Abstract
Depression is a complex, heterogeneous mental disorder. Currently available antidepressants are only effective in about one-third to one-half of all patients. The mechanisms underlying antidepressant response and treatment resistance are poorly understood. Recent clinical evidence implicates the involvement of leptin in treatment response to antidepressants. In this study, we determined the functional role of the leptin receptor (LepRb) in behavioral responses to the selective serotonergic antidepressant fluoxetine and the noradrenergic antidepressant desipramine. While acute and chronic treatment with fluoxetine or desipramine in wild-type mice elicited antidepressant-like effects in the forced swim test, mice null for LepRb (db/db) displayed resistance to treatment with either fluoxetine or desipramine. Fluoxetine stimulated phosphorylation of Akt(Thr308) and GSK-3β(Ser9) in the hippocampus and prefrontal cortex (PFC) of wild-type mice but not in db/db mice. Desipramine failed to induce measurable changes in Akt, GSK-3β or ERK1/2 phosphorylation in the hippocampus and PFC, as well as hypothalamus of either genotype of mice. Deletion of LepRb specifically from hippocampal and cortical neurons resulted in fluoxetine insensitivity in the forced swim test and tail suspension test while leaving the response to desipramine intact. These results suggest that functional LepRb is critically involved in regulating the antidepressant-like behavioral effects of both fluoxetine and desipramine. The antidepressant effects of fluoxetine but not desipramine are dependent on the presence of functional LepRb in the hippocampus and cortex.
Introduction
Major depression is a highly prevalent and debilitating mental illness with heterogeneity in symptomatology and treatment response. Currently available antidepressants target monoamine neurotransmitter systems. The commonly prescribed classes of antidepressants are the selective serotonin (5-HT) reuptake inhibitors, selective norepinephrine (NE) reuptake inhibitors and combined 5-HT/NE reuptake inhibitors.1, 2 However, only approximately one-third of patients achieve remission after initial treatment.3 The mechanisms underlying the antidepressant response and treatment resistance are poorly understood.
Leptin is a pleiotropic hormone that has diverse central actions.4 Our previous preclinical studies have shown that the action of leptin via the long form of its receptor (LepRb) is both sufficient and necessary for antidepressant-like behaviors in rodents. Circulating leptin levels are reduced in chronic unpredictable stress and chronic social defeat models of depression.5 Systemic administration of leptin produces antidepressant-like responses in both the forced swim and tail suspension behavioral despair tests,5, 6, 7 two procedures commonly used for screening antidepressant effects. Similar behavioral effects were observed after intracerebroventricular infusion or intrahippocampal infusion of leptin.5, 8 Leptin can also reverse depressive-like behavior induced by chronic unpredictable stress.5, 6 In contrast, selective depletion of LepRb in the hippocampus and cortex causes depression-like phenotypes.9, 10 These studies support that leptin-LepRb signaling is critically involved in the development of depressive behavior and antidepressant action.
Recent clinical studies demonstrate that leptin levels are inversely associated with the severity of depressive symptoms in women across the weight spectrum.11 Moreover, it was reported that leptin levels appear to change along the course of antidepressant treatment.12, 13, 14 Polymorphisms in the leptin gene and decreased leptin levels are found to be associated with responses to different classes of antidepressants.15, 16 These findings suggest a role of leptin in depressive symptomatology and antidepressant efficacy.
The Akt, glycogen synthase kinase 3 beta (GSK3β) and ERK1/2 signaling pathways have been implicated in therapeutic effects of classical antidepressant drugs.17, 18, 19, 20 These pathways are also recruited by leptin via LepRb.6, 9, 10, 21 Evidence suggests that Akt signaling mediates antidepressant-like activity of leptin.9, 10, 21 Blockade of Akt in the hippocampus attenuates the antidepressant-like effect of leptin.21 Therefore, we hypothesize that classical antidepressant drugs and leptin may converge on similar intracellular mechanisms of action. The purpose of this study was to investigate the role of functional LepRb in behavioral effects and intracellular signaling induced by the serotonergic antidepressant fluoxetine and the noradrenergic antidepressant desipramine.
Materials and methods
Animals
Mice were housed on a 12 h light/12 h dark cycle with ad libitum access to food and water. The db/db mice and their littermates were obtained by breeding of db/+ mice (Model Animal Research Center of Nanjing University, Nanjing, China). Mice lacking LepRb in hippocampal and cortical neurons (Lepr cKO) were generated as previously desribed.10 Briefly, EMX1-Cre mice were crossed with Leprflox/flox mice to generate Leprflox/+, Emx1-Cre mice, which were subsequently crossed with Leprflox/flox mice to generate Leprflox/flox, Emx1-Cre (Lepr cKO) mice and Leprflox/flox littermate controls (fWT) for this study. All animal procedures were approved by the Binzhou Medical University Hospital Institutional Animal Care and Use Committee and the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Sucrose/saccharin preference test
Mice were habituated to two water bottles for 1 week in their home cages before testing. Then a free choice between plain water and 1% sucrose solution or 0.01% saccharin solution was provided to each animal for 4 days. Water intake and sucrose/saccharin intake were measured daily. The positions of the weighed bottles were reversed every day. Sucrose/saccharin preference was calculated as the percentage of sucrose/saccharin solution intake over the total fluid intake and was regarded as an index of hedonia.
Forced swim test
To evaluate antidepressant-like effects, mice were injected intraperitoneally (i.p.) with fluoxetine (10 mg kg−1), desipramine (10 mg kg−1) or vehicle (saline). Thirty minutes after i.p. injection, animals were placed into a clear Plexiglas cylinder (25 cm in height and 10 cm in diameter) filled with water (24 °C) to a depth of 15 cm. A 6-min swim session was videotaped by a camera mounted above the cylinder. The duration of immobility was measured for the last 4 min. Immobility was defined as the absence of all movements except those required for respiration.
Tail suspension test
Thirty minutes after drug injection, mice were individually suspended by the tail to a vertical bar on the top of a box (30 × 30 × 30 cm) with adhesive tape. A 6-min test session for each mouse was recorded by a camera placed in front of the box. Immobility time during the 6 min was measured. Immobility was defined as the absence of any movements except those caused by respiration.
Locomotor activity
Thirty minutes after i.p. injection with fluoxetine (10 mg kg−1), desipramine (10 mg kg−1) or vehicle (saline), mice were placed into an open arena to monitor locomotor activity. Animals' activity was recorded for 30 min. The total distance traveled within 30 min was analyzed by using ANY-maze video-tracking system (Stoelting, Wood Dale, IL, USA).
Plasma leptin and insulin levels
Blood samples were collected by decapitation. Plasma was diluted 1:10 and 1:100 for wild-type mice and db/db mice, respectively, for measurement of leptin and insulin levels. Plasma concentrations of leptin and insulin were determined by using mouse leptin and insulin ELISA kits (Alpco Diagnostics, Salem, NH, USA).
Western blot assay
Mice were killed by rapid decapitation. The hippocampus, prefrontal cortex (PFC) and hypothalamus were dissected out on ice and immediately homogenized in a lysis buffer containing 50 mM Tris–HCl buffer, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, phenylmethylsulfonyl fluoride and PhosSTOP Phosphatase Inhibitor Cocktail (Roche Applied Science, Penzberg, Germany). The denatured protein was separated on an SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. The membrane was blocked in a solution of Tris-buffered saline with 1% dried milk and 0.1% Tween 20. Membranes were then incubated with the following primary antibodies diluted in the blocking solution: anti-Akt (1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-Akt(Thr308) (1:1000, Cell Signaling), anti-GSK3β (1:1000, Cell Signaling), anti-phospho-GSK3β(Ser9) (1:1000, Cell Signaling), anti-phospho-GSK3β(Ser389) (1:1000, EMD Millipore, Billerica, MA, USA), anti-ERK1/2 (1:1000, Cell Signaling), anti-phospho ERK1/2(Thr202/Tyr204) (1:1000, Cell Signaling), anti-β-actin (1:3000, Cell Signaling), anti-SERT (1:1000, Abcam, Cambridge, MA, USA). After washing, membranes were incubated with secondary antibodies conjugated to horseradish peroxidase (1:5000, Promega, Madison, WI, USA). Signals were visualized using HyGlo-Chemiluminescent HRP-linked antibody detection reagent (Denville Scientific, Metuchen, NJ, USA) followed by exposure to X-ray film. The densities of the bands were analyzed using Image J for quantitative analysis.
Statistical analysis
Statistical analyses were conducted using two-tailed Student's t-test or two-way analysis of variance followed by Tukey/Kramer post hoc comparisons. P-values <0.05 were considered statistically significant. All data are presented as mean±s.e.m. and the western blot data are presented as percentage of control.
Results
The db/db mice display metabolic and depression-like phenotypes
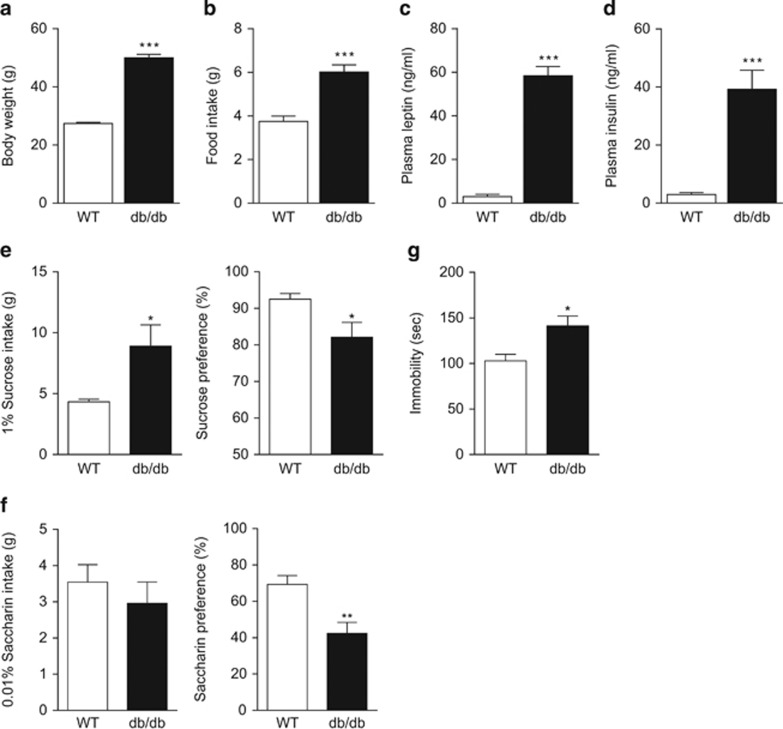
The db/db mice weighed approximately twice as much as wild-type littermate controls at 12 weeks of age (t(17)=17.82, P<0.001, Figure 1a). They consumed 40% more food (t(17)=5.597, P<0.001, Figure 1b) and exhibited hyperleptinemia (t(8)=12.64, P<0.001) and hyperinsulinemia (t(8)=5.504, P<0.001, Figures 1c and d), consistent with expectations. To study depression-related behaviors in db/db mice, sucrose preference was assessed. Wild-type mice and db/db mice were given free access to water and 1% sucrose solution for 4 days. The cage position of the two fluid bottles was switched daily to control for positional preference. The db/db mice showed increased consumption (t(17)=2.760, P<0.05) but reduced preference (t(17)=2.496, P<0.05) for sucrose solution as compared with wild-type littermate controls (Figure 1e). To rule out the potential confounding effect of caloric intake in the sucrose preference test, we tested the mice with 0.01% saccharin, a non-caloric sweetener. The saccharin consumption of db/db mice was similar to that of wild-type mice (t(16)=0.774, P>0.1), but saccharin preference was significantly decreased in db/db mice (t(16)=3.471, P<0.01, Figure 1f). The reduction in preference for both sucrose and saccharin in db/db mice suggests an anhedonic phenotype. Furthermore, in the forced swim test, db/db mice exhibited increased immobility (t(15)=2.867, P<0.05, Figure 1g), an index of behavioral despair. However, as db/db mice have decreased locomotor activity, the increased immobility in the forced swim test could be attributable, in part, to general hypolocomotion.
Figure 1.
Obesity and depression-like phenotypes in db/db mice. (a) Body weight. (b) Food intake. n=9–10 per group, ***P<0.001 compared with wild-type mice. (c) Plasma leptin levels. (d) Plasma insulin levels. n=5 per group, ***P<0.001 compared with wild-type mice. (e) Sucrose preference test. Left panel, sucrose intake; right panel, sucrose preference. n=9–10 per group, *P<0.05 compared with wild-type mice. (f) Saccharin preference test. Left panel, saccharin intake; right panel, saccharin preference. n=9 per group, **P<0.01 compared with wild-type mice. (g) Forced swim test. n=8–9 per group, *P<0.05 compared with wild-type mice.
Antidepressant-like behavioral responses to acute and chronic treatment with fluoxetine and desipramine are attenuated in db/db mice
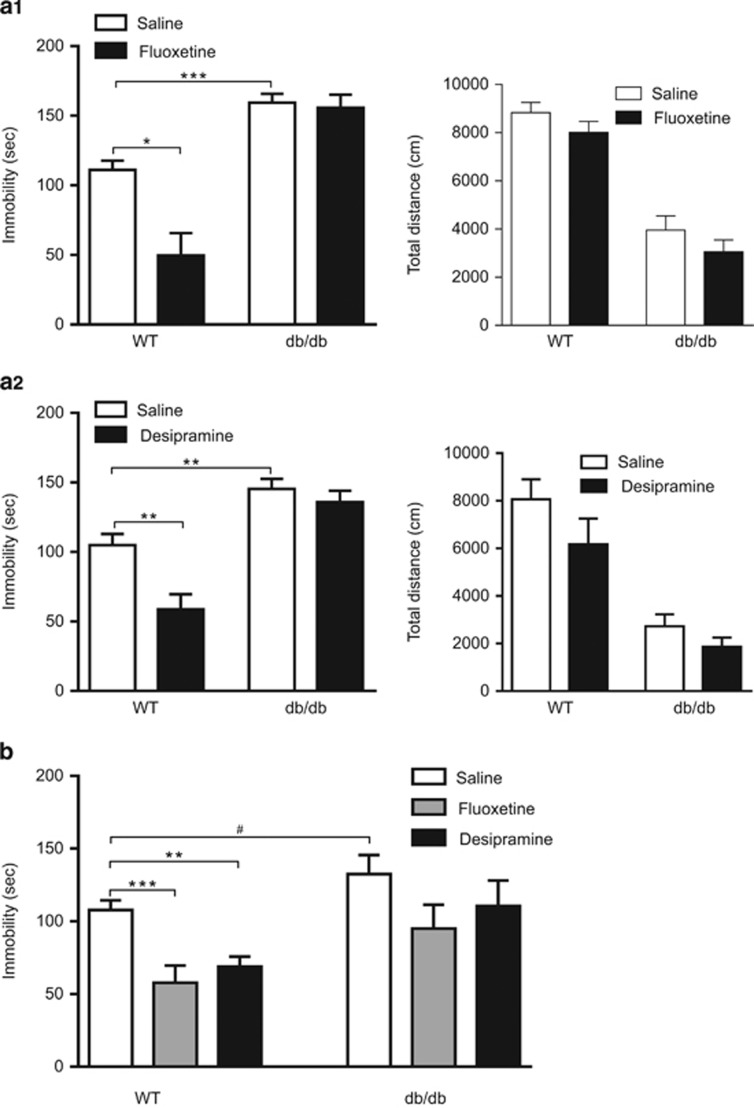
To determine the impact of leptin receptor deficiency on behavioral responses to different classes of antidepressants, the serotonergic antidepressant fluoxetine and the noradrenergic antidepressant desipramine were used to evaluate their antidepressant-like effects in db/db mice in the forced swim test. First, we determined the behavioral effects of acute fluoxetine and desipramine in db/db mice and their wild-type littermate controls. The forced swim test was performed 30 min after acute administration with fluoxetine (10 mg kg−1, i.p.) or desipramine (10 mg kg−1, i.p.). There were significant main effects of treatment, genotype and interaction between drug treatment and genotype (fluoxetine: F(1,35)=9.46, P<0.01 for treatment; F(1,35)=53.23, P<0.001 for genotype and F(1,35)=7.402, P=0.01 for interaction; desipramine: F(1,26)=9.791, P<0.01 for treatment; F(1,26)=43.66, P<0.001 for genotype and F(1,26)=4.25, P<0.05 for interaction). Vehicle-treated db/db mice exhibited increased immobility when compared with vehicle-treated wild-type littermate controls (P<0.01, Figure 2a, left panels), which is consistent with the results above obtained from naive mice. Both fluoxetine and desipramine induced antidepressant-like behavioral effects in wild-type mice, as indicated by dramatic reduction of immobility (P<0.05). However, the behavioral effects of both fluoxetine and desipramine were completely abolished in db/db mice (Figure 2a, left panels). To rule out the possible contribution of differing motoric responses to fluoxetine and desipramine in db/db mice from wild-type mice, locomotor activity was assessed at the time point (that is, 30 min after drug administration) when the mice were subjected to the forced swim test. Fluoxetine and desipramine caused no significant change in locomotion in either wild-type mice or db/db mice (fluoxetine: F(1,33)=3.07, P>0.05 for treatment; F(1,33)=100.13, P<0.001 for genotype and F(1,33)=0.01, P>0.5 for interaction; desipramine: F(1,26)=2.99, P>0.05 for treatment; F(1,26)=36.69, P<0.001 genotype and F(1,26)=0.42, P>0.5 interaction; Figure 2a, right panels).
Figure 2.
Behavioral responses of db/db mice to fluoxetine and desipramine in the forced swim test. (a) Behavioral effects of acute treatment with fluoxetine (10 mg kg−1, i.p.) and desipramine (10 mg kg−1, i.p.). Forced swim test and locomotor activity test were performed 30 min after injection. (a1) Left panel, forced swim test; right panel, locomotor activity test. (a2) Left panel, forced swim test; right panel, locomotor activity test. (b) Behavioral effects of chronic administration with fluoxetine (10 mg kg−1 per day, i.p.), desipramine (10 mg kg−1 per day, i.p.) for 21 days. The forced swim test was carried out 24 h after the last injection. n=7–13 per group. *P<0.05, **P<0.01, ***P<0.001 compared with vehicle-treated wild-type (WT) mice; #P<0.05 compared with vehicle-treated db/db mice. i.p., intraperitoneal.
Because chronic antidepressant administration is required for therapeutic effectiveness,3, 22, 23, 24 we, therefore, examined the responsiveness of db/db mice to the behavioral effects of fluoxetine and desipramine after chronic (21-day) administration (Figure 2b). There were significant main effects of genotype (F(1,52)=12.93, P<0.001) and drug treatment (F(2,52)=7.17, P<0.01) on immobility time in forced swim test. Both fluoxetine and desipramine produced robust effects in reducing immobility in wild-type mice (P<0.01) but not in db/db mice. These data indicate that LepRb deficiency causes resistance to the antidepressant-like behavioral effects of both fluoxetine and desipramine.
Effects of fluoxetine on Akt, GSK3β and ERK1/2 signaling in the hippocampus, PFC and hypothalamus of wild-type mice and db/db mice
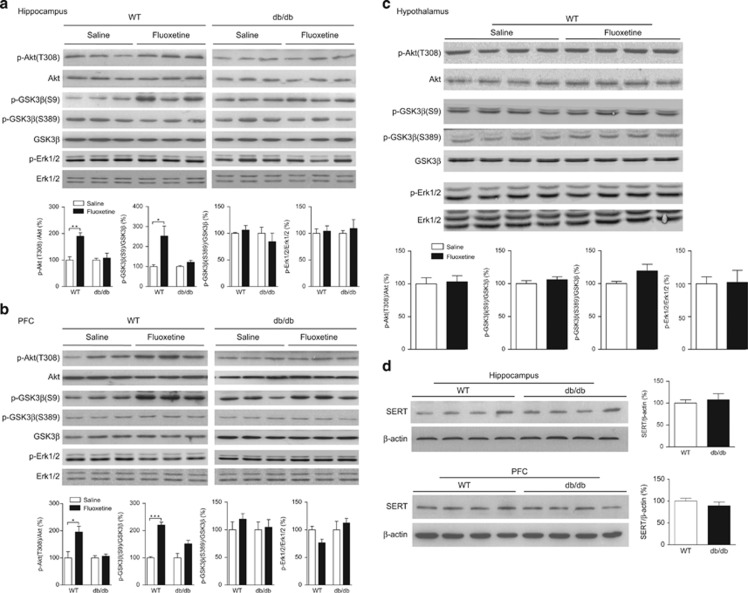
Akt, GSK3β and ERK1/2 phosphorylation was determined in three brain regions that express LepRb,10, 25 30 min following an acute administration of fluoxetine. Fluoxetine stimulated phosphorylation of Akt(Thr308) and GSK3β(Ser9) in the hippocampus (t(8)=4.983, P<0.01 for Akt(Thr308) and t(6)=3.020, P<0.05 for GSK3β(Ser9)) and PFC (t(4)=3.039, P<0.05 for Akt(Thr308) and t(4)=10.78, P<0.001 for GSK3β(Ser9)) of wild-type mice but not db/db mice (hippocampus (t(6)=0.396, P>0.5 for Akt(Thr308) and t(6)=2.084, P>0.05 for GSK3β(Ser9)) and PFC (t(4)=0.559, P>0.5 for Akt(Thr308) and t(4)=2.413, P>0.05 for GSK3β(Ser9)); Figures 3a and b). Following fluoxetine treatment, there was no significant difference in phosphorylation of GSK3β(Ser389) in the hippocampus (t(6)=0.719, P>0.1 for wild-type and t(6)=0.790, P>0.1 for db/db) and PFC (t(4)=1.110, P>0.1 for wild-type and t(4)=0.239, P>0.5 for db/db). Fluoxetine failed to induce phosphorylation of ERK1/2(Thr202/Tyr204) in the hippocampus (t(6)=0.310, P>0.5 for wild-type and t(6)=0.540, P>0.5 for db/db) and PFC (t(4)=2.612, P>0.05 for wild-type and t(4)=0.713, P>0.5 for db/db) in either wild-type or db/db mice. Moreover, we examined these signaling pathways in the hypothalamus, an important leptin target site for feeding and body weight regulation,26, 27 in response to fluoxetine. There were no measurable changes in the phosphorylation of Akt (t(6)=0.235, P>0.5), GSK3β (Ser9 t(6)=0.919, P>0.1 and Ser389 t(6)=1.822, P>0.1) or ERK1/2 (t(6)=0.092, P>0.5) in the hypothalamus of wild-type mice following fluoxetine treatment (Figure 3c).
Figure 3.
Effects of acute fluoxetine treatment on phosphorylation of Akt, GSK3β, ERK1/2 and total protein levels of SERT in the hippocampus, PFC and hypothalamus of db/db mice versus wild-type mice. (a) Phosphorylation of Akt, GSK3β and ERK1/2 was determined 30 min after acute fluoxetine treatment (20 mg kg−1, i.p.). Upper panel showing representative immunoblots of Akt, GSK3β and ERK1/2 in the hippocampus of wild-type (WT) mice and db/db mice. Bottom panel showing quantitative data. (b) Phosphorylation of Akt, GSK3β and ERK1/2 in the PFC of wild-type mice and db/db mice. (c) Phosphorylation of Akt, GSK3β and ERK1/2 in the hypothalamus of wild-type mice. n=3–5 per group. *P<0.05, **P<0.01, ***P<0.001 compared with vehicle-treated wild-type mice. (d) SERT protein levels in the hippocampus and PFC show no genotype difference. n=4 per group. i.p., intraperitoneal; PFC, prefrontal cortex; SERT, serotonin reuptake transporter.
To determine whether the absence of the stimulatory effect of fluoxetine in the hippocampus and PFC of db/db mice is due to loss of its binding sites, the serotonin reuptake transporter (SERT), SERT levels in the hippocampus and PFC were measured using western blotting. The db/db mice showed normal levels of SERT protein in the hippocampus (t(6)=0.464, P>0.5) and PFC (t(6)=1.011, P>0.1), indistinguishable from wild-type control mice (Figure 3d). This finding suggests that the failure of fluoxetine to induce antidepressant behavioral effects and stimulate signal transduction in the hippocampus and PFC is not attributable to a reduced level of SERT in these two brain regions.
Effects of desipramine on Akt, GSK3β and ERK1/2 signaling in the hippocampus, PFC and hypothalamus of wild-type mice and db/db mice
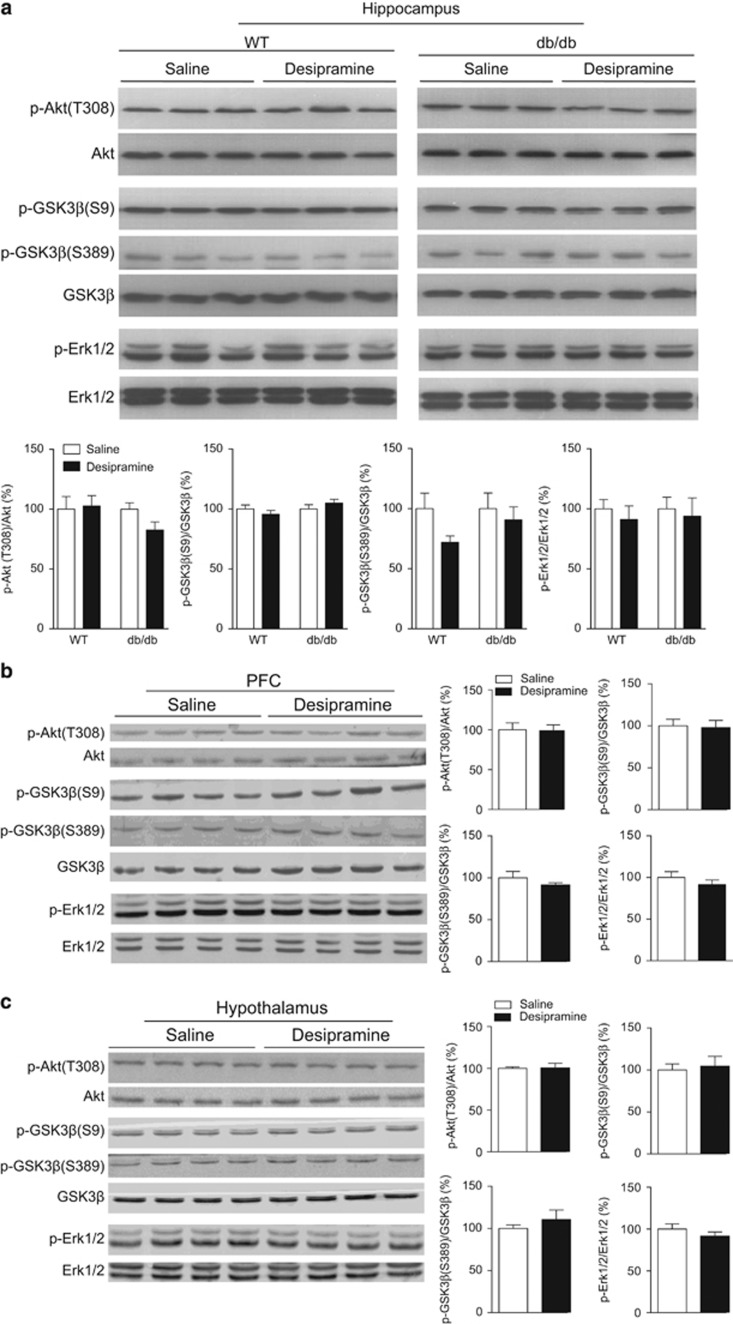
Akt, GSK3β, and ERK1/2 phosphorylation was determined in three brain regions 30 min following an acute administration of desipramine. In the hippocampus and PFC of wild-type mice or db/db mice, desripramine produced no significant effect on Akt(Thr308) (hippocampus (t(6)=0.201, P>0.5 for wild-type and t(6)=1.992, P>0.05 for db/db) and PFC (t(6)=0.098, P>0.5 for wild-type)), GSK3β(Ser9) (hippocampus (t(6)=0.962, P>0.1 for wild-type and t(6)=1.058, P>0.1 for db/db) and PFC (t(6)=0.182, P>0.5 for wild-type)), GSK3β(Ser389) (hippocampus (t(6)=0.539, P>0.5 for wild-type and t(6)=0.548, P>0.5 for db/db) and PFC (t(6)=1.064, P>0.1 for wild-type)) and ERK1/2(Thr202/Tyr204) (hippocampus (t(6)=0.647, P>0.5 for wild-type and t(6)=0.339, P>0.5 for db/db) and PFC (t(6)=0.958, P>0.1 for wild-type); Figures 4a and b). Similar to fluoxetine, phosphorylation of these signaling molecules following desipramine treatment was not significantly different from that of vehicle treatment in the hypothalamus of wild-type mice (Akt(Thr308) t(6)=0.616, P>0.5; GSK3β(Ser9) t(6)=0.335, P>0.5; GSK3β(Ser389) t(6)=0.872, P>0.1; and ERK1/2(Thr202/Tyr204) t(6)=1.064, P>0.1; Figure 4c).
Figure 4.
Effects of acute desipramine treatment on phosphorylation of Akt, GSK3β and ERK1/2 in the hippocampus, PFC and hypothalamus of db/db mice versus wild-type mice. Phosphorylation of Akt, GSK3β and ERK1/2 was determined 30 min after acute desipramine treatment (20 mg kg−1, i.p.). (a) Upper panel showing representative immunoblots of Akt, GSK3β and ERK1/2 in the hippocampus of wild-type (WT) mice and db/db mice. Bottom panel showing quantitative data. (b) Phosphorylation of Akt, GSK3β and ERK1/2 in the PFC of wild-type mice. (c) Phosphorylation of Akt, GSK3β and ERK1/2 in the hypothalamus of wild-type mice. n=4 per group. i.p., intraperitoneal.
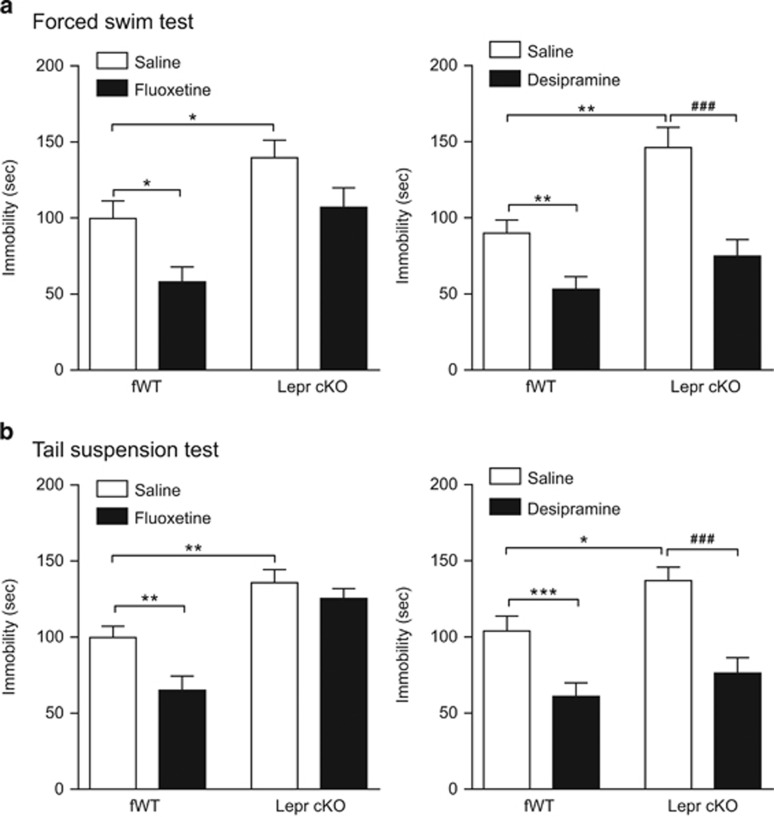
Lepr cKO mice are resistant to the antidepressant-like behavioral effect of fluoxetine but not desipramine
Our previous studies have characterized Lepr cKO mice, showing that LepRb is specifically deleted in the hippocampus and cortex, but LepRb expression is intact in the hypothalamus in this line of conditional knockout mice. Lepr cKO mice display normal body weight and locomotor activity, but exhibit depression-like phenotypes.10 Using this line of mice, we sought to determine whether LepRb in the hippocampus and cortex is necessary for the antidepressant-like behavioral effects of fluoxetine and desipramine. In the forced swim test, a two-way analysis of variance revealed significant effects of genotype (F(1,36)=14.29, P<0.001) and fluoxetine treatment (F(1,36)=10.02, P<0.01) on immobility time. The behavioral effect of acute fluoxetine was blunted in Lepr cKO mice (Figure 5a, left panel). Following desipramine treatment, significant effects of genotype (F(1,26)=14.37, P<0.001) and drug treatment (F(1,26)=27.61, P<0.001) on immobility time were also found. Desipramine treatment effectively reduced immobility time in Lepr cKO mice to an extent comparable to desipramine-treated wild-type mice (Figure 5a, right panel). To confirm these findings, a separate cohort of Lepr cKO mice and their wild-type littermate controls were tested in the tail suspension test following acute treatment with fluoxetine and desipramine. There were significant main effects of genotype (F(1,28)=49.7, P<0.001 for fluoxetine; F(1,52)=7.26, P<0.05 for desipramine) and drug treatment (F(1,28)=10.83, P<0.01 for fluoxetine; F(1,52)=33.35, P<0.001 for desipramine). Similar to the findings obtained from the forced swim test, the behavioral effect of fluoxetine in the tail suspension test was abolished in Lepr cKO mice, whereas desipramine produced equivalent behavioral responses in both Lepr cKO mice and wild-type control mice (Figure 5b).
Figure 5.
Behavioral responses of Lepr cKO mice to fluoxetine and desipramine. The Lepr cKO mice and littermate control (fWT) mice received acute treatment with fluoxetine (10 mg kg−1, i.p.) and desipramine (10 mg kg−1, i.p.). Forced swim test or tail suspension test was performed 30 min after injection. (a) Forced swim test. Left panel, fluoxetine produces antidepressant-like behavioral effect in control mice but not in Lepr cKO mice. Right panel, desipramine produces similar behavioral effects in Lepr cKO mice and control mice. (b) Tail suspension test showing similar results. Left panel, behavioral response of Lepr cKO mice to fluoxetine. Right panel, behavioral response of Lepr cKO mice to desipramine. n=7–15 per group, *P<0.05, **P<0.01, ***P<0.001 compared with vehicle-treated control mice. ###P<0.001 compared with vehicle-treated Lepr cKO mice. i.p., intraperitoneal.
Discussion
A substantial percentage of depressed patients fail to respond to antidepressant treatment.3, 28, 29 The biological mechanisms underlying antidepressant response and treatment resistance, however, remain unknown. Here we have shown that leptin/LepRb signaling is critically involved in the mechanisms of action of antidepressant drugs. The serotonergic antidepressant fluoxetine and the noradrenergic antidepressant desipramine are the two most commonly prescribed drugs used to treat depression. Their antidepressant-like behavioral effects following both acute and chronic administration were abolished or blunted in LepRb-null db/db mice. Akt/GSK3β signaling in response to fluoxetine was impaired in the hippocampus and PFC of db/db mice, whereas no measurable changes in Akt/GSK3β and ERK1/2 signaling molecules were observed following desipramine. Moreover, deletion of LepRb specifically in the hippocampal and cortical neurons reduced behavioral response to fluoxetine but not desipramine. Our results indicate that functional LepRb in the hippocampus and PFC is essential for behavioral efficacy and intracellular signaling of fluoxetine but not desipramine, suggesting separable neural substrates mediating their antidepressant actions.
Epidemiological data suggest that obesity is linked to an increased risk of depressive disorders.30, 31, 32 One possible mechanism involves leptin resistance. The db/db mice, a model of total leptin resistance, are severely obese. Given a decreased preference for both sucrose and saccharin (non-caloric sweetener) solutions observed in db/db mice, this deficit is unlikely due to an effect of the motivational drive for food, but rather a specific anhedonic phenotype. As reported previously,33 db/db mice displayed increased immobility in the forced swim test, usually indicative of enhanced ‘behavioral despair'. Increased adipose tissue mass in db/db mice may result in more floatation in the forced swim test, which could be a potential caveat. However, higher body weight and adiposity in mice do not seem to impair the animal's mobility response to antidepressant effects. It has been reported that a single injection of leptin reduces immobility time to a similar extent in leptin-deficient (ob/ob) obese mice and wild-type mice.8 Moreover, acute desipramine treatment decreases immobility to a similar extent in both diet-induced obese mice and control mice.8 In view of these findings, the resistance to fluoxetine and desipramine observed in db/db mice is likely the outcome of impaired LepRb signaling rather than the consequence of fat status of the mice.
Clinical studies have suggested an association of leptin levels with antidepressant responses. It was reported that leptin levels are increased during successful antidepressant treatment of depressed patients.12 Genome-wide pharmacogenetic analyses demonstrate a strong association of polymorphisms of the leptin gene with response to nortriptyline, a tricyclic antidepressant.16 Moreover, a recent study reported that patients carrying specific polymorphisms in the leptin gene showed lower remission rates or impaired treatment response to tricyclic antidepressants and mirtazapine, a 5-HT and NE reuptake inhibitor.15 Our observations of the resistance of db/db mice to the antidepressant-like behavioral effects of fluoxetine and desipramine suggest an important role of leptin-LepRb in antidepressant response and may represent a mechanism underlying treatment-resistant depression.
Fluoxetine and desipramine function by enhancing neurotransmission of 5-HT and/or NE via inhibition of neurotransmitter reuptake. Possible interactions between LepRb and 5-HT or NE neurotransmission could occur presynaptically and/or postsynaptically. The primary source of NE in the central nervous system derives from the locus coeruleus,34, 35 where there is no LepRb expression.25 LepRb is highly expressed in the raphe nuclei that are densely populated with 5-HT neurons.25, 36 However, 5-HT neurons were found not to express LepRb.37 These observations argue against the possibility of a direct action of LepRb on 5-HT or NE presynaptic neurons. The second possibility is that leptin interacts with 5-HT or NE neurotransmission in terminal fields, such as the hippocampus and PFC.25, 38, 39, 40, 41 In these two brain regions, fluoxetine stimulated phosphorylation of Akt and GSK3β in wild-type mice but not in db/db mice. In light of Akt/GSK3β signaling in therapeutic effects of antidepressants,17, 19, 20 this is consistent with impaired antidepressant-like behavioral responses of db/db mice to fluoxetine. As fluoxetine acts primarily as an inhibitor of SERT to increase 5-HT availability for synaptic transmission, it is possible that the failure of fluoxetine to produce behavioral and signaling effects in db/db mice could be simply due to the downregulation of SERT. However, it seems to not be the case as SERT protein levels were unaltered in the hippocampus and PFC of db/db mice. Together, these data suggest that fluoxetine may interact with LepRb on postsynaptic neurons, targeting at the intracellular Akt/GSK3β signaling pathway. However, whether this is caused by fluoxetine transactivation of LepRb independent of leptin and 5-HT reuptake inhibition remains to be investigated.
In contrast to the well-characterized signaling pathways mediating antidepressants acting on 5-HT neurotransmission, there is not much information available on Akt/GSK3β or ERK1/2 signaling in response to the noradrenergic antidepressant desipramine. The present study demonstrated that desipramine failed to induce phosphorylation of Akt, GSK3β or ERK1/2 in the hippocampus and PFC in either wild-type mice or db/db mice. These signaling pathways were also determined in another brain region, the hypothalamus, and showed no measurable changes in response to desipramine treatment. It is possible that desipramine may activate unique signaling pathways in these brain regions. It is also possible that the hippocampus and PFC may serve as neural substrates mediating the effects of fluoxetine but not desipramine. Our further investigation confirmed the second possibility using mice with deletion of LepRb specifically in hippocampal and cortical neurons. These mice exhibit depression-like phenotypes, such as anhedonia and behavioral despair,10 similar to those seen in db/db mice. In contrast to the obesity syndrome in db/db mice, Lepr cKO mice have normal fat deposition and body weight, as well as plasma levels of leptin and insulin.10 We found that deletion of LepRb in hippocampal and cortical neurons impaired behavioral responses to fluoxetine in both the forced swim test and tail suspension test. In contrast, desipramine produced similar behavioral effects in both conditional LepRb knockout mice and wild-type mice. This interesting finding suggests that functional LepRb in the hippocampus and PFC is necessary for behavioral responses to fluoxetine but not desipramine. The neural substrates that mediate LepRb actions on behavioral responses to desipramine, however, remain to be further explored.
Treatment-resistant depression has been proposed to represent a biologically unique subtype of depression. The present study suggests that functional LepRb may be a determinant of antidepressant efficacy. Whether genetic polymorphisms of the LepRb gene are associated with treatment resistance to antidepressant therapy and the failure to achieve remission in depressed patients awaits future investigations. The nature of the neural circuits and molecular mechanisms involved in therapeutic actions of different classes of antidepressants are still not well understood. Identifying neural substrates underlying leptin action on behavioral effects of classical antidepressants in future studies would provide new insight into the understanding of differential responses to antidepressants and the development of novel therapeutic strategies to overcome treatment resistance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81301164, MG), the Scientific Research Starting Foundation for Returned Overseas Chinese Scholars, Ministry of Education, China (MG), the Taishan Scholar Programs of Shandong Province, China (20120214, X-YL) and the National Institute of Mental Health (MH076929, X-YL).
The authors declare no conflict of interest.
References
- Petersen T, Dording C, Neault NB, Kornbluh R, Alpert JE, Nierenberg AA, et al. A survey of prescribing practices in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:177–187. doi: 10.1016/s0278-5846(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–1950. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17:790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl. 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152:2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- Guo M, Huang TY, Garza JC, Chua SC, Lu XY. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol. 2013;16:857–867. doi: 10.1017/S1461145712000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl Psychiatry. 2012;2:e83. doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf. 2012;76:520–525. doi: 10.1111/j.1365-2265.2011.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esel E, Ozsoy S, Tutus A, Sofuoglu S, Kartalci S, Bayram F, et al. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:565–570. doi: 10.1016/j.pnpbp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Koethe D, Pollmacher T. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry. 2002;35:220–225. doi: 10.1055/s-2002-36390. [DOI] [PubMed] [Google Scholar]
- Laimer M, Kramer-Reinstadler K, Rauchenzauner M, Lechner-Schoner T, Strauss R, Engl J, et al. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry. 2006;67:421–424. doi: 10.4088/jcp.v67n0313. [DOI] [PubMed] [Google Scholar]
- Kloiber S, Ripke S, Kohli MA, Reppermund S, Salyakina D, Uher R, et al. Resistance to antidepressant treatment is associated with polymorphisms in the leptin gene, decreased leptin mRNA expression, and decreased leptin serum levels. Eur Neuropsychopharmacol. 2013;23:653–662. doi: 10.1016/j.euroneuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7:1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation. Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XZ, Zhang D, Lu XY.Dentate gyrus–CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin Mol Psychiatryadvance online publication, 5 August2014. doi: 10.1038/mp.2014.75(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Katz MM, Koslow SH, Maas JW, Frazer A, Bowden CL, Casper R, et al. The timing, specificity and clinical prediction of tricyclic drug effects in depression. Psychol Med. 1987;17:297–309. doi: 10.1017/s0033291700024831. [DOI] [PubMed] [Google Scholar]
- Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. 2001;62:869–877. doi: 10.4088/jcp.v62n1106. [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. 2005;66:974–981. doi: 10.4088/jcp.v66n0803. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring. 2006;14:208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Managing treatment-resistant major depression. J Clin Psychiatry. 2003;64:5–12. [PubMed] [Google Scholar]
- Preskorn SH.Antidepressant drug selection: criteria and options J Clin Psychiatry 1994556–22.discussion 23-24, 98-100. [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Lawlor DA, Singh-Manoux A, Batty GD, Ferrie JE, Shipley MJ, et al. Common mental disorder and obesity: insight from four repeat measures over 19 years: prospective Whitehall II cohort study. BMJ. 2009;339:b3765. doi: 10.1136/bmj.b3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: causal mechanisms and implications for treatment. Clin Psychol Sci Pract. 2008;15:1–20. [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;187:703–724. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE. Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol. 1975;164:171–183. doi: 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]