Abstract
Suicide is one of the leading causes of death in the United States, yet it remains difficult to understand the mechanistic provocations and to intervene therapeutically. Stress is recognized as a frequent precursor to suicide. Psychological stress is well established to cause activation of the inflammatory response, including causing neuroinflammation, an increase of inflammatory molecules in the central nervous system (CNS). Neuroinflammation is increasingly recognized as affecting many aspects of CNS functions and behaviors. In particular, much evidence demonstrates that inflammatory markers are elevated in traits that have been linked to suicidal behavior, including aggression, impulsivity and depression. Lithium is recognized as significantly reducing suicidal behavior, is anti-inflammatory and diminishes aggression, impulsivity and depression traits, each of which is associated with elevated inflammation. The anti-inflammatory effects of lithium result from its inhibition of glycogen synthase kinase-3 (GSK3). GSK3 has been demonstrated to strongly promote inflammation, aggressive behavior in rodents and depression-like behaviors in rodents, whereas regulation of impulsivity by GSK3 has not yet been investigated. Altogether, evidence is building supporting the hypothesis that stress activates GSK3, which in turn promotes inflammation, and that inflammation is linked to behaviors associated with suicide, including particularly aggression, impulsivity and depression. Further investigation of these links may provide a clearer understanding of the causes of suicidal behavior and provide leads for the development of effective preventative interventions, which may include inhibitors of GSK3.
Introduction
In 2010, suicide was the 10th leading cause of death in the United States, accounting for more than 38 000 deaths, the suicide rate increased steadily during the previous 10 years, and an astounding approximately one million people in the US made a suicide attempt (Centers for Disease Control and Prevention website). Thus, it is evident that suicide is a major health problem that is not adequately treated, as well as being poorly understood. Clearly, there is a crucial need to develop improved strategies to understand the conditions that elicit suicidal behavior and to develop effective interventions.
Suicidal behavior is often, but certainly not always, associated with psychiatric illnesses, particularly major depression, bipolar disorder and schizophrenia. For example, a strong association was indicated by the finding that suicide is 60% comorbid with mood disorders,1 and the risk of suicide is at least 15 times higher in patients with bipolar disorder than for the general population.2 However, the perplexing question remains as to what differentiates the suicidal person from those with similar conditions that are not suicidal. This issue has led to numerous studies attempting to identify behavioral characteristics that contribute to suicidal behavior. Among the key characteristics that have been identified to be associated with suicidal behavior, impulsiveness, aggression and feelings of helplessness or depression demonstrate particularly strong links.1, 3, 4, 5, 6, 7, 8, 9 These associations raise the possibility that identification of mechanisms and therapeutic interventions that regulate these characteristics may provide insight into the causes of suicidal behavior and lead to methods for early detection and intervention. In this regard, there is increasing evidence that abnormal activation of the inflammatory system is linked to each of these individual behaviors in animal models, and to suicidal behavior in humans.
Here, we review evidence suggesting that inflammation may be a key factor precipitating suicidal behaviors in response to initiating stressors, we assess key aspects of suicidal behavior-linked endophenotypes that have been studied in rodents, and we examine the effects of lithium intervention that appears to diminish suicide-linked behaviors.
Strategies to study suicidal behavior in animal models
The very nature of suicide limits direct investigation except postmortem, thus gaining a better understanding of suicidal behavior requires the development of indirect strategies. Two feasible approaches include studies in animal models of mechanisms that regulate suicide-associated behaviors, and studies of the mechanism of action of drugs that alter suicidal behavior. Thus, although suicide cannot be directly studied in animal models, rodents can be used to study factors that regulate suicide-relevant behaviors or endophenotypes. Using the endophenotype approach to investigate complex behaviors associated with numerous psychiatric and neurological conditions has been discussed by many investigators in a variety of fields,10, 11 and although not perfect, it remains the primary strategy available for studies in rodents. Thus, a better understanding of suicidal behavior may benefit from studies of endophenotypes in rodents, particularly impulsive behavior, aggression and depression-like behaviors that have been linked to suicidal behavior. Another strategy to examine mechanisms regulating suicidal behavior is to consider the actions of an agent that reduces attempted and completed suicides. Substantial evidence demonstrates that lithium, the classical mood stabilizer used to treat bipolar disorder, reduces suicidal behavior and mortality during long-term treatment.5, 12 This conclusion is supported by several meta analyses and has been reported in patients with unipolar and bipolar depression (the patient populations most often treated with lithium), in responders and nonresponders to the mood stabilizing action of lithium, and the antisuicidal effect of lithium is not matched by other mood stabilizers or antidepressants.6, 8, 13, 14, 15, 16 Furthermore, several studies have found that relatively high levels of lithium in the public drinking water are associated with reduced risk of suicide in the general population.17 Thus, studies in rodents of individual behaviors associated with suicidal behavior, in conjunction with studies of lithium, which is able to diminish suicidal behavior, provide feasible investigative strategies to better understand the underlying causes of suicidal behavior and to develop effective interventions.
Stress induces inflammation which is associated with suicidal behavior
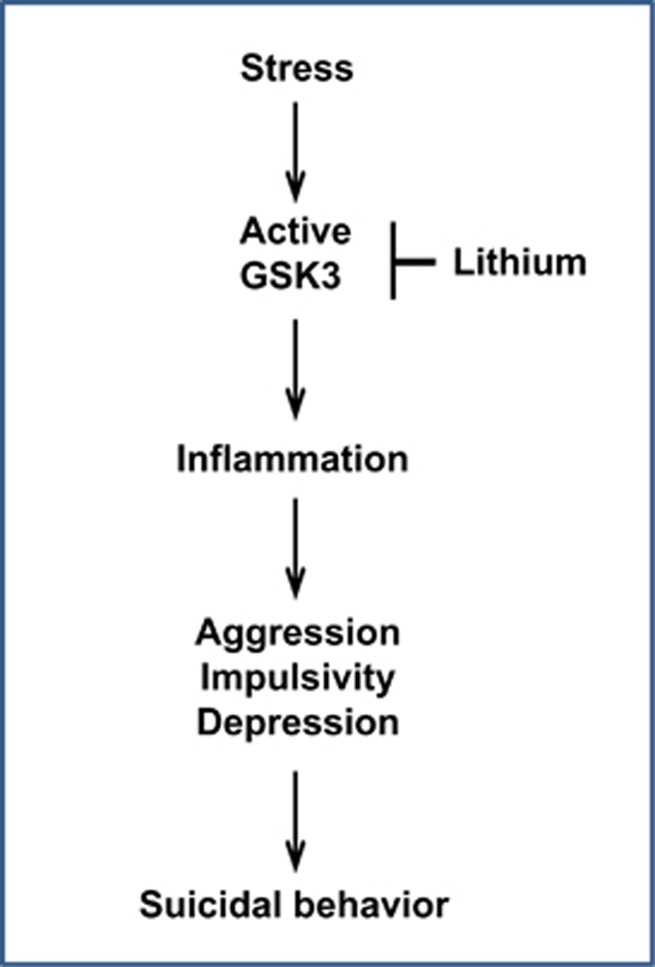
Stress is a common precursor of suicidal behavior.18 Stress also increases inflammation, and inflammation is linked to increased impulsive, aggressive and depressive behaviors, leading to the hypothesis that stress-triggered inflammation has an important role in provoking suicidal behavior (Figure 1). Multiple types of psychological stress have been shown to cause activation of the inflammatory response, which is indicated by elevated levels of inflammatory cytokines.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 However, comparative studies have not been reported to determine if different types of stress induce different patterns of inflammatory cytokine production, and which inflammatory molecules are most closely associated with suicidal behavior. The common finding that stress induces inflammation has been interpreted in evolutionary terms as a logical mechanism to enhance survival. Historically, many stressors had the potential to lead to injury and infection, therefore pre-activation of the immune system would enhance survival and recovery.46 However, as psychological stress has increased in modern societies, and drugs are available to combat life-threatening infections, these evolutionary mechanisms to improve survival after injury may now have deleterious effects on behaviors, including promotion of multiple suicide-linked behaviors, as discussed below.
Figure 1.
Scheme of a potential mechanistic pathway that may lead to suicidal behavior. The scheme displays a hypothetical component of mechanisms contributing to suicidal behavior. Stress is established to cause activation of GSK3 in rodent brain, and lithium is an established inhibitor of GSK3, which we propose may contribute to its antisuicidal actions. Active GSK3 promotes inflammation, and we hypothesize that inflammation, one of multiple signaling systems regulated by GSK3, contributes to provoking components of suicidal behavior, such as aggression, impulsivity and depression. GSK3, glycogen synthase kinase-3.
Except for the initial insult, stress appears to utilize many of the same mechanisms as pathogens to induce an inflammatory response, although much still remains to be learned about the details of the stress-induced signaling pathway. The stress response is most well-characterized for signaling through Toll-like receptor 4 (TLR4), the receptor for lipopolysaccharide (LPS), which is the most widely used agent to study inflammation experimentally and is the major cause of sepsis. TLR4 is activated by both pathogen-associated molecular patterns of microbes, and insult-induced endogenous ligands, called danger- or damage-associated molecular patterns (DAMPs).47 DAMPS induce TLR4 signaling outcomes that respond to the need for rapid danger-recovery and restoration of homeostasis.48 DAMPs that activate TLR4 include a broad range of molecules, such as heat-shock proteins,49 hyaluronan oligosaccharides,50 high-mobility group protein box-1,51 modified lipids52 and several others, which are produced by a variety of stressors in the central nervous system as well as peripherally. Thus, stress-induced DAMPs can set in motion an inflammatory response that appears to be equivalent to that induced by pathogens. TLR4 is expressed by microglia, astrocytes and neurons, as well as immune cells.48, 53, 54, 55, 56, 57 TLR4 expression is dynamic and is often upregulated in conditions that are associated with increased levels of pathogen-associated molecular patterns or DAMPs,52, 58 including evidence of dynamic changes in the expression of TLR4 in the brain. For example, TLR4 expression in rodent brain increased in response to ischemia/reperfusion injury, which was partially attributed to DAMPs arising from oxidative stress.55, 59 Furthermore, chronic mild stress increased TLR4 expression in rat prefrontal cortex,42 and administration of a TLR4 antagonist reduced stress-induced neuroinflammation.45 Thus, TLR4 can be activated in response to stress, not only by pathogens, and is involved in stress-induced inflammation, including in the central nervous system.
There is much evidence linking an activated inflammatory response with suicidal behavior. Elevated levels of inflammatory cytokines, particularly interleukin-6 (IL-6), were found in the blood and CSF of patients who attempted suicide compared with nonsuicidal depressed patients and controls.60, 61, 62, 63, 64 Elevated markers of inflammation and microglial activation also were found in postmortem brains of suicide victims.65, 66, 67 Conversely, therapeutic administration of cytokines increases suicide risk.68, 69, 70, 71 Particularly interesting is the recent finding from a postmortem brain study that protein expression of TLR4 is higher in depressed suicide victims than in depressed nonsuicide subjects and controls.72 Notably, alterations in genes involved in inflammation have been found to be associated with suicidal behavior in multiple studies of potential candidate genes.73, 74, 75, 76 In addition, inflammation activates the enzyme indoleamine-2,3-dioxygenase, which catalyzes the formation of kynurenine, and plasma kynurenine levels were higher in depressed patients with a history of suicide attempts than in nonsuicidal depressed patients and healthy controls.77 The authors suggested that elevated kynurenine levels may be a marker of suicide attempt risk, independent of depression severity, and that kynurenine metabolites may contribute to the aggression/impulsivity and neurocognitive deficits proposed as endophenotypes associated with suicidal behavior.1, 18, 77, 78, 79 Thus, multiple lines of evidence demonstrate a consistent relationship between elevated markers of inflammation and suicidal behavior. Therefore, it is important to identify which components of suicidal behavior may be induced by activation of the inflammatory response.
Aggressive behavior
Aggression has been linked to suicidal behavior in many studies1, 3, 4, 5, 7, 8, 79 and an increasing number of reports demonstrate that inflammation is associated with increased aggressive behaviors.80 Elevated aggressive traits were associated with increased serum TNF,81 with the inflammatory marker C-reactive protein,82, 83, 84 and with multiple cytokines.84, 85 Increased serum IL-6 levels correlated with personality traits of aggression in healthy controls83 and with aggression traits in female patients with eating disorders.86 Furthermore, aggressive traits were increased in patients treated with cytokines therapeutically.87, 88 Thus, in humans, aggressive behaviors are well correlated with increased markers of inflammation.
A few studies of rodents have also examined links between aggression and inflammation. In rodents, aggression is often measured using the social dominance tube test and the aggression test. Mice bred for high aggression had increased cytokine levels89 and knockout of both tumor necrosis factor receptor-1 and tumor necrosis factor receptor-2 resulted in the remarkable absence of aggressive behavior.90 Thus, there appears to be a strong link between aggression and activation of the inflammatory system in rodents, but further studies are needed to verify this association and to delineate which inflammatory molecules mediate the interaction and the mechanisms that are involved.
Substantial evidence shows that lithium can reduce aggressive behavior. Lithium is well documented to reduce aggressive behavior in a variety of human populations, for example, children, adults and the elderly, which has been related to its antisuicidal actions.5, 6, 8, 91, 92 As reviewed in detail previously,5, 92, 93 many studies have shown that aggressive behavior in rodents also is consistently reduced by lithium treatment. Thus, lithium significantly reduces aggressive traits and inflammation, but these two outcomes of lithium administration have not yet been examined together.
Impulsive behavior
As noted in the Introduction, impulsive behavior may frequently be an important component of suicidal behavior. Only a limited number of studies have tested if there is a relationship between inflammation and impulsive behavior. In a study of nearly 5000 individuals, elevated levels of IL-6 were associated with impulsivity-related traits.94 A novel study of 5652 people over a period of 3 years identified a strong correlation between impulsiveness and increased lymphocyte numbers that are indicative of immune activation, and the authors concluded that ‘impulsiveness was a predictor of chronic inflammation'.95
Links between inflammation and impulsive behavior appear not to have been examined in animal models, but there is evidence that lithium administration reduces impulsive behavior in humans and rodents. Three controlled studies of lithium in humans concluded that lithium reduces impulsive behavior, but further studies would strengthen this conclusion.96, 97, 98 In rodents, impulsive behavior exemplified by choosing a small or poor reward that is available immediately, in preference to a larger but delayed reward, is often measured using the three-choice serial reaction time task,99 in which mice are trained to respond to a flash of light occurring in one of three locations with a nose poke, which releases a food reward. In the subsequent test phase, mice are trained to choose between two light cues, one giving a larger food reward than the other. In subsequent trials, the delivery of the larger food reward is delayed, so mice must choose the immediate smaller reward or the delayed larger reward, and wild-type mice predominantly choose the latter. Increased impulsive behavior results in mice choosing the immediate smaller reward rather than the delayed larger reward. There is some indication that lithium treatment reduces impulsive behavior in rodents, but the data are limited. Lithium administration suppressed impulsive behavior in the three-choice serial reaction time task in male Wistar/ST rats, a strain that has been shown to be more impulsive than Lister hooded rats.100 Lithium reduced premature responses and increased the latency of the correct responses in the three-choice serial reaction time task in male Wistar/ST rats, without affecting response latency and without affecting the amount of food consumption or other motivation-related measures.101 Lithium also reduced impulsivity in mice in the delay discounting task in which mice receive larger rewards after a delayed response than after an immediate response.102 Thus, the links between impulsive behavior and inflammation, as well as its control by lithium, remain sparse but supportive of these associations.
Depressive behavior
Depression is often linked with suicidal behavior, although, in contrast to the commonly held assumption, many suicidal patients are not depressed.1, 4, 5, 7, 9, 78, 103 There is abundant evidence that inflammation is associated with the onset and severity of depression, as inflammatory molecules are upregulated in the serum and postmortem brains of depressed patients, as discussed in detail in several reviews.104, 105, 106, 107, 108, 109 Furthermore, administration of interferon-α to bolster immunity induces depression in susceptible people.103, 110 Moreover, LPS administration induces symptoms of depression in humans,111 and a mild stimulation of the primary host defense system has negative effects on emotion, which is thought to be caused by elevated cytokines.110, 112 As noted above, psychological stresses that can induce depression increase inflammatory cytokine production in humans and rodents.113,114 Inflammation in patients with major depression is associated with resistance to antidepressant treatment, and anti-inflammatory drugs can improve antidepressant actions.106, 107, 108,114, 115, 116 Raison and Miller46 recently summarized results demonstrating that many genetic changes identified in patients with major depressive disorder involve the inflammatory system. In rodents, administration of inflammatory cytokines or the inflammation-stimulant LPS causes depression-like behaviors that are attenuated by antidepressants.105 Specific inflammatory cytokines that have been identified as promoters of depression-like behavior in rodents include IL-6,117 TNFα118 and IL-1β.119 Thus, there is much evidence that inflammation can precipitate depression and impair therapeutic responses.
Lithium is not used therapeutically as a direct antidepressant, but is often used to augment antidepressants in treatment-resistant depression, and inflammation is reduced by lithium. In mice, lithium has a wide variety of antidepressant-like effects. For example, in mice, lithium administration produces antidepressant-like effects in the learned helplessness paradigm120 and in the forced swim test.121 The antidepressant actions of lithium are often attributed to its action as an inhibitor of glycogen synthase kinase-3 (GSK3), as discussed in the following section, because pharmacological or molecular inhibition of GSK3 has similar antidepressant effects in animal models.
GSK3 inhibitors reduce inflammation
GSK3 refers to two paralogs, GSK3α and GSK3β, that are encoded by different genes but retain 85% homology and are commonly referred to as isoforms. GSK3 is primarily regulated by phosphorylation on serine-21-GSK3α and serine-9-GSK3β, which inhibits GSK3 activity. Homozygous GSK3α/β21A/21A/9A/9A knockin mice express both GSK3 isoforms with serine-to-alanine mutations at these sites, S9A-GSK3β and S21A-GSK3α. This maintains GSK3 maximally active, since it cannot be inhibited by serine phosphorylation, but within the physiological range because GSK3 is expressed at normal levels.122 GSK3 may be a feasible therapeutic target to diminish suicidal behaviors because it promotes several suicide-linked behaviors in rodents and promotes inflammation, and lithium is a well-established inhibitor of GSK3, which may contribute to the capacity of lithium to reduce suicide.
A variety of evidence has raised the possibility that activated GSK3 may contribute to suicidal behaviors. GSK3 is activated in mouse brain by stress,123 a response that may promote suicide-linked behaviors, and GSK3β activity was found to be elevated in postmortem brains of depressed suicide victims.124 Clear evidence has demonstrated that GSK3 promotes aggressive behaviors, as reduced expression of either GSK3 isoform decreased aggressive behaviors in mice.125, 126 The contribution of GSK3 to impulsive behaviors has yet to be examined, except for the studies of lithium discussed above, but an evaluation of SNPs in the GSK3β gene revealed that a genetic variability in the GSK3β gene is associated with increased impulsive behavior in patients with bipolar disorder.127 Many studies have shown that GSK3 promotes depression-like behaviors in rodents.128 These include clear antidepressant effects of a variety of new small molecule inhibitors of GSK3, in addition to lithium, in rodents,121, 129, 130, 131 ,132 including on depressive behavior exhibited by tryptophan hydroxylase-2 mutant mice with deficient serotonin.125 Also, overexpression of a dominant-negative mutant of GSK3 to reduce GSK3 actions promoted resilience in the social defeat stress test of depression-like behavior.133 In addition, inhibition of GSK3 is required for the rapid antidepressant effect of ketamine in the learned helplessness model of depression in mice.120 Antidepressants increase serotoninergic signaling, which inhibits GSK3 by increasing its serine phosphorylation, and increase signaling by Wnt2, which inhibits GSK3 in the Wnt signaling pathway.134, 135 Importantly, antidepressants inhibit GSK3 in mouse brain after in vivo administration of clinically relevant doses.125, 135 Furthermore, oppositely to inhibiting GSK3, expression of constitutively active GSK3 in mice results in increased susceptibility to stress-induced depression-like behavior in mice.123
Lithium is an established inhibitor of GSK3, and lithium and other GSK3 inhibitors are remarkably effective in reducing inflammation. Therapeutic levels of lithium, ~1 mM, inhibit GSK3 both directly136, 137 and by an indirect mechanism that causes increased inhibitory serine phosphorylation of GSK3.138, 139 GSK3 inhibitors have been shown to be effective anti-inflammatory drugs, reducing by 67–90% inflammatory IL-6, IL-1β and TNFα production by microglia,140 astrocytes,141, 142, 143, 144, 145, 146 human monocytes and peripheral blood mononuclear cells147 and other immune cells.143, 146, 147, 148, 149 Remarkably, in vivo administration of lithium provided protection from endotoxin shock sufficiently enough to allow the survival of most mice from an otherwise lethal (LD100) dose of LPS.147 Thus, GSK3 inhibition effectively reduces inflammation throughout the periphery and the central nervous system.150 Reduced LPS-induced inflammatory cytokines attained by inhibiting GSK3 was found to be due to inhibition of the transcriptional activity of NF-κB, a transcription factor that mediates upregulation of many inflammatory molecules,147 in accordance with reports that GSK3 promotes NF-κB activity, as we reviewed.151 GSK3 inhibitors also block signal transducer and activator of transcription-3 (STAT3) activation, a key transcription factor in inflammatory signaling.141 Remarkably, GSK3 regulates the anti-inflammatory cytokine IL-10 in an opposite manner, so GSK3 inhibition increases anti-inflammatory IL-10 levels three- to fourfold in vivo and in vitro.147 This is mediated by GSK3 inhibition of the CREB and AP-1 transcription factors to reduce their expression of anti-inflammatory IL-10, which underlies the increase in IL-10 levels induced by GSK3 inhibitors.147, 152 The anti-inflammatory actions of GSK3 inhibitors likely contribute to their beneficial effects that have been found in multiple animal models of inflammatory diseases, including endotoxic shock,147 arthritis and peritonitis,152, 153 endotoxemia,154 colitis155 and traumatic brain injury.156 Furthermore, GSK3 inhibitors alleviate inflammatory disease severity in the mouse model of multiple sclerosis.146, 157
In summary, GSK3 may be a feasible therapeutic intervention for suicidal behavior. GSK3 is activated by stress, is a strong promoter of inflammation, promotes in rodents aggressive and depression-like behaviors, and is inhibited by lithium, which diminishes suicidal behavior.
Perspective
Altogether, there is substantial evidence that suicidal behavior and individual impulsivity, aggression and depression are all associated with increased inflammation, which itself can be induced by stress. Thus, we propose the concept that stress activates GSK3 and induces inflammation, which, in turn, promotes the suicide-linked endophenotypes of impulsivity, aggression and depression-like behaviors. We speculate that different inflammatory molecules are produced following different types of stress and that different inflammatory molecules may mediate each of the behavioral outcomes, perhaps accounting, in part, for why not all suicidal patients exhibit each behavior. Furthermore, it is likely that differential effects of inflammatory molecules on the specific brain regions and neural circuits that mediate each of the suicide-linked behaviors influence the cumulative behavioral outcome, which also must be regulated by genetic and epigenetic characteristics of affected subjects. Identification of the inflammatory and behavioral responses to stress that are attenuated by lithium may begin to provide information about its mechanism for reducing suicidal behavior, and why it is not effective in all patients. Furthermore, we suggest that inhibition of inflammatory signaling and inhibition of GSK3 may provide mechanisms to diminish in tandem both the inflammatory response to stress and suicide-related behaviors.
Acknowledgments
Research in the authors' laboratories was supported by grants from the NIMH (MH038752, MH090236, MH095380).
The authors declare no conflict of interest.
References
- Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- Koller G, Preuss UW, Bottlender M, Wenzel K, Soyka M. Impulsivity and aggression as predictors of suicide attempts in alcoholics. Eur Arch Psychiatry Clin Neurosci. 2002;252:155–160. doi: 10.1007/s00406-002-0362-9. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Brown JS, Wingate LR. The psychology and neurobiology of suicidal behavior. Annu Rev Psychol. 2005;56:287–314. doi: 10.1146/annurev.psych.56.091103.070320. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gottesman II, Gould TD. Lithium's antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175–198. doi: 10.1146/annurev.pharmtox.011008.145557. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ. Long-term lithium treatment in the prevention of suicidal behavior in bipolar disorder patients. Epidemiol Psichiatr Soc. 2009;18:179–183. doi: 10.1017/s1121189x00000439. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Pine DS, Tragon T, Austin DR, Henter ID, Chen G, et al. Animal models of suicide-trait-related behaviors. Trends Pharmacol Sci. 2009;30:165–173. doi: 10.1016/j.tips.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oerlinghausen B, Lewitzka U. Lithium reduces pathological aggression and suicidality: a mini-review. Neuropsychobiology. 2010;62:43–49. doi: 10.1159/000314309. [DOI] [PubMed] [Google Scholar]
- Dalca IM, McGirr A, Renaud J, Turecki G. Gender-specific suicide risk factors: a case-control study of individuals with major depressive disorder. J Clin Psychiatry. 2013;74:1209–1216. doi: 10.4088/JCP.12m08180. [DOI] [PubMed] [Google Scholar]
- De Jager PL, Bennett DA. An inflection point in gene discovery efforts for neurodegenerative diseases: from syndromic diagnoses toward endophenotypes and the epigenome. JAMA Neurol. 2013;70:719–726. doi: 10.1001/jamaneurol.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan G, Sawa A, Pletnikov MV. Mouse models of gene-environment interactions in schizophrenia. Neurobiol Dis. 2013;57:5–11. doi: 10.1016/j.nbd.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon S, Chengappa KN, Malhi GS. Lithium specificity in bipolar illness: a classic agent for the classic disorder. Bipolar Disord. 2009;11 (Suppl 2:34–44. doi: 10.1111/j.1399-5618.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162:1805–1819. doi: 10.1176/appi.ajp.162.10.1805. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ. Lithium treatment reduces suicide risk in recurrent major depressive disorder. J Clin Psychiatry. 2007;68:380–383. doi: 10.4088/jcp.v68n0304. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Sacchetti E.Lithium in drinking water and suicide prevention: a review of the evidence Int Clin Psychopharmacol 2014(in press). [DOI] [PubMed]
- Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120537. doi: 10.1098/rstb.2012.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, et al. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, et al. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF, et al. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, et al. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Stark M, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Munhoz C, Madrigal JL, García-Bueno B, Pradillo JM, Moro MA, Lizasoain I, et al. TNF-alpha accounts for short-term persistence of oxidative status in rat brain after two weeks of repeated stress. Eur J Neurosci. 2004;20:1125–1130. doi: 10.1111/j.1460-9568.2004.03560.x. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–1069. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Sá Lima L, Avellar MCW, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-κB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neuroscience. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effect of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;7:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1β and TNF-α production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav. 2009;98:351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T, et al. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, et al. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J Immunol. 2010;184:2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MC, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun. 2011;25:1197–1205. doi: 10.1016/j.bbi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Mays JW, Bailey MT, Hanke ML, Sheridan JF. Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behav Immun. 2011;25:46–52. doi: 10.1016/j.bbi.2010.07.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, et al. Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation. 2011;8:151. doi: 10.1186/1742-2094-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, Audet MC, Jacobson-Pick S, Anisman H. Environmental enrichment influences brain cytokine variations elicited by social defeat in mice. Psychoneuroendocrinology. 2013;38:987–996. doi: 10.1016/j.psyneuen.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Caso JR, Alou L, Gómez-Lus ML, et al. Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflammation. 2014;11:8. doi: 10.1186/1742-2094-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H, et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, et al. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res. 2008;86:1077–1086. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Blitz D, Margolin Z, Vartanian T. A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med. 2010;12:149–163. doi: 10.1007/s12017-009-8094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Nässberger L, Träskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatr Scand. 1993;88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee SW, Kim SH, Shim SH, Han SW, Choi SH, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:356–361. doi: 10.1016/j.pnpbp.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Erhardt S, Träskman-Bendz L, Engström G, Brundin L, et al. CSF biomarkers in suicide attempters—a principal component analysis. Acta Psychiatr Scand. 2011;124:52–61. doi: 10.1111/j.1600-0447.2010.01655.x. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DA, Hardie T, Baron SH. Possible association of interleukin-2 treatment with depression and suicide. J Am Osteopath Assoc. 1993;93:799–800. [PubMed] [Google Scholar]
- Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with α-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML, et al. Suicidal ideation during interferon-α2b and ribavirin treatment of patients with chronic hepatitis C. Gen Hosp Psychiatry. 2004;26:237–240. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, et al. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33:312–318. doi: 10.1097/WNF.0b013e3181f8d513. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Toll-like receptors in the depressed and suicide brain. J Psychiatr Res. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlovic S, Mozes E, Eilat E, Doron A, Lereya J, Zakuth V, et al. Immune activation in non-treated suicidal major depression. Immunol Lett. 1999;67:105–108. doi: 10.1016/s0165-2478(98)00145-x. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, et al. Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- Thalmeier A, Dickmann M, Giegling I, Schneider B, M Hartmann A, Maurer K, et al. Gene expression profiling of post-mortem orbitofrontal cortex in violent suicide victims. Int J Neuropsychopharmacol. 2008;11:217–228. doi: 10.1017/S1461145707007894. [DOI] [PubMed] [Google Scholar]
- Galfalvy H, Zalsman G, Huang YY, Murphy L, Rosoklija G, Dwork AJ, et al. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J Biol Psychiatry. 2013;14:574–582. doi: 10.3109/15622975.2011.597875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–1278. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Currier DM. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur Psychiatry. 2010;25:268–271. doi: 10.1016/j.eurpsy.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65:556–563. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalcman SS, Siegel A. Neurobiology of aggression and rage: role of cytokines. Brain Behav Immunity. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Association of C-reactive protein elevation with trait aggression and hostility in personality disordered subjects: a pilot study. J Psychiatr Res. 2006;40:460–465. doi: 10.1016/j.jpsychires.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22:753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71:158–165. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PM, Vermetten E, Kavelaars A, Geuze E, Heijnen CJ. Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology. 2008;33:1041–1050. doi: 10.1016/j.psyneuen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Ahrén-Moonga J, Lekander M, von Blixen N, Rönnelid J, Holmgren S, af Klinteberg B, et al. Levels of tumour necrosis factor-alpha and interleukin-6 in severely ill patients with eating disorders. Neuropsychobiology. 2011;63:8–14. doi: 10.1159/000321832. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML. Interferon α-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New Eng J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Lysle DT, Gariepy JL, Lewis MH. Association of genetic differences in social behavior and cellular immune responsiveness: effects of social experience. Brain Behav Immun. 1994;8:111–122. doi: 10.1006/brbi.1994.1011. [DOI] [PubMed] [Google Scholar]
- Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav Immun. 2010;24:1276–1280. doi: 10.1016/j.bbi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbrauer J, Nilsson A, Müller-Oerlinghausen B, Bauer M.Therapeutic and prophylactic effects of lithium on pathological aggression Bauer M, Grof P, Müller-Oerlinghausen B.(eds). Lithium in Neuropsychiatry Informa Healthcare, Abingdon, UK; 2006227–236. [Google Scholar]
- Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacol Biochem Behav. 2010;94:380–386. doi: 10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. High neuroticism and low conscientiousness are associated with interleukin-6. Psychol Med. 2010;40:1485–1493. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Milaneschi Y, Cannas A, Ferrucci L, Uda M, Schlessinger D, et al. Impulsivity-related traits are associated with higher white blood cell counts. J Behav Med. 2012;35:616–623. doi: 10.1007/s10865-011-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrego MF, Canevaro L, Kuzis G, Sabe L, Starkstein SE. A randomized, double-blind, crossover study of methylphenidate and lithium in adults with attention-deficit/hyperactivity disorder: preliminary findings. J Neuropsychiatry Clin Neurosci. 2002;14:289–295. doi: 10.1176/jnp.14.3.289. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD. Pattern of response to divalproex, lithium, or placebo in four naturalistic subtypes of mania. Neuropsychopharmacology. 2002;26:530–536. doi: 10.1016/S0893-133X(01)00390-6. [DOI] [PubMed] [Google Scholar]
- Hollander E, Pallanti S, Allen A, Sood E, Baldini Rossi N. Does sustained-release lithium reduce impulsive gambling and affective instability versus placebo in pathological gamblers with bipolar spectrum disorders. Am J Psychiatry. 2005;162:137–145. doi: 10.1176/appi.ajp.162.1.137. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Breysse N.Rodent model of attention: the 5-choice serial reaction time task Curr Protoc Pharmacol 2008Chapter 5Unit 5.49. [DOI] [PubMed] [Google Scholar]
- Galtress T, Garcia A, Kirkpatrick K. Individual differences in impulsive choice and timing in rats. J Exp Anal Behav. 2012;98:65–87. doi: 10.1901/jeab.2012.98-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Tsutsui-Kimura I, Kumamoto H, Minami M, Izumi T, Yamaguchi T, et al. Lithium, but not valproic acid or carbamazepine, suppresses impulsive-like action in rats. Psychopharmacology. 2012;219:421–432. doi: 10.1007/s00213-011-2496-9. [DOI] [PubMed] [Google Scholar]
- Halcomb ME, Gould TD, Grahame NJ. Lithium, but not valproate, reduces impulsive choice in the delay discounting task in mice. Neuropsychopharmacology. 2013;38:1937–1944. doi: 10.1038/npp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Poulter M.O, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Lichte P, Kobbe P, Rosenberger C, Banner C, et al. Neural response to emotional stimuli during experimental human endotoxemia. Hum Brain Mapp. 2013;34:2217–2227. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;3:8–35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–954. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs. 2009;10:664–671. [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jové F, Woodgett JR, Maretto S, Piccolo S, et al. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3β in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C, et al. Abnormalities in brain structure and behavior in GSK-3α mutant mice. Mol Brain. 2009;2:35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Arias B, Mitjans M, Goikolea JM, Roda E, Ruíz V, et al. Association between GSK3β gene and increased impulsivity in bipolar disorder. Eur Neuropsychopharmacol. 2014;24:510–518. doi: 10.1016/j.euroneuro.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16–26. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on β-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H, et al. Role of glycogen synthase kinase-3β in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leão P, et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3β. Neuroscience. 2008;152:656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A novel role of the WNT-dishevelled-GSK3β signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti M. J, et al. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS, et al. In vivo regulation of glycogen synthase kinase-3β (GSK3β) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3β phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009;6:9–20. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Glycogen synthase kinase-3 promotes the synergistic action of interferon-γ on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cell Signalling. 2009;21:978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Glycogen synthase kinase-3 controls inflammatory tolerance in astrocytes. Neuroscience. 2010;169:1063–1070. doi: 10.1016/j.neuroscience.2010.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Wen-I Y, Michalek SM, Harrington LE, Jope RS. Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol. 2011;186:1391–1398. doi: 10.4049/jimmunol.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.C, Kai J.I, Huang W.C, Wang C.Y, Wang Y, Chen C.L, et al. Glycogen synthase kinase-3β facilitates IFN-γ-induced STAT1 activation by regulating Src homology-2 domain-containing phosphatase 2. J Immunol. 2009;183:856–864. doi: 10.4049/jimmunol.0804033. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci. 2011;4:18. doi: 10.3389/fnmol.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, et al. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Di Paola R, Muia C, Crisafulli C, Dugo L, et al. Glycogen synthase kinase-3β inhibition attenuates the degree of arthritis caused by type II collagen in the mouse. Clin Immunol. 2006;120:57–67. doi: 10.1016/j.clim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dugo L, Collin M, Allen DA, Patel NS, Bauer I, Mervaala EM, et al. GSK-3β inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C, et al. Reduction of exp erimental colitis in the rat by inhibitors of glycogen synthase kinase-3β. Br J Pharmacol. 2006;147:575–582. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM, et al. Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma. 2012;29:362–374. doi: 10.1089/neu.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Kaidanovich-Beilin O, Yeh WI, Song L, Palomo V, Michalek SM, et al. Regulation of Th1 cells and experimental autoimmune encephalomyelitis (EAE) by glycogen synthase kinase-3. J Immunol. 2013;190:5000–5011. doi: 10.4049/jimmunol.1203057. [DOI] [PMC free article] [PubMed] [Google Scholar]