Abstract
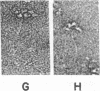
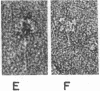
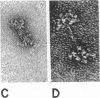
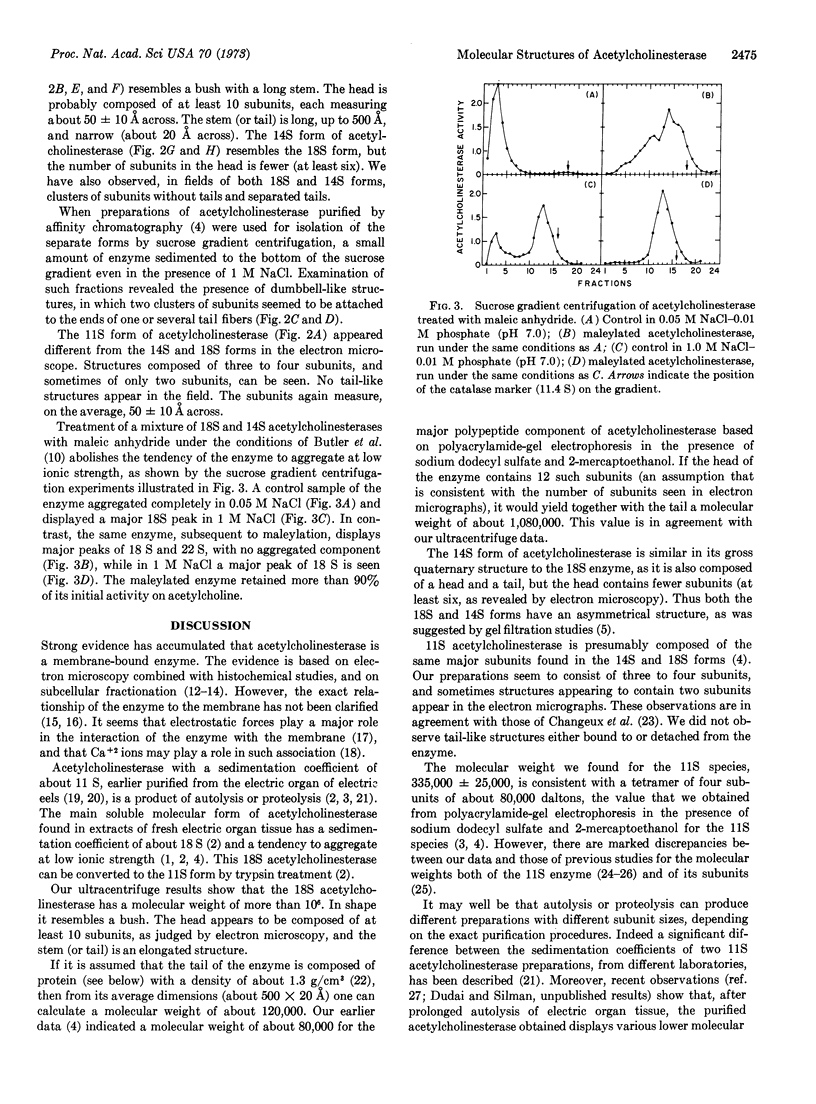
Three purified molecular forms of acetylcholinesterase (EC 3.1.1.7) with sedimentation coefficients of 18 S, 14 S, and 11 S were studied by analytical ultracentrifugation and electron microscopy. The three species have molecular weights of (1.1 ± 0.1) × 106, (7.5 ± 1.5) × 105, and (3.35 ± 0.25) × 105, respectively. Electron micrographs reveal that the 18S and 14S forms are asymmetric, composed of a head, containing a large number of subunits, and an elongated tail. The 11S form of acetylcholinesterase is apparently a tetrameric structure devoid of the tail. Maleylation of 18S and 14S acetylcholinesterases abolishes their tendency to aggregate at low ionic strength.
Keywords: electron microscopy, analytical ultracentrifugation, maleylation, protein subunits, membrane enzymes
Full text
PDF
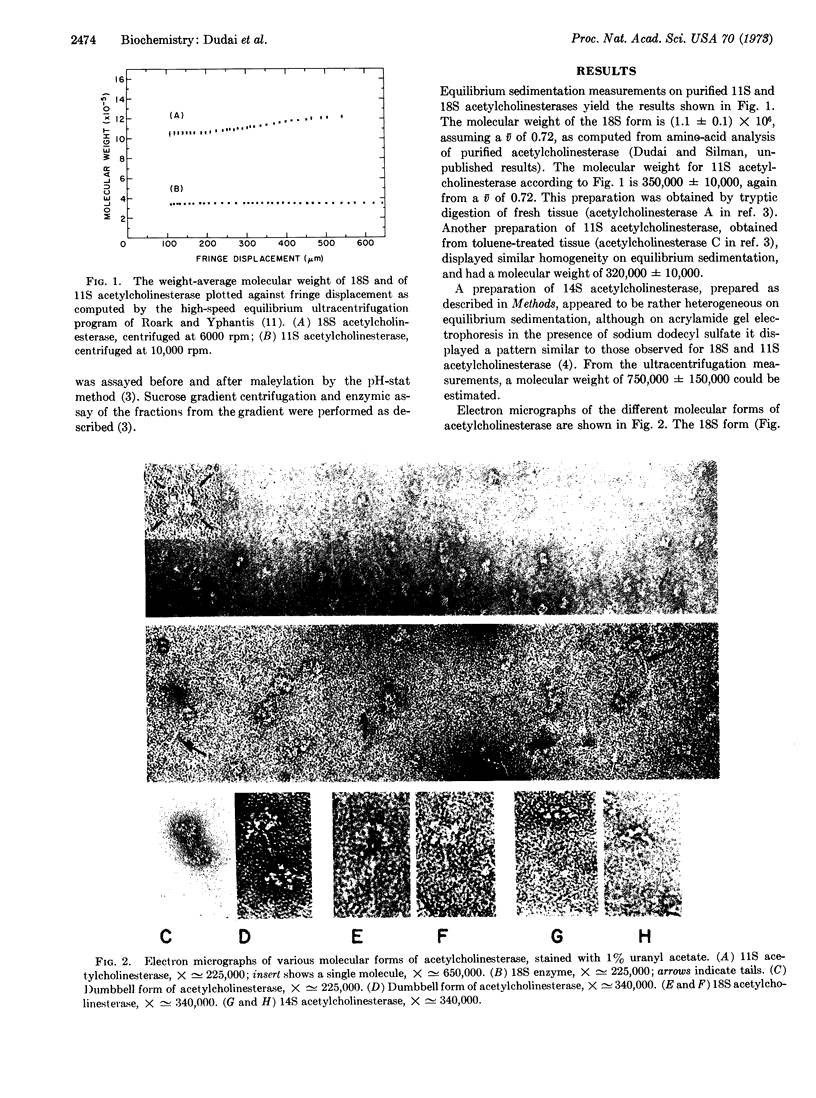

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W., Sakmann B. "Disjunction" of frog neuromuscular synapses by treatment with proteolytic enzymes. Nat New Biol. 1971 Jul 21;232(29):94–95. doi: 10.1038/newbio232094a0. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Barrnett R. J. Fine structural localization of acetylcholinesterase in electroplaque of the electric eel. J Cell Biol. 1966 Jun;29(3):475–495. doi: 10.1083/jcb.29.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Ryter A., Leuzinger W., Barrand P., Podleski T. On the association of tyrocidine with acetylcholinesterase. Proc Natl Acad Sci U S A. 1969 Mar;62(3):986–993. doi: 10.1073/pnas.62.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Weber M., Huchet M., Changeux J. P. Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972 Oct 1;26(1):43–47. doi: 10.1016/0014-5793(72)80538-6. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Kalderon N., Blumberg S. Purification by affinity chromatography of acetylcholinesterase from electric organ tissue of the electric eel subsequent to tryptic treatment. Biochim Biophys Acta. 1972 Apr 7;268(1):138–157. doi: 10.1016/0005-2744(72)90208-2. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Shinitzky M., Blumberg S. Purification by affinity chromatography of the molecular forms of acetylcholinesterase present in fresh electric-organ tissue of electric eel. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2400–2403. doi: 10.1073/pnas.69.9.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Yadin, Silman Israel. The effect of Ca(2+) on interaction of acetylcholinesterase with subcellular fractions of electric organ tissue from the electric eel. FEBS Lett. 1973 Feb 15;30(1):49–52. doi: 10.1016/0014-5793(73)80616-7. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. II. Evidence for the presence of a monomer--dimer equilibrium. Biochemistry. 1970 Feb 17;9(4):894–908. doi: 10.1021/bi00806a026. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. ELECTRON MICROSCOPE STUDIES ON THE STRUCTURE OF NATURAL AND SYNTHETIC PROTEIN FILAMENTS FROM STRIATED MUSCLE. J Mol Biol. 1963 Sep;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Kelly R. B. Enzymatic detachment of endplate acetylcholinesterase from muscle. Nat New Biol. 1971 Jul 14;232(28):62–63. doi: 10.1038/newbio232062a0. [DOI] [PubMed] [Google Scholar]
- KREMZNER L. T., WILSON I. B. A PARTIAL CHARACTERIZATION OF ACETYLCHOLINESTERASE. Biochemistry. 1964 Dec;3:1902–1905. doi: 10.1021/bi00900a020. [DOI] [PubMed] [Google Scholar]
- KREMZNER L. T., WILSON I. B. A chromatographic procedure for the purification of acetylcholinesterase. J Biol Chem. 1963 May;238:1714–1717. [PubMed] [Google Scholar]
- Leuzinger W., Baker A. L. Acetylcholinesterase, I. Large-scale purification, homogeneity, and amino Acid analysis. Proc Natl Acad Sci U S A. 1967 Feb;57(2):446–451. doi: 10.1073/pnas.57.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger W., Goldberg M., Cauvin E. Molecular properties of acetylcholinesterase. J Mol Biol. 1969 Mar 14;40(2):217–225. doi: 10.1016/0022-2836(69)90470-7. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Rieger F., Bon S. Espèces acétylcholinestérasiques globulaires et allongées des organes électriques de poissons. Eur J Biochem. 1971 Aug 25;21(4):542–551. doi: 10.1111/j.1432-1033.1971.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Rieger F. L'acétylcholinestérase des organes électriques de poissons (torpille et gymnote); complexes membranaires. Eur J Biochem. 1969 Dec;11(3):441–455. doi: 10.1111/j.1432-1033.1969.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Rieger F., Tsuji S. Solubilisation de l'acetylcholinestérase des organes électriques de gymnote. Action de la trypsine. Eur J Biochem. 1970 Jul;14(3):430–439. doi: 10.1111/j.1432-1033.1970.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Nachmansohn D. Proteins in excitable membranes: their properties and function in bioelectricity are discussed. Science. 1970 May 29;168(3935):1059–1066. doi: 10.1126/science.168.3935.1059. [DOI] [PubMed] [Google Scholar]
- Rieger F., Bon S., Massoulié J. Observation par microscopie électronique des formes allongées et globulaires de l'acétylcholinestérase de gymnote (Electrophorus electricus. Eur J Biochem. 1973 May 2;34(3):539–547. doi: 10.1111/j.1432-1033.1973.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]
- Shelton E., Yonemasu K., Stroud R. M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc Natl Acad Sci U S A. 1972 Jan;69(1):65–68. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman H. I., Karlin A. Effect of local pH changes caused by substrate hydrolysis on the activity of membrane-bound acetylcholinesterase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1664–1668. doi: 10.1073/pnas.58.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A. J., Moses M. J. The structure of the central region in the synaptonemal complexes of hamster and cricket spermatocytes. J Cell Biol. 1973 Jan;56(1):145–152. doi: 10.1083/jcb.56.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svehag S. E., Manhem L., Bloth B. Ultrastructure of human C1q protein. Nat New Biol. 1972 Jul 26;238(82):117–118. doi: 10.1038/newbio238117a0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]