and D3 separation ranged from 11 mm to 14 mm (mean ± standard deviation (SD): 12 ± 1.41 mm) measured by the Surface method and from 5.39 mm to 9.17 mm (mean ± SD: 7.25 ± 0.96 mm) calculated by the Euclidean method. Within area 1, D2 and D3 separation ranged from 6 mm to 8 mm (mean ± SD: 7.46 ± 1.61 mm) measured by the Surface method and from 3.74 mm to 5.83 mm (mean ± SD: 4.68 ± 0.86 mm) calculated by the Euclidean method. The separation of cortical responses to stimulation of adjacent digits was 1.6 times greater in area 3b than in area 1 across subjects.
Reproducibility within a Single Subject across Multiple Runs
Spatial reproducibility in areas 3b and 1
To evaluate the spatial reproducibility of single-digit activation across runs, we collected multiple functional runs per imaging session (3 – 6). Figure 4 illustrates the spatial overlap of the cortical activations to stimulation of D2 and D4 in the axial image plane between runs (Run1 in blue, Run2 in red, Run3 in yellow, and Run4 in green) in Subject 2. The four panels present the two conditions in both areas 3b and 1: D2 – Rest in area 3b (top panel), D2 – Rest in area 1 (second panel), D4 – Rest in area 3b (third panel), and D4 – Rest in area 1 (bottom panel). The enlarged field of view in Figure 4B illustrates that the spatial variability in across-run responses is minimal.
Figure 4. Spatial reproducibility of BOLD signals.
(A) Single run composite maps of activation clusters are overlaid on the axial plane of the structural images in the radiological orientation for an individual subject, q (FDR) < 0.005. The first and second rows represent activation during stimulation of D2 in area 3b and area 1, respectively. The third and forth rows represent activation during stimulation of D4 in area 3b and area 1, respectively.
(B) The zoomed images illustrate a high degree of spatial agreement between ROIs across 4 runs. The ROIs for Run 1 are outlined in blue, Run 2 red, Run 3 yellow, and Run 4 green. Z-coordinates displayed are in subject’s native space. The white line highlights the boundary between area 3b and area 1, and the white arrow indicates the CS.
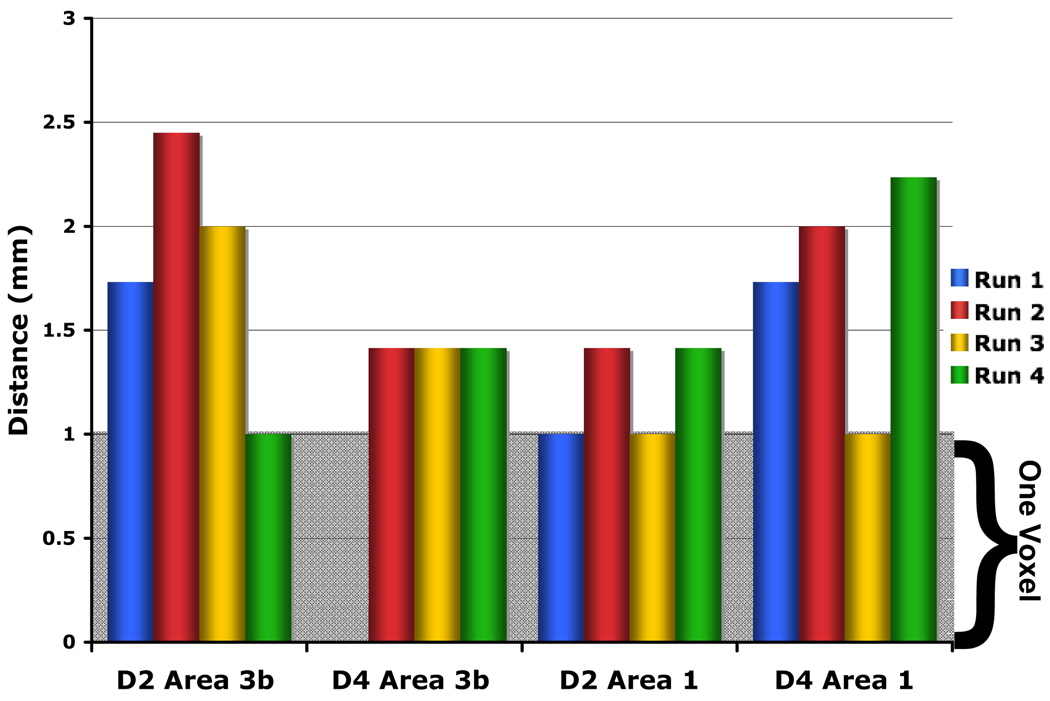
We quantified the extent of spatial reproducibility by calculating the displacement of each ROI’s peak voxel and center of mass (COM) for each of the individual digits. Figure 5 illustrates the calculated COM displacement for all digit-specific ROIs in each run: Run1 (blue), Run2 (red), Run3 (yellow), and Run4 (green), with the grey bar indicating the displacement of one voxel length. The mean peak voxel and COM displacements are 1.84 ± 0.33 mm and 1.45 ± 0.15 mm, respectively. The maximum displacements were 4.12 mm for the peak voxel and 2.45 mm for the COM.
Figure 5. Center of mass displacement across runs.
The center of mass (COM) for each ROI was determined for a single run (as displayed in Figure 4) and for the mean. The displacement of single run COM from the mean COM was calculated. The grey shading represents the displacement by one voxel, 1 mm. The COM displacement was less than 2.5 mm for all runs within an ROI. Run 1 blue, Run 2 red, Run 3 yellow, and Run 4 green.
Temporal reproducibility in areas 3b and 1
To evaluate the cross-run temporal reproducibility of BOLD signals, we compared the magnitude of the BOLD signal by calculating the area under the curve for each run of the digit-specific ROIs defined in Figure 4. An ANOVA was performed on the BOLD signal magnitudes to test whether the variance across runs was greater than the variance across ROIs. The ANOVA showed no main effect of run (F (1, 3) = 0.44, p > 0.73), suggesting a high degree of temporal reproducibility of the BOLD signal.
Discussion
Differentiability of BOLD Signal at Ultra-high Field (7 T)
The differentiability of BOLD signals is constrained by the imaging resolution employed in fMRI experiments. Typical functional imaging studies use “snapshot” imaging techniques of which echo-planar imaging (EPI) is a prime example, in which complete cross sectional images that are sensitive to BOLD effects are obtained in very short times, substantially less than one second. These functional images are of lower resolution than more conventional anatomic MR images, so that activation maps are typically acquired with voxels on the order of 3 × 3 × 3 mm3 (> 20 mm3). At this spatial resolution differentiation between neighboring regions, such as area 3b and 1, or neighboring digits, is uncertain. High-resolution studies (e.g. 1 × 1 × 2 mm3) have been employed to reveal fine-scale functional structures in cortex (Harel et al., 2006; Sanchez-Panchuelo et al.; Schweizer et al., 2008), such as ocular dominance columns in visual cortex and digit somatotopic maps within subregions of SI cortex. In these studies, multiple blocks or runs were combined to compensate for the signal drop resulting from smaller voxel sizes (Schweizer et al., 2008). High-resolution mapping at higher fields benefits from an increased SNR and there is less need to average over as many acquisitions.
In this study, single-run BOLD activations (acquired as 3 blocks each of 12 images with and without the stimulus) were robust, and spatially reproducible across runs. The clear separation of adjacent digits within areas 3b and 1 in individual subjects support the differentiability of BOLD signals at 7 T. A direct comparison with lower fields was not attempted here so we cannot quantify the specific benefits of conducting a BOLD fMRI experiment at 7 T in this study. However, our experience at 3 T and the existent literature supports our observation that the reliability at 7 T is substantially better than at lower fields, and that individual results at higher resolution are obtainable with significantly fewer acquisitions and runs than are commonly achieved (van der Zwaag et al., 2009).
The present study has demonstrated that BOLD signals at high field can be focal and spatially specific such as for individual digit representations in area 3b. This observation is consistent with our findings obtained with an identical approach in anesthetized monkeys at high field (9.4 T). In area 3b of monkeys, BOLD activation maps (obtained with 0.625 × 0.625 mm2 in plane resolution) correlate well with maps identified by the receptive fields of neurons (Chen et al., 2007). Our observations in humans and monkeys seem to be inconsistent with previous reports (e.g. (Kim et al., 2004)) where BOLD signals appeared to be less focal and had a poor correlation with underlying electrical activity. We believe that the stimulus and model systems (visual versus somatosensory) contribute to the discrepancy between their study and our studies. In our present study, natural and subtle vibrotactile stimulation of a single distal finger pad was used. In this condition, only a small piece of cortex was activated (~ 1 mm2 in New World primates and ~ 10 mm2 in human area 3b), and the vascular response may be much more constrained for such a subtle stimulus.
Technical Challenges of High-Field BOLD mapping
Ultra-high field fMRI faces several challenges. The first is related to macroscopic field variations caused by increased inhomogeneities of magnetic susceptibility, which can introduce image distortion. Secondly, the performance of radiofrequency (RF) coils is also affected at higher fields, and it is more difficult to create uniform RF fields within large objects (i.e. the human brain). To meet these challenges, we employed the use of parallel array coils (16 channels) for data acquisition, reduced the imaging volume to partial brain coverage, and applied B0 distortion correction in data preprocessing. Additionally, we took extra efforts in defining the shim volume for each subject. With these approaches, the susceptibility problems in SI areas around the CS appear minimal. There is, however, some degree of signal drop in brain regions near air-tissue interfaces, specifically the inferior temporal lobes. Other techniques such as dynamic shimming during functional scans for high-resolution fMRI studies are currently under development in our laboratory (Sengupta et al., 2009). The data presented in this paper highlight the current potential 7 T BOLD imaging can provide in the understanding of brain function.
Functional Imaging of Individual Subject
While many previous studies of digit topography in SI have relied on group analysis (Gelnar et al., 1998; Maldjian et al., 1999; Weibull et al., 2008), less than a handful have successfully achieved single-subject analysis (Nelson and Chen, 2008; Sanchez-Panchuelo et al.; Schweizer et al., 2008). Nelson and Chen identified the somatotopic organization of all five digits in both area 3b and area 1 of SI, where the thumb (D1) was represented most laterally, anteriorly, and inferiorly in both areas, similar to our findings, but their spatial resolution was limited to 2.08 × 2.08 × 2.4 mm3 (> 10 mm3) voxel size (Nelson and Chen, 2008). Similarly, Schweizer, et al, achieved a single-subject analysis of the topography of all five digits in SI area 3b (Schweizer et al., 2008). Their voxel resolution was 1 × 1 × 2 mm3, but also employed a 0.4 mm gap thickness, decreasing the effective sampling of the cortical tissue. In addition, while fine-scale somatotopic maps were obtained, the overall imaging time required to achieve these was considerably longer. Two imaging sessions were conducted in order to complete the study. Each digit required approximately 12 min 18 s to scan, and with 5 digits the total functional scan time was 61 min 30 s. In our study, the functional scan time varied between subjects, but on average the scan time per digit was 7 min 12 s.
Single-subject somatotopic maps are achievable at 3 T (Nelson and Chen, 2008; Schweizer et al., 2008), but Sanchez-Panchuelo, et al, demonstrated how the increase in CNR at 7 T can be utilized in order to increase spatial resolution (1 mm3) and decrease the total scan time necessary to achieve topographic maps of digit topography (Sanchez-Panchuelo et al.). In their study, however, the employed stimulus paradigm consisting of a “traveling wave,” and the data analysis generating a “digit-phase correspondence,” do not allow for mapping of discrete digit representations. Instead the authors present a spectrum of continuous distal finger pad representations along SI cortex. Here we were able to measure and delineate digit representations in both areas 3b and 1 of SI cortex.
Although individual subject analysis can be accomplished at lower MRI field strengths (Nelson and Chen, 2008; Schweizer et al., 2008), the greater signal sensitivity at 7 T could provide extra benefits. For instance, reliable BOLD signal in a single subject can be acquired in as little as 2 min 30 s per stimulus, given that our reproducibility analysis indicates that a scan time of only 2 min 24 s is necessary to accurately map a single digit response. This improvement could negate the need for more across-run averaging, ultimately reducing the total scan time to collect functional data. The across-run temporal reproducibility implies 7 T imaging has a potential application in examining dynamic cortical changes within one imaging session in an individual subject, such as immediate plastic changes following a manipulation of the stimulus. Measurements of the variability of the magnitude of the BOLD response provide another quantification for characterizing the cortical response strength at a specific cortical region (e.g. areas 3b and 1). Finally, the detection of subtle variations of digit representation could have significant potential for detecting small cortical changes in disease conditions such as plastic changes following spinal cord injury.
Cortical Magnification: Interdigit Distance
It has been suggested that the cortical magnification factor (M) of the cortex is proportional to sensory acuity (Cowey and Rolls, 1974; Duncan and Boynton, 2003). There have only been a few studies that have examined the somatosensory cortical magnification factor in human or non-human primates (Nelson et al., 1980; Schwartz et al., 2004; Sur et al., 1982). In primates area 3b has a greater M factor than area 1, implying an important role of area 3b on the representation of tactile acuity (Friedman et al., 2008; Sur et al., 1982). Our data along with other studies suggest that the same rule also exists in human SI cortex (Kurth et al., 2000; Nelson and Chen, 2008). A greater digit separation in area 3b than in area 1 was observed, with a ratio of 1.6. Specifically in our study, the measured interdigit distance was 12 mm (Surface) and 7.46 mm (Euclidian) in area 3b, and 7.25 mm (Surface) and 4.68 mm (Euclidian) in area 1. These observations are comparable to previous data, where the reported cortical distance between D1 and D5 varied from 16.05 to 17.09 mm in area 3b and varied from 14.26 to 14.9 mm in area 1 (Kurth et al., 2000; Nelson and Chen, 2008). Our data suggest that calculating digit distance based on the Euclidian formula underestimates cortical distance measurements that could affect the interpretations of digital distance measurements.
The degree of intersubject variability was large, a finding consistent within the literature (Francis et al., 2000; Kurth et al., 2000; Nelson and Chen, 2008). Our decision to analyze subjects in their native space may have added to the degree of variability; however we found that the use of the “Surface” method for calculating digit separation did not decrease the intersubject variability. Thus the inconsistency in digit-distance measurements across subjects is at least partially due to a unique somatotopic representation of each subject. This observation stresses the need for analyzing subjects on an individual level in their native space.
Conclusion
This study demonstrates that ultra-high field BOLD fMRI at 7 T is capable of resolving fine-scale digit topography within areas 3b and 1 of SI cortex. These maps are comparable to nonhuman primate somatotopic maps derived from high-resolution electrophysiological and intrinsic optical methods and are in agreement with human digit somatotopic maps derived from fMRI and intraoperative imaging studies. BOLD activation is robust and reproducible across runs, highlighting its potential application in mapping cortical dynamic changes in individual subjects.
Acknowledgements
The authors thank Drs. Robert Barry and Baxter Rogers for their thoughtful comments and suggestions during the review process, Robin Avison for her technical assistance, and Lauren Holroyd for her assistance. This project was supported by NIH grants IS10 RR17799 (NCRR), EB002326 (NIBIB), and EB000461 (NIBIB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini PA. What's new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruben J, Meyer R, Schwiemann J, Villringer A. Evidence for a rostral-to-caudal somatotopic organization in human primary somatosensory cortex with mirror-reversal in areas 3b and 1. Cereb Cortex. 2003;13:987–993. doi: 10.1093/cercor/13.9.987. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M, Papinicolas L, Ramachandran VS, Gonzalez RG, Breiter H. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9:1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Brodmann K. VergleichendeLocalisationslehre der Gro Hhirnrinde. Leipzig: Barth; 1909. [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J Neurosci. 2005;25:7648–7659. doi: 10.1523/JNEUROSCI.1990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, Avison MJ. High-resolution maps of real and illusory tactile activation in primary somatosensory cortex in individual monkeys with functional magnetic resonance imaging and optical imaging. J Neurosci. 2007;27:9181–9191. doi: 10.1523/JNEUROSCI.1588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Rolls ET. Human cortical magnification factor and its relation to visual acuity. Exp Brain Res. 1974;21:447–454. doi: 10.1007/BF00237163. [DOI] [PubMed] [Google Scholar]
- Dula A, Welch E, Creasy J, Gatenby J, Stringer E, Chen L, Anderson A, Avison M, Gore J. New Frontiers in Biomedical Engineering; Proceedings of the 3rd International Conference on the Development of BME in Vietnam; Ho Chi Minh City, Vietnam: Springer and IFMBE; 2010. [Google Scholar]
- Duncan RO, Boynton GM. Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron. 2003;38:659–671. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Lotze M, Wietek B, Amunts K, Enck P, Zilles K. Segregation of visceral and somatosensory afferents: an fMRI and cytoarchitectonic mapping study. Neuroimage. 2006;31:1004–1014. doi: 10.1016/j.neuroimage.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Fox K. Experience-dependent plasticity mechanisms for neural rehabilitation in somatosensory cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:369–381. doi: 10.1098/rstb.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis ST, Kelly EF, Bowtell R, Dunseath WJ, Folger SE, McGlone F. fMRI of the responses to vibratory stimulation of digit tips. Neuroimage. 2000;11:188–202. doi: 10.1006/nimg.2000.0541. [DOI] [PubMed] [Google Scholar]
- Friedman RM, Chen LM, Roe AW. Responses of areas 3b and 1 in anesthetized squirrel monkeys to single- and dual-site stimulation of the digits. J Neurophysiol. 2008;100:3185–3196. doi: 10.1152/jn.90278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gati JS, Menon RS, Ugurbil K, Rutt BK. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med. 1997;38:296–302. doi: 10.1002/mrm.1910380220. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV. Fingertip representation in the human somatosensory cortex: an fMRI study. Neuroimage. 1998;7:261–283. doi: 10.1006/nimg.1998.0341. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Schormann T, Mohlberg H, Bodegard A, Roland PE, Zilles K. Integration of microstructural and functional aspects of human somatosensory areas 3a, 3b, and 1 on the basis of a computerized brain atlas. Anat Embryol (Berl) 2001;204:351–366. doi: 10.1007/s004290100200. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical Princliples and Sequence Design. New York: Wiley-Liss; 1999. [Google Scholar]
- Harel N, Ugurbil K, Uludag K, Yacoub E. Frontiers of brain mapping using MRI. J Magn Reson Imaging. 2006;23:945–957. doi: 10.1002/jmri.20576. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Krubitzer LA, Nagarajan SS, Disbrow EA. Sensorimotor integration in S2, PV, and parietal rostroventral areas of the human sylvian fissure. J Neurophysiol. 2007;97:1288–1297. doi: 10.1152/jn.00733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Thompson RM, Butts RK, Sharbrough FW, Kelly PJ, Hanson DP, Riederer SJ, Ehman RL, Hangiandreou NJ, Cascino GD. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology. 1994;190:85–92. doi: 10.1148/radiology.190.1.8259434. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Augath M, Logothetis NK. Integration of touch and sound in auditory cortex. Neuron. 2005;48:373–384. doi: 10.1016/j.neuron.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ronen I, Olman C, Kim SG, Ugurbil K, Toth LJ. Spatial relationship between neuronal activity and BOLD functional MRI. Neuroimage. 2004;21:876–885. doi: 10.1016/j.neuroimage.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Krause T, Kurth R, Ruben J, Schwiemann J, Villringer K, Deuchert M, Moosmann M, Brandt S, Wolf K, Curio G, Villringer A. Representational overlap of adjacent fingers in multiple areas of human primary somatosensory cortex depends on electrical stimulus intensity: an fMRI study. Brain Res. 2001;899:36–46. doi: 10.1016/s0006-8993(01)02147-3. [DOI] [PubMed] [Google Scholar]
- Kurth R, Villringer K, Curio G, Wolf KJ, Krause T, Repenthin J, Schwiemann J, Deuchert M, Villringer A. fMRI shows multiple somatotopic digit representations in human primary somatosensory cortex. Neuroreport. 2000;11:1487–1491. [PubMed] [Google Scholar]
- Kurth R, Villringer K, Mackert BM, Schwiemann J, Braun J, Curio G, Villringer A, Wolf KJ. fMRI assessment of somatotopy in human Brodmann area 3b by electrical finger stimulation. Neuroreport. 1998;9:207–212. doi: 10.1097/00001756-199801260-00006. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Detre JA, Alsop DC. The sensory somatotopic map of the human hand demonstrated at 4 Tesla. Neuroimage. 1999;10:55–62. doi: 10.1006/nimg.1999.0448. [DOI] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM. Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol. 2000;84:558–569. doi: 10.1152/jn.2000.84.1.558. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Chen R. Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cereb Cortex. 2008;18:2341–2351. doi: 10.1093/cercor/bhm257. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH. Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980;192:611–643. doi: 10.1002/cne.901920402. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, Servos P. Distributed digit somatotopy in primary somatosensory cortex. Neuroimage. 2004;23:462–472. doi: 10.1016/j.neuroimage.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Ruben J, Krause T, Taskin B, Blankenburg F, Moosmann M, Villringer A. Sub-area-specific Suppressive Interaction in the BOLD responses to simultaneous finger stimulation in human primary somatosensory cortex: evidence for increasing rostral-to-caudal convergence. Cereb Cortex. 2006;16:819–826. doi: 10.1093/cercor/bhj025. [DOI] [PubMed] [Google Scholar]
- Sanchez-Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J Neurophysiol. 103:2544–2556. doi: 10.1152/jn.01017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vanduffel W, Knutsen T, Tyler C, Tootell R. Symmetry activates extrastriate visual cortex in human and nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:3159–3163. doi: 10.1073/pnas.0500319102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Chen LM, Friedman RM, Spencer DD, Roe AW. Intraoperative optical imaging of human face cortical topography: a case study. Neuroreport. 2004;15:1527–1531. doi: 10.1097/01.wnr.0000131006.59315.2f. [DOI] [PubMed] [Google Scholar]
- Schweizer R, Voit D, Frahm J. Finger representations in human primary somatosensory cortex as revealed by high-resolution functional MRI of tactile stimulation. Neuroimage. 2008;42:28–35. doi: 10.1016/j.neuroimage.2008.04.184. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Welch E, Zhao Y, Foxall D, Starewicz P, Anderson A, Gore J, Avison M. Dynamic B0 Shimming at 7 Tesla; Hawaii, USA. Proceedings of the 17th Annual Meeting, International Society for Magnetic Resonance in Medicine; 2009. p. #777. [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DD, Bradley WG. Magnetic Resonance Imaging. C. V. Mosby; 1999. [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982;211:177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Devaney KJ, Young JC, Postelnicu G, Rajimehr R, Ungerleider LG. fMRI mapping of a morphed continuum of 3D shapes within inferior temporal cortex. Proc Natl Acad Sci U S A. 2008;105:3605–3609. doi: 10.1073/pnas.0712274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag W, Francis S, Head K, Peters A, Gowland P, Morris P, Bowtell R. fMRI at 1.5, 3 and 7 T: characterising BOLD signal changes. Neuroimage. 2009;47:1425–1434. doi: 10.1016/j.neuroimage.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeiner Ergebnisse unserer Hinforschung. Journal of Psychology and Neurology. 1919;25:279–462. [Google Scholar]
- Weibull A, Bjorkman A, Hall H, Rosen B, Lundborg G, Svensson J. Optimizing the mapping of finger areas in primary somatosensory cortex using functional MRI. Magn Reson Imaging. 2008;26:1342–1351. doi: 10.1016/j.mri.2008.04.007. [DOI] [PubMed] [Google Scholar]
- White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D. Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cereb Cortex. 1997;7:18–30. doi: 10.1093/cercor/7.1.18. [DOI] [PubMed] [Google Scholar]