Abstract
Prescription opiate use by adolescent girls has increased significantly in the past decade. Preclinical studies using rats report alterations in morphine sensitivity in the adult offspring of adolescent morphine-exposed females (MOR-F1) when compared with the offspring of adolescent saline-exposed females (SAL-F1). To begin to elucidate the development of these next generation modifications, the present study examined the effects of acute morphine administration on sedation and corticosterone secretion in prepubescent SAL-F1 and MOR-F1 male and female rats. In addition, alterations in proopiomelanocortin (POMC) gene expression in the arcuate nucleus, as well as in tyrosine hydroxylase (TH) and μ-opioid receptor (OPRM1) gene expressions in the ventral tegmental area, were analyzed using quantitative PCR, to determine whether differential regulation of these genes was correlated with the observed behavioral and/or endocrine effects. Increased morphine-induced sedation, coupled with an attenuation of morphine-induced corticosterone secretion, was observed in MOR-F1 males. Significant alterations in both POMC and OPRM1 gene expressions were also observed in MOR-F1 males, with no change in TH mRNA expression. Overall, these data suggest that the transgenerational effects of adolescent morphine exposure can be discerned before pubertal development and are more pronounced in males, and suggest dysregulation of the hypothalamic–pituitary–adrenal axis in the offspring of adolescent morphine-exposed females.
Keywords: arcuate nucleus, corticosterone, gene expression, locomotor activity, mu-opioid receptor, proopiomelanocortin, transgenerational, ventral tegmental area
Introduction
The use and misuse of prescription opiates in adolescent populations has increased significantly in the past decade, increasing the percentage of people exposed to these powerful narcotics during a key developmental period (Johnston et al., 2010). Interestingly, recent data indicate that adolescent girls are more likely to be prescribed opiates than their male counterparts (McCabe et al., 2007), whereas rates of nonmedical opiate use are equivalent between the sexes (McCabe et al, 2011; SAMHSA, 2011). Thus, when one considers both medical and nonmedical use, it is clear that a large number of female individuals are being exposed to opiates during adolescent development. Currently, the potential long-term effect of this increased exposure remains unknown.
We have previously demonstrated significant transgenerational effects following adolescent opiate exposure using a rat model. Specifically, Sprague–Dawley female rats exposed to intermittent, increasing doses of morphine for 10 days during adolescent development produced offspring that showed enhanced behavioral sensitivity to morphine as adults (Byrnes, 2005; Byrnes et al., 2011). As all of the adolescent exposed female rats were drug-free for at least 3 weeks before mating, these findings indicate that a history of adolescent opiate exposure in the absence of continued use during pregnancy can impact the next generation. These offspring effects, however, were sexually dimorphic, with males demonstrating enhanced sensitivity, whereas females were less affected. The point at which developmental alterations in opiate sensitivity emerge in F1 animals is unknown. If significant sex differences are observed before puberty, this would argue against the possibility that circulating ovarian hormones act to mitigate transgenerational effects in F1 females. Such findings would suggest an early developmental effect that is sex-specific. Indeed, recent data demonstrate that male offspring of adolescent morphine-exposed females show decreased play behavior when tested as juveniles, an effect that is not observed in female offspring (Johnson et al., 2011). Whether similar prepubertal effects would be observed with regard to an acute response to direct opiate receptor stimulation in F1 animals remains to be determined.
Acutely, morphine has a biphasic, dose-dependent effect on locomotor activity, with initial sedation superseded by hyperactivity. At lower doses, hypoactivity is not observed and prolonged hyperactivity is the primary effect. The effects of morphine-induced sedation, as measured by a decrease in activity, are critically dependent upon μ-receptor activation (Calenco-Choukroun et al, 1991; Bardo et al., 2003). In addition, infusions of morphine into either the nucleus accumbens or ventral tegmental area (VTA) decrease locomotor activity (Costall et al., 1978; Havemann et al., 1982; Calenco-Choukroun et al., 1991). Thus, morphine-induced sedation provides a functional measure of μ-opioid sensitivity mediated within the mesoaccumbens dopamine pathway.
The acute effect of morphine on locomotor activity is also modulated by glucocorticoids. Specifically, in the rat, adrenalectomy prevents the increased locomotor activity that is normally observed following low-dose morphine administration and can even result in locomotor hypoactivity in response to subthreshold morphine doses (Deroche et al., 1992). Moreover, stress has been shown to potentiate locomotor hyperactivity following acute morphine administration, an effect that is prevented by adrenalectomy and reinstated by corticosterone replacement (Deroche et al., 1992, 1993a, 1993b; Marinelli et al., 1994; Stohr et al., 1999). Thus, activation of the hypothalamic–pituitary–adrenal (HPA) axis, resulting in increased corticosterone secretion, modulates morphine-induced locomotor activity. Previous findings in the adult male offspring of adolescent exposed females revealed significant alterations in the regulation of corticosterone secretion (Byrnes et al., 2013). Therefore, the current study included analysis of morphine-induced changes in corticosterone secretion as one of the endpoints.
Proopiomelanocortin (POMC) neurons within the arcuate nucleus represent the primary source of β-endorphin within the forebrain. Arcuate POMC gene expression can be regulated directly by μ-opioid receptor activation, as well as by circulating corticosterone. Moreover, POMC activity has been shown to regulate the response of corticotropin releasing hormone within the paraventricular nucleus (PVN). We previously observed a significant decrease in POMC gene expression in adult females exposed to morphine during adolescent development (Byrnes, 2008). As shifts in the regulation of this gene could impact the sensitivity to both locomotor and endocrine responses to morphine, the current study also examined potential alterations in morphine-induced modifications in POMC gene expression in male and female offspring.
The purpose of the current study was to examine the effects of a novel environment on general locomotor activity, as well as the effects of acute morphine administration (0, 0.3, 1.0, 3.0, or 10.0 mg/kg) on sedation and corticosterone secretion in prepubescent male and female offspring of adolescent saline-exposed and morphine-exposed females (SAL-F1 and MOR-F1, respectively). As significant differences in activity and corticosterone secretion were observed between SAL-F1 and MOR-F1 groups after administration of the largest dose of morphine (10 mg/kg), additional data on POMC gene expression in the arcuate nucleus, as well as on μ-opioid receptor (OPRM1) and tyrosine hydroxylase (TH) gene expressions in the VTA, were collected from a random subset of subjects injected acutely with either saline or morphine (10 mg/kg). Overall, the findings indicate that female adolescent exposure to morphine induces significant effects in offspring, which vary by sex even when examined before pubertal development.
Methods
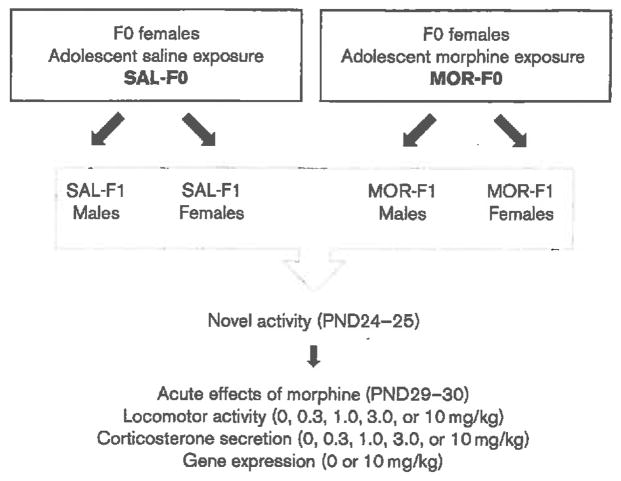
An overview of experimental conditions and dependent measures is presented in Fig. 1.
Fig. 1.
Overview of experimental conditions and dependent measures. MOR-F1, offspring of morphine-exposed females; PND, postnatal day; SAL-F1, offspring of saline-exposed females.
Adolescent morphine exposure
A total of 96 Sprague–Dawley female rats were administered either morphine (MOR-F0; n = 48) or saline (SAL-F0; n = 48) during adolescence. Adolescent morphine exposure included daily injections with an intermittent, increasing dose regimen of morphine that increased every other day (5, 10, 15, 20, 25 mg/kg). All injections were subcutaneous and were administered daily between 9:00–10:00 h. SAL-F0 females were injected with sterile saline at volumes matching morphine injections. This dosing regimen is based on allometric scaling and is intended to model the intermittent, escalating use pattern often observed with opiate abuse; however, the route of administration clearly does not mimic human use. We have chosen to use morphine as a prototypical opiate (as opposed to using more commonly abused opiates such as oxycontin or vicodin) because of the wealth of information that is available on the neural and endocrine effects of this compound in both humans and rats. The regimen began on postnatal day 30 (PND30) and continued for 10 days as we were interested in effects unique to this period, which would correspond to the early-mid adolescent phase in humans (Spear, 2004). One SAL-F0 female had to be removed from the study because of an unrelated animal housing issue.
Mating
All F0 females were mated 3 weeks after the final morphine injection (PND60); this was done by housing three females from the same adolescent exposure condition with a drug-naive male for 20 days. Females were then single-housed and monitored daily for the onset of parturition (designated as PND0). On the day after parturition (PND1), all F1 litters were weighed and culled to five males and five females. On PND21 all F1 litters were weaned. Male and female F1 subjects were housed with same sex littermates. A total of 44 SAL-F1 litters and 47 MOR-F1 litters were generated, with only one male and one female from each litter used in the current set of studies.
Novel activity
On PND24–25, F1 males and females were tested for novel activity. Between 07:00 and 10:30 h (lights on 06:00 h), one subject per litter was removed from the home cage and immediately placed in our standard activity chamber (Motor Monitor; Hamilton-kinder) in a quiet behavior testing room. Locomotor activity was recorded for 60 min.
Morphine dose–response
On PND29–30, between 07:00 and 12:30 h, juveniles that were previously tested for novel activity were weighed, injected (subcutaneously) with morphine (0, 0.3, 1.0, 3.0, or 10.0 mg/kg), and placed in the activity chamber for 60 min. At the end of the behavioral session, subjects were immediately transferred to a procedure room (transit < 60 s), exposed briefly to CO2, and subsequently decapitated. Trunk blood was collected in tubes pretreated with 100 μl heparin. Brains were removed, rapidly frozen in −20°C methylbutane, and stored at −80°C. Blood was spun at 2700 rpm for 10 min and plasma was stored at −20°C.
Corticosterone radioimmunoassay
Plasma concentrations of corticosterone (ng/ml) were determined using commercially available radioimmunoassay kits (Coat-a-Count Rat Corticosterone; Siemens Medical Solutions, Los Angeles, California, USA) according to the manufacturer’s protocol. The detection limit of the assay was 20 pg/ml, and all samples were run in duplicate with an intra-assay variation of 3.2%.
Gene expression
For gene expression, frozen brains were mounted on a cryostat and 1 mm3 bilateral micropunches of the arcuate nucleus (A/P, −2.30 mm; M/L, ± 1 mm; D/V −8 mm) and VTA (A/P, −4.7; M/L, ±1.5; D/V, −8.2; all coordinates relative to the bregma) were obtained. Tissue punches were homogenized in lysis buffer, and total RNA was extracted using the RNeasy kit from Qiagen (Valencia, California, USA) according to the manufacturer’s protocol. Complementary DNA was synthesized using the RETRO script kit from Applied Biosystems (Carlsbad, California, USA). PCR was performed on an AB 7500 system (Applied Biosystems) under standard amplification conditions. All PCR primers were TaqMan Gene Expression Assays purchased from Applied Biosystems (Assay ID numbers were as follows: GAPDH: Rn01775763_g1; POMC: Rn00595020_m1; OPRM1: Rn01430371_m1; TH: Rn00562500_m1). The amplification efficiency of each of these assays has been validated by Applied Biosystems and averages 100% (±10). GAPDH was chosen as the housekeeping gene following validation studies indicating similar expression of this gene between experimental groups. All samples were run in duplicate. All data are presented as ΔΔCt, which was calculated by subtracting the POMC cycle threshold (Ct) from GAPDH Ct and then and subtracting individual ΔCt values from the mean ΔCt of the acute saline SAL-F1 group within sex.
Statistical analyses
Data were analyzed by analysis of variance using both within-subject and between-subject factors depending upon the measure. Given the presence of significant sex differences in basal levels of locomotor activity and corticosterone, males and females were analyzed separately. Thus, for the majority of the data, the between-subject factors were adolescent exposure (SAL-F0/F1 compared with MOR-F0/F1, respectively) and dose (locomotor activity, corticosterone, and gene expression in F1 offspring), whereas the within-subject factors were either day (body weight data in F0 females) or time (novel activity in F1 offspring). Tukey’s multiple comparison tests were used following all significant main effects or interactions and significance was defined as a P-value of less than 0.05.
Results
As a result of daily morphine exposure during adolescent development, a significant difference in body weight gain was observed [day × adolescent exposure interaction; F(2,186) = 8.17, P < 0.001]. As expected, all adolescent females gained weight throughout the 10-day exposure period; however, morphine-exposed females (MOR-F0) gained significantly less than their saline-exposed (SAL-F0) counterparts, as measured on PND40 (47.6±0.7 vs. 56.9±0.9 g, respectively). Reduced body weight gain could still be observed a week after the final injection (94.9±1.4 vs. 101.1±2.0 g), but was no longer apparent 2 weeks later (124.8±1.9 vs. 126.7±2.2 g). Thus, no differences in body weight were observed before mating.
No significant differences in litter size (SAL-F1 = 12.2 ±0.9; MOR-F1 = 12.9 ±0.7) or male/female distribution (SAL-F1 = 5.9±0.6/6.5±0.5; MOR-F1 = 6.7 ± 0.5/6.1 ±0.4) were observed between SAL-F1 and MOR-F1 groups, as measured on PND1. In addition, no differences in litter body weights were observed on PND1 (SAL-F1 = 79.5 ±5.0; MOR-F1 = 87.7 ±3.9). Finally, when individual male and female subjects from each litter were weighed before testing on PND29–30, there was a significant sex difference [main effect of sex; F(1,178) = 37.4, P < 0.001], but there was no maternal exposure effect and no sex × maternal exposure interaction (both P’s > 0.1). These data are shown in Table 1.
Table 1.
Mean (±SEM) body weight (g) as measured before testing on PND30
| SAL-F1 (n=44) | MOR-F1 (n=47) | |
|---|---|---|
| Males | 100.1 ± 1.6 | 102.4 ± 1.5 |
| Females* | 89.9 ± 1.8 | 92.6 ± 1.7 |
MOR-F1, offspring of morphine-exposed females; PND, postnatal day; SAL-F1, offspring of saline-exposed females.
P < 0.01 compared with males collapsed across maternal exposure.
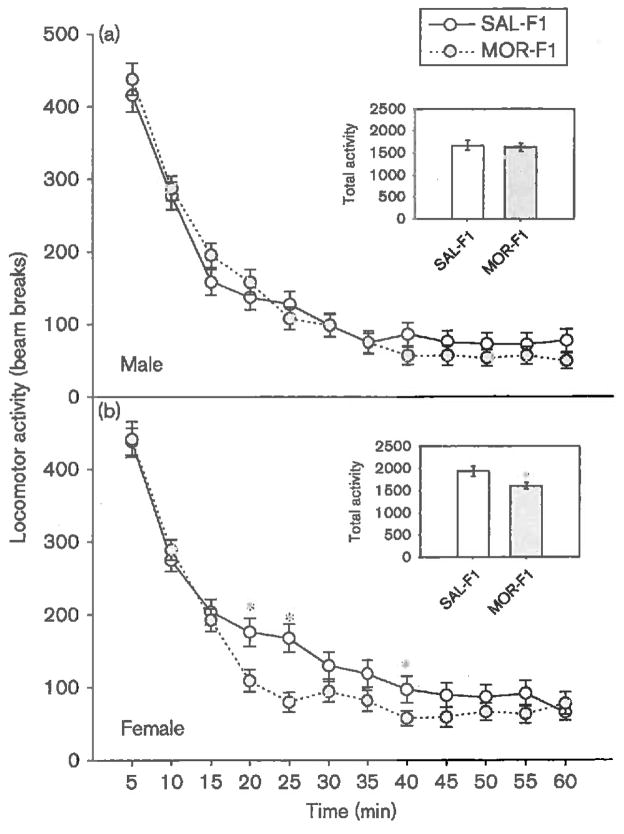
When tested as juveniles for their locomotor response to a novel environment (PND24–25), as expected, all F1 males demonstrated high levels of exploratory activity that decreased over time [main effect of time; F(11,979) = 115, P < 0.001]. Among males, there was no effect of maternal exposure and no time × maternal exposure interaction (both P’s > 0.2). These data are shown in Fig. 2a. The response to a novel environment among F1 females, however, was significantly influenced by maternal exposure [main effect of time; F(11,979) = 112, P < 0.001; main effect of maternal exposure; F(1,89) = 6.3, P < 0.05; time × maternal exposure interaction; F(11,979) = 2.15, P < 0.05]. Thus, as illustrated in Fig. 2b, although the initial response to the novel environment was similar between SAL-F1 and MOR-F1 females, MOR-F1 females appear to habituate to the environment more quickly, and overall, to have lower levels of activity.
Fig. 2.
Mean (±SEM) number of photobeam breaks in response to placement in a novel environment in PND24–25 (a) male and (b) female offspring of adolescent saline-exposed and morphine-exposed females (SAL-F1 and MOR-F1, respectively). Inset graph represents total number of photobeam breaks collapsed across time. *P < 0.05 as compared with SAL-F1; n = 44-47 per group. PND, postnatal day.
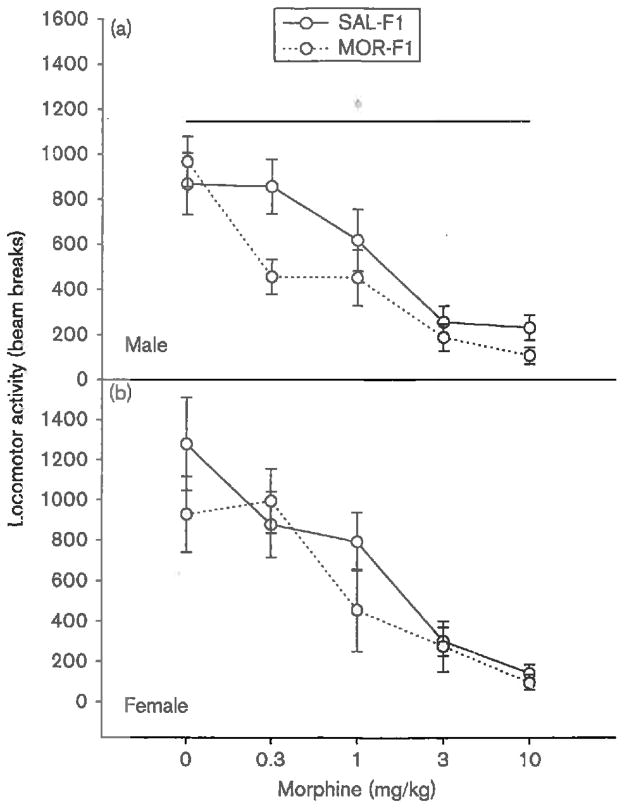
When tested for their locomotor response following an acute morphine injection, F1 males demonstrated significant differences as a function of maternal exposure [main effect of maternal exposure; F(1,91) = 4.2, P < 0.05]. These data are illustrated in Fig. 3a. Overall, morphine dose- dependently decreased locomotor activity [main effect of dose; F(4,91) = 18.8, P < 0.001]. This sedative effect was augmented in MOR-F1 males, although there was no significant dose × maternal exposure interaction (P = 0.16). As shown in Fig. 3a, MOR-F1 males demonstrate a significant reduction in locomotor activity in response to morphine. As shown in Fig. 3b, no significant main effect of maternal exposure on locomotor activity in response to an acute morphine injection was observed in F1 females (P = 0.2). Again, morphine dose-dependently decreased locomotor activity [main effect of dose; F(4,91) = 14.0, P < 0.001]; however, this dose effect was not influenced by maternal exposure (P = 0.5).
Fig. 3.
Mean (±SEM) number of photobeam breaks measured for 60 min in PND30 F1 (a) males and (b) females following an acute injection of either saline or morphine (0.3, 1.0, 3.0, or 10.0 mg/kg). *P < 0.05 compared with SAL-F1; n = 9-11 per group. MOR-F1, offspring of morphine-exposed females; PND, postnatal day; SAL-F1, offspring of saline-exposed females.
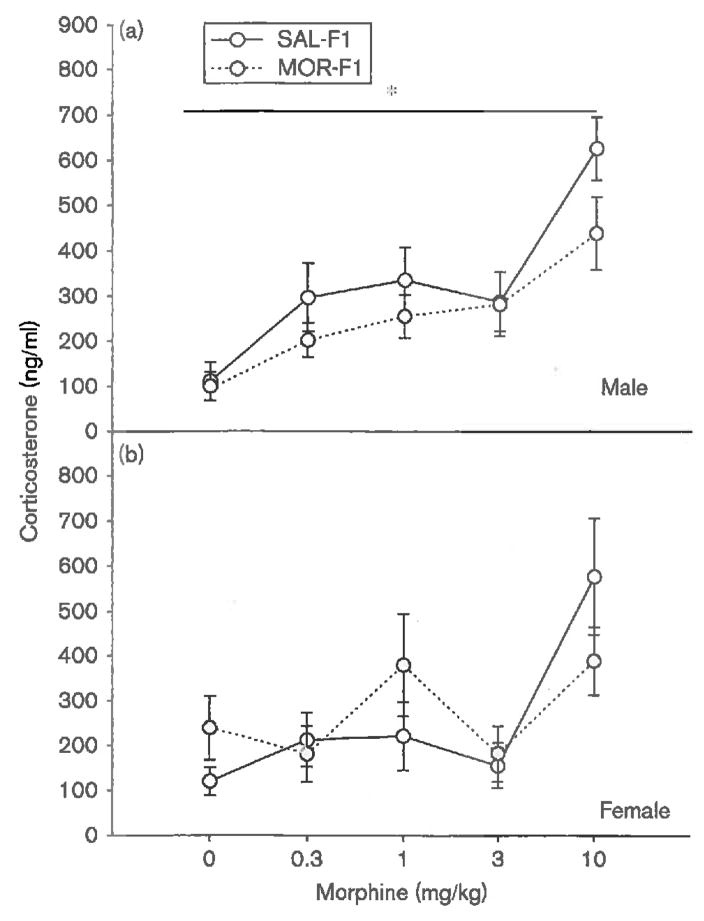
Analogous effects were observed with regard to corticosterone secretion. As shown in Fig. 4a, F1 males showed a dose-dependent increase in corticosterone secretion [main effect of dose; F(4,91) = 12.8, P < 0.001]. In addition, although there was no significant dose × maternal exposure interaction (P = 0.6), there was a significant main effect of maternal exposure [F(1,91) = 3.9, P < 0.05], with MOR-F1 males demonstrating a blunted corticosterone response. In contrast, no effect of maternal exposure on corticosterone secretion was observed in F1 females (P = 0.7), with all F1 females showing a dose-dependent increase in corticosterone [main effect of dose; F(4,91) = 5.8, P < 0.001], which was not further influenced by maternal exposure (P = 0.2). These data are shown in Fig. 4b.
Fig. 4.
Mean (±SEM) levels of plasma corticosterone (ng/ml) as measured in PND30 F1 (a) males and (b) females 60 min after an acute injection of either saline or morphine (0.3, 1.0, 3.0, or 10.0 mg/kg). *P < 0.05 compared with SAL-F1; n = 9-11 per group. MOR-F1, offspring of morphine-exposed females; PND, postnatal day; SAL-F1, offspring of saline-exposed females.
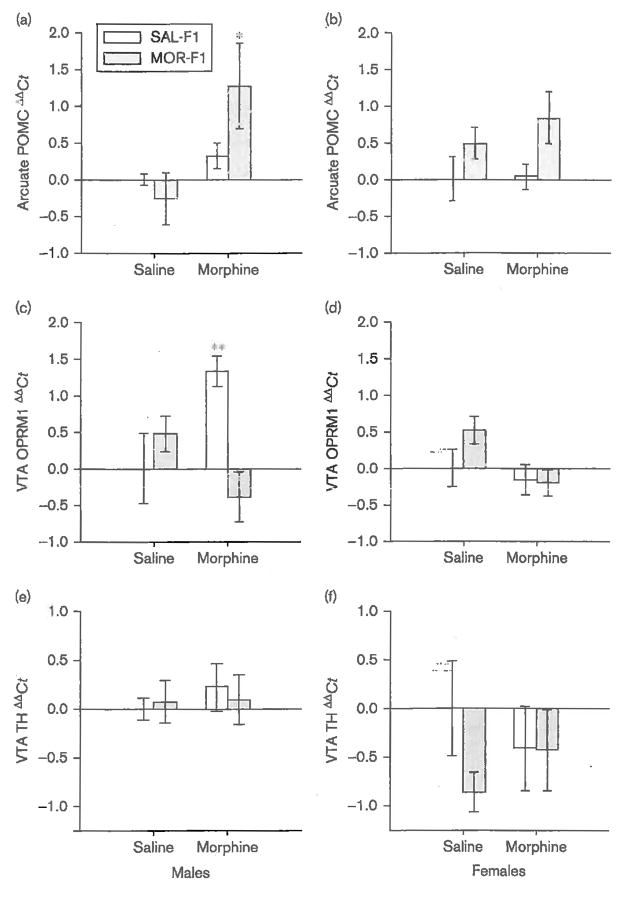
Finally, significant effects on gene expression within both the arcuate nucleus and the VTA were observed in response to a 10 mg/kg dose of morphine. Among F1 males (Fig. 5a), morphine significantly increased POMC gene expression [main effect of dose; F(1,34) = 4.8, P < 0.05]. Although there was no significant main effect of maternal exposure (P = 0.5) or the dose × maternal exposure interaction (P = 0.18), post-hoc analysis of the main effect of dose revealed that a significant increase in POMC gene expression was only observed in MOR-F1 males (P < 0.05). Among F1 females, there was no significant effect of morphine on POMC gene expression (P = 0.2) or the dose × maternal exposure interaction (P = 0.9). However, the main effect of maternal exposure approached significance [F(1,34) = 3.9, P = 0.057], which appeared to be because of increased expression of POMC mRNA in MOR-F1 females (Fig. 5b).
Fig. 5.
Mean (±SEM) ΔΔCt of (a, b) POMC, (c, d) OPRM1 and (e, f) TH mRNA expression as measured in tissue punches from the arcuate nucleus (POMC) and VTA (OPRM1 and TH) of PND30 F1 males (left panels) and females (right panels) 60 min after an acute injection of either saline or morphine (10.0 mg/kg). *P < 0.05 compared with saline-treated MOR-F1 and morphine-treated SAL-F1 males; **P ≤ 0.01 compared with saline- treated SAL-F1 and morphine-treated MOR-F1 males; n = 7-10 per group for POMC and 5-6 per group for OPRM1 and TH. MOR-F1, offspring of morphine-exposed females; OPRM1, μ-opioid receptor; PND, postnatal day; POMC, proopiomelanocortin; SAL-F1, offspring of saline-exposed females; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
In the VTA of F1 males, maternal exposure significantly altered OPRM1 gene transcription in response to an acute morphine injection [main effect of maternal exposure; F(1,23) = 5.6, P < 0.05; maternal exposure × dose interaction; F(1,23) = 7.5, P < 0.01]. As shown in Fig. 5c, OPRM1 expression in the VTA was significantly increased in SAL-F1 males (P < 0.01 as compared with saline-treated SAL-F1 males), but no such effect was observed in MOR-F1 males. Indeed, MOR-F1 males tended to have decreased OPRM1 expression following an acute morphine injection, resulting in significantly lower expression when compared with morphine-injected SAL-F1 males (P < 0.01). No significant effects on OPRM1 expression were observed in F1 females (Fig. 5d), although there was a weak trend toward a main effect of dose [F(1,25) = 3.5, P = 0.08], which was clearly driven by decreased OPRM1 expression observed in MOR-F1 females following acute morphine administration (P = 0.03 compared with saline-injected MOR-F1 females). Thus, the pattern of OPRM1 expression in the VTA in response to acute morphine injection was similar in MOR-F1 males and females, but not in SAL-F1 males and females. Finally, no significant effects on TH gene expression were observed in any F1 animal (Fig. 5e and f).
Discussion
The current set of findings shows significant effects on both male and female offspring of females exposed to morphine during adolescence. Moreover, some of these effects appear to be sex-specific even when measured before the onset of puberty. Among females, these effects include more rapid habituation to a novel environment in the absence of any initial effects on exploratory behavior (i.e. activity measure during the first 5-15 min in the test chamber). Alterations in intrasession habituation have been associated with a number of neurotransmitter systems, including glutamate, serotonin, and dopamine. Moreover, there appears to be a genetic component to modifications in this behavior (Leussis and Bolivar, 2006; Bolivar, 2009). As epigenetic modifications often occur in gene coding regions (Choi et al., 2009), it is possible that differences in intrasession habituation are due to changes in the expression of genes associated with this behavior induced by prior maternal exposure to morphine. Whether such alterations are due to effects at the germline or are experience-dependent in nature remains to be determined.
In male offspring, no significant differences in baseline locomotor activity were observed; however, significant differences emerged following the administration of morphine. Overall, these effects are not dose-dependent, but rather suggest a general shift in both behavioral and endocrine effects of μ-opioid receptor activation. With regard to sedation, MOR-F1 males demonstrated enhanced sensitivity to morphine, which resulted in prolonged sedation in response to an acute morphine injection. These same subjects, however, demonstrated a more attenuated glucocorticoid response to morphine when compared with SAL-F1 controls. This effect was not due to a change in baseline corticosterone, as no differences were observed in saline-treated subjects. It is possible that the changes in locomotor activity are related to the blunted corticosterone secretion. Indeed, increased corticosterone levels are correlated with increased locomotor activation in response to morphine (Deroche et al., 1992, 1993a, 1993b, 1995; Spanagel et al., 1996).
The effects of morphine on locomotor activity are biphasic with initial hypoactivity or sedation followed by a period of hyperactivity. We measured locomotor activity for 60 min, a period that should capture the sedative phase and, perhaps, the initial stages of hyperactivity. Although this time period limited our ability to examine morphine-induced hyperactivity, it was selected to allow assessment of dynamic changes in morphine-induced corticosterone secretion, which may have been missed at a later time-point. Given that corticosterone has been shown to enhance morphine-induced hyperactivity, lower levels of morphine-induced corticosterone might attenuate this effect. Thus, increased sedation in MOR-F1 males may have been due to a failure to transition into this more active phase. Additional studies examining the direct effects of glucocorticoids on locomotor responding in MOR-F1 animals are needed to determine the role of the HPA axis in mediating the observed differences in morphine-induced sedation.
The activity of the HPA axis is modulated by a number of positive and negative feedback mechanisms, including inhibition of corticotropin releasing hormone-producing neurons in the PVN by POMC-derived β-endorphin (Hellbach et al., 1998). In the brain, POMC cell bodies are primarily located in the arcuate nucleus, and both upregulation of POMC mRNA in this region and increased β-endorphin immunoreactivity have been reported following acute morphine administration (Seo et al., 2009). The effects of morphine may be mediated in part by corticotropin-releasing factor, which has been demonstrated to be a potent regulator of POMC expression and associated β-endorphin release (Almeida et al., 1986; Nikolarakis et al., 1986). However, μ-opioid receptors are located both presynaptically and postsynaptically on POMC neurons within the arcuate nucleus, and thus morphine administration may also have direct effects on POMC activity (Pennock et al., 2012).
Previous studies on the effects of prenatal morphine exposure described long-term effects on the expression of the POMC gene within the arcuate nucleus of adult offspring. Moreover, these effects were both sex-specific and subject to regulation by gonadotropins (Slamberova et al., 2004). Specifically, prenatal morphine-exposed males demonstrated decreased POMC mRNA expression, whereas female offspring had increased POMC mRNA expression. The effects in females, however, were only observed following ovariectomy, and could be eliminated by estrogen replacement. In the current study, there was a trend for MOR-F1 females to have increased POMC mRNA expression regardless of their acute treatment. As these studies were performed before puberty, it is unknown whether similar effects would be observed in adult MOR-F1 females. Moreover, as all subjects were subjected to both the stress of an injection and exposure to a novel environment, additional studies will be needed to dissociate the possible interaction between stress and the observed changes in POMC gene expression.
Among males, no differences were observed in saline-treated animals but a significant increase in POMC mRNA expression was observed in response to morphine. These effects were largely driven by the response in MOR-F1 males. This elevated POMC mRNA expression following acute morphine injection in MOR-F1 males suggests that one possible mechanism underlying the observed behavioral effects may be increased release of POMC-derived β-endorphin. Indeed, increased β-endorphin release into the nucleus accumbens and PVN would be predicted to result in increased hypoactivity and blunted corticosterone production, respectively. If one of the transgenerational effects of adolescent morphine exposure in females is enhanced β-endorphin secretion in response to morphine, then MOR-F1 males may also be more sensitive to the rewarding effects of morphine. Future studies are needed to examine shifts in reward- related behaviors in response to morphine in these offspring and to determine whether any observed behavioral effects are modulated by changes in β-endorphin release in specific brain regions.
No significant differences in TH gene expression were observed in the VTA. Changes in OPRM1 expression, however, were observed in F1 males. Activation of μ-opioid receptors in the VTA results in increased locomotor activity through inhibition of GABAergic input on dopamine neurons, which results in their increased firing (Vezina and Stewart, 1984; Vezina et al., 1989; Johnson and North, 1992; Georges et al., 2006). No significant differences in OPRM1 expression were observed in saline-treated animals; however, acute challenge with morphine resulted in increased OPRM1 expression in SAL-F1 males, whereas lower OPRM1 expression was observed in MOR-F1 males. A similar rapid induction of OPRM1 expression in the VTA, but not the substantia nigra, was reported previously following a resident-intruder stress paradigm (Nikulina et al., 1999). Thus, the increased OPRM1 expression in SAL-F1 males may be related to the significant morphine-induced increase in corticosterone secretion observed in this group. Of note, OPRM1 expression following morphine administration in MOR-F1 males was quite different from that in SAL-F1 males and instead was similar to the response observed in MOR-F1 females in that OPRM1 expression was lower following morphine administration. These findings suggest that MOR-F1 animals may not show the same types of sex differences as in SAL-F1 animals. As all of these studies were performed before puberty, such results suggest significant alterations in sex-specific organization effects on maternal exposure to morphine before conception. Moreover, these data indicate that changes in both POMC and OPRM1 gene transcription in response to acute morphine administration are significantly modified in F1 males on the basis of their mother’s adolescent drug history.
The mechanism underlying maternal transmission of altered opiate responding to the F1 generation in the absence of in-utero exposure is unknown. However, in addition to the well-known effects on pain transmission and reward processes, endogenous opioids are critical regulators of the neuroendocrine axis. Exposure to opiates can alter both the hypothalamic–pituitary–gonadal (HPG) and the HPA axes (Morley, 1981; Little and Kuhn, 1995; Zhou et al., 1999; Kreek et al., 2009). Modifications in either of these systems can induce persistent neural plasticity in various brain regions (Conrad and Bimonte-Nelson, 2010; Levy and Tasker, 2012). Moreover, in females, altering HPG and/or HPA processes can significantly impact prenatal and/or postnatal physiology and behavior. It is possible that exposure to morphine during adolescence, a period of significant reorganization of both the HPG and the HPA axes, could result in persistent modifications in maternal physiology. Indeed, we have observed significant effects of adolescent morphine exposure on gene expression within the mediobasal hypothalamus several weeks after cessation of drug administration (Byrnes, 2008). Indirect effects of morphine administration may also play a role. A reduction in body weight gain is one consequence of morphine exposure in these adolescent females. Although this effect is transitory, it could induce more long-lasting effects, particularly during this sensitive developmental period. Future studies using pair-fed controls may be useful in dissociating direct and indirect effects of adolescent opiate exposure. Regardless of the mechanisms involved, developmental exposure to opiates could modify the HPG and/or HPA axes, resulting in neurodevelopmental changes in future offspring. Much of what we understand about the interplay between the neuroendocrine axis and neurodevelopment is based on studies examining the impact of prenatal stress on offspring development. Increased activation of the HPA axis through exposure to prenatal stress results in high levels of free corticosterone in both maternal and fetal plasma (Takahashi et al., 1998). As detailed in several recent reviews, stress-induced changes in the uterine environment play a significant role in long-term developmental changes observed in offspring (Charil et al., 2010; Glover et al., 2010; Bale, 2011; Glover, 2011; Pryce et al., 2011). Administration of morphine during pregnancy also alters offspring neurodevelopment. The extent to which such offspring effects are due to direct activation of fetal opiate receptors or to morphine-induced changes in maternal physiology remains unclear. However, it is interesting to note that many of the effects of prenatal morphine administration are similar to those observed following prenatal stress, including shifts in the regulation of the HPA axis in the adult offspring of both prenatal morphine and prenatal stress exposed subjects. Moreover, both of these prenatal manipulations enhance the sensitivity of offspring to opiates (Kinsley et al., 1988; Insel et al., 1990; Spear, 1996).
Overall, the current data indicate that female adolescent morphine exposure significantly alters the development of future progeny, resulting in differences in physiological responses to the same drug that their mothers received. These effects can be observed before puberty and yet appear to be sex-specific. The nature of the observed effects implicates dysregulation of the HPA axis, which could have an impact on the risk for addiction or other stress-related disorders. Future studies are needed to investigate these long-term effects; however, it is clear from the current findings that maternal drug use before conception can impact the development of future offspring in a sex-specific manner.
Acknowledgments
This work was supported by NIH R01DA25674 (E.M.B.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Almeida OF, Nikolarakis KE, Herz A. Regulation of hypothalamic beta- endorphin and dynorphin release by corticotropin-releasing factor (CRF) NIDA Res Monogr. 1986;75:401–402. [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Gehrke BJ, Shortridge BE, Rauhut AS. Effects of beta- funaltrexamine and naloxonazine on single-trial morphine-conditioned place preference and locomotor activity. Pharmacol Biochem Behav. 2003;74:617–622. doi: 10.1016/s0091-3057(02)01049-3. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol Learn Mem. 2009;92:206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats; effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty induces long- lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Res. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227:263–272. doi: 10.1007/s00213-012-2960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenco-Choukroun G, Dauge V, Gacel G, Feger J, Roques BP. Opioid delta agonists and endogenous enkephalins induce different emotional reactivity than mu agonists after injection in the rat ventral tegmental area. Psychopharmacology (Berl) 1991;103:493–502. doi: 10.1007/BF02244249. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Choi JK, Bae JB, Lyu J, Kim TY, Kim YJ. Nucleosome deposition and DNA methylation at coding region boundaries. Genome Biol. 2009;10:R89. doi: 10.1186/gb-2009-10-9-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary- adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Costall B, Fortune DH, Naylor RJ. The induction of catalepsy and hyperactivity by morphine administered directly into the nucleus accumbens of rats. Eur J Pharmacol. 1978;49:49–64. doi: 10.1016/0014-2999(78)90221-2. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon H. Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993a;611:352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Le Moal M, Simon H. Individual differences in the psychomotor effects of morphine are predicted by reactivity to novelty and influenced by corticosterone secretion. Brain Res. 1993b;623:341–344. doi: 10.1016/0006-8993(93)91451-w. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Havemann U, Winkler M, Kuschinsky K. Is morphine-induced akinesia related to inhibition of reflex activation of flexor alpha-motoneurones? Role of the nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:101–104. doi: 10.1007/BF00506308. [DOI] [PubMed] [Google Scholar]
- Hellbach S, Gartner P, Deicke J, Fischer D, Hassan AH, Almeida OF. Inherent glucocorticoid response potential of isolated hypothalamic neuroendocrine neurons. FASEB J. 1998;12:199–207. doi: 10.1096/fasebj.12.2.199. [DOI] [PubMed] [Google Scholar]
- Insel TR, Kinsley CH, Mann PE, Bridges RS. Prenatal stress has long-term effects on brain opiate receptors. Brain Res. 1990;511:93–97. doi: 10.1016/0006-8993(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2009. Vol 1: Secondary School Students. Bethesda, MD: National Institute on Drug Abuse NIH publication; 2010. pp. 10–7584. [Google Scholar]
- Kinsley CH, Mann PE, Bridges RS. Prenatal stress alters morphine- and stress-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1988;30:123–128. doi: 10.1016/0091-3057(88)90434-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, et al. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology. 2009;56(Suppl 1):32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary- adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci. 2012;6:24. doi: 10.3389/fncel.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM. Ontogenetic studies of tolerance development: effects of chronic morphine on the hypothalamic-pituitary-adrenal axis. Psychopharmacology (Berl) 1995;122:78–84. doi: 10.1007/BF02246445. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV, Deroche V, Maccari S, Le Moal M, Simon H. Corticosterone circadian secretion differentially facilitates dopamine- mediated psychomotor effect of cocaine and morphine. J Neurosci. 1994;14(5 Pt 1):2724–2731. doi: 10.1523/JNEUROSCI.14-05-02724.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Young A. Medical and nonmedical use of prescription drugs among secondary school students. J Adolesc Health. 2007;40:76–83. doi: 10.1016/j.jadohealth.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Cranford JA, Ross-Durow P, Young A, Teter CJ, Boyd CJ. Medical misuse of controlled medications among adolescents. Arch Pediatr Adolesc Med. 2011;165:729–735. doi: 10.1001/archpediatrics.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. The endocrinology of the opiates and opioid peptides. Metabolism. 1981;30:195–209. doi: 10.1016/0026-0495(81)90172-4. [DOI] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta- endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399:152–155. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr, Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. Multiple inhibitory G-protein- coupled receptors resist acute desensitization in the presynaptic but not postsynaptic compartments of neurons. J Neurosci. 2012;32:10192–10200. doi: 10.1523/JNEUROSCI.1227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology (Berl) 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. NSDUH Series H-41 HHS Publication No (SMA) 11-4658. 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, Substance Abuse and Mental Health Services Administration, 2011. [Google Scholar]
- Seo YJ, Kwon MS, Choi SM, Lee JK, Park SH, Jung JS, et al. Possible involvement of the hypothalamic pro-opiomelanocortin gene and beta- endorphin expression on acute morphine withdrawal development. Brain Res Bull. 2009;80:359–370. doi: 10.1016/j.brainresbull.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Hnatczuk OC, Vathy I. Expression of proopiomelanocortin and proenkephalin mRNA in sexually dimorphic brain regions are altered in adult male and female rats treated prenatally with morphine. J Pept Res. 2004;63:399–408. doi: 10.1111/j.1399-3011.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- Spanagel B, Stohr T, Barden N, Holsboer F. Morphine-induced locomotor and neurochemical stimulation is enhanced in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1996;8:93–97. doi: 10.1111/j.1365-2826.1996.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of the effects of developmental toxicants: pharmacological and stress vulnerability of offspring. NIDA Res Monogr. 1996;164:125–145. [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann N Y Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Stohr T, Almeida OF, Landgraf R, Shippenberg TS, Holsboer F, Spanagel R. Stress- and corticosteroid-induced modulation of the locomotor response to morphine in rats. Behav Brain Res. 1999;103:85–93. doi: 10.1016/s0166-4328(99)00027-3. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, Stewart J. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav. 1989;32:581–584. doi: 10.1016/0091-3057(89)90201-3. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioning and place-specific sensitization of increases in activity induced by morphine in the VTA. Pharmacol Biochem Behav. 1984;20:925–934. doi: 10.1016/0091-3057(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]