Abstract
Serotonin receptor 1A gene (HTR1A) knockout mice show pronounced defensive behaviour and increased fear conditioning to ambiguous conditioned stimuli. Such behaviour is a hallmark of pathological human anxiety, as observed in panic disorder with agoraphobia (PD/AG). Thus, variations in HTR1A might contribute to neurophysiological differences within subgroups of PD/AG patients. Here, we tested this hypothesis by combining genetic with behavioural techniques and neuroimaging. In a clinical multicentre trial, patients with PD/AG received 12 sessions of manualized cognitive-behavioural therapy (CBT) and were genotyped for HTR1A rs6295. In four subsamples of this multicentre trial, exposure behaviour (n=185), defensive reactivity measured using a behavioural avoidance test (BAT; before CBT: n=245; after CBT: n=171) and functional magnetic resonance imaging (fMRI) data during fear conditioning were acquired before and after CBT (n=39). HTR1A risk genotype (GG) carriers more often escaped during the BAT before treatment. Exploratory fMRI results suggest increased activation of the amygdala in response to threat as well as safety cues before and after treatment in GG carriers. Furthermore, GG carriers demonstrated reduced effects of CBT on differential conditioning in regions including the bilateral insulae and the anterior cingulate cortex. Finally, risk genotype carriers demonstrated reduced self-initiated exposure behaviour to aversive situations. This study demonstrates the effect of HTR1A variation on defensive behaviour, amygdala activity, CBT-induced neural plasticity and normalization of defence behaviour in PD/AG. Our results, therefore, translate evidence from animal studies to humans and suggest a central role for HTR1A in differentiating subgroups of patients with anxiety disorders.
Introduction
Albeit animal studies showed genetic modulation of fearful behaviour by the serotonin receptor 1a gene (Htr1a), translational approaches towards anxiety disorders are missing. The present study aimed to close this gap by investigating behavioural and neural consequences of HTR1A variation in panic disorder with agoraphobia (PD/AG).
In rodents, disruption of Htr1a has been linked to increased defensive behaviour,1, 2, 3, 4 particularly with regard to ambiguous, potential threat indicating stimuli.5, 6 In these studies, ambiguous cues have been created, for example, by combining unaffected tactile and olfactory cues with spatial cues that were already present in a context in which a fear-conditioning procedure was previously conducted. During that fear training, knockout (KO) mice showed significantly more freezing behaviour than the wild-type mice and, more important, the freezing behaviour was comparably high in the case of ambiguous stimuli in KO mice whereas the freezing behaviour decreased during the test as compared with the conditioning period in wild-type mice.5 Generalization of fear from fearful to neutral or safety signals has been described as a potential mechanism in PD with or without AG.7, 8, 9 Thus, variation in HTR1A might be relevant for the etiopathogenesis of PD/AG.10 The G allele of HTR1A rs6295 has been proposed to convey risk for the development of PD/AG.11, 12, 13, 14 However, despite strong evidence for the role of HTR1A in fear processing and PD/AG, the mechanisms underlying altered behavioural and neural responses are largely unknown.
The 5-HT1A receptor acts as a presynaptic inhibitory auto- and postsynaptic heteroreceptor mediating serotonin regulation.10 rs6295, in the transcriptional control region of HTR1A (−1019C/G), modulates the expression of 5-HT1A receptors and hence auto-inhibitory feedback on the presynaptic serotonergic neuron. While the G allele increases receptor expression at the presynapse and thereby reduces serotonergic neurotransmission due to enhanced auto-inhibitory feedback, it also reduces the expression of postsynaptic 5-HT1A leading to an overall reduction in serotonergic neurotransmission,15 especially in neuronal structures characterized by postsynaptic 5-HT1A heteroreceptors such as frontal cortex, hippocampus and amygdala.16
Elevated defensive behaviours including escape and avoidance have been demonstrated in Htr1a KO mice,2, 16 and are also important characteristics of patients with PD/AG.17 Healthy subjects show shortened reaction times during the anticipation of threat stimuli if carrying the rs6295 GG genotype,18 probably as a result of sensitized neural circuits predisposing to enhanced processing of fear stimuli. Furthermore, in healthy subjects, a reduced amygdala activity has been observed in GG homozygotes during face processing (face > shapes19), which could reflect an inhibition process. However, in PD/AG patients we recently observed distinct defensive behaviours depending upon threat imminence.17 During a standardized behavioural avoidance test (BAT), acute panic and associated escape behaviour was accompanied by intense autonomic mobilization, previously associated with imminent threat processing.20 Variation across patients in escape behaviour during the BAT17 could be partly explained by a hitherto unidentified genetic predisposition regarding the serotonergic system, for example, in HTR1A.
In addition to defence mechanisms, PD/AG was linked to aberrant fear conditioning, overgeneralization of fear21, 22, 23 and dysfunction of related neural networks.9, 24, 25, 26, 27 Findings in anxiety disorders paralleled increased fear conditioning found in Htr1a KO mice mediated by hippocampus and amygdala.5, 6 The neural network implicated in fear conditioning28, 29, 30 overlaps with brain regions that are affected by 5-HT1A-mediated serotonergic neurotransmission (specifically amygdala,31 PAG and ACC32). However, the effect of genetic variations in HTR1A on the neural correlates of fear conditioning in PD/AG is unknown.
With regard to treatment, cognitive-behavioural therapy (CBT) has proven its efficacy for most mental disorders, and particularly PD.33, 34, 35 More recently, neurofunctional brain changes related to psychotherapy, particularly CBT, have been investigated.27, 36, 37, 38, 39 However, despite first evidence indicates that specific genetic polymorphisms may contribute to CBT outcome and changes on the neural and behavioural level,40, 41, 42, 43, 44 the effect of variation of HTR1A on changes in context of psychotherapeutic interventions are unknown. Considering, however, the converging evidence suggesting a central role of HTR1A for fear processing, it is likely that variation in HTR1A contributes to CBT effects in PD/AG.
In summary, animal studies have demonstrated that reduced Htr1a expression goes along with a bias towards threat stimuli predominantly mediated by hippocampus and amygdala. Variations in HTR1A might be of relevance to PD/AG, as increased defence reactivity and an overgeneralization of conditioned fear is an important mechanism in this disorder. rs6295 GG genotype—going along with reduced serotonergic tone in frontal cortex, amygdala and hippocampus—has been associated with PD/AG. Deviations on the functional level, that is, defence reactivity and fear conditioning and effects of exposure-based CBT, might thus be influenced by rs6295. To test this empirically, we used a multilevel strategy to link HTR1A genotype to behaviour, neurofunctional activation and its changes in the course of cognitive-behavioural therapy, respectively. We hypothesized that the rs6295 GG genotype (a) facilitates escape behaviour during the BAT, (b) goes along with increased fear responses reflected by enhanced amygdala activity towards not fully predictive conditioned stimuli (CS+ and CS− during early acquisition where initial pairings of unconditioned stimulus and CS occur) and (c) reduced effects of CBT on neural correlates of fear conditioning and behavioural defence reactivity.
Materials and methods
Participants
All patients with PD/AG investigated in this study participated in the Mechanism of Action in CBT study (see Table 1, Supplementary Figure S1) that has been described in detail earlier.27 Inclusion criteria were: (a) a current primary diagnosis of PD/AG; (b) a clinical interview score >18 on the structured interview for the Hamilton anxiety scale (SIGH-A in anxiety and depression); (c) a score >4 on the clinical global impressions scale; (d) an age of 18–65 years; and (e) the ability and availability to regularly attend treatment sessions.35, 45 Exclusion criteria were (a) comorbid DSM-IV-TR psychotic or bipolar I disorder; (b) current alcohol dependence/current abuse or dependence on benzodiazepine and other psychoactive substances; (c) current suicidal intent; (d) borderline personality disorder; (e) concurrent ongoing psychotherapeutic or psychopharmacological treatment for PD/AG or another mental disorder; (f) antidepressant or anxiolytic pharmacotherapy; and (g) physician-verified contraindications of exposure-based CBT (that is, severe cardiovascular, renal or neurological diseases).45 Additional exclusion criteria were applied to fMRI subjects: cardiac pacemaker, ferromagnetic metal implants, tattoos or permanent make-up with ferromagnetic colours.
Table 1. Demographic and clinical characteristics of the fMRI and BAT samples according to rs6295 (−1019C/G HTR1A) genotype.
|
Genotype |
Differences (CC vs GG) | ||||
|---|---|---|---|---|---|
| CC | CG | GG | |||
| Genetic-BAT-sample (N=245) | |||||
| N | 60 | 120 | 65 | χ2/F | P |
| Female (n (%)) | 42 (70.21) | 82 (68.33) | 54 (83.08) | 2.99a | 0.08 |
| Age (years) | 36.38 (10.88) | 35.58 (11.50) | 35.46 (10.23) | 0.24 | 0.63 |
| Clinical characteristics at baseline | |||||
| SIGH-A total | 24.53 (5.04) | 23.71 (5.23) | 24.46 (5.55) | 0.01 | 0.94 |
| PAS total | 26.96 (9.64) | 26.14 (9.96) | 28.23 (9.33) | 0.57 | 0.45 |
| CGI | 5.17 (0.74) | 5.18 (0.72) | 5.29 (0.61) | 1.09 | 0.30 |
| ASI total | 31.15 (9.96) | 30.51 (11.60) | 32.28 (12.61) | 0.31 | 0.58 |
| BDI II total | 16.19 (8.82) | 16.56 (8.24) | 15.87 (9.02) | 0.04 | 0.84 |
| MI7 | 2.07 (0.92) | 1.93 (0.99) | 1.92 (0.99) | 0.69 | 0.41 |
|
Genotype |
Differences (CC/CG vs GG) | ||||
|---|---|---|---|---|---|
| CC | CG | GG | |||
| Genetic-BAT-treatment-sample (N=171) | |||||
| N | 43 | 85 | 43 | χ2/F | P |
| Female (n (%)) | 29 (67.44) | 57 (67.06) | 34 (79.07) | 2.17a | 0.34 |
| Age (years) | 36.28 (11.31) | 36.60 (12.09) | 33.28 (9.40) | 1.31 | 0.27 |
| Clinical characteristics at baseline | |||||
| SIGH-A total | 25.09 (5.37) | 23.62 (5.07) | 25.19 (5.76) | 1.74 | 0.18 |
| PAS total | 26.39 (9.18) | 25.78 (9.85) | 29.33 (9.37) | 2.04 | 0.13 |
| CGI | 5.26 (0.76) | 5.16 (0.72) | 5.28 (0.63) | 0.46 | 0.63 |
| ASI total | 32.81 (9.95) | 30.72 (11.35) | 33.12 (12.62) | 0.84 | 0.43 |
| BDI II total | 16.67 (9.00) | 16.34 (8.56) | 15.51 (9.14) | 0.21 | 0.81 |
| MI7 | 1.97 (0.89) | 1.83 (0.88) | 1.87 (0.89) | 0.35 | 0.70 |
| Clinical characteristics at post-treatment | |||||
| SIGH-A total | 13.65 (7.89) | 11.78 (7.57) | 12.60 (6.90) | 0.89 | 0.41 |
| PAS total | 13.68 (8.93) | 14.21 (9.52) | 14.18 (8.14) | 0.05 | 0.95 |
| CGI | 3.42 (0.85) | 3.46 (1.11) | 3.40 (1.00) | 0.06 | 0.94 |
| ASI total | 17.23 (10.36) | 15.86 (10.42) | 16.47 (10.04) | 0.26 | 0.77 |
| BDI II total | 8.49 (8.36) | 8.62 (7.99) | 8.44 (8.26) | 0.01 | 0.99 |
| MI7 | 1.53 (0.68) | 1.29 (0.49) | 1.47 (0.68) | 2.43 | 0.09 |
|
Genotype |
Differences (CC vs GG) | ||||
|---|---|---|---|---|---|
| CC | CG | GG | |||
| Genetic-fMRI-treatment-sample (N=39) | |||||
| N | 9 | 21 | 9 | F | P |
| Female (n (%)) | 7 (77.78) | 14 (66.67) | 5 (55.56) | 1.00a | 0.62 |
| Age (years) | 30.11 (11.47) | 37.67 (10.01) | 36.11 (7.57) | 1.76 | 0.20 |
| Clinical characteristics at baseline | |||||
| SIGH-A total | 22.89 (4.68) | 23.62 (5.34) | 26.22 (5.93) | 1.75 | 0.20 |
| PAS total | 22.99 (6.72) | 24.19 (9.33) | 31.90 (7.80) | 6.74 | 0.02 |
| CGI | 5.00 (0.71) | 5.38 (0.59) | 5.67 (0.50) | 5.33 | 0.04 |
| ASI total | 30.44 (6.50) | 29.14 (9.08) | 36.00 (11.41) | 1.61 | 0.22 |
| BDI II total | 14.00 (7.63) | 16.05 (7.40) | 18.67 (11.51) | 1.03 | 0.33 |
| MI7 | 1.84 (0.71) | 1.84 (0.93) | 1.65 (1.00) | 0.22 | 0.65 |
| Clinical characteristics at post-treatment | |||||
| SIGH-A total | 9.78 (3.99) | 12.38 (6.66) | 13.44 (9.38) | 1.16 | 0.29 |
| PAS total | 9.15 (5.28) | 13.57 (8.96) | 18.54 (8.79) | 7.54 | 0.01 |
| CGI | 3.22 (0.97) | 3.62 (1.24) | 3.78 (0.67) | 2.00 | 0.18 |
| ASI total | 13.00 (7.81) | 15.05 (7.41) | 19.11 (11.94) | 1.65 | 0.22 |
| BDI II total | 4.78 (6.40) | 9.67 (6.16) | 9.89 (11.17) | 1.42 | 0.25 |
| MI7 | 1.45 (0.71) | 1.24 (0.38) | 1.20 (0.41) | 0.82 | 0.38 |
Abbreviations: ASI, Anxiety Sensitivity Index; BDI II, Beck Depression Inventory II; CGI, Clinical Global Impressions Scale; PAS, Panic and Agoraphobia Scale; MI7, 7-day version of the Movement Inventory (accompanied); SIGH-A, Hamilton Anxiety Scale.
Pearson's Chi-square.
Means (s.d.) except where noted. Due to missing values, MI7 scores were available in the BAT total sample only in 229 patients (CC: 57, CG: 112, GG: 60) and in the BAT treatment group sample only in 160 patients (CC: 41, CG: 80, GG: 39).
Eight treatment centres in Germany participated in the clinical multicentre trial including BAT procedure as part of the baseline diagnostics (Aachen, Berlin-Adlershof, Berlin-Charité, Bremen, Dresden, Greifswald, Münster, Würzburg). In the study, exposure-based CBT was administered in 12 twice-weekly sessions based on a highly standardized and controlled treatment protocol.35, 45 The treatment procedure was shown to be highly effective.35
In total, n=369 patients were enrolled in the clinical study.35 Here, we refer to four different subgroups of this clinical sample to investigate genotype effects on (1) exposure behaviour, (2) on BAT before and (3) after CBT, as well as (4) on the neural correlates of fear conditioning (see Supplementary Figure 1 and ref. 40 for further details).
Exposure sample
For the investigation of genotype effects on exposure behaviour, data of 184 patients were available (CC=45; CG=91; GG=48).
BAT t1 sample
In total, 364 patients performed the BAT. From 306 patients, who entered the BAT box and were not re-randomized from the waiting list group, blood samples were available in 245 patients (CC=60; CG=120; GG=65).
BAT t2 sample
Of the 245 patients from the BAT t1 sample, 171 were randomized to one of two active treatment conditions35, 45 and also repeated BAT during post-assessment (CC=43; CG=85; GG=43).
fMRI sample
In total, 89 patients took part in the neuroimaging study, because only four (Aachen, Berlin, Dresden and Münster) of the eight treatment centres had fMRI technique assessable. Quality-controlled fMRI data were available before and after CBT from 42 patients. Blood samples for genotyping were obtained from 39 of these 42 patients (CC=9; CG=21; GG=9).
Clinical and demographic data of the BAT and fMRI subcohorts are comparable to the scores of the whole sample (n=369) of the clinical trial (compare Table 1 with Gloster et al.35, 45).
Genotyping of rs6295 (HTR1A −1019C/G)
Genomic DNA was extracted from blood by using a standard de-salting procedure. A 163-bp fragment was amplified by polymerase chain reaction (PCR). The PCR reaction mix included 25 ng of genomic DNA in 2.1 μl Gold Star buffer, 25 mM MgCl2, 2.5 mM of each nucleotide, 10 μM of each forward and modifying primer and 0.5 μl of Taq polymerase. Primer sequences were 5′-GGAAGAAGACCGAGTGTGTCAT-3′ and 5′-GGCTGGACTGTTAGATGATAACG-3′. After an initial denaturation step for 5 min at 95 °C, 38 cycles of denaturating at 95 °C for 30 s, annealing at 59.5 °C for 40 s and extension at 72 °C for 50 s were performed, followed by a final extension step at 72 °C for 5 min. PCR products were digested with BseGI and visualized on a 5% agarose gel containing ethidium bromide.
The rs6295 genotype groups did not significantly differ in age, gender and clinical characteristics between the different subsamples (see Table 1). Genotypes in the total cohort, the BAT and fMRI subcohort did not deviate from Hardy–Weinberg equilibrium (P>0.2).
Treatment intervention
For detailed information of the clinical and treatment aspects of the study, please see Gloster et al.35, 45 and Straube et al.46 Sessions 1–3 consisted of psychoeducation and an individualized behavioural analysis of the patient's symptoms and coping behaviours. Sessions 4–5 provided the treatment rational for exposure and implemented interoceptive exposure exercises in the therapy room identically for both groups. Sessions 6–8 consisted of standardized in situ exposure exercises (bus, shopping mall and forest), which were implemented after the patient agreed to enter the situation without engaging in safety behaviours and waiting for the anxiety to take its natural course. Session 9 reviewed progress to date and addressed anticipatory anxiety. Sessions 10–11 again consisted of in situ exposures but now targeted the patients' two most significant feared situations. Session 12 repeated crucial elements of the manual and instructed patients to continue exposing themselves to feared situations. Since effects of genotype were expected specifically on exposure behaviour, data of the exposure sessions (Sessions 6–8 and 10–11), where patients where specifically motivated to do exposure homework, had been collapsed for respective analyses (see below; and Gloster et al.35 for an identical approach).
Behavioural avoidance test (BAT)
BAT procedure is described in detail elsewhere.17 Briefly, patients were instructed first to sit in front of an open test chamber (75 × 120 × 190 cm) while defensive reactivity during anticipation of the upcoming exposure was measured (for 10 min). Afterwards, patients were asked to sit in the dark and locked chamber as long as possible (maximum 10 min). Stopping exposure in the test chamber was always possible. Defensive reactivity was measured by self-reports of anxiety on a visual analogue scale, and by observable behaviour (premature escaping behaviour during exposure). Defensive reactivity during anticipation and exposure was analysed as a function of rs6295 HTR1A genotype.
fMRI
Parallel versions of a previously validated differential conditioning paradigm were applied during fMRI data acquisition (Figure 1, details in Reinhardt et al.30) before and after CBT (see Kircher et al.27 for methodological details). The fMRI brain images were acquired using a 3T Philips Achieva (Muenster and Aachen, Germany), a 3T Siemens Trio (Dresden, Germany) and a 3T General Electric Healthcare (Berlin, Germany) scanner (for acquisition parameters see Kircher et al.27). MR images were analysed using standard procedures of the software Statistical Parametric Mapping (SPM5; www.fil.ion.ucl.ac.uk) implemented in MATLAB 7.1 (the Mathworks, Sherborn, MA, USA).
Figure 1.
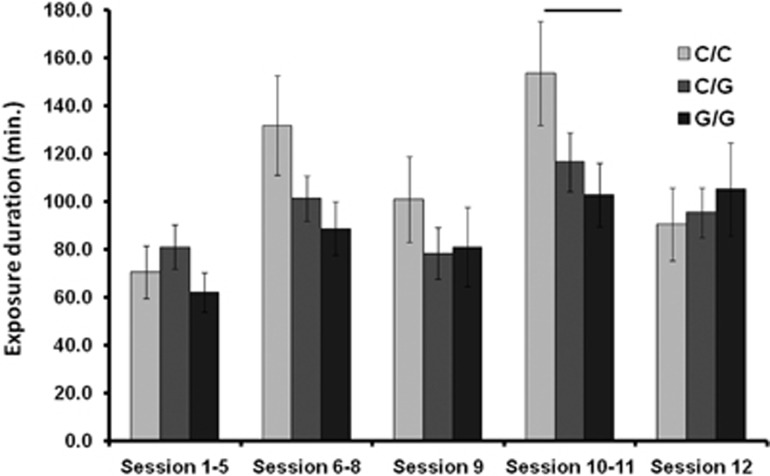
Exposure behaviour. Every CBT session patients were asked how long they exposed their self to an anxiety-related situation. HTR1A CC genotype (light grey; n=45) in contrast to GG (dark grey; n=48) genotype carriers reported longer exposure times, especially during later sessions of CBT (session 10–11). The significant difference (P>0.05) between bars is illustrated by a black line. Thus, the comparable clinical outcome between groups might be a result of different exposure behaviour as a specific mechanism of CBT. CBT, cognitive-behavioural therapy.
At the single-subject level, the realignment parameters of each patient were included as regressors into the model to account for movement artefacts. The BOLD response for each event type (CS+paired, CS+unpaired, CS−, unconditioned stimulus) and each phase (familiarization phase (F): early (F1) and late (F2); acquisition phase (A): early (A1) and late (A2); extinction phase: early (E1) and late (E2)) was modelled by the canonical haemodynamic response function used by SPM5 within the framework of the general linear model to analyse brain activation differences related to the onset of the different stimuli.27 Parameter estimates (β−) and t-statistic images were calculated for each subject.
Group analyses were performed by entering contrast images into flexible factorial analyses as implemented in SPM5, in which subjects are treated as random variables. The fMRI centre was introduced as a covariate to account for scanner differences. To investigate the influence of rs6295 on neural activity, we compared the genetic subgroups during the processing of CS+unpaired and CS− in the early acquisition phase of the fear-conditioning paradigm (where the most pronounced effects and the neural plasticity induced by CBT in PD/AG were detected, see Kircher et al.27). Analyses were performed by contrasting the extreme groups of the three genetic subgroups GG (n=9), CG (21) and CC (n=9). Due to the small sample size, these analyses should be considered as preliminary. To explore general effect of genotype on the neural processing of not fully conditioned stimuli in the early acquisition phase, the genotype main effect (GG>CC) independent of time point (t1/t2) and stimulus type (CS+/CS−) had been calculated. To test for genotype-specific effects on CBT-related changes, interaction analyses had been performed (GG/CC × t1/t2 × CS+/CS−).
The identical cluster threshold of at least 142 voxels at SPM significance level of P<0.005 uncorrected (based on a Monte Carlo simulation for correction of multiple testing47), as in previous investigations of this multicentre trial has been applied.9, 27, 40 For the anatomical localization, functional data were referenced to probabilistic cytoarchitectonic maps48 and the AAL toolbox.49
Results
Clinical characteristics
There was no significant effect of genotype on baseline characteristics (t1) and post-treatment characteristics (t2) in the BAT and fMRI samples (see Table 1).
Despite absence of effects on primary clinical outcome variables, we found variation in HTR1A (GG vs CC) to be related to differences in exposure behaviour during CBT (interaction effect of HTR1A × CBT session: F(1,91)=3.976, P<0.05), indicating that CC in contrast to GG homozygotes performed more exposure on their own during later exposure sessions of therapy; specifically during the exposure sessions 10 and 11 (CC>GG, t91=2.025, P<0.05; linear effect CC>CG>GG: F(1,181)=4.203; P<0.05, see Figure 1). Importantly variation in HTR1A is not related (P>0.2) to treatment variants (therapist vs self-guided exposure), which has been previously shown to be related to exposure behaviour35 and the neural correlates of conditioning.50
Behavioural avoidance test
Effect of HTR1A
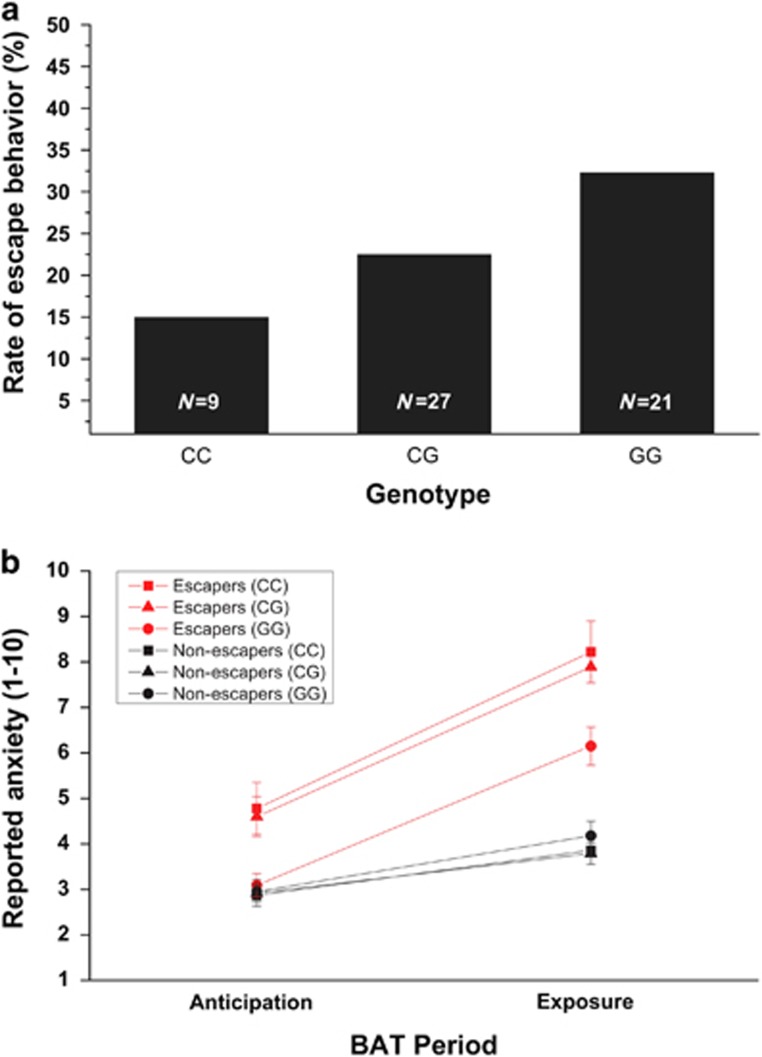
Risk genotype was significantly associated with acute flight behaviour before therapy (t1): GG genotype carriers escaped more often during the exposure to the test chamber as compared with CC carriers (χ2=5.12, P<0.05; see Figure 2a). Univariate analysis of variance with genotype (GG carriers vs CC carriers) and behaviour (escapers vs non-escapers) as between-subjects variables revealed significant interaction effects between genotype and behaviour on reported anxiety during anticipation period (F(1,121)=5.42, P<0.05) and exposure period (F(1,121)=6.40, P<0.05). Post hoc analysis displayed that CC carriers who showed escaping behaviour during the exposure already reported significantly more anticipatory anxiety as compared with non-escaping patients at the anticipation period (behaviour F(1,58)=8.57, P<0.01) while anticipatory anxiety between escaping and non-escaping G allele homozygotes was comparable (behaviour F(1,63)=0.11, P=0.75; see Figure 2b) suggesting that pronounced self-reported anticipatory anxiety preceded escape behaviour only if carrying the CC gene variant. During exposure, reported anxiety was significantly increased in escaping patients as compared with non-escaping patients in both, C allele (behaviour F(1,58)=31.03, P<0.001) and G allele homozygotes (behaviour F(1,63)=12.88, P<0.01). However, escaping CC carriers reported significantly higher anxiety than G allele homozygous escapers (genotype F(1,28)=6.96, P<0.05) while no significant difference between genotypes was observed in non-escaping patients (genotype F(1,93)=0.53, P=0.47; see Figure 2b).
Figure 2.
BAT baseline assessment. Rate of escaping behaviour during exposure period ((a) GG=32% (n=21 of 65); CG=23% (n=27 of 120); CC=15% (n=9 of 60)) and (b) means and s.e. of reported anxiety during anticipation and exposure period as a function of rs6295 [C(−1019)G] genotype and defensive behaviour in 245 PD/AG patients during baseline assessment before therapy. BAT, behavioural avoidance test; PD/AG, panic disorder/agoraphobia.
Effect of CBT
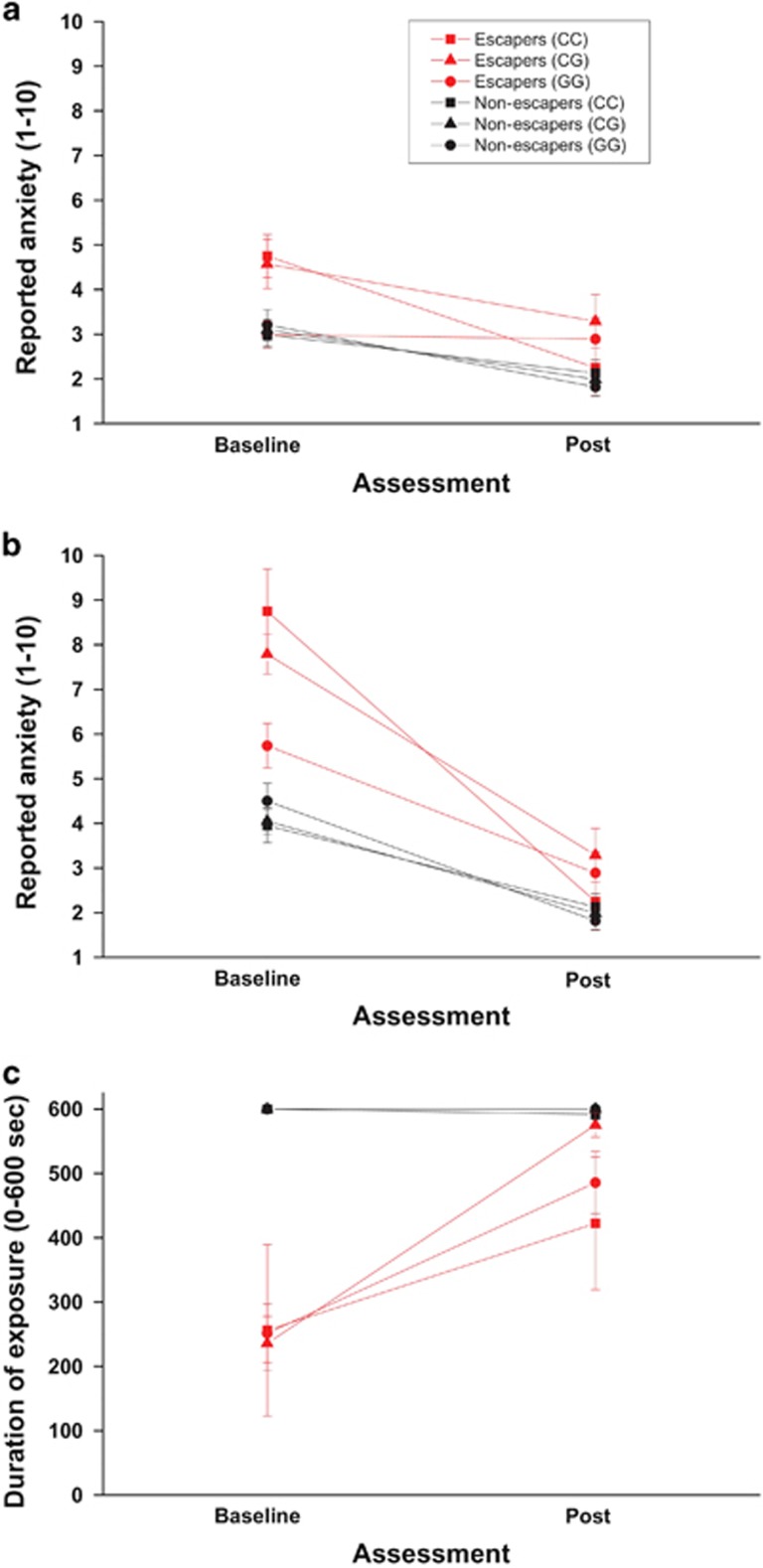
Of those 43 patients who were included in the following analyses and who were carrying the CC genotype variant, only four patients showed escape behaviour during t1 disallowing to conduct planned analyses. Since CC and CG genotype carriers did not differ in any of the performed analyses below (see Supplementary Material), we collapsed both groups for the following analyses. Univariate analysis of variance with genotype (GG carriers vs C carriers) and behaviour (escapers vs non-escapers) as between-subjects variables and time (t1 vs t2) as within-subject variable revealed significant interaction effects between genotype, behaviour and time on anxiety during anticipation period (F(1,167)=6.34, P<0.05) and exposure period (F(1,167)=10.14, P<0.05), and exposure duration (F(1,167)=3.84, P=0.05) observed in those 171 patients who obtained active treatment. Post hoc analyses displayed significant larger fear reductions from t1 to t2 in pretreatment BAT escapers carrying the C allele during both, anticipation period (time × genotype F(1,31)=6.91, P<0.05; see Figure 3a) and exposure period (time × genotype F(1,31)=8.18, P<0.01; see Figure 3b). In contrast, no significant genotype effect on fear reduction in pretreatment BAT non-escaping patients was observed (anticipation period: time × genotype F(1,136)=0.24, P=0.62; exposure period: time × genotype F(1,136)=2.27, P=0.13). As a result, initial differences in reported anxiety depending on genotype in escaping patients during t1 (anticipation: genotype × behaviour F(1,167)=6.26, P<0.05, post hoc escapers: genotype F(1,31)=8.53, P<0.01, post hoc completers: genotype F(1,136)=0.17, P=0.68; exposure: genotype × behaviour F(1,167)=8.92, P<0.01, post hoc escapers: genotype F(1,31)=12.72, P=0.001, post hoc completers: genotype F(1,136)=0.97, P=0.33) were no longer observable during t2 (anticipation period: genotype F(1,167)=0.13, P=0.72; genotype × pretreatment behaviour F(1,167)=0.10, P=0.75; exposure period: genotype F(1,167)=0.34, P=0.56; genotype × pretreatment behaviour F(1,167)=0.01, P=0.93). In line with the results above, no significant differences between genotype in the frequency of escape behaviour were observed during t2 (CC/CG: N=5, 3.9% GG: N=5, 11.6% exact Fisher's P=0.12).
Figure 3.
BAT baseline to post-assessment. Means and s.e. of reported anxiety during anticipation period (a) and exposure period (b) and of tolerated duration of exposure during baseline and post-assessment (c) in 171 PD/AG patients randomized to one of two active treatment groups. BAT, behavioural avoidance test; PD/AG, panic disorder/agoraphobia.
fMRI results
Effect of HTR1A
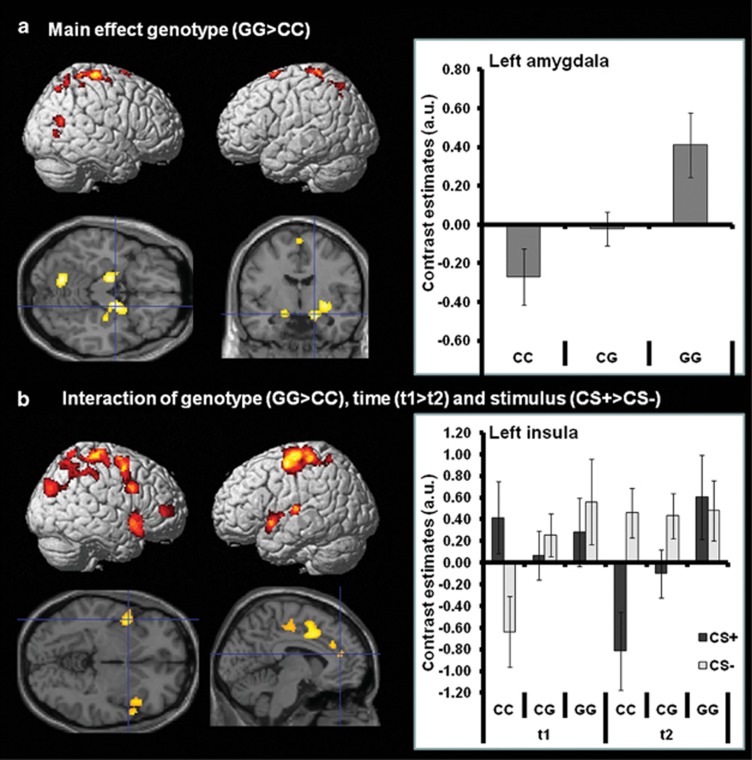
The main effect of genotype (GG>CC) for the processing of CS+unpaired and CS− during early acquisition phase of the conditioning paradigm baseline (t1) and post-assessment (t2) revealed activity in the bilateral amygdalae, hippocampi as well as distributed regions including predominantly parietal, temporal and cerebellar structures (see Figure 4a; Table 2). Risk genotype carriers (GG; N=9) in contrast to CC genotype carriers generally demonstrated higher activity in these regions independent of time point or stimulus type. Bar graphs in Figure 4a illustrate the contrast estimates for the activity in the left amygdala. Contrast estimates for all other activation clusters demonstrate a similar pattern of increased activity in GG carriers independent of measurement point.
Figure 4.
Main effects and interactions of rs6295 (−1019C/G HTR1A) during early fear acquisition in patients with PD/AG. (a) Main effect of genotype (GG>CC) for the processing of CS+unpaired and CS− during early acquisition phase of the conditioning paradigm at pre- (t1) and post-treatment (t2). Risk type carriers (GG) generally demonstrated more activity in the illustrated regions independent of time point or stimulus type. Bar graphs illustrate the contrast estimates for the activity in the left amygdala (collapsed across CS+unpaired and CS− at t1 and t2; the cluster was restricted to the amygdala using a ROI defined by the anatomy toolbox of SPM.48, 59 Cluster extension: 272 voxels). Contrast estimates for all other activation clusters demonstrate a similar pattern of increased activity in the GG group. (b) Interaction of genotype, the processing of CS+unpaired vs CS− during early acquisition phase and pre- (t1) vs post-treatment effects (t2). Bar graphs illustrate the contrast estimates for the activity in the left insula (whole cluster: 330 voxels). Contrast estimates for all other activation clusters demonstrate similar patterns. Risk type carriers demonstrated relatively stable activity in the illustrated regions independent of time point or stimulus type. By contrast, patients with the protective genotype (CC) showed a reduced activation for the CS+unpaired after treatment and an opposite effect for the CS−. For coordinates and statistics, see Table 2. CS, conditioned stimulus; PD/AG, panic disorder/agoraphobia; ROI, region of interest; SPM, statistical parametric mapping.
Table 2. fMRI results (coordinates and statistics).
| Contrast/region | Cluster extensions/submaxima | x | y | z | t-value | P uncorrected | Cluster size |
|---|---|---|---|---|---|---|---|
| Main effect: GG>CC | |||||||
| Right Amy/HC | Amy (SF, 69.7% CM, 80.3%), HC (CA, 8.5%) | 18 | −6 | −16 | 4.05 | <0.001 | 837 |
| Right putamen | 30 | −8 | −6 | 3.54 | <0.001 | ||
| Right insula | 32 | −18 | 20 | 3.51 | <0.001 | ||
| Left SPL | −18 | −64 | 54 | 4.01 | <0.001 | 865 | |
| Left postcentral gyrus | −18 | −40 | 72 | 3.69 | <0.001 | ||
| Right postcentral gyrus | Right postcentral gyrus | 32 | −38 | 52 | 3.80 | <0.001 | 802 |
| Right precentral gyrus | 28 | −26 | 68 | 3.53 | <0.001 | ||
| Right calcarine gyrus | 16 | −58 | 12 | 3.44 | <0.001 | 695 | |
| Right precuneus | 18 | −54 | 16 | 3.26 | 0.001 | ||
| Right thalamus | 8 | −14 | 24 | 3.80 | <0.001 | 460 | |
| Right SPL | 24 | −66 | 52 | 3.20 | 0.001 | 303 | |
| Right cuneus | 18 | −76 | 38 | 3.17 | 0.001 | ||
| Left HC/Amy | Amy (SF, 31.5%), HC (CA, 7.4% FD, 13.3%) | −14 | −12 | −14 | 3.81 | <0.001 | 279 |
| Left HC | −28 | −20 | −12 | 3.30 | 0.001 | ||
| Left SMA | BA 6 | −6 | 10 | 70 | 3.56 | <0.001 | 223 |
| Right SMA | 2 | 0 | 66 | 2.94 | 0.002 | ||
| Thalamus | −20 | −14 | 8 | 3.17 | 0.001 | 202 | |
| Left insula | −34 | −20 | 4 | 3.06 | 0.001 | ||
| Left cerebellum | −12 | −68 | −16 | 3.74 | <0.001 | 143 | |
| Interaction: genotype (CC>GG) × time (t1>t2) × stimulus (CS+unpaired>CS−) | |||||||
| Left precentral gyrus | −38 | −12 | 58 | 4.37 | <0.001 | 4471 | |
| Right SMA | 8 | 6 | 60 | 4.36 | <0.001 | ||
| Left precentral gyrus | −28 | −18 | 72 | 4.18 | <0.001 | ||
| Right middle occipital gyrus | 30 | −74 | 30 | 4.34 | <0.001 | 2360 | |
| Right postcentral gyrus | 34 | −32 | 68 | 3.94 | <0.001 | ||
| Right precentral gyrus | 30 | −28 | 74 | 3.94 | <0.001 | ||
| Right temporal pole | 54 | 18 | −16 | 3.69 | <0.001 | 455 | |
| Right temporal pole | 60 | 14 | −4 | 3.17 | 0.001 | ||
| Right insula | 46 | 18 | −4 | 2.99 | 0.002 | ||
| Left insula | −46 | 8 | −4 | 3.47 | <0.001 | 330 | |
| Left temporal pole | −54 | 10 | −10 | 3.22 | 0.001 | ||
| Left IFG (pars opercularis) | −40 | 8 | 8 | 2.93 | 0.002 | ||
| Right MFG | 48 | 48 | 6 | 3.71 | <0.001 | 149 | |
| Right MFG | 40 | 56 | 8 | 3.12 | 0.001 | ||
| Left ACC | −10 | 34 | 26 | 3.18 | 0.001 | 144 | |
| Left superior medial gyrus | −2 | 32 | 34 | 2.98 | 0.002 | ||
| Left ACC | −6 | 42 | 18 | 2.86 | 0.002 | ||
| Left STG | −52 | −18 | 10 | 3.10 | 0.001 | 142 | |
Abbreviations: ACC, anterior cingulate gyrus; Amy, amygdala; CA, cornu amonis; CM, centromedial group; FD, fascicular dentata; HC, hippocampus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SF, superficial group; SPL, superior parietal lobe; STG, superior temporal gyrus.
Significance level, t-values, uncorrected P-value and the size of the respective cluster (voxels) at P<0.05, corrected (MC), were mentioned. Coordinates are listed in MNI atlas space. Contrasts are named in italic letters. Cluster extensions denominate activated regions for larger voxel clusters encompassing different brain areas and should be considered approximate. Anatomical regions have been defined by the anatomy toolbox of statistical parametric mapping.48, 59
Effect of CBT
The interaction of genotype (GG<CC), processing of CS+unpaired vs CS− during early acquisition phase and baseline (t1) vs post-assessment effects (t2) revealed activation in the bilateral insulae, the middle cingulate cortex and distributed regions of the parietal and occipital lobe (see Figure 4b, Table 2). Bar graphs in Figure 4b illustrate the contrast estimates for the activity in the left insula. Contrast estimates for all other activation clusters show similar patterns. Risk genotype carriers demonstrated relatively stable activity in the illustrated regions independent of time point or stimulus type. By contrast, patients with the protective genotype (CC; N=9) showed a reduced activation for the CS+unpaired after treatment and an opposite effect for the CS−.
Exploratory correlation analyses were performed to reveal the association of BAT anxiety ratings, genotype and fMRI activity. While amygdala activity was correlated with numbers of G alleles (left amygdala: r=0.450, P=0.004 uncorrected, P=0.036 corrected for multiple comparisons; right amygdala: r=0.513, P=0.001 uncorrected, P=0.008 corrected for multiple comparisons), no association between amygdala activity and anxiety ratings from anticipation and exposure phase of the BAT task could be observed (for all P>0.20). For differential conditioning (CS+unpaired>CS−), the right insula correlated negatively with anxiety ratings during the anticipation of BAT exposure before treatment (r=−0.344, P=0.032 uncorrected, P=0.324 corrected for multiple comparisons). Activation change (t2−t1) for the differential conditioning (CS+unpaired>CS−) in the right insula was positively correlated with the number of G alleles (r=0.404, P=0.011 uncorrected, P=0.099 corrected for multiple comparisons) and negatively correlated with changes in the anxiety reports during BAT exposure after CBT (t2−t1; r=−0.339, P=0.035 uncorrected, P=0.315 corrected for multiple comparisons).
Discussion
The rationale of this study was built upon conclusive evidence from animal research suggesting that lack of Htr1a in hippocampus and amygdala neurons leads to increased fear response to ambiguous stimuli.5, 6 Thus, genetic variation in human HTR1A should also be of relevance for the pathophysiology of PD/AG, as generalization of fear to ambiguous or even safety signals is an important aetiological mechanism for the disorder.7, 9 In translating evidence from rodent models to humans with PD/AG, we found that HTR1A rs6295 risk genotype (GG) carriers display increased threat-related defensive reactivity (escape behaviour) during BAT and increased amygdala activity—measured with fMRI—for both threat as well as safety cues during fear conditioning. Both behavioural styles can be interpreted as increased fear-related flight behaviour in response to ambiguous cues, just as observed in Htr1a knockout mice. In contrast, we found the CC allele carriers to be associated with pronounced decreases of defensive response during the BAT as well as neurofunctional changes with regard to differential conditioning activity after 12 sessions of CBT.27, 35 Despite these differences, both groups demonstrated clinical improvement. However, these might be obtained by different components of CBT as indicated by increased exposure behaviour in CC genotype carriers. Synthesizing this data, we argue that HTR1A genotype contributes to predisposing a patient to preferentially utilize different neural pathways of fear (supported by escape behaviour and amygdala activity in GG carriers and subjective anxiety and CBT effects on fear conditioning in C allele carriers). Our data suggest that there are neurogenetic subgroups of PD/AG patients and, depending on genotype, CBT may act upon different pathways of fear. These findings might be useful in the future for informing clinical decisions regarding CBT treatment.
In line with the hypothesis that the GG genotype of HTR1A should facilitate flight behaviour, GG homozygotes more often escaped from a small, dark and closed test chamber during a standardized BAT. Extensive animal research suggests that defensive reactivity is dynamically organized as a function of threat proximity51, 52 resulting in different patterns of defensive reactions, for example, increased autonomic arousal, and related brain circuit activation. In the case of imminent threat, the dorsal periaqueductal grey was shown to mediate the expression of defensive behaviour53, 54, 55 and is also relevant for fear conditioning in PD/AG.9 Electric or chemical stimulation of the PAG in animals induces strong increases in autonomic arousal and fight/flight behaviour, which are the dominant characteristics of defensive responses during acute threat in general, but also during acute panic states and escape behaviour in PD/AG patients.17 As 5-HT inhibits PAG-mediated panic and escape behaviour,56 decreased serotonergic neurotransmission as a consequence of the HTR1A GG genotype might well contribute to increased escape behaviour during the standardized BAT. Interestingly, escape behaviour was preceded by increased anticipatory anxiety in CC but not GG genotype carriers. Moreover, reported anxiety immediately before escape was more pronounced in CC carriers as compared with GG carriers. Although it remains speculative, our results suggest that acute escape in C allele homozygotes might be driven by the motivation to reduce anxious apprehension. In contrast, escape behaviour in GG carriers might be less depending on previous subjective distress. Future research has to clarify whether G allele-associated flight behaviour in humans is indeed associated to a more sensitive PAG as supposed by animal models and how functionality of that brain structure might be affected by anticipatory anxiety.
In line with the BAT data and the assumption that the presence of G alleles goes along with increased fear reactions towards not fully predictive conditioned stimuli, our preliminary neuroimaging data suggest that HTR1A GG homozygotes show increased activation of the bilateral amygdalae upon presentation of conditioned stimuli (CS+unpaired and CS−) as an indicator of potential threat (unconditioned stimuli) detection. Evidence for increased activation of the amygdala can also be found in response to viewing emotional stimuli (faces) in patients with panic disorder carrying the rs6295 GG genotype,13 whereas in healthy subjects, even reduced amygdala activity has been reported for the processing of faces.19 Intriguingly, increased amygdala activation in GG homozygotes in our small fMRI sample was highly stable and remained constant even after successful CBT.
Previously, we have shown that PD/AG patients exhibit altered top-down (prefrontal regions) and bottom-up processing (midbrain regions) of conditioned stimuli compared with healthy individuals.9 Further, we also demonstrated that CBT predominantly influences top-down processes, as differential conditioning activity in the left inferior frontal gyrus (IFG) was reduced after CBT treatment.27 Here, we extend these findings in demonstrating preliminary evidence for the effects of HTR1A on the neural correlates of fear conditioning and related changes in the context of CBT (in the CC group only). Amygdala activity upon CS+unpaired and CS− presentation in the GG group suggest a dysfunctional differential conditioning or general increased reactivity reflected in a hyper-reactivity to both fear and safety cues in these PD/AG risk genotype carriers, paralleling the reaction towards ambiguous cues in Htr1a KO mice. Although this effect was not affected by CBT, HTR1A CC homozygotes demonstrated effects of CBT on the differential processing of CS+unpaired and CS− in the early acquisition phase, as indicated by a significant interaction of genotype group (GG vs CC), treatment (t1 vs t2) and stimulus (CS+unpaired vs CS−). After CBT, only the HTR1A CC group demonstrated reduced activation in response to the CS+unpaired in a network including the bilateral insulae, the anterior/middle cingulate cortex and more distributed regions of the parietal and occipital lobe. Especially the involvement of the bilateral insulae might indicate successful differential conditioning29 and a reduced interoceptive attention after CBT in this genotype patient group.57, 58 Thus, our exploratory fMRI data suggest that CBT influenced the neural correlates of fear learning only in the CC group, maybe as a result of longer durations of exposure training in this patient subgroup. In line with this finding, stronger CBT-related improvements (reduced anxiety; longer exposure toleration) in pretreatment escapers carrying the C allele compared with G allele homozygotes were observed during BAT. Thus, CBT might predominantly act on aversive expectations and avoidance in the CC group, leading to a more efficient encoding in the conditioning paradigm.
Our findings can only provide a starting point for further investigations on the role of HTR1A in PD/AG and its treatment and should be interpreted in light of some limitations. Especially, the results of the fMRI analyses have to be interpreted with caution because of the small sizes of the genotype subgroups. Due to the small sample size, we cannot exclude that our results either represent false positive effects or that important differences might have been missed due to false negative findings. Especially, activation of the parietal lobe has to be interpreted with caution since activation change in this region could also be observed in healthy subjects (see Supplementary Material) and might be unrelated to CBT. Replications of such gene by treatment interactions in larger fMRI samples are necessary to support our findings and interpretations. On the other hand, our data benefit from coming from a large and controlled trial and from converging lines of evidence that strengthen our findings. For example, here we had the opportunity to perform correlations between anxiety ratings during BAT and fMRI activity. Such exploratory analyses indicate, for example, that activity predominantly in the right insular cortex is associated with the subjective experience and evaluation of anxiety in context of the BAT, whereas amygdala activity was unrelated to subjective anxiety ratings. Another issue to be kept in mind is that variation in HTR1A, which causes rather subtle molecular changes, is not identical to a corresponding knockout in animals. Therefore, it is even more remarkable that we still observe paralleling defensive behaviour and fear conditioning to ambiguous conditioned stimuli in humans and animals on neural and behavioural level.
Taken together, we demonstrated the effect of HTR1A on mechanisms of fear, reflected in increased threat-related defensive reactivity and dysfunctional differential conditioning processes indicated by amygdala activity for both threat and safety cues in GG homozygotes. On the other hand, in CC genotype carriers, we found increased subjective anxiety as a precursor of escape behaviour during BAT. Furthermore, only the latter group demonstrated neurofunctional changes with regard to differential conditioning activity due to CBT. Our results, therefore, translate evidence from animal studies to humans and suggest a central role for HTR1A in differentiating subgroups of patients with anxiety disorders. Because therapy was effective for all patients investigated with fMRI and BAT (see Table 1), our data could be explained by the fact that distinct components of CBT influence the processing of fear in different ways, as manualized CBT embraced several interventions (such as cognitive strategies, exposure therapy and so on.) with the overall goal of helping as many patients as possible. Longer exposure times in CC homozygote carriers suggest that exposure is the important component of CBT, which might be responsible for the neurofunctional changes within this patient subgroup. If future studies are able to identify further components of CBT, a more effective and personalized therapy for the individual patient might ultimately be possible.
Acknowledgments
We gratefully acknowledge expert technical assistance by C Gagel and I Reck. We are indebted to all the patients for participating in the MAC trial. This work is part of the German multicentre trial ‘Mechanisms of Action in CBT (MAC)'. The MAC study is funded by the German Federal Ministry of Education and Research (BMBF; project no. 01GV0615) as part of the BMBF Psychotherapy Research Funding Initiative. Principal investigators (PIs) with respective areas of responsibility in the MAC study are VA (Münster: Overall MAC Program Coordination), H-UW (Dresden: PI for the Randomized Clinical Trial (RCT) and Manual Development), AH (Greifswald: PI for Psychophysiology), ALG (Münster: PI for Psychophysiology and Panic subtypes), AS (Berlin: PI for Experimental Pharmacology), TK (Marburg: PI for Functional Neuroimaging) and JD (Würzburg: PI for Genetics). Additional site directors in the RCT component of the program are GWA (Würzburg), TF and L Fehm (Berlin-Adlershof) and TL (Bremen). All principal investigators take responsibility for the integrity of the respective study data and their components. All the authors and co-authors had full access to all the study data. The following are the staff members by site: Greifswald (coordinating site for psychophysiology): Christiane Melzig, Jan Richter, Susan Richter, Matthias von Rad; Berlin-Charite (coordinating centre for experimental pharmacology): Harald Bruhn, Anja Siegmund, Meline Stoy, Andre Wittmann; Berlin-Adlershof: Irene Schulz; Münster (Overall MAC Program Coordination, Genetics and Functional Neuroimaging): Andreas Behnken, Katharina Domschke, Adrianna Ewert, Carsten Konrad, Bettina Pfleiderer, Christiana Sehlmeyer, Peter Zwanzger; Münster (coordinating site for psychophysiology and subtyping): Judith Eidecker, Swantje Koller, Fred Rist, Anna Vossbeck-Elsebusch; Marburg/ Aachen (coordinating centre for functional neuroimaging): Barbara Drüke, Sonja Eskens, Thomas Forkmann, Siegfried Gauggel, Susan Gruber, Andreas Jansen, Thilo Kellermann, Isabelle Reinhardt, Nina Vercamer-Fabri; Dresden (coordinating site for data collection, analysis, and the RCT): Franziska Einsle, Christine Fröhlich, Andrew T. Gloster, Christina Hauke, Simone Heinze, Michael Höfler, Ulrike Lueken, Peter Neudeck, Stephanie Preiß, Dorte Westphal; Würzburg Psychiatry Department (coordinating centre for genetics): Andreas Reif; Würzburg Psychology Department: Julia Dürner, Hedwig Eisenbarth, Antje B. M. Gerdes, Harald Krebs, Paul Pauli, Silvia Schad, Nina Steinhäuser; Bremen: Veronika Bamann, Sylvia Helbig-Lang, Anne Kordt, Pia Ley, Franz Petermann, Eva-Maria Schröder. Additional support was provided by the coordinating centre for clinical studies in Dresden (KKS Dresden): Xina Grählert and Marko Käppler. The study was registered with the ISRCTN: ISRCTN80046034. The RCT project was approved by the Ethics Committee of the Medical Faculty of the Technical University of Dresden (EK 164082006). The neuroimaging components were approved by the Ethics Committee of the Medical Faculty of the Rheinisch-Westfälische Hochschule University Aachen (EK 073/07). The experimental pharmacology study was approved by the Ethics Committee of the state of Berlin (EudraCT: 2006-00-4860-29). Further support was provided by Deutsche Forschungsgemeinschaft (DFG; German Research Foundation; Collaborative Research Centre ‘Fear, Anxiety, Anxiety Disorders' SFB-TRR-58) project C2 to KD and JD, project Z2 to JD, AR and PP, project B1 to PP and project C1 to PZ.
VA is a member of the advisory boards and/or gave presentations for the following companies: AstraZeneca, Janssen-Organon, Lilly, Lundbeck, Servier, Pfizer and Wyeth. He also received research grants from AstraZeneca, Lundbeck and Servier. He chaired the committee for the ‘Wyeth Research Award Depression and Anxiety'. JD received in the past 3 years honoraria by Janssen, Bristol-Myers Squibb, Wyeth, Lundbeck, AstraZeneca and Pfizer and Grant Support by Medice, Lundbeck and AstraZeneca. TK received fees for educational programs from Janssen-Cilag, Eli Lilly, Servier, Lundbeck, Bristol-Myers Squibb, Pfizer and AstraZeneca; travel support/sponsorship for congresses from Servier; speaker's honoraria from Janssen-Cilag; and research grants from Pfizer and Lundbeck. CK received fees for educational programs from Esparma GmbH/Aristo Pharma GmbH, Lilly Deutschland GmbH, Servier Deutschland GmbH and MagVenture GmbH. AR has received research support from PsyNova, and AR and KD have received research grants from AstraZeneca. KD has received honoraria for scientific talks from Pfizer, Lilly and Bristol-Myers Squibb and has been a consultant for Johnson & Johnson. AS received research funding from Lundbeck, and speaker honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lundbeck, Pfizer, Wyeth and UCB. Educational grants were given by the Stifterverband für die Deutsche Wissenschaft, the Berlin Brandenburgische Akademie der Wissenschaften, the Boehringer Ingelheim Fonds and the Eli Lilly International Foundation. H-UW has served as a general consultant (non-product related) for Pfizer, Organon, Servier and Essex Pharma and has received grant funding for his institution from Sanofi Aventis, Pfizer, Lundbeck, Novartis, Essex Pharma, Servier and Wyeth. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo IL, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Straube B, Konrad C, Wittchen HU, Ströhle A, Wittmann A, et al. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am J Psychiatry. 2013;170:1345–1355. doi: 10.1176/appi.ajp.2013.12111484. [DOI] [PubMed] [Google Scholar]
- Lueken U, Straube B, Reinhardt I, Maslowski NI, Wittchen HU, Ströhle A, et al. Altered top-down and bottom-up processing of fear conditioning in panic disorder with agoraphobia. Psychol Med. 2014;44:381–394. doi: 10.1017/S0033291713000792. [DOI] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, et al. Association of a functional 1019C>G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia. Int J Neuropsychopharmacol. 2004;7:189–192. doi: 10.1017/S1461145703004061. [DOI] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, et al. Association of the functional −1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol. 2006;9:349–355. doi: 10.1017/S1461145705005869. [DOI] [PubMed] [Google Scholar]
- Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, et al. Human 5-HT1A receptor C(−1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Richter J, Hamm AO, Pané-Farré CA, Gerlach AL, Gloster AT, Wittchen HU, et al. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biol Psychiatry. 2012;72:512–520. doi: 10.1016/j.biopsych.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Kirsch P, Reuter M, Alexander N, Kozyra E, Kuepper Y, et al. The 5-HT1A C(−1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. Int J Neuropsychopharmacol. 2009;12:383–392. doi: 10.1017/S1461145708009401. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, et al. Effects of HTR1A C(−1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Rachman S. The conditioning theory of fear-acquisition: a critical examination. Behav Res Ther. 1977;15:375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2009;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- de Carvalho MR, Dias GP, Cosci F, de-Melo-Neto VL, Bevilaqua MC, Gardino PF, et al. Current findings of fMRI in panic disorder: contributions for the fear neurocircuitry and CBT effects. Expert Rev Neurother. 2010;10:291–303. doi: 10.1586/ern.09.161. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Kircher T, Arolt V, Jansen A, Pyka M, Reinhardt I, Kellermann T, et al. Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol Psychiatry. 2013;73:93–101. doi: 10.1016/j.biopsych.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt I, Jansen A, Kellermann T, Schuppen A, Kohn N, Gerlach AL, et al. Neural correlates of aversive conditioning: development of a functional imaging paradigm for the investigation of anxiety disorders. Eur Arch Psychiatry Clin Neurosci. 2010;260:443–453. doi: 10.1007/s00406-010-0099-9. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Smits JA, Otto MW. Empirically supported treatments for panic disorder. Psychiatr Clin North Am. 2009;32:593–610. doi: 10.1016/j.psc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Meca J, Rosa-Alcazar AI, Marin-Martinez F, Gomez-Conesa A. Psychological treatment of panic disorder with or without agoraphobia: a meta-analysis. Clin Psychol Rev. 2010;30:37–50. doi: 10.1016/j.cpr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Gloster AT, Wittchen HU, Einsle F, Lang T, Helbig-Lang S, Fydrich T, et al. Psychological treatment for panic disorder with agoraphobia: a randomized controlled trial to examine the role of therapist-guided exposure in situ in CBT. J Consult Clin Psychol. 2011;79:406–420. doi: 10.1037/a0023584. [DOI] [PubMed] [Google Scholar]
- Prasko J, Horacek J, Zalesky R, Kopecek M, Novak T, Paskova B, et al. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinol Lett. 2004;25:340–348. [PubMed] [Google Scholar]
- Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, et al. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33:218–226. doi: 10.1016/j.neuroimage.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, et al. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biol Psychiatry. 2009;66:594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Reif A, Richter J, Straube B, Höfler M, Lueken U, Gloster AT, et al. MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry. 2014;19:122–128. doi: 10.1038/mp.2012.172. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry. 2011;1:e41. doi: 10.1038/tp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester KJ, Eley TC. Therapygenetics: using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol Mood Anxiety Disord. 2013;3:4. doi: 10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kircher T, Straube B. The neural correlates of cognitive behavioral therapy: Recent progress in the investigation of patients with panic disorder. Behav Res Ther. 2014;62:88–96. doi: 10.1016/j.brat.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Lueken U, Straube B, Wittchen HU, Konrad C, Ströhle A, Wittmann A, et al. Therapygenetics: anterior cingulate cortex-amygdala coupling is associated with 5-HTTLPR and treatment response in panic disorder with agoraphobia. J Neural Transm. 2014. [DOI] [PubMed]
- Gloster AT, Wittchen HU, Einsle F, Hofler M, Lang T, Helbig-Lang S, et al. Mechanism of action in CBT (MAC): methods of a multi-center randomized controlled trial in 369 patients with panic disorder and agoraphobia. Eur Arch Psychiatry Clin Neurosci. 2009;259:155–166. doi: 10.1007/s00406-009-0065-6. [DOI] [PubMed] [Google Scholar]
- Straube B, Lueken U, Jansen A, Konrad C, Gloster AT, Gerlach AL, et al. Neural correlates of procedural variants in cognitive-behavioral therapy: a randomized, controlled multicenter FMRI study. Psychother Psychosom. 2014;83:222–233. doi: 10.1159/000359955. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Straube B, Lueken U, Jansen A, Konrad C, Gloster AT, Gerlach AL, et al. Neural correlates of procedural variants in cognitive behavioral therapy: a randomized, controlled multicentre fMRI study. Psychother Psychosom. 2014;83:222–233. doi: 10.1159/000359955. [DOI] [PubMed] [Google Scholar]
- Timberlake W. Behavior systems and reinforcement: an integrative approach. J Exp Anal Behav. 1993;60:105–128. doi: 10.1901/jeab.1993.60-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Graeff FG. Panic disorder: is the PAG involved. Neural Plast. 2009;2009:108135. doi: 10.1155/2009/108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, et al. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Hilbert K, Stolyar V, Maslowski NI, Wittchen H-U. Neural substrates of defensive reactivity in two subtypes of specific phobia. Soc Cogn Affect Neurosci. 2014;9:1668–1675. doi: 10.1093/scan/nst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem. 2010;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.