Abstract
The hemostatic system plays pleiotropic roles in cancer progression by shaping the tumor microenvironment and metastatic niches through thrombin-dependent fibrin deposition and platelet activation. Expanding experimental evidence implicates coagulation protease receptors expressed by tumor cells as additional players that directly influence tumor biology. Pro-angiogenic G protein-coupled signaling of TF through protease activated receptor 2 and regulation of tumor cell and vascular integrins through ligation by alternative spliced TF are established pathways driving tumor progression. Our recent work shows that the endothelial protein C receptor (EPCR), a stem cell marker in hematopoietic, neuronal and epithelial cells, is also crucial for breast cancer growth in the orthotopic microenvironment of the mammary gland. In aggressive triple-negative breast cancer cells, EPCR expression is a characteristic of cancer stem cell-like populations that have tumor initiating properties in vivo. Blocking antibodies to EPCR attenuate in vivo tumor growth and proliferation specifically of EPCR+ cells on defined integrin matrices in vitro. We also showed that tumor-associated macrophages are a source for upstream coagulation proteases that can activate TF- and EPCR-dependent cellular responses, suggesting that tumor cells utilize the tumor microenvironment for tumor promoting coagulation protease signaling.
Keywords: cancer stem cell, coagulation, tumor microenvironment, macrophage, protease
Links of coagulation initiation and tumor progression
The tissue factor (TF) initiated extrinsic coagulation pathway is essential for responses to vascular and tissue injury, coupling hemostatic clot formation (1;2) with innate immune host defense (3–5) and initiating repair mechanisms and their pathological consequences (6–13). TF association with cytoskeletal structures (14–17), the interaction of the TF extracellular domain with integrins (18;19), and TF-dependent activation of the pro-migratory G protein-coupled receptor, protease activated receptor (PAR) 2 provide molecular connections by which TF supports not only tissue repair, but also cancer progression. TF-FVIIa cleaves PAR2 and stimulates cell migration though chemokine induction (20–23). PAR2 activation also recruits β-arrestin adaptor proteins (24) that act as scaffolds for MAP kinase ERK1/2 and cofilin activation in cell migration (25–28). TF-FVIIa-PAR2 signaling induces phosphorylation of the TF cytoplasmic domain and thereby reverses inhibitory effects of TF on integrin α3β1-dependent migration (18;29). In clinical breast cancer, upregulated TF and PAR2 expression is associated with TF cytoplasmic domain phosphorylation and relapse (30), indicating that pathological TF-FVIIa signaling is turned on in aggressive cancers.
TF-FVIIa signaling elicits a transcriptional program that broadly influences angiogenesis and innate immune cell recruitment (22;31;32). TF-FVIIa-PAR2 signaling can be selectively blocked by an antibody that prevents the association of TF-FVIIa with integrins, but has no major effect on coagulation (19;33). Inhibition of tumor growth and angiogenesis by this antibody provided the first compelling evidence that TF-FVIIa proangiogenic signaling promotes tumor progression independent of coagulation activation in vivo, a conclusion confirmed by a delayed “angiogenic switch” in tumor prone mice lacking PAR2, but not PAR1 (34). Clinical (30) and transfection (35;36) studies implicated the TF cytoplasmic domain in tumor progression. Consistently, cytoplasmic domain deleted (TFΔCT) mice showed the same delay in angiogenesis-dependent spontaneous cancer development as PAR2−/− mice (37). Reconstitution of PAR2−/− breast cancer cells with PAR2 or with a PAR2 mutant defective in β-arrestin recruitment restored proangiogenic signaling and tumor growth (37), supporting the current model of tumor cell TF-FVIIa-PAR2 signaling promoting angiogenesis and tumor progression.
The requirement for TF in embryonic vascular development (38–42) led to diverse studies on coagulation-dependent (43;44) and –independent (45–51;51–55) functions of the TF pathway in angiogenesis. While these studies were largely focused on roles of the TF-FVIIa complex, recent data show that alternatively spliced TF (asTF) regulates angiogenesis independent of initiating coagulation or PAR signaling (56). The TF mRNA is spliced in two gene products, transmembrane-anchored full-length TF (flTF) or asTF that lacks exon 5 encoding an extracellular region involved in substrate FX binding. Instead asTF has a unique carboxyl-terminus translated from an alternative reading frame of exon 6 and is a soluble protein (57). While TF procoagulant function is crucial for tumor cell metastasis, asTF does not contribute to this process (58). Instead, purified asTF was shown to induce endothelial cell sprouting by ligating endothelial cell-expressed integrins α6β1 and αvβ3 (59) and upregulates leukocyte adhesion molecules to enhance monocyte transmigration (60;61). In addition, inhibitory antibodies specific for asTF have shown a role for asTF in regulating tumor cell proliferation in vivo (62). Broader roles of coagulation receptors in tumor progression were uncovered by our recent studies in mice deficient of the endothelial protein C receptor (EPCR) (63) which will be reviewed here.
EPCR as a stem cell marker
The procoagulant effects of the TF pathway are counterbalanced by the protein C (PC) anticoagulant pathway to avoid intravascular thrombosis (64). The CD1d–like immune receptor EPCR binds the γ-carboxyl glutamic acid-rich (Gla) domain of PC to markedly improve PC activation by thrombin bound to endothelial cell-expressed thrombomodulin. The protein C pathway has an important role in balancing the prometastatic effects of thrombin generation initiated by tumor cell-expressed TF. For example, vascular overexpression of EPCR or treatment with activated protein C (aPC) reduces metastasis, while protein C blockade, protein C resistance due to the factor VLeiden mutation or thrombomodulin dysfunction increase metastasis (65–68). In contrast, EPCR deficiency has minimal effects on metastasis (58), suggesting that direct neutralization of thrombin by thrombomodulin is a dominant mechanism by which metastatic spread is prevented once tumor cells have entered the bloodstream.
In addition to these roles of the anticoagulant pathway on vascular cells, tumor cell-expressed EPCR has been implicated in tumor progression. EPCR-dependent PAR1 activation by aPC stimulates cell migration of breast cancer cells or prevents apoptosis of lung cancer cells leading to enhanced metastasis (69;70). In contrast to these tumor promoting functions of EPCR in epithelial tumors, EPCR counteracts TF and PAR1 dependent metastasis of mesothelioma in the pleural cavity (71). It is unclear whether EPCR exerts the profound anti-proliferative and pro-apoptotic effects on TF-expressing mesothelioma through anticoagulant or signaling pathways. We became interested in the role of EPCR in breast cancer progression, because EPCR also binds FVIIa and FXa and contributes to TF-dependent and independent signaling by these proteases (72–74). In addition, EPCR is a potential marker for breast cancer stem cells and was used to isolate these subpopulations implicated in cancer recurrence (75;76).
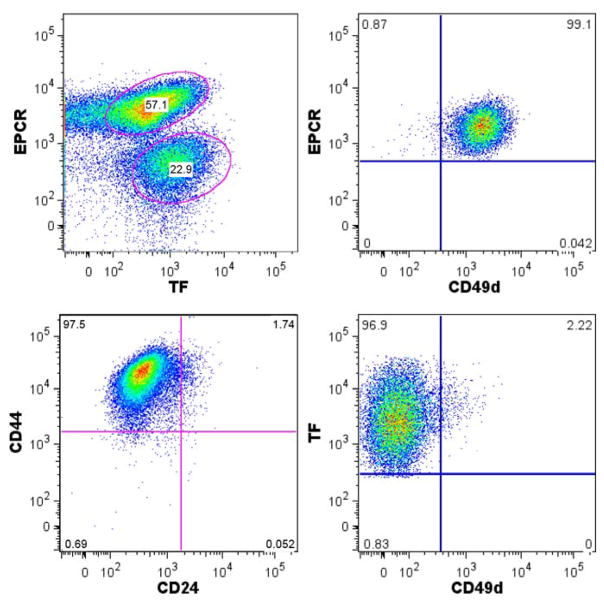
EPCR is expressed by hematopoietic, neuronal and epithelial progenitor populations (77–80), but functional roles of EPCR in stem cell and cancer stem cell biology are poorly understood. EPCR is expressed by highly aggressive basal-like breast cancer subtypes (81). Cancer tissue from patients contain stem cell-like subpopulations that can be enriched by several markers, including a CD44high/CD24− surface phenotype (82), expression of aldehyde dehydrogenase (ALDH1) (83), and EPCR (76). We found that in a triple negative, aggressive breast cancer line (84), EPCR defined a subpopulation of CD44high/CD24− cells (Fig. 1) and went on to characterize the unique properties of these cells.
Fig. 1.
TF and EPCR expression levels reveal distinct subpopulations of highly aggressive triple negative MDA-MB-231mfp breast cancer cells.
EPCR expression influences tumor cell biological properties
Comparison of this population with another relatively stable subpopulation with high TF expression revealed distinct differences. The subpopulation with high TF expression showed upregulated PAR2 expression and of a concerted repertoire of genes previously implicated in proangiogenic TF-dependent signaling. In the EPCR expressing subpopulation, we documented expression of markers associated with an aggressive or stem cell-like phenotype, including ALDH1B1 and ALDH1A3 (83), the hematopoietic stem cell marker integrin α4 (85), and the pan stem cell maker integrin α6 (86). However, these cells did not match previously established gene signatures, for example found in integrin α6high skin cancer stem cells or other stem cells (87;88), suggesting that plasticity of tumors include partial escape from developmentally defined regulation of stem cell gene expression patterns. Importantly, selection for EPCR enriched a subpopulation with improved growth properties in suspension culture and the ability to produce tumor growth in vivo at very low doses of injected tumor cells.
Most importantly, we found that inhibitory antibodies to EPCR attenuated the tumor initiation capacity of these cells injected at low doses and also reduced tumor growth of a mixed aggressive breast cancer population. In a mouse model of EPCR deficiency, we further documented diminished oncogene-induced spontaneous tumor progression. In addition, deletion of EPCR from murine mammary tumors reduced tumor growth, providing multiple lines of evidence for a functional role of the stem cell marker EPCR in breast cancer growth. Genetic overexpression of EPCR in endothelial cells reduces lung metastasis (65), indicating a role for EPCR in tumor cell survival in vascular niches. However, we found no differences in spontaneous lung metastasis in the spontaneous breast cancer models when EPCR expression was severely reduced.
Proteases ligands for EPCR and TF are provided by innate immune cells in the tumor microenvironment
The tumor promoting roles of EPCR in murine and human breast cancer growth and the attenuation of tumor initiation and growth by inhibitory antibodies to EPCR suggested that cancer stem cell-like populations sense EPCR ligands in the tumor microenvironment. EPCR binds three coagulation proteases, activated PC, FVIIa, and FXa that could conceivably enter the tumor microenvironment due to the hyper-permeability of the tumor vasculature. However, this pathway was difficult to rationalize when very low numbers of EPCR-expressing cancer stem cells were injected for tumor growth. Although FVII is synthesized by certain cancers (89;90), the TF-and PAR2-dependent breast cancer models studied by us showed no cell autonomous production of FVII or FX. However, we detected mRNA expression of FVII and FX in tumor-associated macrophages recovered from the tumor stroma of the PyMT breast cancer model of spontaneous breast cancer progression (63). It is important to point out that human lung macrophages have been shown previously to synthesize FVIIa macrophages (91), excluding that extrahepatic synthesis of upstream coagulation factors is a peculiarity of rodent models. Thus, these experiments uncovered a potential pathway by which cancer stem cell expressed EPCR may transmit cues from the innate immune system to regulate stem cell survival, retention, and/or differentiation (Fig. 2). These reciprocal links between tumor cells and the host innate immune system in tumor initiation and angiogenesis will require validation by additional genetic mouse models with altered expression of coagulation proteases and their receptors in the tumor microenvironment.
Fig. 2.
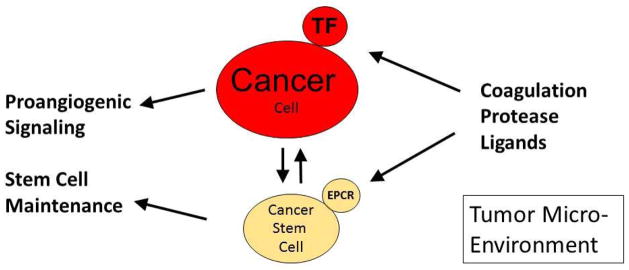
Upstream coagulation protease interactions with tumor cell populations in the tumor microenvironment.
These experiments should also stimulate further research in the pharmacological modulation of coagulation protease activity in preclinical models and potentially in clinical cancer therapy. The mechanisms of novel small molecule oral anticoagulants targeting specific coagulation proteases are finding wider acceptance for thrombosis indications. Identifying EPCR’s protease ligand will be instrumental to select an optimal anticoagulant strategy that may be beneficial in cancer therapy by interrupting the function of EPCR-bound proteases in maintaining cancer stem cell population.
Acknowledgments
The studies reviewed in this grant were supported by NIH grant HL-60742.
Abbreviations
- EPCR
endothelial Protein C Receptor
- TF
tissue factor
- TME
tumor microenvironment
- TAM
tumor associated macrophages
Footnotes
The authors have no conflicting financial interests.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 2.Luther T, Flössel C, Mackman N, Bierhaus A, Kasper M, Albrecht S, et al. Tissue factor expression during human and mouse development. Am J Pathol. 1996;149:101–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010 Aug;16(8):887–96. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 4.Imamura T, Iyama K, Takeya M, Kambara T, Nakamura S. Role of macrophage tissue factor in the development of the delayed hypersensitivity reaction in monkey skin. Cell Immunol. 1993;152:614–22. doi: 10.1006/cimm.1993.1317. [DOI] [PubMed] [Google Scholar]
- 5.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004 Jun;113(11):1596–606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009 Jan 15;113(3):705–13. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmur JD, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, et al. Tissue factor is rapidly induced in arterial smooth muscle after balloon injury. J Clin Invest. 1993;91:2253–9. doi: 10.1172/JCI116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyo RT, Sato Y, Mackman N, Taubman MB. Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration. Thromb Haemost. 2004;92:451–8. doi: 10.1160/TH04-02-0122. [DOI] [PubMed] [Google Scholar]
- 9.Schecter AD, Giesen PLA, Taby O, Rosenfield C-L, Rossikhina M, Fyfe BS, et al. Tissue factor expression in human arterial smooth muscle cells. TF is present in three cellular pools after growth factor stimulation. J Clin Invest. 1997;100:2276–85. doi: 10.1172/JCI119765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speidel CM, Eisenberg PR, Ruf W, Edgington TS, Abendschein DR. Tissue factor mediates prolonged procoagulant activity on the luminal surface of balloon-injured aortas in rabbits. Circulation. 1995;92(11):3323–30. doi: 10.1161/01.cir.92.11.3323. [DOI] [PubMed] [Google Scholar]
- 11.Hasenstab D, Lea H, Hart CE, Lok S, Clowes AW. Tissue factor overexpression in rat arterial neointima models thrombosis and progression of advanced atherosclerosis. Circulation. 2000;101:2651–7. doi: 10.1161/01.cir.101.22.2651. [DOI] [PubMed] [Google Scholar]
- 12.Anthoni C, Russell J, Wood KC, Stokes KY, Vowinkel T, Kirchhofer D, et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007 Jul 9;204(7):1595–601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Xu H, Ploplis VA, Castellino FJ. Factor VII deficiency impairs cutaneous wound healing in mice. Mol Med. 2010 May;16(5–6):167–76. doi: 10.2119/molmed.2009.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin binding protein 280. J Cell Biol. 1998;140(5):1241–53. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luther T, Dittert DD, Kotzsch M, Erlich J, Albrecht S, Mackman N, et al. Functional implications of tissue factor localization to cell-cell contacts in myocardium. J Pathol. 2000;192:121–30. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH667>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Müller M, Albrecht S, Gölfert F, Hofer A, Funk RHW, Magdolen V, et al. Localization of tissue factor in actin-filament-rich membrane areas of epithelial cells. Exp Cell Res. 1999;248:136–47. doi: 10.1006/excr.1999.4395. [DOI] [PubMed] [Google Scholar]
- 17.Fischer EG, Riewald M, Huang HY, Miyagi Y, Kubota Y, Mueller BM, et al. Tumor cell adhesion and migration supported by interaction of a receptor-protease complex with its inhibitor. J Clin Invest. 1999;104(9):1213–21. doi: 10.1172/JCI7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Crosstalk of integrinα3β1 and tissue factor in cell migration. Mol Biol Cell. 2004;15(10):4416–25. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111(1):190–9. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006 Jan 1;66(1):307–14. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 21.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98(14):7742–7. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, et al. Tissue factor-factor VIIa specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated via PAR-2 and results in increased cell migration. Blood. 2004 Jan 8;103(8):3029–37. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–60. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett N. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148(6):1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L, Ly Y, Hollenberg M, DeFea K. A β-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2 induced chemotaxis. J Biol Chem. 2003 Jun 23;278(36):34418–26. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 26.Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem. 2004 Dec 31;279(53):55419–24. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- 27.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta -arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007 May 11;282:20634–46. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 28.Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010 May 7;285(19):14318–29. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279(22):23038–44. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 30.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126(10):2330–40. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albrektsen T, Sorensen BB, Hjortoe GM, Fleckner J, Rao LVM, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–97. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J Biol Chem. 2000;275:6580–5. doi: 10.1074/jbc.275.9.6580. [DOI] [PubMed] [Google Scholar]
- 33.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103(38):13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, et al. Protease activated receptor (PAR)2, but not PAR1 signaling promotes the development of mammary adenocarcinoma in PyMT mice. Cancer Res. 2008;68(17):7219–27. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Deng Y, Luther T, Müller M, Ziegler R, Waldherr R, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–7. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe K, Shoji M, Chen J, Bierhaus A, Danave I, Micko C, et al. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci USA. 1999;96(15):8663–8. doi: 10.1073/pnas.96.15.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaffner F, Versteeg HH, Schillert A, Yokota N, Petersen LC, Mueller BM, et al. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010 Dec 23;116(26):6106–13. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–5. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 39.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmbäck K, Danton MJS, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93:6258–63. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–7. [PubMed] [Google Scholar]
- 41.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Jr, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–9. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 42.Griffin CT, Srinavasan Y, Zheng Y-W, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–70. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- 43.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006 Nov;10(5):355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Reinhardt C, Bergantall M, Greiner TU, Schaffner F, Ostergren-Lunden G, Petersen LC, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012 Apr 11;483(7391):627–31. doi: 10.1038/nature10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arderiu G, Pena E, Aledo R, Juan-Babot O, Badimon L. Tissue Factor Regulates Microvessel Formation and Stabilization by Induction of Chemokine (C-C motif) Ligand 2 Expression. Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2607–15. doi: 10.1161/ATVBAHA.111.233536. [DOI] [PubMed] [Google Scholar]
- 46.Siegbahn A, Johnell M, Rorsman C, Ezban M, Heldin C-H, Rönnstrand L. Binding of factor VIIa to tissue factor on human fibroblasts leads to activation of phospholipase C and enhanced PDGF-BB-stimulated chemotaxis. Blood. 2000;96:3452–8. [PubMed] [Google Scholar]
- 47.Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nature Med. 2004;10(5):502–9. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 48.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nature Med. 1996;2:209–15. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Kasper M, Heck T, Nakagawa K, Humpert PM, Bai L, et al. Tissue factor as a link between wounding and tissue repair. Diabetes. 2005 Jul;54(7):2143–54. doi: 10.2337/diabetes.54.7.2143. [DOI] [PubMed] [Google Scholar]
- 50.Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of Protease Activated Receptor 1 and 2 Signaling in Hypoxia-Induced Angiogenesis. Arterioscler Thromb Vasc Biol. 2007 Mar 15;27(6):1456–62. doi: 10.1161/ATVBAHA.107.142539. [DOI] [PubMed] [Google Scholar]
- 51.Hembrough TA, Swartz GM, Papathanassiu A, Vlasuk GP, Rote WE, Green SJ, et al. Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 2003 Jun 1;63(11):2997–3000. [PubMed] [Google Scholar]
- 52.Hembrough T, Ruiz J, Papathanassiu A, Green S, Strickland DK. Tissue factor pathway inhibitor inhibits endothelial cell proliferation via association with the very low density lipoprotein receptor. J Biol Chem. 2001;276(15):12241–8. doi: 10.1074/jbc.M010395200. [DOI] [PubMed] [Google Scholar]
- 53.Hembrough TA, Ruiz JF, Swerdlow BM, Swartz GM, Hammers HJ, Zhang L, et al. Identification and characterization of a very low density lipoprotein receptor-binding peptide from tissue factor pathway inhibitor that has antitumor and antiangiogenic activity. Blood. 2004 May 1;103(9):3374–80. doi: 10.1182/blood-2003-07-2234. [DOI] [PubMed] [Google Scholar]
- 54.Holroyd EW, Delacroix S, Larsen K, Harbuzariu A, Psaltis PJ, Wang L, et al. Tissue factor pathway inhibitor blocks angiogenesis via its carboxyl terminus. Arterioscler Thromb Vasc Biol. 2012 Mar;32(3):704–11. doi: 10.1161/ATVBAHA.111.243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh R, Pan S, Mueske CS, Witt TA, Kleppe LS, Peterson TE, et al. Tissue factor pathway inhibitor deficiency enhances neointimal proliferation and formation in a murine model of vascular remodelling. Thromb Haemost. 2003 Apr;89(4):747–51. [PubMed] [Google Scholar]
- 56.Signaevsky M, Hobbs J, Doll J, Liu N, Soff GA. Role of alternatively spliced tissue factor in pancreatic cancer growth and angiogenesis. Semin Thromb Hemost. 2008 Mar;34(2):161–9. doi: 10.1055/s-2008-1079256. [DOI] [PubMed] [Google Scholar]
- 57.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003 Apr;9(4):458–62. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 58.Yokota N, Zarpellon A, Chakrabarty S, Bogdanov VY, Gruber A, Castellino FJ, et al. Contributions of thrombin targets to tissue factor-dependent metastasis in hyperthrombotic mice. J Thromb Haemost. 2013 Nov;1:10. doi: 10.1111/jth.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19497–502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godby RC, van den Berg YW, Srinivasan RS, Sturm R, Hui DY, Konieczny SF, et al. Non-Proteolytic Properties of Murine Alternatively Spliced Tissue Factor: Implications for Integrin-Mediated Signaling in Murine Models. Mol Med. 2012 Jul 18;18(1):771–9. doi: 10.2119/molmed.2011.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srinivasan R, Ozhegov E, van den Berg YW, Aronow BJ, Franco RS, Palascak MB, et al. Splice Variants of Tissue Factor Promote Monocyte-Endothelial Interactions by Triggering the Expression of Cell Adhesion Molecules via Integrin-Mediated Signaling. J Thromb Haemost. 2011 Oct;9(10):2087–96. doi: 10.1111/j.1538-7836.2011.04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kocaturk B, van den Berg YW, Tieken C, Mieog JS, de Kruijf EM, Engels CC, et al. Alternatively spliced Tissue Factor promotes breast cancer growth in a B1 integrin-dependent manner. PNAS. 2013 Jul 9;110(28):11517–22. doi: 10.1073/pnas.1307100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaffner F, Yokota N, Carneiro-Lobo TC, Kitano M, Schaffer M, Anderson GM, et al. Endothelial Protein C Receptor Function in Murine and Human Breast Cancer Development. PLoS ONE. 2013 Apr 9;8(4):e61071. doi: 10.1371/journal.pone.0061071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006 Sep;13(5):382–5. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 65.Bezuhly M, Cullen R, Esmon CT, Morris SF, West KA, Johnston B, et al. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009 Apr 2;113(14):3371–4. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 66.Van Sluis GL, Niers TM, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, et al. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1-mediated vascular endothelial barrier enhancement. Blood. 2009 Aug 27;114(9):1968–73. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruggemann LW, Versteeg HH, Niers TM, Reitsma PH, Spek CA. Experimental melanoma metastasis in lungs of mice with congenital coagulation disorders. J Cell Mol Med. 2008 Dec;12(6B):2622–7. doi: 10.1111/j.1582-4934.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horowitz NA, Blevins EA, Miller WM, Perry AR, Talmage KE, Mullins ES, et al. Thrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domain. Blood. 2011 Sep 8;118(10):2889–95. doi: 10.1182/blood-2011-03-341222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beaulieu LM, Church FC. Activated protein C promotes breast cancer cell migration through interactions with EPCR and PAR-1. Exp Cell Res. 2007 Feb 15;313(4):677–87. doi: 10.1016/j.yexcr.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anton I, Molina E, Luis-Ravelo D, Zandueta C, Valencia K, Ormazabal C, et al. Receptor of Activated Protein C Promotes Metastasis and Correlates with Clinical Outcome in Lung Adenocarcinoma. Am J Respir Crit Care Med. 2012 Jul 1;186(1):96–105. doi: 10.1164/rccm.201110-1826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keshava S, Sahoo S, Tucker TA, Idell S, Rao LV, Pendurthi UR. Endothelial cell protein C receptor opposes mesothelioma growth driven by tissue factor. Cancer Res. 2013 Jul 1;73(13):3963–73. doi: 10.1158/0008-5472.CAN-12-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Disse J, Petersen HH, Larsen KS, Persson E, Esmon N, Esmon CT, et al. The Endothelial Protein C Receptor Supports Tissue Factor Ternary Coagulation Initiation Complex Signaling through Protease-activated Receptors. J Biol Chem. 2011 Feb 18;286(7):5756–67. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sen P, Gopalakrishnan R, Kothari H, Keshava S, Clark CA, Esmon CT, et al. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011 Mar 17;117(11):3199–208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Disse J, Ruf W. Endothelial protein C receptor is required for tissue factor ternary complex signaling in the mouse. J Thromb Haemost. 2011 Dec;9(12):2516–8. doi: 10.1111/j.1538-7836.2011.04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS ONE. 2009 Dec 21;4(12):e8377. doi: 10.1371/journal.pone.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007 Mar;11(3):259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 77.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002 Oct 18;298(5593):601–4. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 78.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004 Sep 3;118(5):635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002 Oct 18;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 80.Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2005 Nov 22;107(6):2317–21. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res. 2010 Feb 1;16(3):876–87. doi: 10.1158/1078-0432.CCR-09-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007 Nov;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jessani N, Humphrey M, McDonald WH, Niessen S, Masuda K, Gangadharan B, et al. Carcinoma and stromal enzyme activity profiles associated with breast tumor growth in vivo. Proc Natl Acad Sci U S A. 2004 Sep 21;101(38):13756–61. doi: 10.1073/pnas.0404727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006 May 1;107(9):3503–10. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 86.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10544–9. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008 May;40(5):499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anastassiou D, Rumjantseva V, Cheng W, Huang J, Canoll PD, Yamashiro DJ, et al. Human cancer cells express Slug-based epithelial-mesenchymal transition gene expression signature obtained in vivo. BMC Cancer. 2011 Dec 30;11:529, 529. doi: 10.1186/1471-2407-11-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010 Aug 5;116(5):815–8. doi: 10.1182/blood-2009-10-250639. [DOI] [PubMed] [Google Scholar]
- 90.Koizume S, Jin M-S, Miyagi E, Hirahara F, Nakamura Y, Piao J-H, et al. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 2006 Oct 16;66(19):9453–60. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 91.Chapman HA, Jr, Allen CL, Stone OL, Fair DS. Human alveolar macrophages synthesize factor VII in vitro. Possible role in interstitial lung disease. J Clin Invest. 1985 Jun;75(6):2030–7. doi: 10.1172/JCI111922. [DOI] [PMC free article] [PubMed] [Google Scholar]