Abstract
Background
Blepharitis, an inflammatory condition associated with itchiness, redness, flaking, and crusting of the eyelids, is a common eye condition that affects both children and adults. It is common in all ethnic groups and across all ages. Although infrequent, blepharitis can lead to permanent alterations to the eyelid margin or vision loss from superficial keratopathy (abnormality of the cornea), corneal neovascularization, and ulceration. Most importantly, blepharitis frequently causes significant ocular symptoms such as burning sensation, irritation, tearing, and red eyes as well as visual problems such as photophobia and blurred vision. The exact etiopathogenesis is unknown, but suspected to be multifactorial, including chronic low‐grade infections of the ocular surface with bacteria, infestations with certain parasites such as demodex, and inflammatory skin conditions such as atopy and seborrhea. Blepharitis can be categorized in several different ways. First, categorization is based on the length of disease process: acute or chronic blepharitis. Second, categorization is based on the anatomical location of disease: anterior, or front of the eye (e.g. staphylococcal and seborrheic blepharitis), and posterior, or back of the eye (e.g. meibomian gland dysfunction (MGD)). This review focuses on chronic blepharitis and stratifies anterior and posterior blepharitis.
Objectives
To examine the effectiveness of interventions in the treatment of chronic blepharitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2012, Issue 1), MEDLINE (January 1950 to February 2012), EMBASE (January 1980 to February 2012), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We searched the reference lists of included studies for any additional studies not identified by the electronic searches. There were no date or language restrictions in the electronic searches for trials. The electronic databases were last searched on 9 February 2012.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐randomized controlled trials (CCTs) in which participants were adults aged 16 years or older and clinically diagnosed with chronic blepharitis. We also included trials where participants with chronic blepharitis were a subset of the participants included in the study and data were reported separately for these participants. Interventions within the scope of this review included medical treatment and lid hygiene measures.
Data collection and analysis
Two authors independently assessed search results, reviewed full‐text copies for eligibility, examined risk of bias, and extracted data. Data were meta‐analyzed for studies comparing similar interventions and reporting comparable outcomes with the same timing. Otherwise, results for included studies were summarized in the text.
Main results
There were 34 studies (2169 participants with blepharitis) included in this review: 20 studies (14 RCTs and 6 CCTs) included 1661 participants with anterior or mixed blepharitis and 14 studies (12 RCTs and 2 CCTs) included 508 participants with posterior blepharitis (MGD). Due to the heterogeneity of study characteristics among the included studies, with respect to follow‐up periods and types of interventions, comparisons, and condition of participants, our ability to perform meta‐analyses was limited. Topical antibiotics were shown to provide some symptomatic relief and were effective in eradicating bacteria from the eyelid margin for anterior blepharitis. Lid hygiene may provide symptomatic relief for anterior and posterior blepharitis. The effectiveness of other treatments for blepharitis, such as topical steroids and oral antibiotics, were inconclusive.
Authors' conclusions
Despite identifying 34 trials related to treatments for blepharitis, there is no strong evidence for any of the treatments in terms of curing chronic blepharitis. Commercial products are marketed to consumers and prescribed to patients without substantial evidence of effectiveness. Further research is needed to evaluate the effectiveness of such treatments. Any RCT designed for this purpose should separate participants by type of condition (e.g. staphylococcal blepharitis or MGD) in order to minimize imbalances between groups (type I errors) and to achieve statistical power for analyses (prevent type II errors). Medical interventions and commercial products should be compared with conventional lid hygiene measures, such as warm compresses and eyelid margin washing, to determine effectiveness, as well as head‐to‐head to show comparative effectiveness between treatments. Outcomes of interest should be patient‐centered and measured using validated questionnaires or scales. It is important that participants be followed long‐term, at least one year, to assess chronic outcomes properly.
Keywords: Humans, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Blepharitis, Blepharitis/pathology, Blepharitis/therapy, Chronic Disease, Hygiene, Randomized Controlled Trials as Topic, Steroids, Steroids/therapeutic use
Plain language summary
Interventions for blepharitis
Blepharitis, defined as inflammation of the eyelids, is a common eye condition and affects both children and adults. Blepharitis can be categorized in several different ways. First, categorization is based on the length of disease process: acute or chronic blepharitis. Second, categorization is based on the anatomical location of disease: anterior, or front of the eye (e.g. staphylococcal and seborrheic blepharitis), and posterior, or back of the eye (e.g. meibomian gland dysfunction (MGD)). This review focuses on chronic blepharitis and stratifies anterior and posterior blepharitis. There were 34 studies (2169 participants with blepharitis) included in the review, 20 of which included participants with anterior blepharitis and 14 of which included participants with posterior blepharitis. For anterior blepharitis, topical antibiotics provided some symptomatic relief and were effective in clearing bacteria from the eyelid margins. There was no difference between the types of topical antibiotics used. Topical steroids also provided some symptomatic relief; however, they were ineffective in eliminating bacteria. Lid hygiene, including warm compresses and lid scrubs, showed some symptomatic relief in both anterior and posterior blepharitis. Overall, there was no strong evidence for any of the treatments in terms of curing chronic blepharitis. Further research should be done to evaluate the effectiveness of treatments for blepharitis, with particular attention paid to adequate diagnosis and classification of the disease.
Summary of findings
for the main comparison.
| Topical antibiotics compared with placebo for anterior/mixed blepharitis (7 studies) | ||||
|
Population: participants with anterior/mixed blepharitis Intervention: topical antibiotics Comparison: placebo | ||||
| Outcomes | Studies and outcomes* | Comments | ||
| Definition of outcome |
Mean difference, IV, Fixed (95% CI) or RR, M‐H, Fixed (95% CI) |
No of participants (studies) | ||
|
Clinical outcomes: overall clinical improvement Follow‐up: 3 to 14 days |
Day 3 (2 studies) | |||
| Mean scores based on 5‐point rating scale (Hyndiuk 1990) | ‐0.90 (‐1.47 to ‐0.33) | 39 (1 study) | 1 additional study reported no significant difference between groups in mean change from baseline of total scores based on 4‐point rating scale (Shulman 1982) | |
| Proportion cured or improved (Hyndiuk 1990) | 1.53 (0.98 to 2.38) | 39 (1 study) | ||
| Proportion cured (Hyndiuk 1990) | 15.75 (0.96 to 258.08) | 39 (1 study) | ||
| Day 7 (4 studies) | ||||
| Mean change from baseline in clinical scores based on 5‐point rating scale for signs and VAS for symptoms (Behrens‐Baumann 2006); mean scores based on 5‐point rating scale (Hyndiuk 1990); and mean scores based on 4‐point rating scale of signs and symptoms, and presence or absence of additional problems (Jackson 1982) | ‐0.76 (‐1.30 to ‐0.23) | 264 (3 studies) | 1 additional study reported no significant difference between groups in mean change from baseline of total scores based on 4‐point rating scale (Shulman 1982) | |
| Proportion cured or improved (Hyndiuk 1990) | 1.35 (1.00 to 1.84) | 39 (1 study) | ||
| Proportion cured (Hyndiuk 1990) | 2.46 (1.19 to 5.05) | 39 (1 study) | ||
| Day 14 (4 studies) | ||||
| Mean change from baseline in clinical scores based on 5‐point rating scale for signs and VAS for symptoms (Behrens‐Baumann 2006); and mean scores based on 4‐point rating scale of signs and symptoms, and presence or absence of additional problems (Jackson 1982) | ‐1.37 (‐2.43 to ‐0.30) | 225 (2 studies) | 1 additional study reported no significant difference between groups in per cent of participants with improvement based on 4‐point rating scale (Donshik 1983); 1 additional study reported no significant difference between groups in mean change from baseline of total scores based on 4‐point rating scale (Shulman 1982) | |
| Other follow‐up times (1 study) | ||||
| Clinical evaluation and participant questionnaires (no time point or further details provided) (Laibovitz 1991) | Not estimable | Not reported (1 study) | Study reported that participants receiving topical antibiotics were more likely to describe themselves as cured (P = 0.024); clinical improvement detected in participants with moderate disease (P = 0.034) | |
|
Clinical outcomes: improvement in signs Follow‐up: 3 days to 8 weeks |
Day 3 (2 studies) | |||
| Mean change in scores based on 4‐point rating scale for lid discharge (Donshik 1983) | Not estimable | Not reported (1 study) | Study reported no significant difference between groups | |
| Mean change from baseline based on 4‐point rating scale for individual signs: lid edema, lid hyperemia (Shulman 1982) | Not estimable | 35 (1 study) | Study reported no significant difference between groups | |
| Day 7 (2 studies) | ||||
| Mean scores based on 4‐point rating scale of signs, and presence or absence of additional problems (Jackson 1982) | ‐0.06 (‐1.36 to 1.24) | 30 (1 study) | ||
| Mean change in scores based on 4‐point rating scale for lid discharge (Donshik 1983) | Not estimable | Not reported (1 study) | Study reported no significant difference between groups | |
| Day 14 (3 studies) | ||||
| Mean scores based on 4‐point rating scale of signs, and presence or absence of additional problems (Jackson 1982) | ‐0.29 (‐1.60 to 1.02) | 28 (1 study) | ||
| Mean change in scores for based on 4‐point rating scale for lid discharge (Donshik 1983) | Not estimable | Not reported (1 study) | Study reported no significant difference between groups | |
| Proportion with severe or very severe grading: lid edema (Behrens‐Baumann 2006) | 0.35 (0.14 to 0.87) | 75 (1 study) | ||
| Proportion with severe or very severe grading: lid erythema (Behrens‐Baumann 2006) | 0.46 (0.27 to 0.80) | 108 (1 study) | ||
| Proportion with severe or very severe grading: lid debris (Behrens‐Baumann 2006) | 0.40 (0.15 to 1.08) | 104 (1 study) | ||
| Proportion with severe or very severe grading: meibomitis (Behrens‐Baumann 2006) | 0.42 (0.24 to 0.74) | 109 (1 study) | ||
| Other follow‐up times (1 study) | ||||
| Proportion with improvement based on 5‐point rating scale of signs during first 4 weeks of cross‐over trial (More 1968) | 1.14 (0.77 to 1.69) | 13 (1 study) | ||
| Proportion with improvement based on 5‐point rating scale of signs during second 4 weeks of cross‐over trial (More 1968) | 0.21 (0.03 to 1.43) | 13 (1 study) | ||
|
Clinical outcomes: improvement in symptoms Follow‐up: 7 days to 8 weeks |
Day 7 (1 study) | |||
| Mean scores based on 4‐point rating scale of symptoms (Jackson 1982) | 0.19 (‐0.65 to 1.03) | 30 (1 study) | ||
| Day 14 (2 studies) | ||||
| Mean scores based on 4‐point rating scale of symptoms (Jackson 1982) | 0.04 (‐0.75 to 0.83) | 28 (1 study) | ||
| Mean change in VAS rating from baseline for ocular discomfort (Behrens‐Baumann 2006) | Not estimable | 197 (1 study) | Study reported significant difference between groups (P = 0.011) | |
| Other follow‐up times (1 study) | ||||
| Proportion with improvement based on 5‐point rating scale of symptoms during first 4 weeks of cross‐over trial (More 1968) | 1.14 (0.77 to 1.69) | 13 (1 study) | ||
| Proportion with improvement based on 5‐point rating scale of symptoms during second 4 weeks of cross‐over trial (More 1968) | 1.29 (0.31 to 5.31) | 13 (1 study) | ||
|
Bacteriologic outcomes Follow‐up: 3 to 28 days |
Day 3 (2 studies) | |||
| Mean bacterial colony counts (Hyndiuk 1990) | ‐426.00 (‐539.94 to ‐312.06) | 39 (1 study) | 1 additional study reported that topical antibiotics were significantly more effective than placebo in rendering lid cultures negative (Shulman 1982) | |
| Day 7 (1 study) | ||||
| Mean bacterial colony counts (Hyndiuk 1990) | ‐454.00 (‐659.68 to ‐248.32) | 39 (1 study) | ||
| Day 14 (2 studies) | ||||
| Lid cultures (Donshik 1983; Jackson 1982) | 4.21 (2.10 to 8.44) | 70 (2 studies) | ||
| Other follow‐up times (2 studies) | ||||
| Quantitative cultures (time not reported) (Laibovitz 1991) | Not estimable | Not reported (1 study) | Study reported a reduction in the incidence of positive cultures (P = 0.00000035) relative to placebo | |
| Conjunctival cultures at week 4; end of first cross‐over phase (More 1968) | 0.50 (0.06 to 3.91) | 10 (1 study) | ||
|
Adverse outcomes Follow‐up: up to 8 weeks |
Proportion of total adverse events: bibrocathol (Behrens‐Baumann 2006), mercuric oxide (Hyndiuk 1990), and penotrane (More 1968) | 0.91 (0.60 to 1.38) | 268 (3 studies) | Individual analyses for each type of antibiotic were not significant 1 additional study reported that 3 participants receiving gentamicin had increased ocular hyperemia and itching; no increases in IOP were detected in any group (Donshik 1983); and another reported that 3 participants receiving gentamicin had an allergic reaction; no abnormal increases in IOP were detected in any group (Shulman 1982) 1 additional study reported that 1 participant in the placebo group had irritation; 5 participants ended the study with inferior epithelial keratitis; no difference in IOP between groups (Jackson 1982) |
*Of the studies that compared topical antibiotics with placebo 6/7 reported overall clinical outcomes; 4/7 reported outcomes for signs and 3/7 reported outcomes for symptoms separately; 6/7 reported bacteriologic outcomes; and 6/7 reported adverse outcomes. Treatment effects in bold were statistically significant.
95% CI: 95% confidence interval
IOP: intraocular pressure IV, Fixed: generic inverse variance method, fixed‐effect model
MGD: meibomian gland dysfunction M‐H, Fixed: Mantel‐Haenszel method, fixed‐effect model RR: risk ratio
VAS: visual analog scale
Background
Description of the condition
Blepharitis, defined as inflammation of the eyelids, is one of the most common ocular conditions and affects both children and adults (Lemp 2009; Viswalingham 2005). Blepharitis can be categorized in several different ways. First, categorization is based on the length of disease process: acute and chronic blepharitis. Acute blepharitis, referred to by some as lid infection, may be bacterial, viral, or parasitic in etiology (Eliason 2005) and is beyond the scope of this review. The more common form is chronic blepharitis, or lid inflammation. Though McCulley 1982 previously classified chronic blepharitis into six categories, it more recently has been divided into three categories: staphylococcal, seborrheic, and meibomian gland dysfunction (MGD) (AAO 2008). Further, many clinicians prefer to classify blepharitis based on anatomic location where anterior blepharitis causes inflammation primarily at the base of the eyelashes (staphylococcal and seborrheic blepharitis are often grouped together and referred to as anterior blepharitis), posterior blepharitis affects the posterior lid margin (the section of the eyelid that comes into contact with the cornea and bulbar conjunctiva), and marginal blepharitis includes both anterior and posterior blepharitis (Nelson 2011). MGD affects primarily the oil glands located on the posterior lid and therefore it is included as a subset of posterior blepharitis.
Staphylococcal blepharitis is believed to be associated with staphylococcal bacteria on the ocular surface. However, the mechanism by which the bacteria cause symptoms of blepharitis is not fully understood. Comparisons in bacterial flora between normal eyes and those diagnosed with staphylococcal blepharitis have identified some differences. Only 8% of normal patients had cultures positive for Staphylococcus aureus as compared to 46% to 51% of those diagnosed with staphylococcal blepharitis (Dougherty 1984; McCulley 1984). Patients with staphylococcal blepharitis were found to be similar dermatologically to matched controls (McCulley 1985). Hordeolum, a nodular inflammatory lesion of the eyelid arising from either the hair follicles or the meibomian gland, is often associated with staphylococcal blepharitis (Probst 2005). On the other hand, in two studies, 92% to 97% of patients with blepharitis had cultures positive for Staphylococcus epidermis, proportions not significantly different from control populations (Dougherty 1984; McCulley 1984).
Since only half of patients diagnosed with staphylococcal blepharitis had positive cultures for S. aureus it is likely that there are additional contributing factors. Some researchers have hypothesized that toxins produced by certain strains of S. aureus or S. epidermis may be a cause of irritation (Valenton 1973). However, a specific toxin more associated with clinically blepharitic lids than controls has not been identified (Seal 1990). Enhanced cell‐mediated immunity to S. aureus was found in 40% of patients with blepharitis and these patients more often required topical corticosteroid therapy (Ficker 1991). The significance of these findings is poorly understood.
Seborrheic blepharitis is characterized by less inflammation than staphylococcal blepharitis but with more oily or greasy scaling. Some patients with seborrheic blepharitis also exhibit characteristics of MGD. Since the meibomian glands are derived from the sebaceous glands of the skin, the finding of MGD in patients with generalized sebaceous gland abnormality is not surprising (Raskin 1992).
Posterior blepharitis is characterized by inflammation of the posterior lid margin and has various causes, such as MGD, infectious or allergic conjunctivitis, and systemic conditions such as acne rosacea (Nelson 2011). MGD is a condition that affects the glands on the posterior lid margin that are responsible for secreting meibum, the outermost oily layer of the tear film. This substance has several functions important in normal eye health and comfort. Meibum is responsible for slowing evaporation of the tear film, preventing contamination of the tear film, thickening the tear film, and smoothing the tear film to provide an even optical surface (Driver 2005). Patients with MGD have tears that evaporate more quickly than controls (Mathers 1993; Rolando 1985), leaving the eye susceptible to ocular surface damage and discomfort.
Quantitative or qualitative deficiencies in meibum may be responsible for the symptoms experienced in MGD blepharitis. Hyperkeratinization of the meibomian gland epithelium (thickening of the lining of the glands) may lead to obstruction and a decrease in the quantity of meibomian gland secretions (Jester 1989a; Jester 1989b). Meibomian gland obstruction has been found to be associated with increased tear evaporation and ocular surface damage and discomfort (Shimazaki 1995) due to a quantitative decrease in the protective oil layer.
Qualitative differences in the composition of meibum between patients with MGD and controls have also been reported. Dougherty 1986a and Dougherty 1991b found that patients with MGD had significant differences in free fatty acids in the secretions of their meibomian glands as compared to controls. Similarly, Shine 1991 found cholesterol esters in all patients with MGD but only half of normal controls. It is not known whether these differences are present in endogenous secretions or whether bacterial enzymes may modify the secretions on the surface of the eye (Dougherty 1986b; Dougherty 1991a; Probst 2005). Changes in these protective portions of the tear film may decrease their effectiveness and contribute to inflammation and irritation.
Demodex mites have also been considered a causative factor for blepharitis (Czepita 2007). The mites, which infest the eyelid margin around the lash follicles and sebaceous glands, may have a role in both anterior and posterior blepharitis. It is theorized that the infestation and waste of the mites causes blockage of the follicles and glands and/or an inflammatory response.
Epidemiology
Though not sight‐threatening, chronic blepharitis is one of the most common ocular disorders encountered by ophthalmologists (McCulley 2000). In a survey of US ophthalmologists and optometrists, 37% to 47% of patients seen by those surveyed had signs of blepharitis (Lemp 2009). In 1982 blepharitis was responsible for 590,000 patient visits in the USA (NDTI 1982). However, few epidemiologic data exist that estimate the true prevalence of blepharitis.
In a case‐control study conducted in the San Francisco Bay area and Texas, staphylococcal blepharitis occurred more commonly in women and had an average age of onset of 42 years (McCulley 1982; McCulley 1985). Also, it was postulated that staphylococcal blepharitis occurred more frequently in warmer climates (Bowman 1987). Approximately 25% to 50% of cases were associated with keratoconjunctivitis sicca (KCS), a class of dry eye syndrome (McCulley 1982; McCulley 1985). KCS is associated with a reduced aqueous tear film production in contrast to dry eyes from abnormal evaporation.
In the same study the mean age of participants with seborrheic blepharitis was 50 years (McCulley 1985). There was no difference in prevalence between men and women. Ninety‐five per cent of participants with seborrheic blepharitis also had seborrheic dermatitis that presents with symptoms of flaking and greasy skin on the scalp, retroauricular area, glabella, and nasolabial folds (McCulley 1982). Approximately one third of those participants had KCS (McCulley 1984).
The incidence of MGD increases with age (Driver 2005). The average age in the McCulley 1982 group of MGD blepharitis patients was 50 years and prevalence was equal between men and women (McCulley 1984). MGD may be more common in cooler climates (Bowman 1987). MGD seems to be more common in fair‐skinned individuals but this may be due to its association with acne rosacea, which is also more prevalent in this population (Driver 1996). Acne rosacea is characterized by skin telangiectasias (dilated superficial blood vessels), erythema, papules, and pustules. It was diagnosed in 20% of MGD patients (McCulley 1982). Also, 46% were diagnosed with seborrheic dermatitis (McCulley 1982). Chalazia are more common in patients with MGD. Since a chalazion (a sterile, chronic, nodular inflammation of the meibomian glands) is thought to be due to obstruction of the gland orifice it is expected that patients with MGD would be at risk.
In a study conducted in Florida, Groden 1991 found that the prevalence of acne rosacea was 44% and the prevalence of KCS was 30% in a cohort of participants with all types of blepharitis. In an Austrian study of 407 patients with chronic blepharitis, 14.5% of participants had KCS, 32.9% had seborrheic dermatitis, and 26.7% had acne rosacea (Huber‐Spitzy 1991).
Presentation and diagnosis
Symptoms of blepharitis include burning, itchiness, gritty feeling of the eyes, contact lens intolerance, photophobia (light‐sensitivity), and redness and crusting of the eyelid margins. Symptoms are usually worse in the mornings and a patient may have several exacerbations and remissions.
Staphylococcal blepharitis is characterized on examination by erythema and edema of the eyelid margin. Telangiectasia may be present on the anterior eyelid. Brittle scales may be seen in the eyelashes and these may form collarettes, which encircle the lash at the base or further up as the lash grows. In severe and long‐standing cases trichiasis (misdirection of eyelashes toward the eye), poliosis (depigmentation of the eyelashes), madarosis (loss of eyelashes), eyelid ulceration, and eyelid and corneal scarring may occur (AAO 2008).
Seborrheic blepharitis is differentiated by less erythema, edema, and telangiectasia of the lid margins as compared to staphylococcal blepharitis but an increased amount of oily scale and greasy crusting on the lashes (McCulley 1985).
Posterior blepharitis may be seen clinically by examining the posterior eyelid margin. The meibomian glands may appear capped with oil, be dilated, or be visibly obstructed. The secretions of the glands are usually turbid and thicker than normal. Telangiectasias and lid scarring may also be present in this area. In all forms of blepharitis examination of the tear film may show instability and rapid evaporation.
Description of the intervention
Though the pathophysiology of anterior and posterior blepharitis may be different, the treatment options are similar. Current practice is such that patients generally are offered treatment if they report discomfort or experience visual symptoms. Initial treatment is eyelid hygiene, which includes warm compresses, eyelid massage, and eyelid scrubs (AAO 2008; Geerling 2011). McCulley 1984 recommends that warm compresses be applied two to four times daily with a warm facecloth for 5‐ to 10‐minute intervals in the acute phase of blepharitis. The warm compresses raise the temperature of the eyelid above the melting point for meibomian gland secretions and thus aid in expression. Eyelid massage, which consists of pressing the eyelid against the eyeball, is thought to help milk excess secretions from the meibomian glands. Eyelid scrubs, which consist of gently scrubbing the eyelids with a wet washcloth and detergent such as baby shampoo or one of a number of commercially available products, are performed after the warm compresses to clear away scale and debris that have accumulated on the eyelid margin. As blepharitis is a chronic disease, eyelid hygiene must be performed even after an acute exacerbation has resolved. Adverse effects of lid hygiene treatment are few but may include mechanical irritation from overly vigorous scrubbing or sensitivity reaction to the detergents used.
All forms of blepharitis may benefit from a course of treatment with topical corticosteroid drops to decrease inflammation in an acute exacerbation. The American Academy of Ophthalmology (AAO 2008) recommends applying drops several times daily, tapered to discontinuation over one to three weeks. However, corticosteroids may have significant adverse effects over the long‐term such as increased intraocular pressure (IOP), posterior subcapsular cataract formation, and superinfection. For this reason they are not recommended for long‐term use.
Staphylococcal and seborrheic blepharitis may be treated with topical antibiotics, preferably in ointment form in order to coat the lids better. Ointment is applied after lid hygiene maneuvers once or twice daily depending on the severity of the inflammation (Raskin 1992). Erythromycin and bacitracin are commonly prescribed. Antibiotic therapy may be discontinued in two to eight weeks or once symptoms resolve. Some patients require chronic therapy in order to remain symptom free (McCulley 1984).
In patients with posterior blepharitis, oral tetracycline or doxycycline may be effective (AAO 2008). Though clinical improvement requires several weeks, once it is achieved therapy may be discontinued or tapered to maintenance doses. Improvement in posterior blepharitis with tetracyclines may be related to inhibition of bacterial lipases in both S. aureus and S. epidermidis (Dougherty 1991a). Tetracyclines are also effective in the treatment of facial acne rosacea (Driver 2005). Adverse effects include photosensitization, gastrointestinal upset, vaginitis, and hypersensitivity (AAO 2008). Tetracyclines should not be used orally in pregnant or lactating women or children younger than eight years old because of the risk of tooth enamel abnormalities (Driver 1996). Also they interact with some medicines such as coumadin and oral cholesterol‐lowering drugs. Oral minocycline, a broad‐spectrum tetracycline antibiotic, showed some benefit in treating MGD in two case series (Aronowicz 2006; Shine 2003).
A report from the International Workshop on MGD recommends increasing dietary intake of essential fatty acids, specifically omega‐3 fatty acid, in cases of mild‐to‐severe MGD (Geerling 2011). The recommendation was added to the overall treatment algorithm for MGD because essential fatty acids may be beneficial to anti‐inflammatory processes and because oral supplements have been associated with reduced dry eye symptoms.
Why it is important to do this review
Blepharitis is a common chronic disease whose etiology is poorly understood. Commercial products are available and marketed to patients, but it is not clear whether or not they are effective. The AAO Preferred Practice Pattern Guidelines (AAO 2008) rate the strength of evidence to support lid hygiene, topical antibiotics, topical corticosteroids, and oral tetracyclines as treatment for blepharitis as level III ‐ a consensus opinion in the absence of substantial controlled evidence. Since the literature search by the AAO encompassed only English‐language articles published between 1997 and 2007 a more complete systematic approach is warranted to identify trials and to highlight any evidence gaps in the literature. This review focuses on the evidence to support therapeutic interventions for blepharitis.
Objectives
The objective of this review was to examine the effectiveness of medical and mechanical interventions in improving patient symptoms, as well as clinical signs, for the treatment of chronic blepharitis. For the purposes of this review, mechanical interventions include any nonmedical and nonsurgical intervention aimed to physically treat the condition, such as eyelid hygiene (washing or scrubbing of the eyelid margin) and the application of warm compresses.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs) and quasi‐randomized controlled trials (CCTs). CCTs were defined as studies that did not use randomization to allocate participants to treatment groups, but that attempted to use a nonbiased method of treatment assignment such as birth date, social security number, or medical record number of a consecutive sample of eligible patients.
Types of participants
We included trials in which participants were adults aged 16 years or older, clinically diagnosed with chronic blepharitis, inclusive of staphylococcal, seborrheic, or MGD. Because there were no standardized diagnostic protocols for chronic blepharitis or for the three subtypes we also included studies where the type of chronic blepharitis was not specified and studies that categorized chronic blepharitis using a different classification (e.g. meibomitis, primary meibomitis, meibomian keratoconjunctivitis). We included trials where participants with chronic blepharitis were a subset of the participants included in the study as long as outcomes were reported separately for these participants.
Types of interventions
We included studies in which the following comparisons were made: (1) one medicine (topical antibiotics, topical corticosteroids, combinations of topical antibiotics and corticosteroids, systemic antibiotics, systemic corticosteroids, or other pharmacologic treatments) was compared to a different medicine, a different dose of the same medicine, placebo, or no treatment; (2) lid hygiene (hot compress, warm compress, eyelid massage, eyelid scrubbing with dilute baby shampoo, sodium bicarbonate solution, saline, and commercially available eyelid scrubbing/cleansing solution or detergent) alone was compared to lid hygiene plus medicines, medicines alone, placebo, or no treatment.
Types of outcome measures
Primary outcomes
The primary outcomes for this review included:
subjective improvement in symptoms as judged by patient symptom report measured by questionnaire, interview or visual analog scale (VAS), including but not limited to: irritation, burning, tearing, itching, eyelid sticking, photophobia, and increased frequency of blinking
improvement in clinical exam findings as judged by examiners, including but not limited to: injection/erythema of eyelid margins, scaling, abnormalities of eyelashes, abnormalities in quality or quantity of tear film, and abnormalities of posterior eyelid margin and meibomian orifices
Since there are no standardized diagnostic criteria for blepharitis and no standardized scales on which to judge symptom severity, clinical improvement in symptoms was expected to vary among studies. Although it would have been ideal for studies to use validated scales, all scales used in included studies were considered for inclusion since standardized information was unavailable.
Secondary outcomes
The secondary outcome measures included:
measurements of eradication or decrease in numbers of colonies of positive cultures of bacteria
adverse events measured by patient report or changes in clinical findings
quality‐of‐life measures
economic costs and benefits of different interventions
Follow‐up
Some treatments often were used for short periods of time. Other treatments, such as oral antibiotics, often were used chronically. There were, therefore, no minimum or maximum periods of follow‐up required for inclusion. Because of the possibility of difficulty in differentiating between persistent blepharitis symptoms and recurrent exacerbations, sensitivity analyses were performed to gauge the impact on review findings of studies with follow‐up greater than four weeks.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 1, part of The Cochrane Library. www.thecochranelibrary.com (accessed 9 February 2012), MEDLINE (January 1950 to February 2012), EMBASE (January 1980 to February 2012), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 9 February 2012.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), mRCT (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We searched the reference lists of included studies for any additional studies not identified by the electronic searches. We contacted experts in the field for information on current, past, or unpublished trials. We did not specifically handsearch any conference proceedings or journals for the purpose of this review.
Data collection and analysis
Selection of studies
Two review authors independently assessed search results and selected those that possibly fit the 'Criteria for considering studies for this review' as defined in the published protocol for this review. We obtained full‐text copies of all reports that were selected by at least one review author. Two review authors independently reviewed the full‐text copies for eligibility. Reports that were excluded at this stage were documented and the reasons for exclusion were noted. We resolved discrepancies by consensus.
Data extraction and management
Two review authors independently extracted data from reports from eligible trials onto data extraction forms. Study characteristics extracted for each trial included methods, participants, interventions, and outcomes. Any relevant data not included in these fields were placed in the category labeled 'notes'. We presented in table format the study characteristics extracted. One review author entered the data into Review Manager (Review Manager 2011) and a second review author verified the data entered. We resolved any discrepancies by discussion. We extracted continuous and dichotomous data that were pertinent to the outcomes described in this review.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included trials according to the methods published in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the studies on five parameters: selection bias (sequence generation and allocation concealment), performance bias (masking of participants and researchers), detection bias (masking of those responsible for assessing outcomes), attrition bias (rates of follow‐up between groups and intention‐to‐treat (ITT) analysis), and reporting bias (selective outcome reporting) as well as other sources of bias. For each bias domain, two review authors independently judged the study to have a low risk of bias, an unclear risk of bias, or a high risk of bias. We presented descriptive documentation of the details of each parameter for each study in table format also. We contacted trial authors when additional data were necessary to evaluate bias parameters or when the risk of bias was judged to be unclear. When we were unable to contact the trial authors, the parameter was judged on the information that was available.
Measures of treatment effect
We anticipated that the included studies would use different rating scales for assessing clinical outcomes. For each included study we assessed the validity and reliability of each rating scale as supported by previous studies. Data from valid rating scales with more than 10 categories were to be treated as continuous variables with a normal distribution. When this assumption could not be made we planned to dichotomize using a clinically relevant cut‐off point (e.g. reduction in patient symptom report score by one unit) and treat it as a dichotomous variable. When the included studies used different cut‐off points for valid rating scales we adopted their definitions in the meta‐analysis.
We reported the weighted mean difference for all continuous outcomes and rating scales. We reported the standardized mean difference when different valid rating scales were reported in the included studies. In addition, we reported a risk ratio (RR) for all dichotomous outcomes including any rating scales that were dichotomized based on a clinically relevant cut‐off point.
Unit of analysis issues
The unit of analysis was the eye. For systemic interventions (such as oral medications) the unit of analysis was the individual. Studies that included both eyes of study participants were analyzed as they were reported.
Dealing with missing data
We contacted study authors for additional information when data were missing or incomplete. We set the response time at four weeks; if no reply was received in that time we used the data available in the published report.
Assessment of heterogeneity
We examined the Chi2 test and the I2 statistic for identifying heterogeneity. A Chi2 P value less than 0.05 or an I2 greater than 60% was interpreted as substantial heterogeneity.
Assessment of reporting biases
We examined the symmetry of funnel plots to assess reporting biases when more than three studies were included in a meta‐analysis.
Data synthesis
We conducted meta‐analyses of studies with comparable outcomes and timing of outcomes. We used a random‐effects model to combine study results in meta‐analyses. When there were fewer than three studies and there was no heterogeneity detected, a fixed‐effect model was used. We documented study results that were not compatible for meta‐analysis and summarized the overall treatment effects as reported by each study.
Subgroup analysis and investigation of heterogeneity
Anterior and posterior blepharitis were analyzed separately according to the classifications provided by the authors of the included studies. There were insufficient data to conduct subgroup analyses based on other study or clinical characteristics.
Sensitivity analysis
We conducted sensitivity analyses to examine the impact of excluding unpublished studies, industry‐funded studies, and studies with lower methodologic quality when sufficient data were available.
Results
Description of studies
Results of the search
The electronic search of the literature identified 1801 records, of which 1726 were excluded and 75 were assessed as relevant or possibly relevant for this review (Figure 1). Of the 75 full‐texts assessed, 42 reports from 40 studies were excluded, 32 reports from 31 studies were included, and one report for one study is awaiting classification. Manual searching yielded 15 additional reports assessed at the full‐text level. Of these 15 reports, 11 were excluded, three were included, and one was a report from an already included study. Thus, overall there were 53 reports from 51 studies excluded by full‐text assessment and 36 reports from 34 studies included in the review.
1.
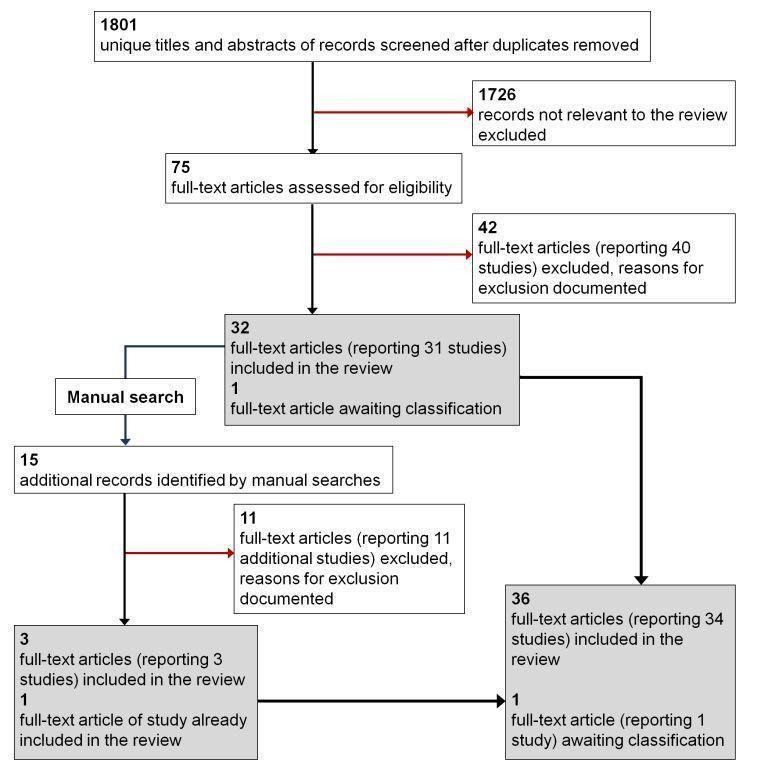
Flow diagram: Results from searching for studies for inclusion in the review.
We could not classify the eligibility of the one study awaiting assessment based on the available information, but we attempted to contact the primary investigators for clarification (John 2008). We will update the review with information on this study as it becomes available.
Included studies
There were 34 studies included in the review (Characteristics of included studies). Twenty‐six (76%) of the studies were RCTs and the remainder were CCTs. A summary of the study participants, interventions, and follow‐up periods from the included studies is presented in Table 2. The included studies were published between 1956 and 2011, enrolled 13 to 464 participants with blepharitis each, and followed participants from 30 minutes to 12 months. Individual trials typically were small; only seven enrolled 100 or more participants. Overall 2383 participants, of which 2169 had blepharitis or blepharoconjunctivitis, were enrolled in the included studies. For the purposes of this review, studies were stratified by the anatomic location of blepharitis: anterior/mixed (e.g. staphylococcal and seborrheic) and posterior (e.g. MGD).
1. Summary of included studies.
|
Study ID Study Design |
Condition(s) included | Number of participants (n) | Interventions studied | Follow‐up period(s) | |
| Treatment(s) | Comparison(s) | ||||
| Anterior/mixed staphylococcal or seborrheic blepharitis | |||||
|
Laibovitz 1991 CCT |
Blepharitis | NR | Topical antibiotic (1% tetracycline ointment) |
Placebo (placebo ointment) |
NR |
|
Behrens‐Baumann 2006 RCT |
Blepharitis | 203 | Topical antibiotic (5% bibrocathol (Noviform) ointment) |
Placebo (vehicle ointment) |
7 and 14 days |
|
Hyndiuk 1990 RCT |
Bacterial blepharitis | 58 | Topical antibiotic (1% mercuric oxide (yellow) ophthalmic ointment) |
Placebo (anhydrous ointment base without active ingredient) |
3 and 7 days |
|
More 1968 CCT |
Chronic or recurrent blepharitis | 13 | Topical antibiotic (0.033% penotrane lotion in a Lissapol and glycerin base and 0.033% penotrane hydroxymethylcellulose gel) |
Placebo (lotion base and gel base without penotrane) |
two 4‐week phases; cross‐over trial |
|
Nguyen 1990 CCT |
Blepharitis | 29 | Topical antibiotic (ciprofloxacin ophthalmic solution) |
Topical antibiotic (tobramycin ophthalmic solution) |
7 days |
|
Adenis 1996a RCT |
External ocular disease: acute conjunctivitis or acute or chronic blepharitis | Acute conjunctivitis (n = 44), chronic blepharitis (n = 21), acute blepharitis (n = 10), and others (n = 2) | Topical antibiotic (0.3% ciprofloxacin ophthalmic solution + eyelid margin scrub for participants with blepharitis) |
Topical antibiotic (1% fusidic acid gel plus eyelid margin scrub for participants with blepharitis) |
7 days |
|
Bloom 1994 RCT |
Blepharitis and blepharoconjunctivitis | 464 | Topical antibiotic (0.3% ciprofloxacin ophthalmic solution) |
Topical antibiotic (0.3% tobramycin ophthalmic solution) |
7 days |
|
Seal 1995 RCT |
Chronic blepharitis with and without associated rosacea | 61 | Topical antibiotic + oral antibiotic (topical 1% fusidic acid in a carbomer gel made isotonic by adding mannitol, buffered to pH 5.5, and preserved plus oral 250 mg oxytetracycline) |
1. Topical antibiotic + oral placebo (topical 1% fusidic acid plus placebo tablet) 2. Topical placebo + oral antibiotic (placebo gel plus oral 250 mg oxytetracycline) |
4 and 8 months |
|
Aragones 1973 RCT |
Infectious blepharitis | 30 | Topical antibiotic + steroid (10% sodium sulfacetamide plus 0.2% prednisolone acetate suspension) |
Topical antibiotic alone (10% sodium sulfacetamide) |
NR |
|
Donshik 1983 RCT |
Chronic staphylococcal blepharoconjunctivitis | 100 | Topical antibiotic + steroid (0.3% gentamicin sulfate and 0.1% betamethasone phosphate ophthalmic solution) |
1. Topical steroid (0.1% betamethasone phosphate ophthalmic solution) 2. Topical antibiotic (0.3% gentamicin sulfate ophthalmic solution) 3. Placebo (sterile vehicle placebo solution) |
3, 7, and 14 days |
|
Jackson 1982 RCT |
Symptomatic infective blepharitis or blepharoconjunctivitis | 46 | Topical antibiotic + steroid (0.3% gentamicin sulfate and 0.1% betamethasone sodium phosphate ointment plus lid margin scrub) |
1. Topical antibiotic (0.3% gentamicin sulfate ointment plus lid margin rub) 2. Placebo (ointment or mineral oil plus lid margin scrub) |
7 and 14 days |
|
Shulman 1982 RCT |
Chronic staphylococcal blepharoconjunctivitis | 87 | Topical antibiotic + steroid (0.3% gentamicin sulfate and 0.1% betamethasone phosphate ointment plus lid margin scrub) |
1. Topical antibiotic (0.3% gentamicin sulfate ophthalmic ointment plus lid margin scrub) 2. Topical steroid (0.1% betamethasone phosphate ointment plus lid margin scrub) 3. Placebo (vehicle ointment plus lid margin scrub) |
3, 7, and 14 days |
|
Goldberg 1960 CCT |
External ocular disease: inflammatory and/or infectious eye diseases | Multiple conditions (n = 185) including blepharoconjunctivitis (16 unilateral and 11 bilateral) | Topical antibiotic + steroid (1 mg/cc triamcinolone acetonide and 2.5 mg/cc gramicidin ophthalmic solution) |
Topical steroid (1 mg/cc triamcinolone acetonide) |
40 days |
|
White 2008 RCT |
Blepharokeratoconjunctivitis | 276 | Topical antibiotic + steroid (0.3% tobramycin and 0.5% loteprednol etabonate ophthalmic suspension) |
Topical antibiotic + steroid (0.1% tobramycin and 0.3% dexamethasone ophthalmic suspension) |
3, 7, and 14 days |
|
Nelson 1990 RCT |
Seborrheic and mixed seborrheic/staphylococcal blepharitis | 40 | Topical antifungal (2% ketoconazole cream) |
Placebo (lanolin base only cream) |
5 weeks on treatment |
|
Wong 1956 RCT |
Marginal blepharitis | 60 | Topical antifungal (0.5% selenium sulfide ophthalmic ointment) |
Topical antibiotic (0.5% ammoniated mercury ophthalmic ointment) |
4 weeks |
|
Collum 1984 RCT |
Chronic blepharitis | 40 | Anti‐inflammatory (4% disodium cromoglycate ointment) |
Placebo (placebo ointment of yellow paraffin and acetylated lanolin) |
4 weeks |
|
Key 1996 CCT |
Chronic blepharitis | 26 | Lid scrub with OCuSoft pad | Lid scrub with Neutrogena bar soap (replaced with baby shampoo during extension period) | 4 months, 3‐month extension |
|
Wasserman 1989 RCT |
Chronic blepharitis | 20 | Scrubs + compress (lid hygiene with commercial eye makeup remover, application of adrenocorticosteroid ointment (0.1% fluorometholone) to lid margin, followed by placement of lyophilized collagen eye pads) |
1. Scrubs (lid hygiene with commercial eye makeup remover and application of adrenocorticosteroid ointment (0.1% fluorometholone) to lid margin) 2. Baby shampoo (lid hygiene with 1:2 dilution of baby shampoo and application of adrenocorticosteroid ointment (0.1% fluorometholone) to lid margin) |
10 days |
|
Sore 2002 CCT |
Blepharitis | 60 | Astringent compress (0.1% isotonic zinc sulfate solution) |
Selenium compress (natural selenium‐rich thermal water) |
1 month |
| Posterior blepharitis/MGD | |||||
|
Luchs 2008 RCT |
MGD | 21 | Topical antibiotic + warm compress (1% topical azithromycin ophthalmic solution) |
Warm compress alone | 14 days |
|
Yoo 2005 RCT |
Chronic MGD | 150 | 1. High‐dose oral antibiotic (200 mg systemic doxycycline monohydrate) 2. Low‐dose oral antibiotic (20 mg systemic doxycycline hyclate) |
Placebo (placebo pill) |
1 month |
|
Perry 2006 RCT |
MGD | 33 | Topical immunosuppressant (topical 0.05% cyclosporine A) |
Placebo (Refresh Plus preservative‐free artificial tears) |
1 and 3 months |
|
Rubin 2006 RCT |
MGD | 30 | Topical immunosuppressant (0.05% topical cyclosporine ophthalmic emulsion) |
Topical antibiotic + steroid (0.3% tobramycin plus 0.1% dexamethasone ophthalmic solution) |
2, 4, 6, 8, 10, and 12 weeks |
|
Yalçin 2002 RCT |
Chronic MGD | 40 | Oral mucolytic agent + control treatment (100 mg oral N‐acetylcysteine) |
Control treatment (topical steroid (prednisone acetate) and antibiotic (tobramycin sulfate), plus warm compress and artificial tears (polyvidone)) |
4 months |
|
Akyol‐Salman 2010 RCT |
MGD | 20 | Topical mucolytic agent (5% N‐acetylcysteine ophthalmic solution) |
Preservative‐free artificial tears | 1 month |
|
Macsai 2008 RCT |
MGD | 38 | Dietary supplement (1000 mg flaxseed oil capsules (55% omega‐3 fatty acid, 15% omega‐6 fatty acid, and 19% omega‐9 fatty acid)) |
Placebo (olive oil capsules) |
1 year |
|
Pinna 2007 RCT |
MGD | 57 | Dietary supplement + lid hygiene (28.5 mg oral linoleic acid and 15 mg γ‐linolenic acid + eyelid hygiene consisting of warm eyelid compresses, massage, and scrubbing) |
1. Lid hygiene (warm eyelid compresses, massage, and scrubbing) 2. Dietary supplement (28.5 mg oral linoleic acid and 15 mg γ‐linolenic acid) |
6 months |
|
Goto 2002 RCT |
Noninflamed obstructive MGD | 20 | Oil eyedrops (2% castor oil, 5% polyoxyethylene castor oil, 0.3% sodium chloride, 0.15% potassium chloride, and 0.5% boric acid emulsion) |
Placebo (normal saline solution) |
2, 4, and 6 weeks |
|
Mori 2003 CCT |
MGD | 25 | Eye warmer (disposable eyelid warming device) |
Control (no treatment) |
2 weeks |
|
Olson 2003 RCT |
MGD | 20 | Warm compress (white cotton napkins saturated with tap water and warmed to 40 °C) |
Control compress (white cotton napkins saturated with tap water and left at room temperature) |
5, 15, and 30 minutes, 5 minutes post‐therapy |
|
Ishida 2008 CCT |
MGD | 20 | Carbon fiber eye warmer (eye mask applied overnight during sleeping) |
Conventional eye warmer (eye mask applied overnight during sleeping) |
2 weeks |
|
Matsumoto 2006 CCT |
MGD | 20 | Warm moist air (warm moist air device with 60 °C air) |
Warm compress control (towels heated and wetted with 60 °C water) |
2 weeks |
|
Friedland 2011 RCT |
MGD | 14 | Automated and heated massage device (lid warmer and massaging eye cup) |
Automated and heated massage device followed by manual expression | 3 months |
CCT: controlled clinical trial (quasi‐randomized controlled trial) mg: milligram NR: not reported RCT: randomized controlled trial
Anterior/mixed staphylococcal and seborrheic blepharitis
Twenty (59%) of the 34 included studies examined the effectiveness of interventions for the treatment of anterior or mixed blepharitis (1661 participants). Of these 20 studies, 14 (70%) studies were RCTs and six (30%) studies were CCTs. In 13 (65%) studies all participants had blepharitis, whereas the remaining seven studies included participants with varying ocular conditions, a subset of whom had blepharitis. The number of participants with blepharitis enrolled in each study ranged from 13 to 464. The follow‐up periods ranged from seven days to eight months; the majority of which were four weeks or less.
The interventions and comparisons investigated varied across studies. Four studies were two‐arm trials comparing topical antibiotics (Laibovitz 1991) or antibacterial agents (Behrens‐Baumann 2006; Hyndiuk 1990; More 1968) with placebo. Three studies were two‐arm trials comparing ciprofloxacin ophthalmic solution with another topical antibiotic, one of which used the same doses for each treatment (Bloom 1994), one used differing doses (Adenis 1996a), and one did not report the doses (Nguyen 1990). One study was a partial cross‐over trial investigating a topical antibiotic and an oral antibiotic, using topical and oral placebos as controls (Seal 1995). Five studies compared combinations of topical antibiotics/antibacterial agents and corticosteroids with the same dose of topical antibiotics (Donshik 1983; Jackson 1982; Shulman 1982) or antibacterial agents (Aragones 1973) alone, corticosteroids alone (Donshik 1983; Goldberg 1960; Shulman 1982), and/or placebo (Donshik 1983; Jackson 1982; Shulman 1982), with or without lid hygiene. One study compared combination topical antibiotic and corticosteroid with another combination of topical antibiotic and corticosteroid (White 2008). Three other studies investigated drug interventions: Nelson 1990 and Wong 1956 compared topical antifungal drugs with placebo and the same dose of topical antibacterial agent, respectively, and Collum 1984 compared a topical anti‐inflammatory agent with placebo. The three remaining studies evaluated lid hygiene interventions: two studies (Key 1996; Wasserman 1989) compared different types of lid scrub regimens with/without compresses and Sore 2002 compared astringent compresses with selenium compresses.
Clinical assessments, including subjective and/or clinical improvement of signs and/or symptoms, were performed as outcome measurements in all studies. With the exception of five studies (Behrens‐Baumann 2006; Goldberg 1960; Key 1996; Seal 1995; White 2008), bacteriologic outcomes also were reported. Fourteen (70%) of 20 studies reported adverse events.
Posterior blepharitis/MGD
Fourteen studies (41% of included studies), including 12 RCTs and two CCTs, assessed the effectiveness of interventions among participants with MGD. All studies were limited to participants with MGD (508 participants). Follow‐up periods varied from one day to one year. Sample sizes ranged from 14 to 150 participants in each study.
There was considerable variation in the types of interventions investigated. Two studies investigated treatment with antibiotics: one studied topical antibiotics plus warm compresses versus warm compresses alone (Luchs 2008) and the other studied high and low doses of oral antibiotic versus placebo (Yoo 2005). Two studies compared a topical immunosuppressant agent with placebo (Perry 2006) or with topical antibiotics plus steroids (Rubin 2006). One study evaluated a treatment regimen of topical antibiotics and steroids plus warm compresses and artificial tears with or without supplementation with an oral mucolytic agent (Yalçin 2002). A topical mucolytic agent was compared with artificial tears in another study (Akyol‐Salman 2010). One long‐term study compared a dietary supplement with placebo (Macsai 2008). One study compared a dietary supplement and lid hygiene with either the supplements or lid hygiene alone (Pinna 2007). One study looked at oil eyedrops versus normal saline solution as control (Goto 2002). Four studies compared warm compresses with no treatment (Mori 2003), control compresses at room temperature (Olson 2003), another type of warm compress (Ishida 2008), or warm moist air treatment at the same temperature (Matsumoto 2006). The final study investigated an automated heater and massaging device with or without additional manual meibomian gland expression (Friedland 2011).
All studies performed clinical assessments, such as tear break‐up time (BUT), Schirmer's score, and improvement of symptoms. None of the studies performed bacteriologic assessments. Five studies reported adverse events (Akyol‐Salman 2010; Goto 2002; Luchs 2008; Yalçin 2002; Yoo 2005).
Excluded studies
See the 'Characteristics of excluded studies' table.
There were 51 potentially relevant studies excluded from this review after full‐text assessment. Of the 51 studies excluded, 10 studies were excluded because they were not RCTs or CCTs; 20 studies were excluded because they did not include populations of interest; 18 studies were excluded because multiple ocular conditions were included in the study population, but cases with blepharitis were not reported separately; one study was excluded because it did not evaluate an intervention of interest; one study was excluded because it did not evaluate any outcomes of interest; and the final study was excluded because a copy of the conference abstract could not be obtained and the available information was insufficient to include in the review.
Risk of bias in included studies
Allocation
Twenty‐six (76%) of the included studies were RCTs. Fourteen of the RCTs (54%) had adequate sequence generation methods (such as random numbers lists, randomization schemes, or independent coordinating centers) and were, therefore, judged to have a low risk of sequence generation bias (Figure 2). Further, eight of these RCTs also were judged to have adequately concealed allocation by using coded prescription bottles (Aragones 1973; Nelson 1990), sealed envelopes (Jackson 1982), or sequentially numbered treatment kits (White 2008); allocating participants to treatment groups after study enrollment (Behrens‐Baumann 2006; Donshik 1983); or assigning treatment groups through a pharmacy department or individual separate from the recruiting department (Mori 2003; Seal 1995). Investigators of one RCT did not conceal allocation following randomization (Pinna 2007). Authors of the other five studies did not report methods for allocation concealment and we assessed these studies as having an unclear risk of bias for this parameter (Akyol‐Salman 2010; Luchs 2008; Macsai 2008; Perry 2006; Shulman 1982). Treatment groups were divided randomly by a co‐author for one RCT (4%); however, it was not clear what method of randomization was used (Goto 2002). The method of allocation concealment for this study was not reported. Methods for randomization and allocation concealment were not reported for the remaining 11 RCTs (44%).
2.
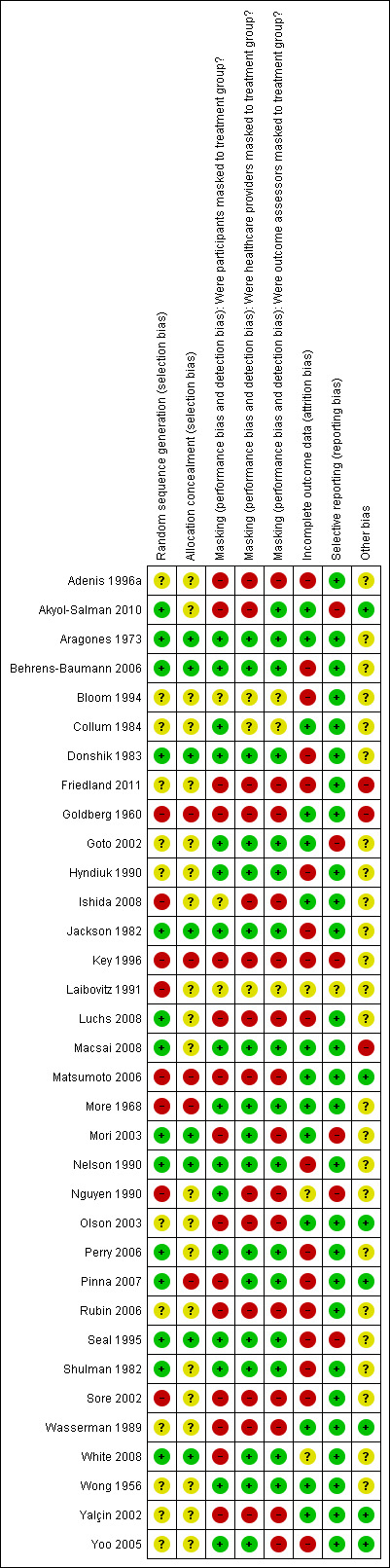
Risk of bias summary: review authors' judgments about each 'Risk of bias' item for each included study.
There were eight (24%) CCTs included in the review. Three studies reported how treatment groups were divided: left eye versus right eye (Key 1996), odd versus even birth date (More 1968), or alternate allocation (Matsumoto 2006). Based on these grouping methods, allocation could not be concealed for these studies. The authors of the remaining five studies did not give details on how groups were assigned or whether allocation was concealed.
Masking (performance bias and detection bias)
Participants, healthcare providers, and outcome assessors were masked in 13 (38%) of the 34 included studies (Figure 2). Two studies reported being double‐masked, but no details were given as to how masking was done or who was masked (Bloom 1994; Laibovitz 1991). As such these studies were judged to have an unclear risk of bias for masking for all study participants and personnel. Two studies were reported as open trials and thus no masking was done (Adenis 1996a; Friedland 2011). One study did not report masking (Sore 2002). Studies that were unmasked or did not report masking were considered to have a high risk for bias for these parameters.
In addition to the 13 studies for which all study participants and personnel were masked, there were three studies in which participants were masked to treatment groups (Collum 1984; Nguyen 1990; Yoo 2005). Using identically packaged, coded bottles and/or distributing placebo to nonactive treatment groups was considered adequate masking of participants. Due to differences between the interventions under investigation, participants could not be masked to treatment allocation in nine studies (26%) (Goldberg 1960; Key 1996; Luchs 2008; Matsumoto 2006; Mori 2003; Olson 2003; Pinna 2007; Wasserman 1989; Yalçin 2002). Authors of one study reported that participants were not masked (White 2008) and authors of two studies did not report masking participants (Akyol‐Salman 2010; Rubin 2006). Studies that were unmasked or for which masking was not reported were considered to have a risk for bias for this parameters. Authors of one study reported that participants were masked to treatment groups, but that the two eye warming masks being studied "had obvious design and appearance differences" (Ishida 2008). Since there were observable differences between the interventions for the two groups, we assessed the risk of bias in masking participants as unclear for this study.
There were an additional four studies in which healthcare providers were masked (Mori 2003; Pinna 2007; White 2008; Yoo 2005). Masking of healthcare providers was classified as adequate when masked codes were used to assign treatment, identically packaged and coded bottles were dispensed, treatment was dispensed through a pharmacy, and/or masking of healthcare providers was reported specifically in the paper. In five studies healthcare providers were unmasked due to allocation, study methods or differences between the interventions under investigation (Akyol‐Salman 2010; Goldberg 1960; Key 1996; Olson 2003; Rubin 2006).
Authors of one study that also reported to be double‐masked provided methods for masking of participants, but did not specify who else was masked or how (Collum 1984). Hence, masking for healthcare providers and outcome assessors was classified as unclear for this study. There was one study in which masking of participants was reported, but masking for other study personnel was not reported (Nguyen 1990). For five studies in which masking of participants could not be done or was not done, masking was not reported or not done for either healthcare providers or outcome assessors (Ishida 2008; Luchs 2008; Matsumoto 2006; Wasserman 1989; Yalçin 2002).
Outcome assessors were masked in three additional studies (Akyol‐Salman 2010; Pinna 2007; White 2008). Masking of outcome assessors was judged as adequate when study treatments were coded and/or masking of outcome assessors was reported specifically in the paper. In four studies outcome assessors were unmasked due to allocation, study outcomes, or differences between the interventions under investigation (Key 1996; Mori 2003; Olson 2003; Rubin 2006). Masking of outcome assessors was not reported in two additional studies (Goldberg 1960; Yoo 2005).
Incomplete outcome data
Incomplete outcome data due to exclusions or losses to follow‐up (attrition) were documented for each study (Characteristics of included studies). Studies that followed ITT analysis were judged to have a low risk of bias for this parameter. ITT analysis was defined as 1) keeping participants by the intervention groups to which they were randomized, regardless of the intervention they actually received; 2) measuring outcome data on all participants; and 3) including all randomized participants in the analysis (Higgins 2011). Analysis was based on ITT in 14 studies (41%) (Figure 2).
One study (3%) reported using ITT analysis, however three participants were not included in the ITT analysis; thus this study was judged to have an unclear risk of bias for this parameter (White 2008). For two studies (6%), reported only in abstracts, it was unclear whether there were incomplete outcome data or whether ITT analysis was followed (Laibovitz 1991; Nguyen 1990). The remaining 17 studies (50%) had incomplete outcome data and did not use ITT analysis.
Selective reporting
Since none of the included studies published protocols previous to publishing the results of the trial, outcomes listed in the methods sections of the articles or from clinical trial registries were compared with the reported results to assess for risk of selective reporting bias. Twenty‐seven (79%) studies reported outcome results as described in the methods of their papers (Figure 2). For one study (3%), reported only in an abstract, study outcomes were unclear (Laibovitz 1991). In the remaining six studies (18%), at least one study outcome that was described in the methods was not reported by treatment group and/or at the specified follow‐up time (Akyol‐Salman 2010; Goto 2002; Key 1996; Mori 2003; Nguyen 1990; Seal 1995).
Other potential sources of bias
Other potential sources of bias were assessed in the included studies. For seven studies (21%) no other potential sources of bias were identified (Akyol‐Salman 2010; Matsumoto 2006; Olson 2003; Pinna 2007; Wasserman 1989; Yalçin 2002; Yoo 2005). In the remaining 27 studies (79%) at least one of the following sources of potential bias were identified:
15 studies (44%) were funded or supported by industry (Adenis 1996a; Aragones 1973; Behrens‐Baumann 2006; Friedland 2011; Goto 2002; Hyndiuk 1990; Ishida 2008; Jackson 1982; Luchs 2008; Macsai 2008; More 1968; Mori 2003; Perry 2006; Rubin 2006; White 2008)
10 studies (29%) included at least one author who was employed by or affiliated with industry (Behrens‐Baumann 2006; Collum 1984; Friedland 2011; Goto 2002; Nelson 1990; Perry 2006; Seal 1995; Shulman 1982; Sore 2002; White 2008)
conditions of study participants were not limited to blepharitis in seven studies (21%) (Adenis 1996a; Bloom 1994; Donshik 1983; Goldberg 1960; Jackson 1982; Shulman 1982; White 2008). Including participants with multiple conditions was considered to introduce potential bias when allocation was not stratified by condition leading to an imbalance between groups (type I errors) or insufficient power for subgroup analyses (type II errors)
in one study (3%), every effort was made to recruit and enroll participants wearing contact lenses (Key 1996). The concurrent use of contact lenses during treatment for blepharitis typically is not recommended and could introduce bias if proportionately different between treatment groups. Among the 20/26 participants who wore contact lenses, eight participants wore soft contact lenses and 12 participants wore rigid gas‐permeable contact lenses. The distribution of use of lenses or type of lenses was not reported by treatment group
three studies (9%) used a cross‐over study design and may have had potential carry‐over in cross‐over phases (Goto 2002; More 1968; Seal 1995). Moreover, the Seal 1995 study implemented placebo‐treatment periods that were not concurrent with the active‐treatment periods. In the first and third phases of the study all participants received placebo, and in the second and fourth phases of the study, participants received one of three active‐treatment regimens. Thus, active treatments were not compared concurrently with placebo for this study
in four studies (12%), the unit of analysis (each eye per individual) differed from the unit of randomization (the individual) (Goto 2002; Luchs 2008; Macsai 2008; Wong 1956). In another study the unit of analysis was unclear (Sore 2002)
in one study (3%), the intervention for both treatment groups was prepared differently during the study (Goldberg 1960). It was unclear whether the study investigators intended for two types of preparations to be used from the beginning, or if the second preparation was added after the trial began since it was easier to administer. It was also not clear why the dosage was prescribed on an individual basis and what effect this may have had on the results
two studies (6%) were not published as full‐text, peer‐reviewed articles (Laibovitz 1991; Nguyen 1990)
Effects of interventions
See: Table 1
Anterior/mixed staphylococcal and seborrheic blepharitis
Medical (drug) interventions
Topical antibiotics versus placebo (7 studies)
In five RCTs (Behrens‐Baumann 2006; Donshik 1983; Hyndiuk 1990; Jackson 1982; Shulman 1982) and two CCTs (Laibovitz 1991; More 1968) topical antibiotics were compared with placebo for the treatment of blepharitis and/or blepharoconjunctivitis. Four studies were two‐arm trials in which the active treatments included bibrocathol ointment (Behrens‐Baumann 2006), mercuric oxide ointment (Hyndiuk 1990), tetracycline ointment (Laibovitz 1991), and penotrane gel (More 1968). Two studies used a 2 x 2 factorial design to investigate the therapeutic effectiveness of gentamicin (antibiotic) and betamethasone (steroid) (Donshik 1983; Shulman 1982). The last study had three treatment groups including combination gentamicin‐betamethasone, gentamicin only, and placebo (Jackson 1982). This section compares the gentamicin‐treated group with the placebo‐treated group for the multi‐arm studies.
Risk of bias
Three of the five RCTs were at low risk of selection bias (sequence generation and allocation concealment), performance bias (masking of participants and healthcare providers), and detection bias (masking of outcome assessors) (Behrens‐Baumann 2006; Donshik 1983; Jackson 1982). Allocation concealment for Hyndiuk 1990 and Shulman 1982 was unclear, but these two RCTs were at low risk of performance bias and detection bias. By not using or reporting randomization, the two CCTs were assessed at high risk of selection bias (Laibovitz 1991; More 1968). Masking of participants, healthcare providers, and outcome assessors was done in the More 1968 study, but was not reported in Laibovitz 1991. More 1968 was the only study in which all participants enrolled were included in the analyses. No study was assessed to be at high risk of reporting bias.
All seven studies had unclear risk of bias for at least one of the following reasons. Five of the seven studies were funded or affiliated with industry (Behrens‐Baumann 2006; Hyndiuk 1990; Jackson 1982; More 1968; Shulman 1982). Three studies included participants with blepharoconjunctivitis (Donshik 1983; Jackson 1982; Shulman 1982). One study was a cross‐over study with no washout period (More 1968). One study was reported as an abstract only (Laibovitz 1991).
Primary outcomes
Five studies reported clinical outcomes as final mean scores or mean changes in scores from baseline for signs and/or symptoms, although outcome definitions and timing varied between studies (Behrens‐Baumann 2006; Donshik 1983; Hyndiuk 1990; Jackson 1982; Shulman 1982). Two studies reported the proportion of participants in each treatment group who were cured or had clinical improvement in signs and/or symptoms (Hyndiuk 1990; More 1968). One study reported per cent improvement of clinical signs based on a 4‐point rating scale (Donshik 1983). One study reported only P values for patient‐ and clinician‐reported clinical outcomes (Laibovitz 1991).
At day 3, one study (Hyndiuk 1990) found overall mean sign and symptom scores to be significantly lower for the antibiotic group compared with placebo (mean difference (MD) ‐0.90; 95% confidence interval (CI) ‐1.47 to ‐0.33) and another study (Shulman 1982) reported mean change in overall scores was not significantly different between treatment groups (MD ‐1.03; 95% CI not estimable) (Analysis 1.1). Donshik 1983 noted that improvement in lid discharge was not different between groups and Shulman 1982 reported that neither lid edema nor lid erythema were different between groups at day 3. The Hyndiuk 1990 study, which favored antibiotics when comparing mean scores, did not show a significant effect when comparing the proportion of participants cured or improved in the antibiotic group with the placebo group (RR 1.53; 95% CI 0.98 to 2.38).
1.1. Analysis.
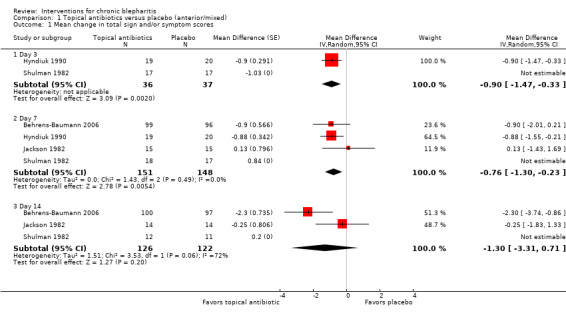
Comparison 1 Topical antibiotics versus placebo (anterior/mixed), Outcome 1 Mean change in total sign and/or symptom scores.
At day 7, five studies reported final means or mean changes from baseline in overall sign and symptom scores, three of which provided sufficient data for meta‐analysis. The summary estimate (MD ‐0.76; 95% CI ‐1.30 to ‐0.23) suggests topical antibiotics are more effective than placebo in reducing signs and symptoms of blepharitis (Analysis 1.1); however, this estimate is heavily influenced by the Hyndiuk 1990 study. Shulman 1982 reported overall mean change in scores were not significantly different between treatment groups (MD 0.84; 95% CI not estimable) and Donshik 1983 noted that improvement in lid discharge did not differ significantly between groups. The Hyndiuk 1990 study, which was the only study to favor antibiotics at day 7, also found a statistically significant effect when comparing the proportion of participants cured or improved in the antibiotic group with the placebo group (RR 1.35; 95% CI 1.00 to 1.84).
At day 14, two studies comparing mean improvement in overall sign and symptom scores suggested a beneficial effect for topical antibiotic use compared with placebo, but were not combined in meta‐analysis due to significant statistical heterogeneity (I2 = 72%; Analysis 1.1). Shulman 1982 reported overall mean change in scores were not significantly different between treatment groups (MD 0.20; 95% CI not estimable) and Donshik 1983 noted that improvement in lid discharge did not differ significantly between groups. The Behrens‐Baumann 2006 study, which was the only individual study to favor antibiotics at day 14, reported that the proportion of participants with severe or very severe grading was significantly lower in the antibiotic group compared with the placebo group for separate assessments of lid edema, lid erythema, and meibomitis. Also, the proportion of participants with severe or very severe grading for lid debris were lower in the antibiotic group compared with the placebo group, but there was no statistically significant difference between groups. This study reported that participants rated greater improvements in ocular discomfort with antibiotic treatment than with placebo as well (P = 0.011). In Donshik 1983, a 62% improvement for clinical rating of signs and symptoms was observed for the antibiotic group compared with 57% in the placebo group. This difference was reported as not statistically different. Jackson 1982 also noted that participants in placebo group had a higher risk of recurrence within six weeks of end of study (75% with placebo versus 5% with other groups combined); however, the measure of significance was not reported.
One study reported that participants receiving topical antibiotics were more likely to describe themselves as cured (P = 0.024) and clinical improvement was detected in participants with moderate disease (P = 0.034) (Laibovitz 1991). No further details were provided as the study was reported as an abstract only.
Results of an eight‐week cross‐over trial showed no significant difference between topical antibiotics and placebo in treating chronic blepharitis (More 1968). At the end of the first four‐week phase, 6/6 participants in the antibiotic group had improvement in both signs and symptoms compared with 6/7 in the placebo group (RR 1.14; 95% CI 0.77 to 1.69).
Secondary outcomes
Of the six studies that measured bacteriologic outcomes, five studies favored topical antibiotics over placebo. Due to heterogeneity in outcome definitions and timing, most studies could not be included in meta‐analysis.
At day 3, Shulman 1982 reported that topical antibiotics were significantly more effective than placebo in rendering lid cultures negative. Hyndiuk 1990 reported that mean bacterial colony counts were significantly lower for the antibiotic group than the placebo group at days 3 and 7. At day 14, topical antibiotics were significantly more effective than placebo in eradicating bacteria from the lid margin in two studies (Donshik 1983; Jackson 1982), but was not statistically significant using a random‐effects model (summary RR 4.21; 95% CI 2.10 to 8.44) (Analysis 1.2). Laibovitz 1991 reported a significant reduction of the incidence of positive cultures in the antibiotic group relative to placebo (P = 0.00000035).
1.2. Analysis.
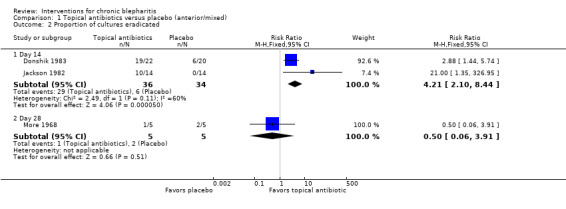
Comparison 1 Topical antibiotics versus placebo (anterior/mixed), Outcome 2 Proportion of cultures eradicated.
Conjunctival cultures taken at week 4 (end of first cross‐over phase) by More 1968 suggested no significant difference between antibiotic (1/5 negative) and placebo (2/5 negative) in eradicating bacteria. Bacteriologic outcomes were not measured by Behrens‐Baumann 2006.
Adverse events
There was no significant difference in the number of adverse events between antibiotic and placebo groups for the six studies that reported adverse events. Three studies with sufficient data to include in a meta‐analysis estimated a summary RR of 0.99 (95% CI 0.62 to 1.57) for the risk of total adverse events with antibiotic compared with placebo during the study periods (Analysis 1.3). Donshik 1983 reported that three participants receiving gentamicin had increased ocular hyperemia and itching and Shulman 1982 reported that three participants receiving gentamicin had an allergic reaction. Both studies stated that with these events, the antibiotic groups did not statistically differ from the placebo group. One participant in the placebo group who had increased irritation discontinued use in the Jackson 1982 study.
1.3. Analysis.
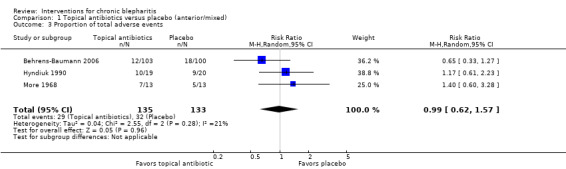
Comparison 1 Topical antibiotics versus placebo (anterior/mixed), Outcome 3 Proportion of total adverse events.
Adverse events were not reported by Laibovitz 1991.
Topical ciprofloxacin versus another topical antibiotic (3 studies)
Two RCTs (Adenis 1996a; Bloom 1994) and one CCT (Nguyen 1990) each evaluated two types of topical antibiotics for the treatment of blepharitis or blepharoconjunctivitis. The three studies compared ciprofloxacin ophthalmic solution with another topical antibiotic: fusidic acid gel (Adenis 1996a) or tobramycin ophthalmic solution (Bloom 1994; Nguyen 1990). The studies included 29 to 464 participants and follow‐up was 7 days. Adenis 1996a included participants with acute conjunctivitis or acute or chronic blepharitis; only data for participants with chronic blepharitis were analyzed for this review.
Risk of bias
Risk of selection bias in the three studies was generally unclear. Methods of randomization and allocation concealment were not reported in the two RCTs (Adenis 1996aBloom 1994) and randomization was not reported in Nguyen 1990. Risk of performance and detection bias was generally high or unclear: Adenis 1996a was an open‐label study; Bloom 1994 was reported to be double‐masked, but details about who was masked were not reported; participants were masked in Nguyen 1990, but masking of healthcare providers and outcome assessors was not reported. Data were missing from the analyses in Adenis 1996a and Bloom 1994. Reported only as an abstract, Nguyen 1990 did not provide information necessary to assess attrition bias. Risk of selective reporting bias was low for Adenis 1996a and Bloom 1994, but high for Nguyen 1990. Adenis 1996a was industry‐funded. Study enrollment eligibility was not limited to only blepharitis for Adenis 1996a and Bloom 1994.
Primary outcomes
All three studies clinically assessed the participants' responses to treatment. At day 7, there was no significant difference in the proportion of participants cured or improved between groups (summary RR 0.98; 95% CI 0.88 to 1.09) (Analysis 2.1). Limiting the analysis to the two studies that compared ciprofloxacin with tobramycin did not change the summary estimate or CIs. In Bloom 1994, between‐group comparisons for individual signs and symptoms did not show any significant differences in effectiveness between groups.
2.1. Analysis.
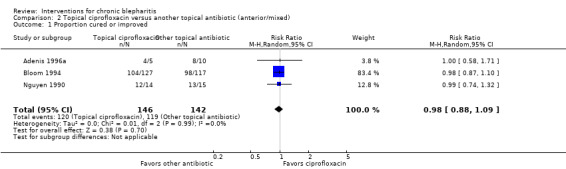
Comparison 2 Topical ciprofloxacin versus another topical antibiotic (anterior/mixed), Outcome 1 Proportion cured or improved.
Secondary outcomes
Two studies measured bacteriologic responses to treatment with lid cultures. At day 7, there was no significant difference in the proportion of cultures eradicated or reduced in the ciprofloxacin group compared with the tobramycin group (summary RR 1.03; 95% CI 0.85 to 1.26) (Analysis 2.2). Adenis 1996a assessed bacteriologic outcomes, but did not report results separately for participants with chronic blepharitis.
2.2. Analysis.
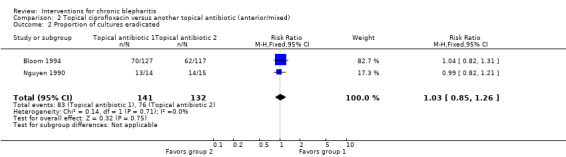
Comparison 2 Topical ciprofloxacin versus another topical antibiotic (anterior/mixed), Outcome 2 Proportion of cultures eradicated.
Adverse events
In Bloom 1994, 1/230 participants in the ciprofloxacin group discontinued treatment due to adverse events compared with 8/234 participants in the tobramycin group (RR 0.13; 95% CI 0.02 to 1.01). In the ciprofloxacin group, 16 participants reported ocular discomfort and 5 reported metallic taste in mouth, and in the tobramycin group 9 participants reported ocular discomfort and 2 reported increased lid erythema or conjunctival injection. None of these participants discontinued treatment. For total adverse events through day 14 the RR was 1.18 (95% CI 0.66 to 2.12).
Nguyen 1990 reported that a few participants in both treatment groups experienced ocular burning and a few participants in the ciprofloxacin group noticed the ciprofloxacin taste. Adenis 1996a did not report adverse events separately for chronic blepharitis participants; however, four adverse events were reported for the entire study population, two events occurred in each group.
Topical antibiotics versus oral antibiotics (1 study)
One RCT compared topical antibiotics with oral antibiotics using a combined cross‐over and parallel‐group design (Seal 1995). The study consisted of four two‐month long phases in which placebo treatment was administered to all 61 study participants during phase 1 and phase 3 and active treatment protocols were administered during phase 2 and phase 4. During the active treatment phases, half the participants received combination topical fusidic acid and oral oxytetracycline and the other half of participants received either topical fusidic acid or oral oxytetracycline. At the end of the eight‐month study period 18 participants were excluded or lost to follow‐up. Data were reported by treatment received regardless of the order in which it was received.
Risk of bias
Seal 1995 was at low risk of selection, performance, and detection bias. ITT analysis was not followed in the study and results were not reported for the end of each treatment phase. One of the study authors was affiliated with industry.
Primary outcomes
Clinical outcomes were measured by patient‐reported changes in symptoms and clinician‐assessed changes in signs. After topical treatment with fusidic acid 6/18 participants noted improvement in symptoms compared with 8/22 participants following treatment with oral oxytetracycline (RR 0.92; 95% CI 0.39 to 2.16). Similarly, 14/16 participants were assessed by the physician to have improved or shown no change in signs following topical treatment compared with 16/19 participants following oral treatment (RR 1.04; 95% CI 0.79 to 1.36).
Secondary outcomes
Bacteriologic outcomes were not measured by Seal 1995.
Adverse events
No serious adverse events were reported.
Combined topical and oral antibiotics versus topical antibiotics alone or oral antibiotics alone (1 study)
This section compares results from the combination treatment phases of the Seal 1995 study to the topical antibiotic alone and oral antibiotic alone phases. The study did not distinguish between initial treatment outcomes (phase 2) and cross‐over treatment outcomes (phase 4); thus results were reported as changes from placebo to active phase.
Risk of bias
The risk of bias for Seal 1995 is reported in the section above.
Primary outcomes
Clinical outcomes were measured by patient‐reported changes in symptoms and clinician‐assessed changes in signs. At the end of active phases of treatment, symptoms improved for 11/34 participants in the combination group compared with 6/18 participants in the topical antibiotic only group (RR 0.97; 95% CI 0.43 to 2.19) and 8/22 participants in the oral oxytetracycline only group (RR 0.89; 95% CI 0.43 to 1.86). Further, signs improved or were unchanged for 30/35 participants in the combination group compared with 14/16 participants in the topical antibiotic only group (RR 0.98; 95% CI 0.78 to 1.23) and 16/19 participants in the oral oxytetracycline only group (RR 1.02; 95% CI 0.80 to 1.29).
Secondary outcomes
Bacteriologic outcomes were not measured by Seal 1995.
Adverse events
No serious adverse events were reported.
Topical antibiotics versus topical steroids (2 studies)
Two RCTs evaluated topical antibiotics and topical steroids for treating chronic staphylococcal blepharoconjunctivitis using a 2 x 2 factorial design (Donshik 1983; Shulman 1982). The topical antibiotic used in both studies was 0.3% gentamicin sulfate and the topical steroid was 0.1% betamethasone phosphate. Study duration was two weeks for both studies. The Donshik 1983 study included 100 participants, 82 of whom were eligible for the efficacy analyses and 3 who were lost to follow‐up. The Shulman 1982 study included 87 participants, 71 of whom were eligible for the efficacy analyses and 2 who were lost to follow‐up.
Risk of bias
Both studies were RCTs in which participants, healthcare providers, and outcome assessors were masked. Allocation concealment was not reported in Shulman 1982. Neither study included all randomized participants in the analysis. Both studies were assessed to be at low risk of reporting bias. Two study authors of Shulman 1982 were affiliated with industry.
Primary outcomes
Both studies assessed clinical outcomes using a 4‐point rating scale of signs. Neither study reported sufficient data for analysis. A 62% improvement was observed for the gentamicin group in Donshik 1983 compared with 76% in the betamethasone group. This difference was reported as not statistically different. Physicians' overall evaluation in the same study significantly favored betamethasone over gentamicin. Shulman 1982 reported no significant difference in treatment effect between gentamicin and betamethasone.
Secondary outcomes
Lid cultures were used to assess bacteriologic outcomes for both studies. At final visit, gentamicin‐treated participants were significantly more likely to have negative cultures compared with betamethasone‐treated participants (summary RR 4.16; 95% CI 2.02 to 8.57) (Analysis 3.1). Shulman 1982 stated that gentamicin was significantly more effective than betamethasone in rendering lid cultures negative as early as day 3 of treatment.
3.1. Analysis.
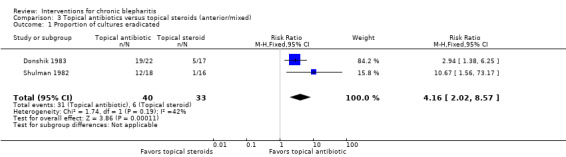
Comparison 3 Topical antibiotics versus topical steroids (anterior/mixed), Outcome 1 Proportion of cultures eradicated.
Adverse events
Three participants receiving gentamicin had increased ocular hyperemia and itching in the Donshik 1983 study and three participants receiving gentamicin had an allergic reaction in the Shulman 1982 study; however, both studies reported no significant differences between treatment groups for adverse events. No abnormal increases in IOP were detected in any group for either study.
Topical steroids versus placebo (2 studies)
The two RCTs described above evaluated topical antibiotics and topical steroids for treating chronic staphylococcal blepharoconjunctivitis using a 2 x 2 factorial design (Donshik 1983; Shulman 1982). This section compares the topical steroid (betamethasone) groups with the placebo groups.
Risk of bias
The risks of bias for these two studies are reported in the section above.
Primary outcomes
Both studies assessed clinical outcomes using a 4‐point rating scale of signs. Neither study reported sufficient data for analysis. A 76% improvement was observed for the betamethasone group compared with 57% in the placebo group in Donshik 1983. This difference was reported as not statistically different. Physicians' overall evaluation in the same study significantly favored betamethasone over placebo. Shulman 1982 reported no significant difference in treatment effect between betamethasone and placebo.
Secondary outcomes
Lid cultures were used to assess bacteriologic outcomes for both studies. At final visit, the number of negative lid cultures between betamethasone‐treated participants and placebo‐treated participants were not significantly different (summary RR 0.86; 95% CI 0.35 to 2.15) (Analysis 4.1).
4.1. Analysis.
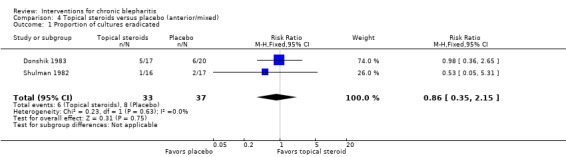
Comparison 4 Topical steroids versus placebo (anterior/mixed), Outcome 1 Proportion of cultures eradicated.
Adverse events
Both studies reported no significant differences between treatment groups for adverse events (Donshik 1983; Shulman 1982). No abnormal increases in IOP were detected in any group for either study.
Combined topical antibiotics and steroids versus placebo (3 studies)
In addition to the two RCTs described above (Donshik 1983; Shulman 1982), another RCT evaluated the combination of topical antibiotic and topical steroid versus placebo for treating blepharitis or blepharoconjunctivitis (Jackson 1982). The Jackson 1982 study was described as a three‐arm, double‐masked, placebo‐controlled study. The three treatment groups in this study were combination 0.3% gentamicin sulfate and 0.1% betamethasone sodium phosphate ointment, 0.3% gentamicin sulfate only ointment, and placebo ointment. After two weeks, 3/46 participants were lost to follow‐up in the Jackson 1982 study. This section compares the combination topical antibiotic and steroid groups with the placebo groups.
Risk of bias
All three studies were RCTs in which participants, healthcare providers, and outcome assessors were masked. Allocation concealment was not reported in Shulman 1982. None of the studies included all randomized participants in the analysis. Risk of reporting bias was assessed as low for all three studies. Two studies were funded or affiliated with industry (Jackson 1982; Shulman 1982). All three studies included participants with blepharoconjunctivitis.
Primary outcomes
The three studies assessed clinical outcomes using a 4‐point rating scale of signs and/or symptoms. Data were insufficient for meta‐analysis. Jackson 1982 reported no significant differences in mean sign and symptom scores between treatment groups on days 7 and 14. A 73% improvement was observed for the combination group compared with 57% in the placebo group in Donshik 1983 at two weeks. This difference of per cent improvement was reported as not statistically different between groups; however, combination‐treated participants had significantly less lid discharge and significantly greater improvements in conjunctival hyperemia than placebo‐treated participants. Shulman 1982 reported that improvements in total sign scores in the combination group were significantly greater than in the placebo group.
Secondary outcomes
Lid cultures were used to assess bacteriologic outcomes for all studies. At final visit, combination‐treated participants were significantly more likely to have negative cultures compared with placebo‐treated participants (summary RR 4.22; 95% CI 1.57 to 11.34) (Analysis 5.1). Shulman 1982 stated that combination treatment was significantly more effective than placebo in rendering lid cultures negative as early as day 3 of treatment.
5.1. Analysis.
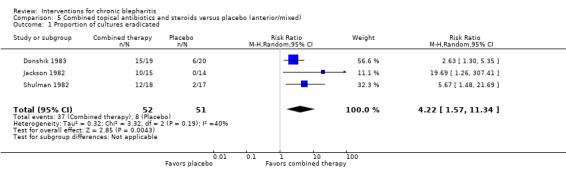
Comparison 5 Combined topical antibiotics and steroids versus placebo (anterior/mixed), Outcome 1 Proportion of cultures eradicated.
Adverse events
Although no significant differences between treatment groups were observed for any study, three participants receiving gentamicin had increased ocular hyperemia and itching in the Donshik 1983 study and three participants receiving gentamicin had an allergic reaction in the Shulman 1982 study. There was one participant in the placebo group who had increased irritation and discontinued use in the Jackson 1982 study. Five participants had inferior epithelial keratitis at the end of the Jackson 1982 study; it was not reported in which groups these participants were assigned. No abnormal increases in IOP were detected in any group for these three studies.
Combined topical antibiotics and steroids versus topical antibiotics alone (4 studies)
In addition to the three studies described in the previous section (Donshik 1983; Jackson 1982; Shulman 1982), one additional RCT compared combination treatment of topical antibiotic plus topical steroid with topical antibiotic alone for treating blepharitis (Aragones 1973). Rather than evaluating gentamicin/betamethasone ointment as with the other three studies, Aragones 1973 studied sulfacetamide/prednisolone eyedrops. The Aragones 1973 study followed 30 hospitalized patients for an unspecified period of time.
Risk of bias
All four studies were RCTs in which participants, healthcare providers, and outcome assessors were masked. Allocation concealment was not reported in Shulman 1982. Aragones 1973 was the only study in which all randomized participants were included in the analysis. Risk of reporting bias was assessed as low for all four studies. Three studies were funded or affiliated with industry (Aragones 1973; Jackson 1982; Shulman 1982). Three studies included participants with blepharoconjunctivitis (Donshik 1983; Jackson 1982; Shulman 1982), whereas Aragones 1973 included only participants with blepharitis.
Primary outcomes
The four studies assessed clinical outcomes using a rating scale of signs and/or symptoms. Data were insufficient for meta‐analysis. Jackson 1982 reported no significant differences in mean sign and symptom scores between treatment groups on days 7 and 14. At two weeks, Donshik 1983 observed a 73% improvement for the combination group compared with 62% in the antibiotic only group. The between‐group difference in per cent improvement was reported as not statistically different; however, combination‐treated participants were reported to have significantly greater relief of ocular itching than participants treated with antibiotic alone. At two weeks, Shulman 1982 reported that improvements in total sign scores in the combination group were significantly greater than in the antibiotic alone group. In Aragones 1973, 15/15 participants treated with combination therapy were judged to have excellent or good therapeutic effectiveness compared with 8/15 participants treated with antibiotic alone (RR 1.82; 95% CI 1.14 to 2.91). For all symptoms assessed (lid edema, lid redness, vessel dilation, loss of cilia, scales, and conjunctival infection), the combination therapy group showed significantly greater improvements than the antibiotic only group. The time point for the Aragones 1973 study was reported as "completion of treatment".
Secondary outcomes
Lid cultures were used to assess bacteriologic outcomes for all studies. At final visit, there was no significant difference in the proportion of negative cultures when comparing participants treated with combination therapy to participants treated with antibiotics alone (summary RR 1.01; 95% CI 0.88 to 1.16) (Analysis 6.1).
6.1. Analysis.
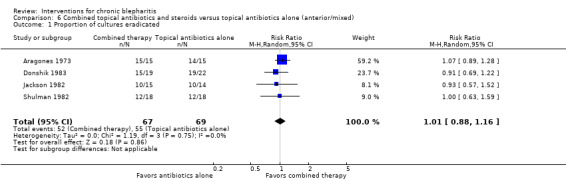
Comparison 6 Combined topical antibiotics and steroids versus topical antibiotics alone (anterior/mixed), Outcome 1 Proportion of cultures eradicated.
Adverse events
Three participants receiving gentamicin had increased ocular hyperemia and itching in the Donshik 1983 study and three participants receiving gentamicin had an allergic reaction in the Shulman 1982 study. These occurrences were reported as not statistically different between treatment groups for both studies. Five participants had inferior epithelial keratitis at the end of the Jackson 1982 study; it was not reported in which groups these participants were assigned. No abnormal increases in IOP were detected in any group for these three studies (Donshik 1983; Jackson 1982; Shulman 1982). The fourth study, Aragones 1973, reported no evidence of adverse events with steroid use during follow‐up examinations.
Combined topical antibiotics and steroids versus topical steroids alone (3 studies)
Three studies compared combined topical antibiotic and topical steroid therapy with topical steroid therapy alone. Two of the studies were RCTs that used a 2 x 2 factorial design to evaluate topical gentamicin plus topical betamethasone for treating chronic staphylococcal blepharoconjunctivitis (Donshik 1983; Shulman 1982). The third study was a CCT that compared triamcinolone acetonide plus antibiotics with triamcinolone acetonide alone for treating inflammatory and/or infectious eye diseases (Goldberg 1960). Although the study included multiple conditions, clinical data were reported separately for 16 participants with unilateral blepharoconjunctivitis.
Risk of bias
Donshik 1983 and Shulman 1982 were RCTs in which participants, healthcare providers, and outcome assessors were masked. Allocation concealment was not reported in Shulman 1982. The authors of Goldberg 1960 did not report randomization and did not use masking. Goldberg 1960 was the only study in which all participants were included in the analysis. Risk of reporting bias was assessed as low for all three studies. Two study authors of Shulman 1982 were affiliated with industry. No study was limited to participants with blepharitis only.
Primary outcomes
Two studies assessed clinical outcomes using a 4‐point rating scale of signs (Donshik 1983; Shulman 1982). Neither study reported sufficient data for analysis. At two weeks, a 73% improvement was observed for the combined therapy group compared with 76% in the betamethasone group in Donshik 1983. This difference was reported as not statistically different. Physicians' overall evaluation in the same study showed no statistical difference between combined therapy and betamethasone only. Shulman 1982 reported that clinical response to combined therapy was comparable to betamethasone alone; however, improvements in total sign scores in the combination group were greater than in the betamethasone group (P < 0.10).
The third study assessed the clinical response to treatment as excellent, good, fair, or poor (Goldberg 1960). The timing of the outcomes for this study was not reported. For the 13 participants with unilateral blepharoconjunctivitis who received topical steroid therapy alone the clinical response classifications were excellent for eight participants, good for two participants, fair for two participants, and poor for one participant. For the three participants with unilateral blepharoconjunctivitis who received combination therapy, all were classified as having good clinical response to treatment.
Secondary outcomes
Lid cultures were used to assess bacteriologic outcomes for Donshik 1983 and Shulman 1982. At final visit, combination‐treated participants were significantly more likely to have negative cultures compared with betamethasone‐treated participants (summary RR 4.02; 95% CI 1.91 to 8.44) (Analysis 7.1). Shulman 1982 stated that combination therapy was significantly more effective than betamethasone in rendering lid cultures negative as early as day 3 of treatment. Bacteriologic outcomes were not measured by Goldberg 1960.
7.1. Analysis.
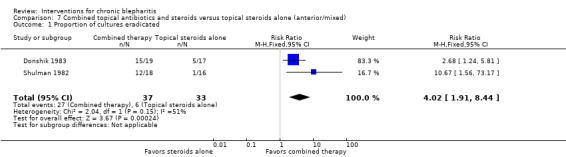
Comparison 7 Combined topical antibiotics and steroids versus topical steroids alone (anterior/mixed), Outcome 1 Proportion of cultures eradicated.
Adverse events
Donshik 1983 and Shulman 1982 reported no adverse events in either treatment group. Both studies also reported no abnormal increases in IOP for any participant regardless of treatment. Adverse events reported by Goldberg 1960 included stinging sensation, sweetish taste, and burning sensation; however, these events occurred in the entire study population and were not limited to participants with blepharitis only.
Combined topical antibiotics and steroids versus other combined topical antibiotics and steroids (1 study)
One RCT compared 0.5% loteprednol etabonate and 0.3% tobramycin ophthalmic suspension with 0.3% dexamethasone and 0.1% tobramycin ophthalmic suspension for blepharokeratoconjunctivitis (White 2008). The study enrolled 276 participants and the treatment and follow‐up periods lasted two weeks. Thirteen participants withdrew from the study and three participants were not included in the ITT analyses.
Risk of bias
The study was at low risk of selection bias as it was adequately randomized and allocation concealment was done. Although participants were not masked, the study was investigator‐masked. Primary and secondary outcomes specified in the methods section and in the clinical trial registration were reported. The study was funded by the company producing a treatment intervention and two study authors were employees of the company producing the treatment intervention.
Primary outcomes
At days 3, 7, and 15, there were no significant differences reported between treatment groups for the investigator's global assessment (cured, improved, not changed, or worsened). The MDs for changes in overall signs and symptoms scores (range 0 to 52) for loteprednol etabonate/tobramycin versus dexamethasone/tobramycin were 0.50 (95% CI ‐0.80 to 1.80) at day 3, 0.90 (95% CI ‐0.90 to 2.70) at day 7, and 0.40 (95% CI ‐1.41 to 2.21) at day 15. Similarly, there were no significant differences for changes in signs composite scores or symptoms composite scores between groups at any follow‐up period.
Two of the 13 individual signs and symptoms scores statistically differed between groups based on least square (LS) mean changes. The LS mean change for chemical chemosis was ‐0.9 for the loteprednol etabonate/tobramycin group and ‐1.0 for the dexamethasone/tobramycin group (90% CI 0.01 to 0.15) at day 15. The LS mean change for ocular burning was ‐0.9 for the loteprednol etabonate/tobramycin group and ‐1.0 for the dexamethasone/tobramycin group (90% CI 0.02 to 0.35) at day 7.
Secondary outcomes
Bacteriologic outcomes were not measured by White 2008.
Adverse events
Nonocular treatment‐emergent adverse events occurred equally in both treatment groups (4/138 in each group). More ocular treatment‐emergent adverse events occurred in the dexamethasone/tobramycin group (9/138) compared with the loteprednol etabonate/tobramycin group (4/138); however the difference between groups was not statistically significant (RR 0.44; 95% CI 0.14 to 1.41). No significant changes or differences between groups were reported for visual acuity, IOP, anterior chamber abnormalities, or cataract.
Topical antifungal versus placebo (1 study)
One RCT compared a topical antifungal agent with placebo for seborrheic and mixed seborrheic/staphylococcal blepharitis (Nelson 1990). All participants used lid hygiene for nine weeks and either ketoconazole cream (antifungal) or lanolin cream (placebo) for five weeks. Three of the 40 study participants were withdrawn during the study and data for all participants were not available for each weekly follow‐up visit.
Risk of bias
Nelson 1990 was at low risk of selection, performance, detection, and selective reporting bias. ITT analysis was not followed. One of the study authors was affiliated with industry.
Primary outcomes
Participants rated signs and symptoms using a VAS (0 to 100). Due to wide variations of scoring within groups, interpretation of results was not statistically meaningful within or between treatment groups for this outcome measure. However, all participants regardless of treatment showed improvement at nine weeks.
At five weeks clinical assessment by a masked examiner suggested greater, but not statistically significant, improvements in ketoconazole‐treated participants compared with placebo‐treated participants (RR 1.63; 95% CI 0.88 to 3.04). Clinician‐assessed scores of signs (scale 0 to 9) were significantly better for both treatment groups at five and nine weeks compared with baseline; however, there was no significant difference between groups at either five weeks (MD 0.60; 95 CI ‐0.15 to 1.35) or nine weeks (MD 0; 95% CI ‐0.85 to 0.85).
Secondary outcomes
The study authors noted no difference between treatment groups in bacterial colonization of the eyelids during the study period. Yeast cultures showed significant reductions in Pityrosporum counts at five weeks for both groups and increased counts after topical treatment ceased. There were no significant differences reported for the mean changes in counts between groups.
Adverse events
Adverse events were not reported by Nelson 1990.
Topical antifungal versus topical antibiotic (1 study)
One RCT compared selenium sulfide ophthalmic ointment (antifungal) with ammoniated mercury ophthalmic ointment (antibiotic) for the treatment of marginal blepharitis (Wong 1956). The study period was six weeks, including four weeks of treatment and two weeks of post‐treatment follow‐up. One participant among the 60 study participants was dropped from the study. Each eye of each participant was analyzed separately resulting in 117 eyes from 59 participants contributing to the final analyses.
Risk of bias
Method of randomization and allocation concealment was not reported in Wong 1956. The study was assessed to have low risks of performance, detection, attrition, and selective reporting biases. However, the unit of randomization (the participant) different from the unit of analysis (the eye) and nonindependence of eyes was not addressed in the analysis.
Primary outcomes
Clinical improvements were assessed by physicians' judgments of clinical responses as either improved or unimproved. At four weeks, 60/76 eyes were classified as improved in the selenium sulfide group compared with 37/41 eyes in the ammoniated mercury group (RR 0.87; 95% CI 0.75 to 1.02).
Secondary outcomes
The study authors noted that no changes in the microbiology of the eye were seen for any participant at any time during the study. No other data were reported for bacteriologic outcomes.
Adverse events
In the selenium sulfide group, two participants developed keratitis, conjunctivitis, and erythematous, swollen eyelids at two weeks. No complications were observed in the ammoniated mercury group.
Topical anti‐allergic versus placebo (1 study)
One RCT compared a topical anti‐allergic agent with placebo in treating chronic blepharitis (Collum 1984). Forty participants were enrolled and randomized to receive either 4% disodium cromoglycate ointment or placebo ointment. After four weeks of treatment two participants, one from each group, were lost to follow‐up.
Risk of bias
Details of randomization, allocation concealment, and masking were not reported in Collum 1984. We assessed the study at low risks of attrition and selective reporting biases. One of the study authors was affiliated with industry.
Primary outcomes
At four weeks, the study authors reported that topical anti‐inflammatory was effective in treating signs (crusting, scaling, hyperemia, and exudate). Clinical assessments of signs and symptoms were reported as percentage values by treatment group, but it was not clear what the percentages represented or how they were computed. Participant and clinician opinions of treatment (full control or no control) also were reported as percentage values by treatment group, but it was not clear how the percentages were computed as we could not reproduce the results with the data available.
Secondary outcomes
Bacteriologic cultures were taken at the start and conclusion of the study. The study authors noted that three participants had moderate bacterial growth after four weeks. No other data were reported for bacteriologic outcomes.
Adverse events
During the four‐week study, 2/19 participants in the anti‐inflammatory group developed mildly red eyes compared with 3/19 participants in the placebo group (RR 0.67; 95% CI 0.13 to 3.55).
Mechanical (hygiene) interventions
Lid scrubs with OCuSoft pads versus bar soap/baby shampoo (1 study)
One intra‐individual comparative study enrolled 26 participants with chronic blepharitis (Key 1996). Lid scrubs with the OCuSoft pad on the right eye and Neutrogena bar soap on the left eye were performed in the morning and evening by each participant. The initial study period lasted four months and one participant was lost to follow‐up. During a three‐month extension of the study, 10 participants remained using the OCuSoft pad on the right eye and switched to diluted Johnson's baby shampoo on the left eye.
Risk of bias
Overall, Key 1996 was at high risk of bias. The allocation method, right versus left eyes, precluded allocation concealment and masking. One participant was excluded from the analysis and not all study outcomes were reported.
Primary outcomes
At four months there were reductions in symptomology in both groups. For patient‐reported improvements in symptoms, 9/25 participants thought the eye treated with OCuSoft pads were symptom‐free compared with 6/25 who thought the Neutrogena treated eyes were symptom‐free (RR 1.50; 95% CI 0.63 to 3.59). Slit‐lamp examinations found 12/25 OCuSoft treated eyes to be completely normal compared with 4/25 Neutrogena treated eyes (RR 3.00; 95% CI 1.12 to 8.05). Of the 20 participants with oily discharge prior to treatment, two had low‐grade discharge at four months. There was no difference between the OCuSoft eyes and Neutrogena eyes for this symptom. Of the 21 participants with crusting prior to treatment, eight had crusting at four months. In five of these participants crusting was only present in the Neutrogena eye after treatment.
After the three‐month study extension, slit‐lamp examinations found 10/10 OCuSoft treated eyes were free of symptoms compared with 8/10 baby shampoo‐treated eyes (RR 1.24; 95% CI 0.87 to 1.75). All the OCuSoft treated eyes were free of hyperemia compared with 7/10 eyes cleaned with baby shampoo. None of the six eyes with crusting had crusting in the OCuSoft group compared with 2/6 eyes in the baby shampoo group at the end of the treatment period.
Secondary outcomes
Bacteriologic outcomes were not measured by Key 1996.
Adverse events
No ocular complications were observed for any treatment. At four months, one participant had dry skin around the eye scrubbed with the OCuSoft pads and did not wish to continue using the product after the study. Two participants experienced stinging with the baby shampoo in the extension period of the study. No discomfort was reported with the Neutrogena bar soap.
Quality‐of‐life outcomes
Quality‐of‐life outcomes were measured by patient questionnaires. Patients' preferences of treatment were based on perception of cleaner lids and ease of use. At four months, 17/25 participants preferred the OCuSoft pads over the Neutrogena bar soap, 2/25 participants preferred the Neutrogena bar soap over the OCuSoft pads, and 6/25 participants had no preference. After the three‐month extension period, 4/10 participants preferred the OCuSoft pads over the baby shampoo, 1/10 participants preferred the baby shampoo over the OCuSoft pads, and 5/10 participants had no preference. The study authors noted that no participant had to discontinue contact lens wear during the study period.
Economic costs and benefits
The study authors reported that the estimated cost of OCuSoft pads was 25 cents per day compared with 7 to 10 cents per day for baby shampoo or Neutrogena bar soap.
Lid scrubs with or without collagen compresses versus baby shampoo (1 study)
One RCT evaluated multiple lid hygiene interventions for the treatment of chronic blepharitis (Wasserman 1989). Twenty participants were randomized to one of three treatment arms: 1) daily lid hygiene with eye makeup remover, collagen compresses, and application of topical steroid; 2) daily lid hygiene with eye makeup remover and application of topical steroid; or 3) daily lid hygiene with baby shampoo and application of topical steroid. The study protocol lasted 10 days and no participants were excluded or lost to follow‐up.
Risk of bias
Methods of randomization, allocation concealment, and masking were not reported in Wasserman 1989. No other risks of bias were identified.
Primary outcomes
Objective and subjective clinical improvements were assessed using a 4‐point rating scale of signs (crusting, conjunctival injection, increased lacrimation, and meibomitis) and symptoms (overall comfort, itching, burning, and gritty sensation). At 10 days, there was 78%, 58%, and 48% resolution from baseline in clinician‐reported objective findings for groups 1, 2, and 3, respectively. Further, there was 79%, 63%, and 62% resolution from baseline in patient‐reported subjective findings for groups 1, 2, and 3, respectively.
At 10 days, mean overall improvement was seen in all groups; however, group 1 showed significantly more improvement than either group 2 (MD 0.45; 95% CI 0.14 to 0.76) or group 3 (MD 0.51; 95% CI 0.17 to 0.85). There was no significant difference in mean overall improvement between groups 2 and 3 (MD 0.06; 95% CI ‐0.17 to 0.29).
Secondary outcomes
Eyelids were cultured at the start and conclusion of the study period. At 10 days, 2/3 culture‐positive eyes were culture‐negative in group 1; 2/4 eyes were culture‐negative in group 2; and 1/3 eyes were culture‐negative in group 3. The number of eligible participants with positive bacterial cultures was too small to yield clinical or statistical meaning.
Adverse events
Adverse events were not reported by Wasserman 1989.
Zinc compress versus selenium compress (1 study)
One parallel‐group study compared zinc compresses with selenium compresses in participants with seborrheic blepharitis (Sore 2002). The zinc compresses were soaked with an isotonic 0.1% zinc sulfate solution (astringent). The selenium compresses were soaked with natural selenium‐rich thermal water. Sixty participants were enrolled in the study, 30 in each treatment group. At four weeks follow‐up data were missing for one participant in the selenium compress group.
Risk of bias
Randomization, allocation concealment, and masking were not reported in Sore 2002. One participant was excluded from the analysis and the unit of analysis was not specified. The study authors were affiliated with pharmaceutical industry.
Primary outcomes
Changes in meibum excretion rates and meibomian gland orifice diameters were evaluated to assess clinical improvements. At baseline, 14/21 participants in the zinc compress group and 14/19 participants in the selenium compress group had a fatty palpebral edge. At four weeks, 6/14 participants in the zinc compress group had a reduction in meibum excretion rate compared with 10/14 participants in the selenium compress group (RR 0.60; 95% CI 0.30 to 1.20). A sampling of five participants from each group showed significant reductions in meibomian gland orifice diameters in both groups at four weeks, although data were not collected for all participants for this outcome.
Secondary outcomes
The mean numbers of bacterial colonies for two types of bacteria were assessed by treatment group at baseline and four weeks. At baseline, 3/30 participants had positive cultures for S. aureus and 12/30 participants had positive cultures for S. epidermidis in the zinc compress group compared with 4/29 participants having positive cultures for S. aureus and 20/29 participants having positive cultures for S. epidermidis in the selenium compress group. At four weeks, the zinc compress group showed a nonsignificant reduction in the mean number of S. aureus colonies and a significant reduction in the mean number of S. epidermidis colonies from baseline. Conversely, at four weeks in the selenium compress group a significant reduction in the mean number of S. aureus colonies was observed and a nonsignificant reduction in the mean number of S. epidermidis colonies was seen. Sufficient data were not available for between‐group analyses.
Adverse events
Clinical and biologic tolerance of the study solutions/compresses were assessed. It was reported that results were identical in both groups. Clinically, there were no functional irritation signs, physical irritation of conjunctiva or cornea, or effects on ocular structures. Further, lacrimal tear film conservation was unchanged and eye comfort indices were greater than 98.5% for both groups. Biologically, there were no infraclinical irritancy or corneal toxicity detected.
Posterior blepharitis/MGD
Medical (drug) interventions
Topical antibiotics versus control (1 study)
One RCT compared azithromycin ophthalmic solution plus warm compresses with warm compresses alone for treating MGD (Luchs 2008). The study period was 14 days. Of the 21 participants enrolled, one participant discontinued treatment and was excluded from the study.
Risk of bias
Computer‐generated randomization was used in the Luchs 2008 study, although allocation concealment was not reported. The industry‐sponsored study was not masked. Data were presented by eyes rather than by the unit of randomization, which was the individual.
Primary outcomes
Subjective improvements were assessed by patient‐rated efficacy scores of treatment regimens (excellent, good, fair, poor, deterioration). After two weeks of treatment, 6/9 participants in the azithromycin group reported excellent or good overall symptomatic relief compared with 2/11 participants in the control group (RR 3.67; 95% CI 0.96 to 13.95).
Mean changes were reported for total clinical outcome scores (scale 0 to 20) as well as for each clinical sign (scale 0 to 4) after two weeks of treatment compared to baseline. Each eye of each participant was studied separately. Mean changes in total clinical outcome scores were significantly better for the azithromycin group than the control group (MD ‐6.20; 95% CI ‐7.18 to ‐5.22). Greater changes in signs were also observed for lid debris (MD ‐0.60; 95% CI ‐1.04 to ‐0.16), lid redness (MD ‐1.90; 95% CI ‐2.28 to ‐1.52), lid swelling (MD ‐0.50; 95% CI ‐0.86 to ‐0.14), meibomian gland plugging (MD ‐1.90; 95% CI ‐2.42 to ‐1.38), and quality of meibomian gland secretion measurements (MD ‐1.40; 95% CI ‐1.73 to ‐1.07) for the azithromycin group compared with the control group.
Secondary outcomes
Bacteriologic outcomes were not measured by Luchs 2008.
Adverse events
After two weeks of treatment, 1/9 participants in the azithromycin group experienced blurred vision and eye irritation compared with 0/11 participants in the control group (RR 3.60; 95% CI 0.16 to 79.01). Visual acuity values, external eye examinations, and slit‐lamp biomicroscopy suggested no ocular safety effects or differences between treatment groups.
Oral antibiotics versus placebo (1 study)
One RCT evaluated the effects of high‐dose (200 mg, twice daily) or low‐dose (20 mg, twice daily) oral doxycycline versus placebo for the treatment of MGD (Yoo 2005). At the end of the one‐month treatment period, 11/150 participants enrolled were lost to follow‐up or stopped medication due to side effects and excluded from analysis.
Risk of bias
Methods of randomization and allocation concealment were not reported. Although participants and nurses dispensing medication were masked to treatment groups, masking of outcome assessors was not reported. ITT analysis was not followed. No other sources of bias were identified.
Primary outcomes
Subjective symptomatic improvements were assessed by the number of symptoms per participant and categorical grading of symptoms (complete remission, partial remission, no change, and aggravation) at one‐month follow‐up. At one month, the number of symptoms per participant significantly decreased from baseline in the high‐dose (MD ‐0.88; 95% CI ‐1.20 to ‐0.56) and low‐dose (MD ‐1.39; 95% CI ‐1.65 to ‐1.13) doxycycline groups, but not in the control group (MD ‐0.09; 95% CI ‐0.55 to 0.37). The mean number of symptoms per participant at one month were significantly lower for the high‐dose (MD ‐0.56; 95% CI ‐0.95 to ‐0.17) and low‐dose (MD ‐0.48; 95% CI ‐0.86 to ‐0.10) groups compared with the control group. Likewise, the number of participants with partial or complete remission of symptoms was significantly higher in the high‐dose (RR 6.54; 95% CI 2.79 to 15.30) and low‐dose (RR 6.74; 95% CI 2.89 to 15.75) doxycycline groups compared with the control group at one‐month follow‐up.
Tear BUTs and Schirmer test scores were used to assess clinical improvements. For these evaluations both eyes of each participant were included. Mean values for both tests were comparable between the three treatment groups at baseline. At one month, mean tear BUTs significantly increased from baseline in the high‐dose and low‐dose doxycycline groups (MD 1.64 s; 95% CI 0.93 to 2.35 s; and MD 1.72 s; 95% CI 1.19 to 2.25 s, respectively), but not in the control group (MD 0.04 s; 95% CI ‐0.54 to 0.62 s). Mean tear BUTs at one month were significantly higher for the high‐dose and low‐dose groups compared with the control group (MD 1.58 s; 95% CI 0.87 to 2.29 s and MD 1.70 s; 95% CI 1.18 to 2.22, respectively). Similar results were observed at one month for Schirmer scores, with significant improvements from baseline observed in the high‐dose and low‐dose groups (MD 1.85 mm; 95% CI 0.73 to 2.97 mm and MD 2.38 mm; 95% CI 1.07 to 3.69 mm, respectively), but not the control group (MD ‐0.68 mm; 95% CI ‐1.91 to 0.55 mm); and significant differences between the high‐dose versus control groups (MD 4.09 mm; 95% CI 2.88 to 5.30 mm) and low‐dose versus control groups (MD 3.76 mm; 95% CI 2.41 to 5.11 mm).
Secondary outcomes
Bacteriologic outcomes were not measured by Yoo 2005.
Adverse events
During the one‐month study, 21 participants reported gastrointestinal problems; seven participants reported itchy skin, urticaria, and erythematous papules; and one participant reported stomatitis. These side effects were more frequent in the high‐dose (18/46 participants; RR 6.13; 95% CI 1.94 to 19.41) and low‐dose (8/46 participants; RR 2.72; 95% CI 0.77 to 9.64) groups compared with the control group (3/47 participants).
Four of 50 participants in the high‐dose group, 2/50 participants in the low‐dose group, and 1/50 participants in the control group discontinued medication due to side effects and were excluded from the study and analyses.
High‐dose versus low‐dose oral antibiotics (1 study)
A three‐arm RCT evaluated the effects of high‐dose oral doxycycline (200 mg, twice daily), low‐dose oral doxycycline (20 mg, twice daily), and placebo for the treatment of MGD (Yoo 2005). This section compares the high‐dose group with the low‐dose group only. At one month, 46/50 participants remained in the high‐dose group and 46/50 participants remained in the low‐dose group.
Risk of bias
The risk of bias for Yoo 2005 is reported in the section above.
Primary outcomes
Subjective symptomatic improvements were assessed by the number of symptoms per participant and categorical grading of symptoms (complete remission, partial remission, no change, and aggravation) at one‐month follow‐up. Although both groups showed significant reductions in the number of symptoms per participant, there was no significant difference between high‐dose and low‐dose treatment groups at one month (MD ‐0.08; 95% CI ‐0.31 to 0.15). The number of participants with partial or complete remission of symptoms was also not significantly different between high‐dose and low‐dose treatment groups at one month (RR 0.97; 95% CI 0.75 to 1.26).
For both tear BUTs and Schirmer test scores significant improvements were observed for both groups compared with baseline values, but mean values at one month were not significantly different between groups (MD ‐0.12 s; 95% CI ‐0.79 to 0.55 s; and MD 0.33 mm; 95% CI ‐0.99 to 1.65 mm, respectively).
Secondary outcomes
Bacteriologic outcomes were not measured by Yoo 2005.
Adverse events
Drug complications occurred more frequently in the high‐dose group (18/46 participants) compared with the low‐dose group (8/46 participants; RR 2.25; 95% CI 1.09 to 4.65). Four participants in the high‐dose group and two participants in the low‐dose group discontinued medication due to side effects and were excluded from the study and analyses.
Topical anti‐inflammatory agents versus placebo (1 study)
One RCT compared topical cyclosporine A drops with placebo drops for treating MGD (Perry 2006). Thirty‐three participants were initially enrolled and randomized in the three‐month study. Five participants, two in the cyclosporine group and three in the placebo group, were excluded from the study due to noncompliance. Two additional participants in the cyclosporine groups discontinued the study due to discomfort instilling the eyedrops.
Risk of bias
Treatment allocation was computer‐generated, although allocation concealment was not reported. All study participants and investigators were masked. The study was funded by the pharmaceutical industry and two study authors were affiliated with industry.
Primary outcomes
Subjective questionnaires were completed by participants to evaluate symptomatic improvements at one, two, and three months. Eight symptoms of MGD were graded 0 to 4, giving a maximum score of 32. At each follow‐up period, both groups had mean improvements from baseline in the overall symptoms scores; however these improvements were not statistically different from baseline scores or significantly different between groups.
Clinical examinations and tests were used to assess improvements in signs of MGD. Data were analyzed using the worse eye of each participant. Mean values for the number of meibomian gland inclusions, fluorescein staining scores, tear BUT, lissamine green staining, and Schirmer scores were comparable between groups at baseline. The mean number of meibomian gland inclusions were not significantly different between groups at one‐month follow‐up (MD ‐2.70; 95% CI ‐8.73 to 3.33), but were significantly lower at two‐month (MD ‐7.20; 95% CI ‐12.77 to ‐1.63) and three‐month follow‐up (MD ‐11.70; 95% CI ‐18.01 to ‐5.39) for the cyclosporine group compared with the placebo group. At three‐month follow‐up, mean fluorescein staining scores were significantly lower for the cyclosporine group compared with the placebo group (MD ‐2.60; 95% CI ‐4.46 to ‐0.74). Comparisons of mean values for tear BUT, lissamine green staining, and Schirmer scores were not statistically different between groups at any of the follow‐up times, although mean changes trended in favor of treatment with cyclosporine.
Secondary outcomes
Bacteriologic outcomes were not measured by Perry 2006.
Adverse events
During the three‐month study period, 2/16 participants in the cyclosporine group and 0/17 participants in the placebo group discontinued the study due to discomfort after instilling drops (RR 5.29; 95% CI 0.27 to 102.49). There were no significant differences in visual acuity, IOP, tear secretion, corneal infiltrates, corneal neovascularization, bulbar conjunctival hyperemia, or lens opacity in either group.
Topical anti‐inflammatory agents versus topical antibiotics and steroids (1 study)
One RCT compared cyclosporine ophthalmic emulsion (Restasis) with tobramycin plus dexamethasone ophthalmic solution for the treatment of posterior blepharitis (Rubin 2006). Thirty participants, 15 in each group, were enrolled. After three months, six participants were lost to follow‐up, three from each group.
Risk of bias
Risks of selection bias and performance bias were unclear. We assessed risk of detection bias as high since investigators were not masked. The study was funded by the pharmaceutical industry.
Primary outcomes
Subjective improvements in symptoms were assessed by participant questionnaires. At three months, more participants in the cyclosporine group than the tobramycin/dexamethasone group reported improvements in burning (7/15 versus 5/15), itching (6/15 versus 5/15), and blurred vision (7/15 versus 6/15), although these differences were not statistically significant. In both groups, 8/15 participants reported improved tearing.
Improvements in signs were assessed by clinical examinations and tests. At three months, lid telangiectasia resolved in 7/15 participants in the cyclosporine group compared with 3/15 participants in the tobramycin/dexamethasone group (RR 2.33; 95% CI 0.74 to 7.35). In both groups, 9/15 participants showed improvements in lid erythema. The study authors also reported that both treatments significantly improved tear lysozyme levels (P ≤ 0.03), although there was no significant between‐group difference (P = 0.86).
At 12 weeks, although Schirmer scores significantly improved for the cyclosporine group (mean change from baseline 2.33 mm) and the tobramycin/dexamethasone group (mean change from baseline 0.90 mm), the mean improvement was greater for the cyclosporine group than the tobramycin/dexamethasone group (MD 1.43 mm, P < 0.001). Similar effects were observed for fluorescein BUT results and meibomian gland secretion quality scores. Mean BUTs in the cyclosporine group improved 1.87 s (standard deviation (SD) 0.74 s) compared with 1.30 s (SD 0.46 s) in the tobramycin/dexamethasone group (MD 0.57 s; 95% CI 0.08 to 1.06 s). Mean secretion scores in the cyclosporine group improved 0.77 (SD 0.56) compared with 0.30 (SD 0.41) in the tobramycin/dexamethasone group (MD 0.47; 95% CI 0.08 to 0.86).
Secondary outcomes
Bacteriologic outcomes were not measured by Rubin 2006.
Adverse events
The study authors reported that no significant, drug‐related adverse events took place during the study period.
Topical mucolytic agents versus artificial tears (1 study)
One RCT compared 5% N‐acetylcysteine ophthalmic solution with artificial tears (Akyol‐Salman 2010). All 20 participants, 10 per group, applied the assigned drops four times a day and performed lid hygiene with a solution (Blepharoshampoo) once daily for one month. There were no exclusions or losses to follow‐up in this study.
Risk of bias
The method of randomization was a random‐number generator and the method of allocation concealment was not reported. Risk of performance bias (i.e. masking of participants and healthcare providers) was unclear; however, outcome assessors were masked. We considered the study to have selective reporting bias as results for changes in the severity of inflammatory symptoms were measured, but not reported.
Primary outcomes
Clinical symptoms, including ocular burning, itching, foreign body sensation, and filmy or blurred vision, were measured at baseline and at one‐month follow‐up by treatment group. The study authors reported statistically significant improvement for ocular burning, foreign body sensation, and filmy or blurred vision at one month for both groups. The N‐acetylcysteine group showed statistically significant improvement for itching at one month. No statistically significant improvement for itching was observed in the artificial tears group; however, due to the low baseline mean for this symptom (mean 0.67, SD 0.78; n = 10) finding a statistically significant improvement was unlikely. No between‐group results were reported.
Clinical tests, including Schirmer‐1 test and fluorescein BUT, were conducted at baseline and at one‐month follow‐up by treatment group. The N‐acetylcysteine group had a statistically significant increase in Schirmer rates compared with the artificial tears group at one month (MD 6.17; 95% CI 1.49 to 10.85). No statistically significant difference was found between groups for fluorescein BUT at one month (MD 3.00; 95% CI ‐0.55 to 6.55).
Secondary outcomes
Bacteriologic outcomes were not measured by Akyol‐Salman 2010.
Adverse events
The study authors reported that "none of the patients developed an allergic reaction to the medications, and IOP measurements were within the normal limits in both groups."
Oral mucolytic agents versus control (1 study)
One RCT evaluated supplemental therapy with or without the oral mucolytic agent, N‐acetylcysteine, for treating chronic posterior blepharitis (Yalçin 2002). All 40 participants in the study were treated with topical steroids and antibiotics, plus warm compresses and artificial tears. The therapy group included 43 eyes of 22 participants and the control group included 36 eyes of 18 participants.
Risk of bias
Method of randomization, allocation concealment, and masking were not reported. We assessed risks of attrition bias, selective reporting bias, and other potential sources of bias to be low for this study.
Primary outcomes
Subjective outcomes were not measured by Yalçin 2002.
Clinical outcomes were assessed by three clinical tests: Schirmer‐1 test, fluorescein BUT, and mucus fern tests. At four months, Schirmer values increased for 23/43 eyes in the therapy group and 10/35 eyes in the control group (RR 1.87; 95% CI 1.03 to 3.39). The mean change in Schirmer values for the therapy group (0.534 mm, standard error of the mean (SE) 8.99 mm) also was greater than the control group (‐7.5 mm, SE 10.52 mm) (MD 8.03 mm; 95% CI 3.63 to 12.44 mm). Fluorescein BUT increased for 35/39 eyes in the therapy group and 17/36 eyes in the control group (RR 1.90; 95% CI 1.32 to 2.73). The mean change in fluorescein BUTs for the therapy group (5.32 s, SE 6.23 s) also was greater than the control group (‐0.5 s, SE 4.56 s) (MD 5.82 s; 95% CI 3.36 to 8.28 s). Mucus fern test results improved for 41/43 eyes in the therapy group and 24/36 eyes in the control group (RR 1.43; 95% CI 1.12 to 1.82). The mean change in mucus fern grading for the therapy group (1.2, SE 0.67) also was greater than the control group (0.64, SE 0.63) (MD 0.56; 95% CI 0.27 to 0.85).
Secondary outcomes
Bacteriologic outcomes were not measured by Yalçin 2002.
Adverse events
Ocular dryness was the most common adverse event among study participants: 6/43 eyes in the therapy group and 8/36 eyes in the control group (RR 0.63; 95% CI 0.24 to 1.64). Additionally, oral N‐acetylcysteine was discontinued in one participant due to diarrhea. One other participant reported minor nausea and another reported minor nasal leakage, both in the therapy group.
Essential fatty acid supplements versus control (2 studies)
Two RCTs compared essential fatty acid supplements with a control. In one study 38 participants, 18 assigned to take flaxseed oil capsules (55% omega‐3 fatty acid, 15% omega‐6 fatty acid, and 19% omega‐9 fatty acid) and 20 assigned to take olive oil capsules (control), were followed for one year (Macsai 2008). At the end one year, eight participants were no longer in the study. The second study was a three‐arm trial evaluating combination therapy of eyelid hygiene and essential fatty acid supplements versus eyelid hygiene alone or supplements alone for treating MGD (Pinna 2007). Dietary supplements of oral linoleic acid and γ‐linolenic acid were taken once daily for 180 days. Fifty‐seven participants were enrolled (19 in each group) and eight were lost to follow‐up. This section compares the combination group with the eyelid hygiene alone (control) group and the supplements alone group with the eyelid hygiene alone group.
Risk of bias
Both studies had adequate randomization and low risks of detection bias and selective reporting bias. Allocation concealment was unclear for Macsai 2008 and not done in Pinna 2007. Participants were masked to treatment in Macsai 2008, but due to the interventions investigated could not be masked in Pinna 2007. Data were imputed for participants lost to follow‐up in Macsai 2008 assuming no change in outcomes. Participants lost to follow‐up in Pinna 2007 were excluded from the analysis. Although participants were randomized to treatment groups in Macsai 2008, the unit of analysis was the eyes.
Primary outcomes
Clinical outcomes were measured by different methods in the two studies, thus no meta‐analysis was performed.
The Ocular Surface Disease Index (OSDI) score was used to assess symptoms by Macsai 2008. There were no significant differences between the flaxseed oil group and the olive oil group at one year for overall OSDI score (MD ‐4.50; 95% CI ‐13.12 to 4.12), ocular symptom score (MD ‐9.60; 95% CI ‐20.00 to 0.80), or visual symptom score (MD ‐6.60; 95% CI ‐13.95 to 0.75).
At one year, mean changes in meibum quality score (graded 0 to 4), per cent meibomian gland blockage, per cent meibomian gland stenosis, and the number of visible ducts were assessed by treatment group in Macsai 2008. Both groups were reported to have improved in meibomian gland health and secretion at one year; however the quantitative results for these measures were reported by eye (both eyes of each participant counted) rather than by individual (unit randomized and receiving treatment) with no adjustment for nonindependence.
Objective clinical measures also were assessed by Macsai 2008. No significant differences in mean changes were found between treatment groups for Schirmer scores, tear BUT, fluorescein staining, rose bengal staining, collarettes, scurf, distichiasis (growth of new row of eyelashes), or madarosis (loss of eyelashes). The number of telangiectasias (dilated blood vessels near the surface of the lid margin) was reported to have decreased more in the flaxseed oil group compared with the olive oil group, but the unit of analysis was not used for the quantitative results.
In Pinna 2007, improvements in symptoms were reported to be statistically significant for all groups after 60 and 180 days. Symptoms were assessed using a 5‐point rating scale questionnaire completed by participants.
Changes in clinical signs were also evaluated by Pinna 2007. Reductions in the number of participants with eyelid edema were observed for all groups at 60 and 180 days compared with baseline, but were statistically significant only for the eyelid hygiene group at 60 (P = 0.02) and 180 days (P = 0.02) and for the combination therapy group at 180 days (P = 0.003). Changes in eyelid margin hyperemia were reported as not statistically significant for all groups. The number of participants with meibomian gland obstruction decreased significantly for all groups at 60 and 180 days compared with baseline. Significant reductions in meibomian secretion turbidity were observed at 180 days for all groups and at 60 days for the supplements only group and combination therapy group. Reductions in the number of participants with foam collection in the tear meniscus and corneal fluorescein staining were observed for all groups at day 180. Data were not available to perform between‐group comparisons for clinical improvement outcomes.
Secondary outcomes
Bacteriologic outcomes were not measured by either study (Macsai 2008; Pinna 2007).
Adverse events
Adverse events were not reported by either study (Macsai 2008; Pinna 2007).
Oil eyedrops versus saline (1 study)
One RCT compared low‐concentration homogenized castor oil eyedrops with saline eyedrops for posterior blepharitis using a cross‐over design (Goto 2002). There was a two‐week wash‐out period with artificial tears prior to the two treatment periods of two weeks each. Forty eyes of 20 participants were treated and no losses to follow‐up were reported.
Risk of bias
Treatment groups were randomly allocated by a study co‐author. It was not clear what method of randomization was used or whether allocation concealment was done. All study participants and personnel were masked and ITT analysis was followed. Results at baseline in the placebo group were not reported. We noted other potential sources of bias including funding by the pharmaceutical industry, potential carry‐over in cross‐over phases, and differing units of randomization and analysis.
Primary outcomes
In this study, results for clinical outcomes were combined for both treatment phases depending on the intervention received. Each eye of each participant was studied separately and analyses were done using Wilcoxon's signed rank test for nonparametric paired data.
Subjective improvements of participants' sensation of lubrication and smoothness during blinking were assessed with face score questionnaires. Face scores were graded 1 (happiest) through 9 (saddest). Following the oil eyedrop period (mean 5.5, SD 1.8) face scores were significantly lower compared with the placebo period (mean 6.7, SD 1.6) (P = 0.004).
Significant differences between groups were observed for clinical tear functions as well. Tear interference grading (scale 1 to 5) was lower following the oil eyedrop period (mean 2.0, SD 0.77) compared with the placebo period (mean 3.1, SD 0.71) (P < 0.0001); tear evaporation rates were lower following the oil eyedrop period (mean 11 x 10‐7 g/s, SD 7.5 x 10‐7 g/s) compared with the placebo period (mean 13 x 10‐7 g/s, SD 6.2 x 10‐7 g/s) (P = 0.01); and tear BUT was longer following the oil eyedrop period (mean 12.0 s, SD 3.5 s) compared with the placebo period (mean 4.6 s, SD 2.8 s) (P < 0.0001). Fluorescein, rose bengal, and meibomian gland orifice obstruction scores were also lower following the oil eyedrop period compared with the placebo period, although the differences were statistically significant for only the rose bengal and orifice obstruction results.
Secondary outcomes
Bacteriologic outcomes were not measured by Goto 2002.
Adverse events
No instances of irritation or severe burning were reported.
Mechanical (hygiene) interventions
Combination therapy with lid hygiene and dietary supplements versus dietary supplements alone (1 study)
One three‐arm RCT evaluated a combination therapy of eyelid hygiene and dietary supplements versus eyelid hygiene alone or supplements alone for treating MGD (Pinna 2007). Eyelid hygiene consisted of warm eyelid compresses, eyelid massage, and eyelid margin scrubbing once daily for 180 days. Dietary supplements of oral linoleic acid and γ‐linolenic acid were taken once daily for 180 days. Fifty‐seven participants were enrolled (19 in each group) and eight were lost to follow‐up. This section compares only the combination group with the supplements alone group in order to show the treatment effect of lid hygiene.
Risk of bias
The Pinna 2007 study had adequate randomization and low risks of detection bias and selective reporting bias. Allocation was not concealed. Due to the interventions investigated, participants could not be masked to treatment group. Participants lost to follow‐up were excluded from the analysis.
Primary outcomes
Improvements in symptoms were reported to be statistically significant for both groups after 60 and 180 days compared with baseline. Symptoms were assessed using a 5‐point rating scale questionnaire completed by participants. Reductions in the number of participants with eyelid edema, meibomian gland obstruction, meibomian secretion turbidity, foam collection in the tear meniscus, and corneal fluorescein staining were observed for both groups at 60 and 180 days compared with baseline. Changes in eyelid margin hyperemia were reported as not statistically significant for either group. Data were not available to perform between‐group comparisons for clinical outcomes.
Secondary outcomes
Bacteriologic outcomes were not measured by Pinna 2007.
Adverse events
Adverse events were not reported by Pinna 2007.
Warm compresses versus control (3 studies)
One RCT (Mori 2003), one intra‐individual comparative RCT (Olson 2003), and one CCT (Ishida 2008) evaluated warm compresses versus a control for the treatment of MGD. In Ishida 2008, a novel eyelid warming mask (Orgahexa eye warmer) was compared with a conventional eye mask among 20 participants. The masks were worn overnight for two weeks. In the study by Mori 2003, 17 participants who applied a disposable eyelid warming device for 5 minutes once a day for two weeks were compared with 8 untreated participants. In Olson 2003, 20 participants had warm compresses applied randomly to one eye and room temperature compresses to the other eye for 30 minutes. No losses to follow‐up were reported in the studies.
Risk of bias
The risks of biases among the three studies were mixed. Mori 2003 had adequate randomization and allocation concealment, Olson 2003 did not report methods of randomization and allocation concealment, and Ishida 2008 was nonrandomized. Clinical examinations were masked in Mori 2003, although participants and, therefore, patient‐reported outcomes, were not masked. Both Ishida 2008 and Olson 2003 were unmasked. All three studies followed ITT analysis. Results of treatment effects for all outcomes were not reported in one study (Mori 2003). Both Ishida 2008 and Mori 2003 had industry funding.
Primary outcomes
Subjective improvements in ocular fatigue and ocular dryness were measured by VAS in the two CCTs (Ishida 2008; Mori 2003). Ishida 2008 reported statistically significant improvements in VAS symptom scores after 10 minutes and 2 weeks of wearing the Orgahexa eye warmer. Statistically significant mean improvement rates of 49.9% for ocular fatigue and 56.2% for dry sensation were reported for the warm compress group by Mori 2003. Results for the control group in each study were reported as not significant and data were not available to compare outcomes between groups. Subjective clinical outcomes were not reported by Olson 2003.
In Ishida 2008, significant improvements in tear film BUT, fluorescein staining, rose bengal staining, and DR‐1 interferometry (measure of the expression of lipids into the tear film) were observed in the Orgahexa eye warmer group compared with baseline. No significant improvement was observed for Schirmer scores in the Orgahexa eye warmer group or for any clinical test in the control group. Data were not available for between‐group comparisons.
In the Mori 2003 study, treatment with warm compresses significantly increased tear BUT by an average of 1 s compared with baseline values. This study also reported that normal tear film lipid layer patterns were observed in 28/34 eyes after treatment with warm compresses compared with 19/34 eyes before treatment; and that the number meibomian gland orifices that were obstructed significantly decreased to 14/34 eyes post‐treatment compared with 26/34 eyes pretreatment. Results for the untreated group were reported as not significant for these outcomes and data were not available to compare outcomes between groups. Fluorescein and rose bengal scores also were measured by Mori 2003, but these results were not reported for either group.
In the Olson 2003 study, mean changes in tear‐film lipid layer thickness (TFLLT) were evaluated during and after compress therapy. At the end of 30 minutes of therapy, TFLLT increased 63.7 nm (P < 0.001) in the warm compress group and 1.5 nm (P = 0.81) in the control group compared with baseline values. Five minutes post‐therapy TFLLTs were still increased compared with baseline in the warm compress group (mean change 38.2 nm, P < 0.001), but unchanged in the control group (‐4.5 nm, P = 0.20). Data were not available to compare outcomes between groups.
Secondary outcomes
Bacteriologic outcomes were not measured by Ishida 2008, Mori 2003, or Olson 2003.
Adverse events
No side effects were observed in Ishida 2008. No decreases in visual acuity were observed and no participant complained of excessive warming of the eye in the Mori 2003 study. Adverse events were not reported by Olson 2003.
Warm air versus warm compresses (1 study)
One CCT evaluated the effects of warm moist air on tear functions and ocular surface compared with warm compresses (Matsumoto 2006). Twenty participants with MGD applied either a warm moist air device or warm compress to their eyes for 10 minutes twice a day for two weeks. The study reported no losses to follow‐up.
Risk of bias
Alternate allocation of participants to treatment groups put the study at high risk of selection bias. The study was not masked. No other risks of bias were identified.
Primary outcomes
Subjective improvements were measured using VAS for dry eye and ocular fatigue symptoms. After two weeks of treatment, participants reported significant improvements for dry eye and ocular tiredness in both the warm air and warm compress groups. Both groups also showed improvements for ocular discomfort, although the effect was significant only for the warm air group. Data were not available to perform between‐group comparisons for symptomatic improvements.
Clinical improvements were assessed using tear function and ocular surface evaluations. After two weeks of treatment, participants treated with warm air had significantly longer tear film BUT compared with baseline values (mean change 3.9 s). Tear film BUT in the warm compress group increased 0.7 s, but this change was not significant. TFLLT increased 13.7 nm in the warm air group and 5.0 nm in the warm compress group, although neither improvement was statistically significant. Fluorescein and rose bengal scores showed no change for either group. Data were not available to compare clinical outcomes between groups.
Secondary outcomes
Bacteriologic outcomes were not measured by Matsumoto 2006.
Adverse events
Adverse events were not reported by Matsumoto 2006.
Automated heating and massaging device versus automated device plus manual expression (1 study)
One intra‐individual RCT investigated a novel automated heating and massaging device (TearScience®) (Friedland 2011). One eye of each participant was treated with the automated device only and the other eye was treated with the automated device followed by heating and manual expression of individual meibomian glands by the clinician.
Risk of bias
Selection bias for Friedland 2011 was unclear as the methods of randomization and allocation concealment were not reported. The study was unmasked and the study was funded by the company producing the treatment intervention and the study authors were consultants and/or employees of the company producing the treatment intervention. Of the 14 participants randomized, 12 completed the three‐month study and were included in the analysis. Results were reported for primary and secondary outcomes specified in the paper.
Primary outcomes
Subjective outcomes were assessed using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire and the OSDI. No significant differences were reported between groups at one week', one month', or three months' follow‐up. Using a discomfort/pain scale from 0 to 10, where 0 equaled no discomfort or pain and 10 equaled intolerable pain, participants judged treatment with the automated device to be less uncomfortable and painful than treatment with manual expression in the same eye (MD ‐1.40; 95% CI ‐2.51 to ‐0.29).
Clinical assessments of the meibomian gland secretion score, the number of meibomian glands yielding liquid secretion across lower eyelid, tear BUT, and corneal fluorescein staining did not significantly differ between groups for any follow‐up time.
Secondary outcomes
Bacteriologic outcomes were not measured by Friedland 2011.
Adverse events
Three adverse events were reported during the study period. One participant experienced discomfort during treatment with the automated device, which resulted in terminating treatment early. One participant developed a chalazion and another participant developed several internal hordeola in one eye. The study authors did not report in which treatment group the adverse events occurred. There were no significant changes in IOP or the fundus observed during the study.
Discussion
Summary of main results
Anterior/mixed staphylococcal and seborrheic blepharitis
The results of interventions for treatment of blepharitis are mixed. Mixed results may be due in large part to the fact that most studies included participants with blepharitis from various etiologies.
When only anterior blepharitis and blepharoconjunctivitis cases were included, there was some suggestion that clinical outcomes were better with topical antibiotic versus placebo (Table 3). However, even when evaluating studies based on anatomical location, there was a mixture of staphylococcal blepharitis and seborrheic blepharitis cases among participants included in the study population. It is likely that staphylococcal blepharitis and seborrheic blepharitis respond to antibiotics differently, leading to the potential for null bias (Woods 1995). Studies measuring microbiologic outcomes demonstrated that topical antibiotics were effective in obtaining negative cultures from the ocular surface, but the clinical significance of this finding was not so clear. In terms of whether one antibiotic was superior to another, there was no difference between different kinds of antibiotics when compared directly. The most common adverse event reported with topical antibiotic use was ocular discomfort, which was reported infrequently.
2. Anterior/mixed blepharitis: summary for topical antibiotics versus placebo.
|
Population: participants with anterior/mixed blepharitis Intervention: topical antibiotic Comparison: placebo | ||||
| Study ID | Study characteristics | Clinical outcomes | Bacteriologic outcomes | Adverse events |
| Behrens‐Baumann 2006 | Antibiotic: bibrocathol Timing: days 7 and 14 | Favors antibiotics | Not measured | No significant difference |
| Donshik 1983 | Antibiotic: gentamicin Timing: days 3, 7, and 14 | No significant difference | Favors antibiotics | No significant difference |
| Hyndiuk 1990 | Antibiotic: mercuric oxide Timing: days 3 and 7 | Favors antibiotics | Favors antibiotics | No significant difference |
| Jackson 1982 | Antibiotic: gentamicin Timing: days 7 and 14 | No significant difference | Favors antibiotics | No significant difference |
| Laibovitz 1991 | Antibiotic: tetracycline Timing: not reported | Favors antibiotics | Favors antibiotics | Not reported |
| More 1968 | Antibiotic: penotrane Timing: two 4‐week phases; cross‐over trial | No significant difference | No significant difference | No significant difference |
| Shulman 1982 | Antibiotic: gentamicin Timing: days 3, 7, and 14 | No significant difference | Favors antibiotics | No significant difference |
Overall, the antibiotic studies were short‐term (most were up to 14 days) and evaluated different types of topical antibiotics. There were no consistently significant differences between antibiotics compared with placebo or another type of antibiotic for clinical outcomes. One problem with assessing the clinical effectiveness of blepharitis therapies is the large placebo effect. Even among the placebo groups, some clinical improvement was observed. Furthermore, not all cultures were negative following treatment with the topical antibiotics. Therefore it is not known how bacteriologic improvements with antibiotic use translate to clinically significant effects on signs and symptoms.
Treatment with steroids may show clinical improvements in the short‐term by masking the primary signs and symptoms of blepharitis, such as inflammation and infection. However, chronic use of steroids is not recommended due to the side effects of long‐term use. Also, bacteriologic outcomes were not affected by topical steroid treatment.
Studies that evaluated both topical antibiotics and topical steroids did not show clinically significant improvements from baseline for either treatment individually or compared with each other. Although these studies also showed that antibiotic therapy significantly decreased bacteriologic cultures compared with steroid therapy, bacteriologic improvement was not associated with clinical improvement. Combined antibiotic plus steroid studies demonstrated the greatest effect in anterior blepharitis and blepharoconjunctivitis cases. Ocular surface cultures were significantly reduced compared with steroid alone or placebo. Side effects were not serious and most were reports of discomfort. However, treatment in these studies was short term, lasting only a few weeks. Long‐term side effects of steroids, such as development of glaucoma and the formation of cataracts, can be potentially harmful.
Topical antifungal agents appear to be ineffective in the treatment of blepharitis as compared with antibiotics or placebo.
Mechanical measures using lid hygiene and/or detergents demonstrated improvements of signs and symptoms in the great majority of the participants with no side effects. However, the two studies assessing these measures used different types of detergents and comparison groups. Compliance to lid hygiene and lid scrubs may also be an issue for long‐term use.
Posterior blepharitis/MGD
Many therapies were studied for the treatment of posterior blepharitis. Due to the variation in medical and mechanical interventions under study, most comparisons of treatment were evaluated only by a single study.
Beneficial effects of topical azithromycin plus warm compresses were observed compared to warm compresses alone in one study; however, the study was small, open‐label, and industry‐funded. Later, multicenter, double‐masked, phase II studies conducted by the same pharmaceutical company did not confirm these results (Inspire 2010).
Oral doxycycline was observed to have an effect on clinical improvements at high (200 mg, twice daily) and low (20 mg, twice daily) doses, with adverse events occurring more frequently in the high‐dose group compared with the low‐dose group. Some side effects of doxycycline can be serious (e.g. liver failure, interaction with other medications, teratogenicity, etc.).
Topical cyclosporine was studied long‐term (3 months) and showed mixed results for clinical tests (e.g. corneal staining scores, Schirmer scores, tear BUT, etc.) when compared with placebo or topical antibiotics plus steroids. However, the clinical significance of changes in test scores is questionable and may not be appreciated by patients.
Castor‐oil‐containing eyedrops were better than saline eyedrops in terms of improving tear function, especially stability. The explanation may be that posterior blepharitis is associated with poor meibum secretion and adding oily substances may help with improving tear film stability.
Heat application showed some benefit in terms of patient symptoms and some effectiveness regarding tear BUT. This finding can be explained by the fact that heat helps express the meibum secretion.
Overall completeness and applicability of evidence
A major problem with blepharitis trials is that it is very difficult to differentiate between various types of anterior blepharitis cases, such as seborrheic, staphylococcal, and demodex‐related blepharitis. Mostly the forms coexist, which is perhaps the reason that studies have failed to show consistent patterns of effectiveness.
Only six (18%) of the 34 included studies were published in or after 2008, when the definitions and classifications for blepharitis were updated by the American Academy of Ophthalmology (AAO 2008). We did not identify any study published since the report by the Definition and Classification subcommittee of the International Workshop on Meibomian Gland Dysfunction was published in 2011 (Nelson 2011). The changing definitions and classifications for blepharitis, as well as improvements in study design and methodology, over the past few years make interpreting the evidence from differing eras difficult.
Also, there were multiple ways outcomes were measured by the studies included in this review (e.g. subjective physician assessment, clinical tests, patient‐reported improvement). Thus results for many studies could not be combined in meta‐analysis.
Most of the studies included in this review were only two weeks or less in duration. For a chronic disease, short follow‐up times do not provide evidence of a lasting effect.
Quality of the evidence
Twenty studies (59%) included in this review were either industry funded or co‐authored by a person affiliated with industry. Sixteen (47%) of the included studies included 30 or fewer participants with blepharitis. Also, 7/34 studies included both eyes of participants in the analyses: three studies were intra‐individual comparative studies (Friedland 2011; Key 1996; Olson 2003), one study was a cross‐over study (Goto 2002), and in three studies the participant was treated and each eye was analyzed separately (Luchs 2008; Macsai 2008; Wong 1956). In only one of these studies (Goto 2002) was paired data analysis used to take into account nonindependence of eyes.
Potential biases in the review process
Of the 20 studies we identified from the search that investigated the effectiveness of interventions for treating participants with clinically related conditions in which blepharitis patients were a subset, 18 studies were excluded from this review because data for the blepharitis subgroup were not reported separately. For the two studies that were included (Adenis 1996a; Goldberg 1960), stratified randomization based on clinical condition (e.g. conjunctivitis, blepharitis, stye) was not part of the allocation process. The implementation of stratified randomization has been shown to prevent type I error and improve power for small trials when the stratifying factor is associated with treatment responsiveness (Kernan 1999).
Agreements and disagreements with other studies or reviews
The findings from this review are consistent with evidence‐based recommendations provided in the AAO's Preferred Practice Guidelines for blepharitis (AAO 2008) and the International Workshop on MGD subcommittee's report on treatment for MGD (Geerling 2011). Consistent high‐level evidence is missing for most treatments and outcomes considered to date. A review by Jackson reported conclusions similar to this review, although that review did not include non‐English language or unpublished studies (Jackson 2008).
Authors' conclusions
Implications for practice.
There is no strong evidence for any of the treatments in terms of curing blepharitis. Treatment of asymptomatic patients with blepharitis remains a topic for discussion. Numerous commercial products are available to patients, although limited evidence are available to support their effectiveness. Mechanical lid hygiene and warm compresses may provide some symptomatic relief for both anterior and posterior blepharitis; however, they have not been shown to cure the condition. For flare‐ups, perhaps combination therapy can be used for short periods of time. Oral antibiotics may not be appropriate for patients with severe problems due to possible side effects. Long‐term treatment is necessary.
Implications for research.
Further research is needed to evaluate the effectiveness of treatments for blepharitis. Substantial heterogeneity between studies in the current literature make drawing conclusions on the effectiveness of current treatments difficult. Better clinical definitions and improved diagnosis are needed. Also, outcomes for future research should be based on clinically relevant outcomes and outcomes important to patients, not bacteriologic outcomes alone since it is unclear how the elimination of bacteria relates to clinical improvement for this condition. Future research aimed at comparing the effectiveness of topical antibiotics with over‐the‐counter lid hygiene products would be informative.
A randomized controlled trial designed to investigate the effectiveness of an intervention for chronic blepharitis should separate participants by type of condition, either by including only a subset of patients (e.g., patients with either staphylococcal, seborrheic, or meibomian gland dysfunction) or by stratifying randomization by type in order to minimize imbalances between groups (type I errors) and to achieve statistical power for analyses (minimize type II errors). Medical interventions and commercial products should be compared with conventional lid hygiene measures, such as warm compresses and washing of the eyelid margin, to determine effectiveness, as well as head‐to‐head to show comparative effectiveness between treatments. Masking of all study participants and personnel should be done when possible. Outcomes of interest should be patient‐centered, such as reduction or elimination of ocular irritation, burning, tearing, or itching, and measured using validated questionnaires or scales. As this is a chronic condition, participants should be followed long‐term, at least one year, to measure time to resolution of the initial episode and rates of recurrence.
History
Protocol first published: Issue 4, 2005 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 19 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge Iris Gordon (Trials Search Co‐ordinator for CEVG) for devising and running electronic search strategies. We acknowledge Kim Miller, Kent Anderson, and Bola Odufuwa for their contributions to the protocol for this review. We acknowledge Ann Ervin and Swaroop Vedula (both CEVG@US) and Anupa Shah (Managing Editor for CEVG) for their contributions to this review.
We thank Barbara Hawkins and other peer reviewers for their meaningful feedback for this review.
We thank the following study investigators for responding to requests for additional information: Akyol‐Salman I, Behrens‐Baumann W, Dogru M, Jackson WB, Korb DR, Luchs J, Nelson ME, Perry HD, Pinna A, Shulman J, and Tsubota K.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Blepharitis #2 blephariti* #3 blepharoconjunctivitis #4 demodex #5 MeSH descriptor Meibomian Glands #6 meibomian near gland* #7 ocular near gland* #8 eye* near inflamm* #9 eye* near infect* #10 eye* near seborrheic #11 eye* near staphylococcal #12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 MeSH descriptor Infection #14 MeSH descriptor Inflammation #15 MeSH descriptor Staphylococcal Infections #16 MeSH descriptor Dermatitis #17 (#13 OR #14 OR #15 OR #16) #18 MeSH descriptor Eyelids #19 (#17 AND #18) #20 (#12 OR #19)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. blepharitis/ 14. blephariti$.tw. 15. blepharoconjunctivitis.tw. 16. demodex.tw. 17. meibomian glands/ 18. (meibomian adj2 gland$).tw. 19. (ocular adj2 gland$).tw. 20. (eye$ adj3 inflamm$).tw. 21. (eye$ adj3 infect$).tw. 22. (eye$ adj3 seborrheic).tw. 23. (eye$ adj3 staphylococcal).tw. 24. or/13‐23 25. exp infection/ 26. exp inflammation/ 27. exp staphylococcal infections/ 28. dermatitis seborrheic/ 29. or/25‐28 30. exp eyelids/ 31. 29 and 30 32. 24 or 31 33. 12 and 32
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. blepharitis/ 34. blephariti$.tw. 35. blepharoconjunctivitis.tw. 36. demodex.tw. 37. meibomian gland/ 38. (meibomian adj2 gland$).tw. 39. (ocular adj2 gland$).tw. 40. (eye$ adj3 inflamm$).tw. 41. (eye$ adj3 infect$).tw. 42. (eye$ adj3 seborrheic).tw. 43. (eye$ adj3 staphylococcal).tw. 44. or/33‐43 45. exp infection/ 46. exp inflammation/ 47. exp staphylococcal infection/ 48. seborrheic dermatitis/ 49. or/45‐48 50. exp eyelids/ 51. 49 and 50 52. 44 or 51 53. 32 and 52
Appendix 4. metaRegister of Controlled Trials search strategy
blepharitis
Appendix 5. ClinicalTrials.gov search strategy
Blepharitis
Appendix 6. ICTRP search strategy
blepharitis
Data and analyses
Comparison 1. Topical antibiotics versus placebo (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total sign and/or symptom scores | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Day 3 | 2 | 73 | Mean Difference (Random, 95% CI) | ‐0.9 [‐1.47, ‐0.33] |
| 1.2 Day 7 | 4 | 299 | Mean Difference (Random, 95% CI) | ‐0.76 [‐1.30, ‐0.23] |
| 1.3 Day 14 | 3 | 248 | Mean Difference (Random, 95% CI) | ‐1.30 [‐3.31, 0.71] |
| 2 Proportion of cultures eradicated | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 14 | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.10, 8.44] |
| 2.2 Day 28 | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 3.91] |
| 3 Proportion of total adverse events | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.57] |
Comparison 2. Topical ciprofloxacin versus another topical antibiotic (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion cured or improved | 3 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 2 Proportion of cultures eradicated | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.85, 1.26] |
Comparison 3. Topical antibiotics versus topical steroids (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cultures eradicated | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [2.02, 8.57] |
Comparison 4. Topical steroids versus placebo (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cultures eradicated | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.15] |
Comparison 5. Combined topical antibiotics and steroids versus placebo (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cultures eradicated | 3 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [1.57, 11.34] |
Comparison 6. Combined topical antibiotics and steroids versus topical antibiotics alone (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cultures eradicated | 4 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.16] |
Comparison 7. Combined topical antibiotics and steroids versus topical steroids alone (anterior/mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of cultures eradicated | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [1.91, 8.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adenis 1996a.
| Methods | Study design: randomized, parallel‐group study Conditions included: acute conjunctivitis or acute or chronic blepharitis Enrollment: 77 participants randomized; 21 with chronic blepharitis Exclusions and loss to follow‐up: 38 participants who were either culture‐negative on day 0 or did not complete follow‐up were excluded from the efficacy analyses Study follow‐up: 7 days |
|
| Participants | Country: France Age: mean 52.8 ± 22.8 years (range 6 to 93 years) Gender: 37 men and 40 women Inclusion criteria: 1) at least 1 year of age, 2) clinical evidence of bacterial acute conjunctivitis or acute or chronic blepharitis Exclusion criteria: 1) allergic to ciprofloxacin, fusidic acid, or components of either formulation; 2) treatment with topical or systemic antimicrobial agents or steroids in the last 48 hours; 3) pregnant or not using adequate birth control methods |
|
| Interventions | Ciprofloxacin (n = 39; 7 with chronic blepharitis; 21 culture‐positive on day 0): 0.3% ciprofloxacin ophthalmic solution, starting with 2 drops every 2 hours for the first 48 hours and followed by 2 drops every 4 hours from days 2 to 6; eyelid margin scrub with 2 drops of ciprofloxacin during treatment period Fusidic acid (n = 38; 14 with chronic blepharitis; 18 culture‐positive on day 0): 1% fusidic acid gel, 1 drop applied twice a day to the conjunctival sac |
|
| Outcomes | Primary outcomes:
1) efficacy of interventions (in participants who were culture‐positive on day 0): bacteriologic response to treatment between day 7 and day 0 (eradication, reduction, persistence, or proliferation); change in clinical sign and symptom scores; patient's response to treatment (cured, improved, unchanged, or worsened)
2) safety of interventions (in all participants): clinical adverse events Measurements taken at baseline and day 7 Unit of analysis: microbiologic outcomes were based on the eye having the least response to treatment, overall signs and symptoms were based on the average of both eyes in cases of bilateral infections, and safety data were reported descriptively for all eyes |
|
| Notes | Study dates: not reported Funding source: Alcon Laboratories Inc., USA Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Reported as an open study. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Reported as an open study. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Reported as an open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Combined results for participants with conjunctivitis or acute or chronic blepharitis. Even with randomization there was an imbalance between treatment groups with respect to diagnosis. Funded by the pharmaceutical industry. |
Akyol‐Salman 2010.
| Methods | Study design: randomized, parallel‐group study Conditions included: MGD (posterior blepharitis) Enrollment: 20 participants randomized Exclusions and loss to follow‐up: none Study follow‐up: 1 month |
|
| Participants | Country: Turkey Age: mean 40 years Gender: 12 men and 8 women Inclusion criteria: 1) thickening or irregularity of the eye lid margins; 2) erythema of posterior lid margin; 3) dilation of blood vessels and telangiectasis around the glandular orifices; 4) reduced or no expulsion of normally thin, oily secretions on digital pressure; 5) expulsion of large amounts of cloudy, turbid, foamy, granular, or semi‐solid secretion on digital pressure; 6) and capping of meibomian gland orifices Exclusion criteria: 1) systemic abnormalities, 2) previous ocular surgery, 3) intraocular pathology, 4) history of allergic reaction to the drugs, 5) current use of therapies for MGD |
|
| Interventions | NAC (n = 10): 5% NAC ophthalmic solution 4 times daily Control (n = 10): preservative‐free artificial tear 4 times daily All participants applied lid hygiene with a solution (Blepharoshampoo) once daily |
|
| Outcomes | Primary outcomes:
1) decrease in severity of inflammatory symptoms 2) change in mean ocular symptoms (ocular burning, itching, foreign body sensation, and intermittent filmy or blurred vision) 3) tear function: Schirmer test and fluorescein BUT Safety outcomes: elevated IOP and allergic reactions Measurements taken at baseline and 1 month Unit of analysis: the individual (mean of both eyes) |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random‐number generator assigned patients to a treatment group. Odd numbers were assigned to the NAC group, and even numbers were assigned to the preservative‐free artificial tear group." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Masking of participants was not reported. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking of healthcare providers was not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | "All patients were examined by the same masked investigator at 1 day and 1 month." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | High risk | Results for changes in the severity of inflammatory symptoms were not reported. |
| Other bias | Low risk | |
Aragones 1973.
| Methods | Study design: randomized, parallel‐group study Conditions included: infectious blepharitis Enrollment: 30 patients hospitalized at the Lapeer State Home and Training School, Michigan, USA Exclusions and loss to follow‐up: none Study follow‐up: not specified |
|
| Participants | Country: USA Age: not specified Gender: not specified Inclusion criteria: 1) clinically diagnosed blepharitis with an infectious component sensitive to sulfacetamide, 2) associated inflammation |
|
| Interventions | Prednisolone/sulfacetamide (n = 15): 10% sodium sulfacetamide plus 0.2% prednisolone acetate suspension, 3 drops in each eye 4 times daily Sulfacetamide alone (n = 15): 10% sodium sulfacetamide, 3 drops in each eye 4 times daily All participants: nurses administered the eyedrops without removing the excess from the eyelids; no concurrent antibiotics or steroids were given |
|
| Outcomes | Primary outcomes:
1) subjective efficacy of interventions: overall response to treatment (excellent, good, no change, worse); rate of therapeutic effect (rapid, normal, slow)
2) objective efficacy of interventions: changes in clinical signs and symptoms Secondary outcomes: 1) bacteriologic eradication rates 2) clinical adverse events Measurements taken at baseline and daily until completion of treatment Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Allergan Pharmaceuticals, USA Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomized numbering technique was used. |
| Allocation concealment (selection bias) | Low risk | Identical opaque white plastic dropper bottles filled with solutions of similar appearance were prepared. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Participants were masked to treatment group by the use of identically prepared solutions that were administered by nurses. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Reported as a double‐blind study; medications were serially dispensed to each participant from the supply of masked containers. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Reported as a double‐blind study; medications were serially dispensed to each participant from the supply of masked containers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed as there were no exclusions or losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry. |
Behrens‐Baumann 2006.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: blepharitis Enrollment: 203 participants enrolled at 14 medical practices Exclusions and loss to follow‐up: 6 participants, 3 from each group, did not complete at least 1 follow‐up exam and were excluded from the analyses Study follow‐up: 2 weeks |
|
| Participants | Country: Germany Age: median 66 years (range 18 to 89 years) Gender: 87 men and 116 women Inclusion criteria: 1) at least 18 years of age, 2) a blepharitis summary score of at least 12 Exclusion criteria: 1) antibiotic therapy was indicated, 2) cases of resistant blepharitis, 3) unusual eyelid anatomy (independent of the blepharitis), 4) surgical intervention in the eye within the last 90 days, 5) severe KCS (dry‐eye syndrome), 6) allergic ocular illnesses, 7) allergies to components of the test medication, 8) heavy systemic dysfunction judged by the treating doctor, 9) rheumatoid arthritis/spondylitis, 10) anamnesis of malignant illnesses within the last 5 years |
|
| Interventions | Bibrocathol (n = 103): bibrocathol (Noviform) 5% ointment Placebo (n = 100): vehicle ointment All participants: applied a 5‐mm long ribbon of ointment on the upper and lower eyelid up to the eyelid edge after eyelid hygiene 3 times daily |
|
| Outcomes | Primary outcomes:
1) change in blepharitis summary score at 2 weeks
2) change in objective signs at 2 weeks
3) change in subjective symptoms at 2 weeks
4) adverse effects Measurements taken 2 days before baseline, at baseline, and days 7 and 14 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Novartis Pharma GmbH, Nuremberg Declarations of interest: 1 study author affiliated with Novartis Pharma GmbH, Nuremberg and 1 study author affiliated with IMEREM Institute for Medical Research Management and Biometrics GmbH, Nuremberg Publication language: German |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization was carried out by the sponsor of the study in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated to treatment groups at the baseline visit, which occurred 2 days after study enrollment. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | The participants were masked to treatment groups and a placebo ointment was used. The medication for both treatment groups was identical concerning packaging, inscription, tube, and size. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | A masked investigator graded the ocular findings at the initial visit, at each follow‐up visit, and at the conclusion of the treatment period. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | A masked investigator graded the ocular findings at the initial visit, at each follow‐up visit, and at the conclusion of the treatment period. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 participants who were randomized to receive treatment, but did not complete at least 1 follow‐up were excluded from the analyses. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry and 2 study authors affiliated with industry. |
Bloom 1994.
| Methods | Study design: randomized, parallel‐group study Conditions included: blepharitis and blepharoconjunctivitis Enrollment: 464 participants from multiple, international specialist eye centers Exclusions and loss to follow‐up: 220 participants who were culture‐negative on day 0 and did not complete follow‐up were excluded from the efficacy analyses Study follow‐up: 7 days |
|
| Participants | Countries: Europe and North America Age: mean 61 years (range 18 to 80 years) Gender: 217 men and 247 women Inclusion criteria: patients with blepharitis or blepharoconjunctivitis with presumed bacterial origin Exclusion criteria: 1) history of allergy to components of medications, 2) treatment with an antimicrobial agent or steroid in previous 48 hours, 3) pregnancy, 4) refusal to stop wearing contact lenses during study period, 5) meibomian disease, 6) frank marginal ulceration or severe pseudomembranous conjunctivitis |
|
| Interventions | Ciprofloxacin (n = 230): 0.3% ciprofloxacin eyedrops, starting with 1 or 2 drops every 2 hours for the first 48 hours and followed by 2 drops every 4 hours from days 2 to 6 Tobramycin (n = 234): 0.3% tobramycin eyedrops, starting with 1 or 2 drops every 2 hours for the first 48 hours and followed by 2 drops every 4 hours from days 2 to 6 All participants: nightly lid scrubs |
|
| Outcomes | Primary outcomes:
1) change in clinical assessment (cured, better, unchanged, worse)
2) changes in clinical signs and symptoms
3) change in bacteriologic assessment (eradication, reduction, persistence, proliferation)
4) clinical adverse events Measurements taken at baseline and day 7 Unit of analysis: the individual, using the worse eye in cases of bilateral disease |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Included participants with blepharitis or blepharoconjunctivitis. |
Collum 1984.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: chronic blepharitis Enrollment: 40 participants Exclusions and loss to follow‐up: 2 participants, 1 from each group, were lost to follow‐up Study follow‐up: 4 weeks during the time of receiving treatment |
|
| Participants | Country: Ireland (or UK not specified) Age: not reported Gender: not reported Inclusion criteria: history of blepharitis for at least 2 years Exclusion criteria: 1) other eye pathology, 2) use of concurrent steroids or antihistamines |
|
| Interventions | DSCG (n = 20): 4% disodium cromoglycate ointment (Opticrom) 4 times daily for 4 weeks Placebo (n = 20): placebo ointment of yellow paraffin and acetylated lanolin 4 times daily for 4 weeks All participants: lid scrub performed at initial visit |
|
| Outcomes | Primary outcomes:
1) clinical assessment of signs and symptoms at 4 weeks
2) change in bacterial cultures at 4 weeks
3) adverse effects
4) patients' and clinicians opinions of treatment
5) skin testing for common allergens Measurements taken at baseline and weekly for 4 weeks Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: 1 of the authors affiliated with Fisons Pharmaceuticals, Loughborough, UK Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Reported as double‐masked and used placebo for control group. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | 1 of the authors affiliated with pharmaceutical industry. |
Donshik 1983.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: chronic staphylococcal blepharoconjunctivitis Enrollment: 100 participants Exclusions and loss to follow‐up: 3 participants were lost to follow‐up, 18 participants were excluded from the efficacy analyses Study follow‐up: 2 weeks |
|
| Participants | Country: USA Age: range 20 to 94 years Gender: 41 men and 56 women (as reported) Inclusion criteria: 1) chronic staphylococcal blepharoconjunctivitis (at least 1 previous episode of acute blepharoconjunctivitis or at least 1 month's duration of the present eye complaint); 2) scores of 2 or more for conjunctival or lid hyperemia or both, and a total score of at least 5 for all signs; 3) staphylococcal infection sensitive to gentamicin Exclusion criteria: 1) use of topical or systemic corticosteroids, antihistamines, or decongestants within 24 hours; 2) known allergies to the study medications; 3) patients with viral infections, fulminant corneal ulcers, uveitis, endophthalmitis, orbital cellulitis, fungal infections, glaucoma, foreign body, postoperative infections, contact lens or other forms of mechanical irritation, trauma, and chemical conjunctivitis |
|
| Interventions | Combination (n = 25): 0.3% gentamicin sulfate and 0.1% betamethasone phosphate ophthalmic solution, 1 drop 4 times daily for 2 weeks Betamethasone (n = 25): 0.1% betamethasone phosphate ophthalmic solution, 1 drop 4 times daily for 2 weeks Gentamicin (n = 25): 0.3% gentamicin sulfate (Garamycin) ophthalmic solution, 1 drop 4 times daily for 2 weeks Placebo (n = 25): sterile vehicle placebo solution, 1 drop 4 times daily for 2 weeks Participants were not allowed to use concomitant topical medications (eye shampoos, tear replacement agents, etc.) or oral or other systemic medications with known effects on the eye; warm compresses, lid hygiene with water, and oral analgesics were allowed |
|
| Outcomes | Primary outcomes:
1) improvement of signs and symptoms at 2 weeks
2) change in bacterial cultures at 2 weeks
3) adverse reactions
4) compliance with treatment Measurements taken at baseline, days 3 to 4, days 7 to 8, and days 14 to 15 Unit of analysis: the eye with the most severe signs |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment of treatment numbers to the 4 groups was randomized equally between groups. |
| Allocation concealment (selection bias) | Low risk | Treatment numbers were assigned pending culture results. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Study was double‐masked and identical packages were used for all solutions, which had similar appearance, color, and consistency. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Study was double‐masked and used treatment numbers on identically packaged bottles. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Study was double‐masked and used treatment numbers on identically packaged bottles. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Included participants with blepharoconjunctivitis. |
Friedland 2011.
| Methods | Study design: randomized, intra‐individual comparative study Conditions included: MGD (posterior blepharitis) Enrollment: 14 participants Exclusions and loss to follow‐up: 2 participants were not included in final analyses Study follow‐up: 3 months |
|
| Participants | Country: USA Age: mean 54.2 years (range 37 to 72 years) Gender: 4 men and 10 women Inclusion criteria: 1) age 18 years or older; 2) written informed consent; 3) willingness and ability to return for all study visits; 4) history of self‐reported dry eye symptoms for 3 months prior to study; 5) need for regular use of artificial tears, lubricants, or rewetting drops; 6) previous diagnosis of moderate‐to‐severe dry eye; 7) meibomian gland obstruction Exclusion criteria: 1) history of recent ocular surgery, ocular trauma, or herpetic keratitis within 3 months of study; 2) chronic or recurrent ocular inflammation; 3) active ocular inflammation or infection; 4) lid surface abnormalities that affect lid function in either eye; 5) grade 3 or 4 meibomitis, and/or blepharitis on a scale of 0 to 4; 6) dry eye related to Steven‐Johnson syndrome, Riley Day syndrome, sarcoidosis, leukemia, ocular trauma, or chemical burns; 7) women who were pregnant, nursing, or not using adequate birth control; 8) patients who had changed the dosing of systemic or ophthalmic medication in past 30 days of study; 9) use of topical or systemic medications known to cause ocular dryness; 10) use of another investigational device or agent within 30 days of study |
|
| Interventions | Automated device (n = 14): TearScience® automated treatment device for 12 minutes; the lid warmer rests on sclera and heats the meibomian glands of the upper and lower eyelids, the eye cup rests on the closed eyelids and massages the eyelids to express the meibomian glands of the upper and lower eyelids Automated device and manual expression (n = 14): TearScience® automated treatment device for 12 minutes followed by heating and manual expression of individual meibomian glands by clinician All eyes received 2 drops of topical anesthetic prior to device insertion; eyes were treated sequentially, not simultaneously |
|
| Outcomes | Primary outcomes:
1) meibomian gland assessment (meibomian gland secretion score and number of meibomian glands yielding liquid secretion across lower eyelid)
2) objective dry eye tests (tear BUT and corneal fluorescein staining)
3) subjective dry eye symptoms (SPEED, OSDI)
4) ocular health examination (anterior segment and retina evaluation, IOP)
5) discomfort/pain evaluation during and after treatment Measurements taken at baseline, 1 day, 1 week, 1 month, and 3 months Unit of analysis: each eye of each participant (intracomparative) |
|
| Notes | Study dates: June 2008 Funding source: Korb Associates (Boston, MA, USA) and TearScience (Morrisville, NC, USA) Declarations of interest: study authors consultants and/or employees of TearScience Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Selection of eyes to receive or not receive manual expression was described as random. It was not clear what method of randomization was used. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Study providers could not be masked to treatment groups. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Outcome assessors were not masked (open study). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants who missed follow‐up visits were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | High risk | The study was funded by the company producing the treatment intervention and the study authors were consultants and/or employees of the company producing the treatment intervention. |
Goldberg 1960.
| Methods | Study design: parallel‐group study (participants with unilateral disease) and intra‐individual comparative study (participants with bilateral disease) Conditions included: inflammatory and/or infectious eye diseases Enrollment: 185 participants (39 participants had bilateral disease); 27 with blepharoconjunctivitis Exclusions and loss to follow‐up: none reported Study follow‐up: 2 to 40 days (majority were treated between 3 to 14 days) |
|
| Participants | Country: USA Age: range 11 to 78 years Gender: 98 men and 87 women Inclusion criteria: variety of inflammatory and/or infectious conditions in the eye for which topical therapy was given |
|
| Interventions | Triamcinolone acetonide:
Preparation #1 (n = 82 participants with unilateral disease; 19 participants with bilateral disease): 1 mg/cc triamcinolone acetonide sodium hemisuccinate eyedrops Preparation #2 (n = 36 participants with unilateral disease; 19 participants with bilateral disease): 1 mg/cc triamcinolone acetonide dipotassium phosphate eyedrops Triamcinolone acetonide plus antibiotics: Preparation #1 (n = 4 participants with unilateral disease; 20 participants with bilateral disease): 1 mg/cc triamcinolone acetonide sodium hemisuccinate, 2.5 mg/cc neomycin sulfate and 0.25 mg/cc gramicidin eyedrops Preparation #2 (n = 24 participants with unilateral disease; 20 participants with bilateral disease): 1 mg/cc triamcinolone acetonide dipotassium phosphate, 2.5 mg/cc neomycin sulfate and 0.25 mg/cc gramicidin eyedrops 1 drop of ophthalmic solution was administrated according to whatever dosage schedule was prescribed in the individual case; other medications or therapeutic measures were used as needed |
|
| Outcomes | Primary outcomes:
1) clinical improvement (changes in the symptoms and inflammatory clinical findings ) at the end of the treatment period
2) adverse reactions Measurements taken at the end of the treatment period Unit of analysis: the individual for participants with unilateral disease and the eye for participants with bilateral disease (intracomparative) |
|
| Notes | Study dates: not reported Funding source: The Squibb Institute for Medical Research, USA Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization was not reported. |
| Allocation concealment (selection bias) | High risk | The assignment scheme for unilateral disease participants was not reported. It was not reported how treatment groups for bilateral disease participants were determined. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Study interventions were prepared differently by the participant. Triamcinolone acetonide sodium hemisuccinate was provided in powder form and reconstituted immediately before use. Triamcinolone acetonide dipotassium phosphate was provided in ready‐to‐use form. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Study investigators prescribed the dosage for individual cases. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intent to treat analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | High risk | Included participants with various inflammatory and/or infectious conditions, not limited to blepharitis. It was unclear if the study intended for 2 types of preparations to be used from the beginning, or if the second preparation was added after the trial began since it was easier to administer. It was also not clear why the dosage was prescribed on an individual basis and what effect this may have had on the results. |
Goto 2002.
| Methods | Study design: randomized, placebo‐controlled cross‐over study Conditions included: noninflamed obstructive MGD Enrollment: 20 participants (40 eyes) Exclusions and loss to follow‐up: none reported Study follow‐up: 6 weeks |
|
| Participants | Country: Japan Age: mean 52.1 ± 11.0 years Gender: 7 men and 13 women Inclusion criteria: patients with MGD who had not improved sufficiently with conventional treatments such as eye lid hygiene, topical therapy with artificial tear, antibiotics, and corticosteroids or systemic antibiotics Exclusion criteria: eyes with anterior blepharitis of more than moderate severity, infectious conjunctivitis, MGD with acute inflammation, eyes with excessive expression of meibum (seborrheic MGD) |
|
| Interventions | Homogenized oil drops: 2% castor oil, 5% polyoxyethylene castor oil, 0.3% sodium chloride, 0.15% potassium chloride, and 0.5% boric acid emulsion Placebo drops: normal saline solution Drops were instilled 6 times daily for weeks; participants used a preservative‐free artificial tear for 2 weeks (wash‐out) before receiving either oil or placebo drops for 2 weeks, then switching for 2 more weeks |
|
| Outcomes | Primary outcomes:
1) change in symptoms (face score)
2) tear interference grading (1 to 5)
3) tear evaporation rates
4) fluorescein score (0 to 9) and rose bengal score (0 to 9)
5) tear BUT
6) meibomian gland orifice obstruction (0 to 3) Secondary outcomes: 1) adverse events 2) stability of emulsion Measurements taken at baseline and weeks 2, 4, and 6 Unit of analysis: each eye of each participant |
|
| Notes | Study dates: not reported Funding source: Japanese Ministry of Education and Science; Medical School Faculty and Alumni Grants of Keio University, Japan; Hightech Research Center at Tokyo Dental College; and Nihon Tenganyaku Kenkyusho Co. Ltd., Japan Declarations of interest: 2 study authors and 1 funding source applied for a patent on the eyedrops tested in this study Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment groups were randomly divided by a co‐author. It was not clear what method of randomization was used. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | "Blinding among participants ... were performed entirely by protocol." |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | "Blinding among ... persons performing the intervention... were performed entirely by protocol." |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | "Blinding among ... outcome assessors were performed entirely by protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | High risk | Results at baseline in placebo group were not reported. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry; company and study authors have patent pending on study intervention. Potential carry‐over in cross‐over phases. Data were presented by eyes rather than by the unit of randomization, which was the individual. |
Hyndiuk 1990.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: bacterial blepharitis Enrollment: 58 participants Exclusions and loss to follow‐up: 19 participants were excluded from the study (6 due to low initial bacterial counts, 5 due to noncompliance, 5 lost to follow‐up, and 3 due to adverse reactions) Study follow‐up: 7 days |
|
| Participants | Country: USA Age: not reported Gender: not reported Inclusion criteria: biomicroscopic evidence of blepharitis Exclusion criteria: 1) other inflammatory pathology of the eye, 2) use of topical medication in previous 72 hours |
|
| Interventions | Mercuric oxide (n = 19): 1% mercuric oxide (yellow) ophthalmic ointment applied twice daily to the eyelid margin for 10 days Placebo (n = 20): anhydrous ointment base without active ingredient applied twice daily to the eyelid margin for 10 days |
|
| Outcomes | Primary outcomes:
1) improvement of clinical score and signs at 1 week
2) change in bacterial colonies at 1 week
3) adverse reactions
4) compliance with treatment Measurements taken at baseline (day 1), day 3, and day 7 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Commerce Drug Co., Inc.; National Institutes of Health; and Research to Prevent Blindness, Inc. Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Used placebo ointment so participants were unaware which treatment they received. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Used randomly coded ointments bottles. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Used randomly coded ointments bottles. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 19 excluded participants. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry. |
Ishida 2008.
| Methods | Study design: controlled clinical trial Conditions included: MGD (posterior blepharitis) Enrollment: 20 participants Exclusions and loss to follow‐up: none Study follow‐up: 2 weeks |
|
| Participants | Country: Japan Age: mean 54.5 years Gender: 8 men and 12 women Inclusion criteria: patients with simple MGD including 1) occluded meibomian gland orifices, 2) cloudy or inspissated glandular secretion with lack of clear meibum secretion after applying moderate pressure, 3) presence of keratinization or displacement of the mucocutaneous junction Exclusion criteria: 1) inflammatory lid disease, 2) history or clinical findings of cicatricial eyelid and conjunctival diseases, 3) excessive meibomian lipid secretion (seborrheic MGD) |
|
| Interventions | Orgahexa eye warmer (n = 10): eye mask made of carbon fiber (body heat warms the fiber, which releases far‐infrared radiation to warm the mask); masks were applied for 10 minutes in the short‐term study and overnight during sleeping for 2 weeks in the long‐term study Conventional eye warmer (n = 10): eye mask; masks were applied for 10 minutes in the short‐term study and overnight during sleeping for 2 weeks in the long‐term study No topical medication were used during the study |
|
| Outcomes | Primary outcome: efficacy of warming device after 2 weeks measured by
1) eyelid temperature
2) slit lamp examinations
3) tear BUT
4) Schirmer test
5) vital staining
6) tear film lipid layer interferometry
7) dry‐eye symptoms Measurements taken at baseline, 10 minutes, and 2 weeks Unit of analysis: the individual (right eyes only) |
|
| Notes | Study dates: not reported Funding source: Therath Medico, Tokyo, Japan supplied the Orgahexa fiber masks Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported as a "prospective unmasked non‐randomized study." The allocation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Unclear risk | It was reported that "patients did not know which type of mask they were using in this study," however, the study authors noted that the two masks being studied "had obvious design and appearance differences." |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Healthcare providers were not masked. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Outcome assessors were not masked. "The type of eye warmer used was masked to the statistician (MK) performing the analyses." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. "All patients completed both short‐ and long‐term trials wearing the masks successfully during sleep." |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the report. |
| Other bias | Unclear risk | Orgahexa eye warmers were provided by industry. |
Jackson 1982.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: symptomatic infective blepharitis or blepharoconjunctivitis Enrollment: 46 participants from 2 study centers Exclusions and loss to follow‐up: 3 participants were lost to follow‐up Study follow‐up: 14 days |
|
| Participants | Country: Canada Age: mean 48 years Gender: 23 men and 23 women Inclusion criteria: 1) symptomatic infective marginal blepharitis or blepharoconjunctivitis with a total symptom/sign score between 5 and 25 and significant growth at 24 hours of S. epidermidis or S. aureus, 2) at least 12 years of age Exclusion criteria: 1) recent therapy, 2) contraindication for topical steroid therapy, 3) signs of associated KCS |
|
| Interventions | Combination (n = 15): 0.3% gentamicin sulfate and 0.1% betamethasone sodium phosphate (Garasone) ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Gentamicin (n = 15): 0.3% gentamicin sulfate (Garamycin) ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Placebo (n = 16): placebo ointment of mineral oil and white petroleum applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Participants were asked to clean the lid margin before reapplying ointment |
|
| Outcomes | Primary outcomes:
1) improvement of signs and symptoms at 2 weeks
2) change in bacterial cultures at 2 weeks
3) adverse reactions Measurements taken at baseline, day 7, and day 14 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Schering Canada Inc. Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by the company, Schering Canada (personal communication with study author). |
| Allocation concealment (selection bias) | Low risk | Participants were assigned to receive gentamicin‐betamethasone, gentamicin, or placebo by opening a sealed envelope that contained the coded study drug number (personal communication with study author). |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Used placebo ointment and coded bottles so participants were unaware which treatment they received. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Used coded ointment bottles. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Used coded ointment bottles. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 3 participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry. Included participants with blepharoconjunctivitis. |
Key 1996.
| Methods | Study design: intra‐individual comparative study Conditions included: chronic blepharitis Enrollment: 26 participants Exclusions and loss to follow‐up: 1 participant was lost to follow‐up Study follow‐up: 4 months with limited 3‐month extension |
|
| Participants | Country: USA Age: mean 37 years Gender: 7 men and 19 women Inclusion criteria: preference for contact lenses wears with concomitant symptoms and signs of blepharitis |
|
| Interventions | OCuSoft (n = 26): lid scrub with the OCuSoft pad on the right eye in the morning and evening Neutrogena (n = 26): lid scrub with Neutrogena bar soap on the left eye in the morning and evening Baby shampoo (n = 10): as part of study extension, 10 participants replaced Neutrogena lid scrubs in the left eye with diluted Johnson's baby shampoo All participants were instructed to minimize use of ocular cosmetics and to keep their scalp, facial skin, and eyebrows clean; all antibiotic medications were discontinued; participants were encouraged to continue wearing contact lenses |
|
| Outcomes | Primary outcomes:
1) change in symptom rankings at 4 months by clinician
2) change in sign rankings at 4 months by slit lamp examination
3) patient rankings of effectiveness and ease of use Measurements taken at baseline, 6 weeks, and 4 months; and at 7 months for extension period Unit of analysis: each eye of each participant (intracomparative) |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization; right versus left eyes. |
| Allocation concealment (selection bias) | High risk | Treatments were allocated by assigning the right and left eyes to receive lid scrubs with OCuSoft or Neutrogena, respectively. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Allocation of right eyes and left eyes was known. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Allocation of right eyes and left eyes was known. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intent to treat analysis was not followed for 1 participant who did not complete follow‐up. |
| Selective reporting (reporting bias) | High risk | Changes in signs and symptoms were not reported by treatment group. |
| Other bias | Unclear risk | In the recruitment process, every effort was made to enroll participants wearing contact lenses: 8 participants wore soft contact lenses, 12 wore rigid gas permeable contact lenses, and 6 did not wear contact lenses. |
Laibovitz 1991.
| Methods | Study design: placebo‐controlled study Conditions included: blepharitis Enrollment: number of participants not reported Exclusions and loss to follow‐up: not reported Study follow‐up: not reported |
|
| Participants | Age: not reported Gender: not reported Inclusion criteria: blepharitis |
|
| Interventions | Tetracycline: 1% tetracycline ointment Placebo: placebo ointment |
|
| Outcomes | Primary outcome: efficacy of treatment determined by quantitative cultures, clinical evaluations, and patient questionnaires Measurements taken before and after treatment Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: not reported This study was reported in abstract form only; no other associated publications have been identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Unclear risk | Reported as double‐masked, but details of masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Description of participants, methods, and exclusions and losses to follow‐up were not reported in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not clearly specified in the abstract. |
| Other bias | Unclear risk | Study reported in abstract form only, no peer reviewed publications were available. |
Luchs 2008.
| Methods | Study design: randomized, parallel‐group, open‐label study Conditions included: posterior blepharitis Enrollment: 21 participants at 1 study center Exclusions and loss to follow‐up: 1 participant who discontinued treatment was excluded Study follow‐up: 14 days |
|
| Participants | Country: USA Age: mean 63.7 ± 16.13 years (range 28 to 85 years) Gender: 9 men and 11 women (as reported) Inclusion criteria: 1) diagnosis of posterior blepharitis by a qualified ophthalmologist, 2) sign severity score of at least 2 for either redness or swelling of the eyelid margin, 3) sign severity score of at least 2 for either eyelid debris or plugging of the meibomian gland, 4) best corrected visual acuity in both eyes of at least +0.7 Exclusion criteria: 1) lid structural abnormalities; 2) inflammation, active structural change, or both in the iris or anterior chamber; 3) suspected ocular fungal or viral infection; 4) penetrating intraocular surgery in the past 90 days; 5) ocular surface surgery within the past year; 6) history of herpes keratitis; 7) known hypersensitivity to azithromycin or other macrolide antibiotic; 8) glaucoma; 9) pregnant or lactating women |
|
| Interventions | Azithromycin (n = 10): topical azithromycin ophthalmic solution 1%, starting with 1 drop twice daily for 2 days and followed by once daily for the next 12 days, plus warm compresses Compress (n = 11): warm compresses alone Compresses were applied to each eye for 5 to 10 minutes twice daily for 14 days Restrictions for topical and systemic medications were enforced prior to and during the study period; unpreserved tear substitutes were allowed; use of contact lenses and eyelid scrubs were discontinued during the study period |
|
| Outcomes | Primary outcomes:
1) change in severity of 5 clinical signs (eyelid debris, eyelid redness, eyelid swelling, meibomian gland plugging, and quality of meibomian gland secretion) at 14 days
2) patients' rating of overall symptom relief at 14 days
3) ocular safety/adverse events Measurements taken at baseline and day 14 Unit of analysis: each eye of each participant (both eyes were included for all participants) |
|
| Notes | Study dates: not reported Funding source: Inspire Pharmaceuticals, Inc., USA Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization was used. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | "While the study was not masked, while examining the patients, I did not have access to the patients chart, nor did I inquire as to which group the patients belonged to. One of my research coordinators was always present to ensure that I was as "blinded" as possible as to which group patients fell into. Not a truly masked study, but I did my best." (personal communication with study author). |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | "While the study was not masked, while examining the patients, I did not have access to the patients chart, nor did I inquire as to which group the patients belonged to. One of my research coordinators was always present to ensure that I was as "blinded" as possible as to which group patients fell into. Not a truly masked study, but I did my best." (personal communication with study author). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 1 participant who discontinued treatment. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry. Data were presented by eyes rather than by the unit of randomization, which was the individual. |
Macsai 2008.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: MGD (posterior blepharitis) Enrollment: 38 participants Exclusions and loss to follow‐up: 1 participant was excluded due to diagnosis of Sjogren syndrome and 7 participants were lost to follow‐up Study follow‐up: 1 year |
|
| Participants | Country: USA Age: mean 50 years Gender: 6 men and 32 women Inclusion criteria: 1) patients with moderate‐to‐severe chronic blepharitis and simple obstructive meibomian gland disease, onset > 3 months' duration; 2) 18 years or older Exclusion criteria: 1) pregnant or nursing, 2) not willing to comply with study procedures, 3) taking aspirin or COX‐2 inhibitors regularly, 4) on anticoagulant therapy or having blood disorder, 5) preexisting ocular disease, 6) long‐term use of nonsteroidal anti‐inflammatory agents or COX‐2 inhibitors, 7) use of dietary fatty acid supplementation 1 month prior to study |
|
| Interventions | Omega‐3 supplement (36 eyes, 18 participants): two 1000 mg flaxseed oil capsules (55% omega‐3 fatty acid, 15% omega‐6 fatty acid, and 19% omega‐9 fatty acid) 3 times a day for 1 year Placebo (40 eyes, 20 participants): 2 olive oil capsules 3 times a day for 1 year Use of artificial tears was allowed during study period; all participants continued daily lid hygiene with dilute baby shampoo |
|
| Outcomes | Primary outcomes (at 1 year):
1) change in tear BUT
2) change in meibum quality score (meibum color and character scores)
3) change in patient symptoms (overall OSDI score) Secondary outcomes: 1) Schirmer score (under anesthesia) 2) fluorescein and rose bengal surface staining 3) meibomian gland health (appearance and number of gland orifices, quality of meibum) Measurements taken at baseline and months 3, 6, 9, and 12 Unit of analysis: each eye of each participant |
|
| Notes | Study dates: not reported Funding source: Pearl Vision Foundation (Dallas, TX, USA); Research for the Prevention of Blindness, Inc. (USA); Ophthalmology Research Fund, Evanston Northwestern Healthcare (USA); and Natrol Corporation (Chatsworth, CA, USA) provided the supplement and placebo capsules Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subject numbers were pre‐assigned to the control or study group with the aid of the random number generator in Microsoft Excel." |
| Allocation concealment (selection bias) | Unclear risk | It is unclear how and when the "subject numbers were preassigned." |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | "Subjects were masked to the contents of the oil capsule" and "capsules were made to look alike as much as possible and were coded by content." |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | "The list was not incorporated into any documentation, and only research staff members not involved in patient care had access to these assignments." |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | "The list was not incorporated into any documentation, and only research staff members not involved in patient care had access to these assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "An intent‐to‐treat analysis has been done by assuming that patients lost to follow‐up had no change." |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | High risk | Data were presented by eyes rather than by the unit of randomization, which was the individual. Supplements were provided by industry. |
Matsumoto 2006.
| Methods | Study design: controlled clinical trial Conditions included: simple MGD (posterior blepharitis) Enrollment: 20 participants Exclusions and loss to follow‐up: none Study follow‐up: 2 weeks |
|
| Participants | Country: Japan Age: mean 65 years (range 48 to 75 years) Gender: 3 men and 17 women Inclusion criteria: 1) the presence of plugging of the meibomian gland orifices, 2) cloudy or inspissated glandular secretion with lack of clear meibum secretion after the application of moderate digital pressure on the tarsus of the upper and lower eye lid Exclusion criteria: 1) displacement or keratinization of the mucocutaneous junction, 2) inflammatory lid disease or inflammatory skin disorders, 3) history or clinical findings of cicatricial eye lid and conjunctival diseases, 4) excessive meibomian lipid secretion (seborrheic MGD) |
|
| Interventions | Warm moist air (n = 10): warm moist air device applied to the eyes for 10 minutes twice a day for 2 weeks; device was set to 60 °C to maintain constant warm moist air Warm compress control (n = 10): towels heated and wetted with 60 °C water applied to the eyes for 10 minutes twice a day for 2 weeks |
|
| Outcomes | Primary outcome: effectiveness of warm moist air device after 2 weeks on TFLLT and ocular surface health measured by changes in the following,
1) symptom scores
2) tear BUT
3) fluorescein score
4) rose bengal score Measurements taken at baseline and week 2 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization; the assignment was consecutive such that if a participant with MGD was eligible to the study the participant was assigned number 1 and received air device treatment where the next coming participant was number 2 and was allocated to the warm compress group (personal communication with study author). |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed, participants were assigned alternately to treatment groups. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking of physicians was not done; "Physician in charge thus knew which device the patients received" (personal communication with study author). |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Participants were not masked, thus patient‐reported outcomes for symptoms were not masked. Masking of clinical outcome assessors was not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results of treatment effects for all outcomes were reported. |
| Other bias | Low risk | |
More 1968.
| Methods | Study design: placebo‐controlled, cross‐over study Conditions included: chronic or recurrent blepharitis Enrollment: 13 participants Exclusions and loss to follow‐up: none Study follow‐up: 8 weeks |
|
| Participants | Country: UK Age: mean 40.8 ± 23.9 years (range 9 to 75 years) Gender: not reported Inclusion criteria: participants with chronic or recurrent blepharitis |
|
| Interventions | Penotrane (n = 6): 0.033% penotrane lotion in a Lissapol and glycerin base and 0.033% penotrane hydroxymethylcellulose gel (Octrane) Placebo (n = 7): lotion base and gel base without Penotrane Participants were instructed to scrub or wipe their lid margins with tissue soaked in the lotion and then to squeeze the gel along the intermarginal strip and lower conjunctival fornix 3 times daily for 4 weeks After 4 weeks of using initial treatment, participants switched to alternate treatment for another 4 weeks |
|
| Outcomes | Primary outcomes:
1) changes in signs and symptoms after treatment periods
2) change in conjunctival cultures after treatment periods
3) adverse reactions Measurements taken at baseline, and weeks 4 and 8 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Ward Blenkinsop and Co. Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to treatment group was not randomized; determined by even or odd birth date. |
| Allocation concealment (selection bias) | High risk | Allocation to treatment group determined by even or odd birth date. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Active and inert preparations were identified by letters "A" or "B" and their true identity remained unknown until the conclusion of the trial. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Active and inert preparations were identified by letters "A" or "B" and their true identity remained unknown until the conclusion of the trial. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Active and inert preparations were identified by letters "A" or "B" and their true identity remained unknown until the conclusion of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Medication was provided by industry. Potential carry‐over in cross‐over phases. |
Mori 2003.
| Methods | Study design: RCT Conditions included: MGD (posterior blepharitis) Enrollment: 25 participants Exclusions and loss to follow‐up: none Study follow‐up: 2 weeks |
|
| Participants | Country: Japan Age: 53 years (range 26 to 78) Gender: 2 men and 23 women Inclusion criteria: patients with 1) MGD (defined as the absence of visible gland structure or the presence of obstruction of meibomian gland orifices), 2) tear BUT ≤ 5 seconds in both eyes, 3) dry eye symptoms Exclusion criteria: 1) eye disorders affecting the ocular surface such as infectious conjunctivitis, allergic diseases, autoimmune diseases, and collagen diseases; 2) contact lens wear; 3) excessive meibomian lipid secretion (seborrheic MGD); 4) reflex tear production ≤ 10 mm by Schirmer II test (nasal stimulation) |
|
| Interventions | Eye warmer (n = 17): disposable eyelid warming device heated by the oxidation of iron contained inside the mask, applied for 5 minutes once a day for 2 weeks Control (n = 8): untreated |
|
| Outcomes | Primary outcome: therapeutic efficacy of warming device after 2 weeks measured by
1) tear film lipid layer interference patterns
2) tear BUT
3) meibomian gland secretion
4) dry‐eye symptoms Measurements taken at baseline and week 2 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: Kao Corporation, Japan Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was not reported in publication, but a "computer automatically assigned the participants to two groups" (email communication with study author). |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment not reported in publication, but "the allocation assignment was conducted by the third person who specialized in computer" (email communication with study author). |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | The study examinations and measurements were done by a masked observer. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | The outcomes for dry eye symptoms were participant reported and therefore not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | High risk | Results of treatment effects for all outcomes were not reported. |
| Other bias | Unclear risk | Funded by industry. |
Nelson 1990.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: seborrheic and mixed seborrheic/staphylococcal blepharitis Enrollment: 40 participants Exclusions and loss to follow‐up: 3 participants were withdrawn during the study; participants who did not attend a follow‐up appointment were excluded from the analysis for that time period Study follow‐up: 9 weeks |
|
| Participants | Country: UK Age: mean 50.5 years (range 20 to 80 years) Gender: 19 men and 21 women Inclusion criteria: patients with seborrheic and mixed seborrheic/staphylococcal blepharitis not currently receiving treatment Exclusion criteria: 1) use of topical or systemic antibiotics or anti‐inflammatory drugs, 2) significant active corneal disease, 3) contact lens wearers, 4) potential pregnancy, 5) known allergy to imidazole antifungals |
|
| Interventions | Ketoconazole (n = 20): 2% ketoconazole cream for 5 weeks Placebo (n = 20): lanolin base only cream for 5 weeks All participants used lid hygiene, using cotton buds moistened with Johnson and Johnson baby shampoo, prior to applying cream; lid hygiene was used for 9 weeks |
|
| Outcomes | Primary outcomes:
1) change in symptoms using a VAS 2) change in yeast counts 3) change in clinical features 4) bacterial growth or reduction Measurements taken at baseline, and weekly for 9 weeks Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: 1 of the authors affiliated with Janssen Pharmaceutical Ltd. Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used (personal communication with study author). |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by use of coded, identically packaged treatment bottles (personal communication with study author). |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Study was double‐masked and a placebo treatment was used. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Study was double‐masked and a placebo treatment was used. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Study was double‐masked and a placebo treatment was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for participants excluded or lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | 1 of the authors affiliated with pharmaceutical industry. |
Nguyen 1990.
| Methods | Study design: parallel‐group study Conditions included: blepharitis Enrollment: 29 participants at 22 study centers Exclusions and loss to follow‐up: none reported Study follow‐up: 7 days |
|
| Participants | Country: not reported Age: not reported Gender: not reported Inclusion criteria: patients with symptoms (such as itching, tearing, foreign body sensation) or signs (such as discharge, papillary response, conjunctival hyperemia) of blepharitis |
|
| Interventions | Ciprofloxacin (n = 14): ciprofloxacin ophthalmic solution Tobramycin (n = 15): Tobrex® ophthalmic solution (3 mg tobramycin base per mL, preserved with 0.01% (m/v) benzalkonium chloride) Participants applied 1 drop of solution every 2 hours for the first 48 hours, then every 4 hours for the next 4 days; lid scrubs using a cotton swab with the solution were also done nightly |
|
| Outcomes | Primary outcomes:
1) patient reported changes in symptoms on day 7
2) clinician evaluated changes in signs and symptoms on day 7
3) bacteriologic cultures on days 0 and 7
4) Patient reported side effects during treatment Measurements taken at baseline and day 7 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: not reported This study was reported in abstract form only, no other associated publications have been identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Participants were given masked solution bottles. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking was not reported for the physicians. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking was not reported for outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Description of participants, methods, and exclusions and losses to follow‐up were not reported in the abstract. |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes were not clearly specified in the abstract. Results for participant reported changes in symptoms were not reported. |
| Other bias | Unclear risk | Study reported in abstract form only, no peer reviewed publications were available. |
Olson 2003.
| Methods | Study design: randomized, intra‐individual comparative study Conditions included: MGD (posterior blepharitis) Enrollment: 20 participants Exclusions and loss to follow‐up: none Study follow‐up: 30 minutes during therapy and 5 minutes post‐therapy |
|
| Participants | Country: USA Age: range 26 to 59 years Gender: 3 men and 17 women Inclusion criteria: patients with a principle complaint of ocular dryness including 1) subjective dry eye score of 6 or more, 2) meibomian gland obstruction determined by biomicroscopic examination of the eyelid margin, 3) baseline TFLLT of ≤ 90 nm determined by interferometry, 4) fluorescein BUT of ≤ 10 s determined by the Dry Eye Test, 5) Schirmer test ≤ 10 mm performed under topical ocular anesthesia, 6) no evidence of other ocular pathology |
|
| Interventions | Warm compresses (20 eyes): white cotton napkins saturated with tap water and warmed to 40 °C; applied to closed eyelids for 30 minutes Control compresses (20 eyes): white cotton napkins saturated with tap water and left at room temperature; applied to closed eyelids for 30 minutes During the 30‐minute therapy session fresh compresses were applied to each eye every 2 minutes to maintain the proper temperature; participants were instructed to not close their eyelids tightly and to apply the compresses with gentle pressure |
|
| Outcomes | Primary outcomes: changes in TFLLT during and after therapy Measurements taken at baseline, at 5, 15, and 30 minutes during therapy, and 5 minutes post‐therapy Unit of analysis: each eye of each participant (intra‐comparative) |
|
| Notes | Study dates: not reported Funding source: Ocular Research of Boston, Inc., USA Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Physicians could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Outcome assessors could not be masked to treatment groups since measurements were taken during the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for the primary outcome at all follow‐up times. |
| Other bias | Low risk | |
Perry 2006.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: MGD (posterior blepharitis) Enrollment: 33 participants Exclusions and loss to follow‐up: 7 participants were excluded due to noncompliance or discomfort with treatment (4 in the cyclosporine group and 3 in the placebo group) Study follow‐up: 3 months |
|
| Participants | Country: USA Age: not reported Gender: not reported Inclusion criteria: 1) at least 18 years of age, 2) slit‐lamp diagnosis of MGD, 3) score of 12 or greater on the patient Ocular Symptoms Scale, 4) ability to understand and give signed informed consent, 5) willing and able to cooperate with study requirements, 6) use of reliable contraception if of childbearing potential Exclusion criteria: 1) use of contact lenses within 30 days of study; 2) active ocular disease, excluding glaucoma, or infections other than blepharitis; 3) ocular surgery within past 3 months; 4) active ocular allergies; 5) use of isotretinoin within past 6 months; 6) autoimmune disease requiring systemic treatment; 7) unwilling or unable to discontinue use of certain medications during or 30 days prior to study; 8) history of hypersensitivity to oral cyclosporine A; 9) pregnant or nursing or not using reliable contraception |
|
| Interventions | Cyclosporine A (n = 16): topical 0.05% cyclosporine A, 1 drop instilled in each eye twice a day for 3 months Placebo (n = 17): Refresh Plus preservative‐free artificial tears, 1 drop instilled in each eye twice a day for 3 months The use of artificial tears was discouraged, but allowed during the study; participants who were practicing lid hygiene prior to the study were allowed to continue; participants not practicing lid hygiene prior to the study were encouraged, but not required, to practice lid hygiene using warm saline soaks |
|
| Outcomes | Primary outcomes:
1) total ocular symptoms score
2) number of meibomian gland inclusions
3) fluorescein staining scores
4) tear BUT
5) lissamine green staining
6) Schirmer scores Measurements taken at baseline, and monthly for 3 months Unit of analysis: the worse eye of each participant |
|
| Notes | Study dates: not reported Funding source: Allergan, Inc. Declarations of interest: 2 study authors consultants for Allergan, Inc. Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel software was used to randomize participants to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Both the participants and the investigators were masked as to which participants were receiving cyclosporine and which were receiving placebo. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Both the participants and the investigators were masked as to which participants were receiving cyclosporine and which were receiving placebo. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Both the participants and the investigators were masked as to which participants were receiving cyclosporine and which were receiving placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for the 7 excluded participants. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry and 2 study authors affiliated with industry. |
Pinna 2007.
| Methods | Study design: randomized, parallel‐group study Conditions included: MGD (posterior blepharitis) Enrollment: 57 participants Exclusions and loss to follow‐up: 8 participants were lost to follow‐up Study follow‐up: 180 days |
|
| Participants | Country: Italy Age: mean 50 ± 16 years (range 18 to 82 years) Gender: 27 men and 30 women Inclusion criteria: participants with diagnosis of MGD (classified as seborrheic with meibomian seborrhea or seborrheic with secondary meibomitis) Exclusion criteria: 1) infectious keratoconjunctivitis or inflammatory disease unrelated to MGD; 2) Schirmer I test < 10 mm/5 min; 3) concomitant ocular pathologies; 4) previous ocular surgery; 5) alterations of the lachrymal drainage system; 6) concomitant topical ophthalmic medications; 7) topical steroids taken during previous 4 weeks; 8) treatment with systemic drugs affecting tearing; 9) pregnancy; 10) diabetes or other systemic, neurologic, or dermatologic disorders affecting the health of the ocular surface |
|
| Interventions | Group A (n = 19): oral linoleic acid (28.5 mg) and γ‐linolenic acid (15 mg) once daily for 180 days Group B (n = 19): eyelid hygiene consisting of warm eyelid compresses, eyelid massage, and eyelid margin scrubbing once daily for 180 days Group C (n = 19): groups A and B combined for 180 days All participants were instructed to follow their usual diet |
|
| Outcomes | Primary outcomes:
1) change in symptoms score
2) change in clinical signs
3) corneal fluorescein staining
4) foam collection in the tear meniscus Measurements taken at baseline, and days 60 and 180 Unit of analysis: the worse eye of each participant at baseline, if equal then the right eye was used |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly divided into 3 treatment groups of 19. The random sequence was computer‐generated (personal communication with study author). |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed (personal communication with study author). |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Healthcare providers were masked to treatment groups. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Outcome assessors were masked to treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 8 participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Low risk | |
Rubin 2006.
| Methods | Study design: randomized, parallel‐group study Conditions included: posterior blepharitis Enrollment: 30 participants Exclusions and loss to follow‐up: 6 participants, 3 in each group, were lost to follow‐up Study follow‐up: 3 months |
|
| Participants | Country: USA Age: mean 51 years Gender: 11 men and 19 women Inclusion criteria: 1) patients with posterior blepharitis (presence of posterior lid erythema and meibomian gland telangiectasia), 2) previous use of traditional therapies without adequate symptom relief Exclusion criteria: 1) treatment with punctual occlusion, oral doxycycline, steroid‐containing drops, or ointments; 2) uncontrolled systemic disease; 3) contraindication to the study medications; 4) women who were pregnant, lactating, planning pregnancy, or not using reliable birth control |
|
| Interventions | Cyclosporine (n = 63): topical 0.05% cyclosporine ophthalmic emulsion (Restasis), 1 drop applied every 12 hours Tobramycin/dexamethasone (n = 62): 0.3% tobramycin/0.1% dexamethasone ophthalmic solution, 1 drop applied every 12 hours |
|
| Outcomes | Primary outcomes:
1) change in Schirmer’s scores
2) change in tear BUT
3) improvement in clinical health
4) improvement in symptoms Measurements taken at baseline and every 2 weeks for 3 months Unit of analysis: the individual (average of both eyes) |
|
| Notes | Study dates: not reported Funding source: Allergan, Inc. and Research to Prevent Blindness Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Masking was not reported for participants. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | The study did not have masked observers. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | The study did not have masked observers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 6 participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Funded by the pharmaceutical industry. |
Seal 1995.
| Methods | Study design: randomized, partial cross‐over study Conditions included: chronic blepharitis with and without associated rosacea Enrollment: 61 participants Exclusions and loss to follow‐up: 18 participants were excluded or lost to follow‐up Study follow‐up: 8 months |
|
| Participants | Country: UK Age: not reported Gender: not reported Inclusion criteria: patients with chronic blepharitis Exclusion criteria: 1) known hypersensitivity to fusidic acid, oxytetracycline, or benzalkonium chloride; 2) simultaneous wearing of contact lenses; 3) pregnant or nursing or having childbearing potential; 4) concurrent use of prescribed anti‐infective drugs; 5) other ophthalmic complications; 6) severe renal impairment |
|
| Interventions | Fusidic acid (n = 18): topical 1% fusidic acid in a carbomer gel made isotonic by adding mannitol, buffered to pH 5.5, and preserved plus placebo tablet every 12 hours Oxytetracycline (n = 22): oral 250 mg oxytetracycline tablet plus placebo gel every 12 hours Combination (n = 34): both topical fusidic acid and oral oxytetracycline every 12 hours Placebo (n = 61): placebo gel and placebo tablet every 12 hours Study was divided into four 2‐month periods: 1) all participants received placebo gel and tablets, 2) 50% randomized to receive combination and 50% to receive either fusidic acid gel and placebo tablet or placebo gel and oxytetracycline tablet, 3) all participants received placebo gel and tablets, 4) participants who previously received combination were randomized to receive either fusidic acid gel and placebo tablet or placebo gel and oxytetracycline tablet and the remaining participants received combination |
|
| Outcomes | Primary outcomes:
1) patients’ subjective improvement of symptoms
2) investigators' assessment of improvement of signs Measurements taken at baseline, and every 2 months for 8 months Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: 1 study author affiliated with Leo Laboratories Ltd. (Bucks, UK) Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by the pharmacy. |
| Allocation concealment (selection bias) | Low risk | The pharmacist distributed the study medications after participants were enrolled. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Drugs were dispensed every 2 months to participants by the pharmacy so that they were unaware whether they were entering the placebo or active treatment phase. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Active treatment and combination assignments were masked by use of placebos and pharmacy distribution. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | Active treatment and combination assignments were masked by use of placebos and pharmacy distribution. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed. |
| Selective reporting (reporting bias) | High risk | Results were not reported for the end of each treatment phase. |
| Other bias | Unclear risk | 1 of the authors affiliated with industry. Placebo periods (1 and 3) were not parallel with active treatment periods (2 and 4). |
Shulman 1982.
| Methods | Study design: randomized, placebo‐controlled study Conditions included: chronic staphylococcal blepharoconjunctivitis Enrollment: 87 participants were enrolled, 71 were eligible for efficacy analyses Exclusions and loss to follow‐up: 2 participants were lost to follow‐up Study follow‐up: 14 days |
|
| Participants | Country: USA Age: range 10 to 86 years Gender: 36 men and 51 women Inclusion criteria: 1) patients with staphylococcal blepharoconjunctivitis with at least 1 prior episode, or duration of symptoms for at least 1 month; 2) signs and symptoms score of 2 for conjunctival, lid, or both hyperemia and a total score of no less than 5 for all other signs; 3) staphylococcal infection sensitive to gentamicin Exclusion criteria: 1) patients receiving topical or systemic antimicrobials, corticosteroids, antihistamines, or decongestants within 24 hours of enrollment; 2) glaucoma patients requiring concomitant topical medications; 3) history of allergy to any study medications; 4) any eye diseases contraindicated to topical corticosteroids |
|
| Interventions | Combination (n = 18): 0.3% gentamicin sulfate and 0.1% betamethasone phosphate ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Gentamicin (n = 19): 0.3% gentamicin sulfate (Garamycin) ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Betamethasone (n = 16): 0.1% betamethasone phosphate ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks Placebo (n = 18): vehicle ointment applied to the lid margin and gently rubbed into the lashes 3 times daily for 2 weeks All participants: use of eye shampoos or tear replacement agents was not permitted; ancillary therapeutic measures (i.e. warm compresses, water for lid hygiene, lid scrubs, oral analgesics) were allowed; systemic medications known to affect the eye were not allowed |
|
| Outcomes | Primary outcomes:
1) clinical improvement of signs at 2 weeks
2) change in bacterial cultures at 2 weeks
3) adverse reactions Measurements taken at baseline, days 3 to 4, 7 to 8, and 14 to 15 Unit of analysis: the eye of each participant with the most severe signs at enrollment |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: 2 study authors from the Schering Corporation (New Jersey, USA) Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization of treatment numbers was in groups of 4 equally divided between the 4 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. "I recall that I would give out unmarked samples and would record the clinical response" (email communication with study author). |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | All ointments were packaged identically and labeled with treatment numbers and dosage only. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | All ointments were packaged identically and labeled with treatment numbers and dosage only. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | All ointments were packaged identically and labeled with treatment numbers and dosage only. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed for 2 participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | 2 study authors affiliated with industry. Included participants with blepharoconjunctivitis, not limited to blepharitis. |
Sore 2002.
| Methods | Study design: parallel‐group study Conditions included: blepharitis Enrollment: 60 participants Exclusions and loss to follow‐up: 1 participant excluded or lost to follow‐up Study follow‐up: 29 days |
|
| Participants | Country: France Age: not reported Gender: 3 men and 56 women (as reported) Inclusion criteria: patients with seborrheic blepharitis, and/or anterior blepharitis, and/or posterior blepharitis with conjunctival irritation |
|
| Interventions | Zinc sulfate (n = 30): isotonic 0.1% zinc sulfate solution Thermal water (n = 30): natural selenium‐rich thermal water (La Roche‐Posay) 1 solution impregnated compress applied to each eye twice a day for 4 weeks; no eye makeup throughout study |
|
| Outcomes | Primary outcomes:
1) ocular safety and clinical tolerance
2) biologic markers of inflammation in the lachrymal film and microbial flora of palpebral edge and meibomian glands Measurements taken at baseline and day 29 Unit of analysis: not reported (both eyes were treated) |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: authors affiliated with La Roche‐Posay Pharmaceutical Laboratories and Laboratoire Péritesco, France Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization was not reported; "volunteers were divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Masking not reported. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed; 1 participant excluded or lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Authors affiliated with pharmaceutical industry. Unit of analysis was not reported. |
Wasserman 1989.
| Methods | Study design: randomized, parallel‐group study Conditions included: chronic blepharitis Enrollment: 20 participants enrolled Exclusions and loss to follow‐up: none reported Study follow‐up: 10 days, duration of protocol treatment |
|
| Participants | Country: USA Age: not reported Gender: not reported Inclusion criteria: patients with subjective and objective complaints of blepharitis |
|
| Interventions | Protocol 1 (n = 7): daily lid hygiene with commercial eye makeup remover, application of adrenocorticosteroid ointment (fluorometholone 0.1%) to lid margin twice daily, followed by placement of lyophilized collagen eye pads for 20 minutes for 10 days Protocol 2 (n = 7): daily lid hygiene with commercial eye makeup remover and application of adrenocorticosteroid ointment (fluorometholone 0.1%) to lid margin twice daily for 10 days Protocol 3 (n = 6): daily lid hygiene with 1:2 dilution of baby shampoo and application of adrenocorticosteroid ointment (fluorometholone 0.1%) to lid margin twice daily for 10 days |
|
| Outcomes | Primary outcomes:
1) mean change in signs and symptoms at day 10
2) change in bacterial cultures at day 10 Measurements taken at baseline and day 10 Unit of analysis: the individual |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Low risk | |
White 2008.
| Methods | Study design: randomized, parallel‐group study Conditions included: blepharokeratoconjunctivitis Enrollment: 276 participants from 17 centers (280 were screened) Exclusions and loss to follow‐up: 13 participants withdrew from the study Study follow‐up: 14 days |
|
| Participants | Countries: USA Age: mean 55 years (range 18 to 89 years) Gender: 105 men and 168 women (gender for 3 participants not reported) Inclusion criteria: 1) 18 years of age or older, 2) clinical diagnosis of blepharokeratoconjunctivitis in at least 1 eye, 3) willing to comply with all treatment and follow‐up procedures and able to self‐administer the drug, 4) informed consent, 5) women of childbearing age who were sexually inactive or using accepted birth control methods, 6) willing to discontinue contact lens use for duration of study and pinhole Snellen visual acuity equal or better than 20/40 in both eyes Exclusion criteria: 1) nursing or pregnant; 2) significant systemic disease; 3) known hypersensitivity to study drugs or their components; 4) contraindications to tobramycin or ocular corticosteroids; 5) use of systemic or topical ophthalmic non‐steroidal anti‐inflammatory agents, analgesics, or antihistamines; 6) use of topical ophthalmic medications within 2 hours of enrollment; 7) use of systemic or topical ophthalmic antibiotic agents within 72 hours of enrollment; 8) use of systemic or topical ophthalmic corticosteroid agents within 7 days of enrollment; 9) use of systemic or topical ophthalmic mast cell stabilizers within 14 days of enrollment; 10) use of topical ophthalmic immunosuppressant agents within 30 days of enrollment; 11) suspected preseptal cellulitis, dacryocystitis, or any other disease that could interfere with the safety and efficacy evaluations of the study drugs; 12) participation in other trials within 30 days prior to study entry; 13) ocular surgery in either eye within past 3 months |
|
| Interventions | LE/T (n = 138): combination 0.5% loteprednol etabonate and 0.3% tobramycin ophthalmic suspension (Zylet®), 1 or 2 drops 4 times a day for 14 days DM/T (n = 138): combination 0.3% dexamethasone and 0.1% tobramycin ophthalmic suspension (Tobradex®), 1 or 2 drops 4 times a day for 14 days |
|
| Outcomes | Primary outcomes: 1) change from baseline in signs (blepharitis, conjunctivitis, and keratitis) and symptoms (itchiness, foreign body sensation, blurred vision, light sensitivity, painful or sore eyes, and burning) composite score at day 15 Secondary outcomes: 1) percentage of eyes cured or not cured at each visit based on the investigators' global clinical assessment (cured, improved, not changed, worsened) 2) change from baseline in signs and symptoms composite score at days 3 and 7 3) change from baseline to each visit in signs composite score and symptoms composite score 4) change from baseline to each visit in blepharitis signs composite score, conjunctivitis signs composite score, and keratitis signs composite score 5) change from baseline to each visit in individual signs and symptoms Safety outcomes: visual acuity, biomicroscopy findings, IOP measurements, and adverse events were assessed at each visit Measurements taken at baseline (day 1) and days 3, 7, and 15 Unit of analysis: the individual, using the worse eye in cases of bilateral disease or the right eye if eyes were equal |
|
| Notes | Study dates: January 2007 to June 2007 Funding source: Bausch & Lomb, Inc (makers of Zylet®) Declarations of interest: 2 study authors employees of Bausch & Lomb, Inc. Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was developed by an independent statistician prior to study enrollment using a computer random number generator..." |
| Allocation concealment (selection bias) | Low risk | Once randomized, subject kit boxes "were to be assigned to sites sequentially"; bottles of the study drugs "were packaged in identical subject kit boxes." |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants were not masked to treatment. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | The study investigators were masked to treatment groups ("investigator‐masked" study). |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | The study investigators were masked to treatment groups ("investigator‐masked" study). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13 participants withdrew from the study: 4 withdrew consent (1 in the LE/T group and 3 in the DM/T group), 2 had adverse events (both in LE/T group), and 7 related to use of disallowed medications and subject ineligibility (3 in LE/T group and 4 in DM/T group). 3 participants were excluded from the ITT analysis due to missing data for all study follow‐up visits. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes specified in the methods section and in the clinical trial registration were reported. |
| Other bias | Unclear risk | The study was funded by the company producing a treatment intervention and 2 study authors were employees of the company producing the treatment intervention. Included participants with blepharokeratoconjunctivitis, not limited to blepharitis. |
Wong 1956.
| Methods | Study design: randomized, parallel‐group study Conditions included: marginal blepharitis Enrollment: 60 participants Exclusions and loss to follow‐up: clinical data were not reported for 1 participant in the selenium group Study follow‐up: 6 weeks, including 4 weeks during the time of treatment and 2 weeks after completion of treatment |
|
| Participants | Country: USA Age: median 20.5 years (range 2.5 to 86 years) Gender: 29 men and 30 women (as reported) Inclusion criteria: patients with marginal blepharitis |
|
| Interventions | Selenium (n = 39): selenium sulfide 0.5% ophthalmic ointment Control (n = 21): ammoniated mercury 0.5% ophthalmic ointment All participants instructed to cleanse lids with warm water and cotton swab prior to applying ointment twice a day for 4 weeks |
|
| Outcomes | Primary outcomes:
1) clinical improvement assessed by physician at 6 weeks
2) bacteriology and mycology of marginal blepharitis
3) adverse reactions Measurements taken at baseline and weekly for 6 weeks Unit of analysis: the eye (117 eyes from 59 participants) |
|
| Notes | Study dates: not reported Funding source: Medical Fluid Research Fund (Yale University, USA) Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | All drugs were identified by code symbol only. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | All clinical observers were without knowledge of the nature of the drug used by each participant and all drugs were identified by code symbol only. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | Low risk | All clinical observers were without knowledge of the nature of the drug used by each participant and all drugs were identified by code symbol only. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Unclear risk | Data were presented by eyes rather than by the unit of randomization, which was the individual. |
Yalçin 2002.
| Methods | Study design: randomized, parallel‐group study Conditions included: chronic posterior blepharitis Enrollment: 40 participants Exclusions and loss to follow‐up: none reported Study follow‐up: 4 months |
|
| Participants | Country: Turkey Age: mean 43 years Gender: 12 men and 28 women Inclusion criteria: patients with chronic posterior blepharitis visiting SSK Okmeydani Education Hospital's Eye Clinic |
|
| Interventions | Therapy group (43 eyes of 22 participants): 100 mg oral NAC 3 times a day for 8 weeks, plus control treatment Control group (36 eyes of 18 participants): topical steroids (prednisone acetate) and antibiotics (tobramycin sulfate) 4 times daily for 4 weeks, plus warm compresses twice daily for 2 months and artificial tears (polyvidone) 4 times daily for 3 months |
|
| Outcomes | Primary outcomes:
1) Schirmer‐1 test increase rate between groups
2) fluorescein BUT increase rate between groups
3) mucus fern test increase rate between groups
4) adverse events Measurements taken at baseline and weekly for 4 months Unit of analysis: the individual (average of both eyes) |
|
| Notes | Study dates: not reported Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | High risk | Participants could not be masked to differences in treatment groups. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | High risk | Masking not reported. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was followed. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Low risk | |
Yoo 2005.
| Methods | Study design: randomized, parallel‐group study Conditions included: chronic MGD Enrollment: 150 participants Exclusions and loss to follow‐up: 11 participants lost to follow‐up or stopped medication due to side effects Study follow‐up: 1 month |
|
| Participants | Country: Korea Age: mean 47.2 ± 12.36 years Gender: 55 men and 95 women Inclusion criteria: 1) patients newly diagnosed with chronic MGD with grade 2 or worse meibomian gland destruction or meibomian gland orifice obstruction; 2) symptoms failed to improve despite warm compression, lid massage, lid scrub, and topical eyedrops or ointment therapy for more than 2 months |
|
| Interventions | High dose (n = 50): 200 mg systemic doxycycline monohydrate twice a day Low dose (n = 50): 20 mg systemic doxycycline hyclate twice a day Placebo (n = 50): placebo pill twice a day All topical therapy was stopped at least 2 weeks prior to beginning study medication |
|
| Outcomes | Primary outcomes:
1) change in tear BUT
2) change in Schirmer test results
3) change in signs and symptoms
4) adverse events Measurements taken at baseline and 1 month Unit of analysis: the individual (average of both eyes) |
|
| Notes | Study dates: January to December 2003 Funding source: not reported Declarations of interest: none reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking (performance bias and detection bias) Were participants masked to treatment group? | Low risk | Participants were masked to medication and treatment group. |
| Masking (performance bias and detection bias) Were healthcare providers masked to treatment group? | Low risk | Baseline exams were conducted prior to randomization. Nurses dispensing the medication were masked to treatment groups. |
| Masking (performance bias and detection bias) Were outcome assessors masked to treatment group? | High risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not followed; 11 participants were excluded or lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Results were reported for primary and secondary outcomes. |
| Other bias | Low risk | |
BUT: breakup time
COX: cyclo‐oxygenase
DM/T: dexamethasone + tobramycin
IOP: intraocular pressure
ITT: intention to treat
KCS: keratoconjunctivitis sicca
LE/T: loteprednol etabonate + tobramycin
MGD: meibomian gland dysfunction
NAC: N‐acetylcysteine
OSDI: Ocular Surface Disease Index
SPEED: Standard Patient Evaluation of Eye Dryness questionnaire
TFLLT: tear‐film lipid layer thickness
VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adenis 1995 | Multiple conditions were included in the study population: RCT of participants with acute conjunctivitis or acute or chronic blepharitis treated with ciprofloxacin or rifamycin ophthalmic solution; 8 of 41 evaluable participants had chronic blepharitis; results for chronic blepharitis were not reported separately. |
| Adenis 1996b | Multiple conditions were included in the study population: RCT of participants with acute conjunctivitis or acute or chronic blepharitis treated with ciprofloxacin or norfloxacin ophthalmic solution; 50 of 131 participants had acute or chronic blepharitis; results for blepharitis were not reported separately. |
| Asano‐Kato 2003 | Not a comparative trial: interventional case series of 8 patients (16 eyes) for the treatment of atopic blepharitis with ceramide gel; intervention was supplemental to face washing. |
| Bahn 1954 | Not a comparative trial: report of a case series of 100 patients with seborrheic blepharitis treated with selenium sulfide ophthalmic ointment. |
| Barnhorst 1996 | Not population of interest: randomized, intra‐individual comparative study of 13 ocular rosacea participants treated with lid hygiene for both eyes and metronidazole topical gel for 1 eye; participants with chronic blepharitis were included, but chronic blepharitis was not required for study participation. |
| Bartholomew 1982 | Not population of interest: limited cross‐over study of 35 ocular rosacea participants treated with systemic oxytetracycline dihydrate or placebo for 6 weeks; participants with blepharitis were included, but blepharitis was not required for study participation. |
| Blackie 2008 | Not population of interest: RCT of healthy participants assigned to 1 of 3 warm compress methodologies. |
| Breakey 1969 | Multiple conditions were included in the study population: RCT of participants with external ocular disease treated with 1 of 2 types of topical steroid‐antibiotics; 7 participants with blepharitis or meibomitis were followed; results for blepharitis were not reported separately. |
| Bron 1991 | Multiple conditions were included in the study population: RCT of participants with conjunctivitis, blepharoconjunctivitis, keratoconjunctivitis or blepharitis treated with ofloxacin or chloramphenicol ophthalmic solution; 6 of 84 evaluable participants had blepharitis; results for blepharitis were not reported separately. |
| Burnside 1966 | Multiple conditions were included in the study population: clinical trial of participants with acute or chronic conjunctivitis treated with topical triamcinolone or hydrocortisone; 1 participant had meibomitis. |
| Cagle 1981 | Not population of interest: RCT of participants with ocular infections treated with topical tobramycin or gentamicin. Scope of conditions for study enrollment was acute inflammations, including conjunctivitis, blepharitis, blepharoconjunctivitis, and blepharokeratoconjunctivitis. |
| Chisari 2003 | Multiple conditions were included in the study population: RCT of participants with external ocular disease treated with 1 of 2 types of topical antibiotics; 62 participants with blepharoconjunctivitis were followed; results for blepharoconjunctivitis were not reported separately. |
| Cohen 1954 | Not a comparative trial: case series of 40 patients with blepharitis marginalis treated with selenium disulfide. |
| Filho 2011 | Not a comparative trial: case series of patients with chronic blepharitis associated with Demodex treated with oral ivermectin |
| Foulks 1988 | Multiple conditions were included in the study population: RCT of participants with bacterial ocular surface infections treated with 1 of 2 types of topical antibiotics; 39 participants with conjunctivitis, blepharitis, or blepharoconjunctivitis were followed; results for blepharitis were not reported separately. |
| Fox 1973 | Not population of interest: RCT of participants with acute or subacute external ocular infections treated with topical gentamicin or placebo. |
| Friedlaender 1998 | Multiple conditions were included in the study population: RCT of participants with blepharitis, conjunctivitis, or blepharoconjunctivitis treated with ofloxacin eyedrops 2 or 4 times per day; 25 of 50 participants had blepharitis; results for blepharitis were not reported separately. |
| Frucht‐Pery 1989 | Not a comparative trial: case series of 16 patients with ocular rosacea treated with oral doxycycline. |
| Frucht‐Pery 1993 | Not population of interest: RCT of 24 ocular rosacea participants treated with systemic oxytetracycline dihydrate or placebo for 6 weeks; participants with blepharitis were included, but blepharitis was not required for study participation. |
| Gordon 1970 | Multiple conditions were included in the study population: RCT of participants with acute, subacute, or chronic external eye infections treated with topical gentamicin or placebo; 14 of 89 participants had blepharitis or blepharoconjunctivitis; results for blepharitis and blepharoconjunctivitis were not reported separately. |
| Gwon 1992a | Multiple conditions were included in the study population: RCT of participants with external ocular infections treated with ofloxacin or tobramycin ophthalmic solution; 51 of 169 evaluable participants had blepharitis; results for blepharitis were not reported separately. |
| Gwon 1992b | Multiple conditions were included in the study population: RCT of participants with external bacterial ocular infections treated with ofloxacin or gentamicin ophthalmic solution; no evaluable participants in the ofloxacin group had blepharitis (2 in the gentamicin group); results for blepharitis were not reported separately. |
| Jacobson 1988 | Multiple conditions were included in the study population: RCT of participants with external ocular infections treated with topical norfloxacin or tobramycin; 1 participant with blepharitis was included; results for blepharitis were not reported separately. |
| Kastl 1987 | Not population of interest: RCT of participants with eyelid infections treated with mercuric oxide or placebo; conditions studied are most likely acute; "screening criteria were hordeolum or eyelash scaling", "suggestive of infectious blepharitis"; did not mention inflammation as selection criteria. |
| Kitano 1998 | Not population of interest: RCT of participants with external bacterial infections treated with norfloxacin or micronomicin ophthalmic solution; ages of participants ranged from 0 to 90+ years; results for participants with blepharitis ages 16 years and older were not reported separately. |
| Korb 1994 | Not a comparative trial: interventional case series of patients with MGD treated with 4 in‐office meibomian gland expressions and daily applications of warm compresses and lid scrubs with baby shampoo for 6 months. |
| Lamberts 1984 | Not population of interest: RCT of participants with acute blepharitis or conjunctivitis treated with 1 of 2 types of topical antibiotics; participants with chronic blepharitis, defined by more than 6 episodes of infection within the previous 12 months, were excluded from the study. |
| Leibowitz 1981 | Multiple conditions were included in the study population: RCT of participants with external eye disease treated with tobramycin or gentamicin ophthalmic solution; 40 of 56 evaluable participants had blepharoconjunctivitis; results for blepharitis were not reported separately. |
| Lin 2004 | Not intervention of interest: RCT of participants with squamous blepharitis treated with liquefacient nitrogen therapy or control. |
| Maxwell 1964 | Not an interventional study: chart review of patients with ocular lesions to compare treatment with Maxitrol suspension versus ointment; Maxitrol is a combination of antibiotics and anti‐inflammatory. |
| Miller 1992a | Not population of interest: RCT of participants with external ocular bacterial infections treated with topical norfloxacin or gentamicin; participants with chronic blepharitis, defined by having symptoms of longer than 7 days' duration, were excluded. |
| Miller 1992b | Not population of interest: RCT of participants with external ocular bacterial infections treated with topical norfloxacin or chloramphenicol; participants were only included if the infection was acute. |
| Mitsui 1986 | Not population of interest: RCT of participants with external bacterial infections treated with ofloxacin or micronomicin ophthalmic solution; ages of participants ranged from 9 to 80+ years; results for participants with blepharitis ages 16 years and older were not reported separately. |
| Nozik 1985 | Multiple conditions were included in the study population: 2 trials of participants with external ocular infections treated with topical combination antibiotics; the number of included participants with blepharitis was not reported. |
| Olson 1969 | Not population of interest: condition of study was trachoma. |
| Pecori Giraldi 1990 | Exhausted all possible resources: copy of conference proceeding could not be obtained. |
| Pettinger 2005 | Not an interventional study: comment on lid scrubs with sodium bicarbonate for the treatment of blepharitis. |
| Portellinha 1983 | Not an RCT: 47 participants with chronic marginal blepharitis used either baby shampoo (n = 39) or boric water solution (n = 8) to scrub eyelids; study authors stated that treatment allocation was random ("foi aleatória"); however, based on the distribution of participants it was unlikely that the sequence generation was randomized (P < 0.000003). |
| Power 1993 | Multiple conditions were included in the study population: RCT of participants with acute conjunctivitis or acute or chronic blepharitis treated with ciprofloxacin or chloramphenicol ophthalmic solution; the number of included participants with chronic blepharitis was not reported. |
| Rhee 2007 | Not population of interest: RCT of participants with acute blepharoconjunctivitis treated with topical antibiotic/steroid combinations; participants with infectious viral or bacterial conjunctivitis, keratitis, blepharitis, or endophthalmitis were excluded. |
| Sawa 1997 | Not population of interest: RCT of participants with ocular surface inflammatory disorders treated with bromfenac sodium or pranoprofen ophthalmic solution; ages of participants ranged from less than 19 to more than 80 years; results for participants with blepharitis ages 16 years and older were not reported separately. |
| Schechter 2009 | Not population of interest: RCT of 37 participants with rosacea‐associated eyelid and corneal pathology treated with cyclosporine ophthalmic solution or artificial tear solution for 3 months; participants with rosacea blepharitis were included, but outcomes were limited to "dry eye findings" and did not include lid findings. |
| Shulman 1996 | Multiple conditions were included in the study population: RCT of participants with chronic blepharitis or conjunctivitis treated with topical antibiotic/steroid or steroid; 80 of 111 participants had chronic blepharitis; results for blepharitis were not reported separately. |
| Souchier 2008 | Not an RCT: study of 20 patients with MGD; all patients were treated with eyelid hygiene and warm compresses, but 10 of these patients who did not respond to lid hygiene were also given oral minocycline. |
| Torkildsen 2011 | Not population of interest: RCT of participants with moderate‐to‐severe acute blepharitis/blepharoconjunctivitis treated with tobramycin/dexamethasone ophthalmic suspension or azithromycin ophthalmic solution. |
| Tovilla 1992 | Not population of interest: RCT of participants with acute bacterial conjunctivitis, blepharitis, or blepharoconjunctivitis treated with norfloxacin or chloramphenicol ophthalmic solution; participants with symptoms for more than 7 days were excluded. |
| Watson 2010 | Not population of interest: RCT of participants with moderate‐to‐severe dry eye treated with TOSM or saline; participants had moderate‐to‐severe dry eye symptoms, but not diagnosed with blepharitis specifically. |
| Wilson 1982 | Multiple conditions were included in the study population: RCT of participants with external eye diseases treated with topical tobramycin or gentamicin; 53 of 93 evaluable participants had blepharitis or blepharoconjunctivitis; results for blepharitis were not reported separately. |
| Wojtowicz 2011 | Not population of interest: RCT of participants with dry eye treated with omega‐3 supplement or placebo; although participants with MGD were included it was not required for study inclusion. |
| Yactayo‐Miranda 2009 | Not outcome of interest: RCT of participants with chronic blepharoconjunctivitis; participants received no treatment, topical levofloxacin alone, or topical levofloxacin plus eyelid scrub; only outcomes measured were bacterial changes from conjunctival swabs, "care was taken not to touch the eyelid margins or lashes" when swabbing. |
| Zhao 2010 | Multiple conditions were included in the study population: participants with dry eye and ocular inflammation were included; inclusion criteria were symptoms of blepharitis such as red eyes, photophobia, and burning sensation, but participants were not specifically diagnosed with blepharitis and results for blepharitis participants were not reported separately. |
MGD: meibomian gland dysfunction
RCT: randomized controlled trial
TOSM: therapeutic ocular surface medium
Characteristics of studies awaiting assessment [ordered by study ID]
John 2008.
| Methods | Study design: unclear Conditions included: clinical chronic mixed anterior blepharitis Enrollment: 150 eyes of 75 participants Exclusions and loss to follow‐up: none Study follow‐up: not specified |
| Participants | Country: USA Age: 66 years Gender: 33 men and 42 women Inclusion criteria: patients with clinical chronic mixed anterior blepharitis Exclusion criteria: not specified |
| Interventions | Azithromycin (n = 67): azithromycin ophthalmic solution 1% applied to the washed, clean finger or to clean applicator and then to apply the medication directly to the eyelids of both eyes Erythromycin (n = 8): erythromycin ophthalmic ointment |
| Outcomes | Primary outcomes: 1) blepharitis grades based on presence of collarettes, ulcerations at the base of eyelashes, matting of eye lashes, and lid margin erythema Measurement taken in 1‐month intervals Unit of analysis: the individual |
| Notes | Study dates: December 2004 to March 2008 Funding source: not reported Declarations of interest: 1 author was a speaker bureau of Inspire pharmaceuticals Publication language: English |
Differences between protocol and review
This protocol was previously published as: Miller K, Odufuwa B, Liew G, Anderson KL. Interventions for blepharitis (Protocol). Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.: CD005556. DOI: 10.1002/14651858.
Contributions of authors
The original concept for this review was developed by the authors of the protocol, Kim Miller, Kent Anderson, and Bola Odufuwa.
KL's contributions included coordinating the review, screening search results, appraising risk of bias of included studies, extracting data from papers, entering data into RevMan, analysis of data, providing a methodologic perspective for the review, responding to editorial and peer review comments, and writing the review.
SM's contributions included screening search results, appraising risk of bias of included studies, extracting data from papers, writing to authors of papers for additional information, entering data into RevMan, analysis of data, providing a methodologic perspective for the review, and commenting on the review.
EH's contributions included screening search results; appraising risk of bias of included studies; extracting data from papers; entering data into RevMan; providing methodologic, clinical, and consumer perspectives; and commenting on the review.
EKA's contributions included adjudicating the selection of studies; providing methodologic, clinical, and consumer perspectives; responding to editorial and peer review comments; and writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Contract N‐01‐EY‐2‐1003 and Grant 1 U01 EY020522‐01, National Eye Institute, National Institutes of Health, USA.
Declarations of interest
None known.
New
References
References to studies included in this review
Adenis 1996a {published data only}
- Adenis JP, Colin J, Verin P, Riss I, Saint‐Blancat P. Ciprofloxacin ophthalmic solution in the treatment of conjunctivitis and blepharitis: a comparison with fusidic acid. European Journal of Ophthalmology 1996;6(4):368‐74. [DOI] [PubMed] [Google Scholar]
Akyol‐Salman 2010 {published data only}
- Akyol‐Salman I, Azizi S, Mumcu U, Baykal O. Efficacy of topical N‐acetylcysteine in the treatment of meibomian gland dysfunction. Journal of Ocular Pharmacology and Therapeutics 2010;26(4):329‐33. [DOI] [PubMed] [Google Scholar]
Aragones 1973 {published data only}
- Aragones JV. The treatment of blepharitis: a controlled double blind study of combination therapy. Annals of Ophthalmology 1973;5(1):49‐52. [PubMed] [Google Scholar]
Behrens‐Baumann 2006 {published data only}
- Behrens‐Baumann W, Niederdellmann C, Jehkul A, Kohnen R. Bibrocathol eye ointment is efficacious in blepharitis. Results from a randomized, double‐blind, controlled clinical trial. Ophthalmologe 2006;103(11):960‐5. [DOI] [PubMed] [Google Scholar]
Bloom 1994 {published data only}
- Bloom PA, Leeming JP, Power W, Laidlaw DA, Collum LM, Easty DL. Topical ciprofloxacin in the treatment of blepharitis and blepharoconjunctivitis. European Journal of Ophthalmology 1994;4(1):6‐12. [DOI] [PubMed] [Google Scholar]
Collum 1984 {published data only}
- Collum LM, Quinlan P, Read B. Opticrom in the management of blepharitis. Bulletin de la Societe Belge d'Ophtalmologie 1984;211:75‐8. [PubMed] [Google Scholar]
Donshik 1983 {published data only}
- Donshik P, Kulvin SM, Mckinley P, Skowron R. Treatment of chronic staphylococcal blepharoconjunctivitis with a new topical steroid anti‐infective ophthalmic solution. Annals of Ophthalmology 1983;15(2):162‐7. [PubMed] [Google Scholar]
Friedland 2011 {published data only}
- Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Current Eye Research 2011;36(2):79‐87. [DOI] [PubMed] [Google Scholar]
Goldberg 1960 {published data only}
- Goldberg B, Kara GB, Zavell S. Topical use of triamcinolone acetonide in inflammatory condition of the eye. American Journal of Ophthalmology 1960;61(1):150‐5. [DOI] [PubMed] [Google Scholar]
Goto 2002 {published data only}
- Goto E, Shimazaki J, Monden Y, Takano Y, Yagi Y, Shimmura S, et al. Low‐concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology 2002;109(11):2030‐5. [DOI] [PubMed] [Google Scholar]
Hyndiuk 1990 {published data only}
- Hyndiuk RA, Burd EM, Hartz A. Efficacy and safety of mercuric oxide in the treatment of bacterial blepharitis. Antimicrobial Agents and Chemotherapy 1990;34(4):610‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishida 2008 {published data only}
- Ishida R, Matsumoto Y, Onguchi T, Kaido M, Iwamuro K, Kobayashi J, et al. Tear film with "Orgahexa EyeMasks" in patients with meibomian gland dysfunction. Optometry and Vision Science 2008;85(8):684‐91. [DOI] [PubMed] [Google Scholar]
Jackson 1982 {published data only}
- Jackson WB, Easterbrook WM, Connolly WE, Leers WD. Treatment of blepharitis and blepharoconjunctivitis: comparison of gentamicin‐betamethasone, gentamicin alone and placebo. Canadian Journal of Ophthalmology 1982;17(4):153‐6. [PubMed] [Google Scholar]
Key 1996 {published data only}
- Key JE. A comparative study of eyelid cleaning regimens in chronic blepharitis. CLAO Journal 1996;22(3):209‐12. [PubMed] [Google Scholar]
Laibovitz 1991 {published data only}
- Laibovitz RA, Hyndiuk RA, Yee RW, McCulley JP, McGuigan L, Randall PK. A placebo‐controlled trial of topical tetracycline ointment in blepharitis. American Academy of Ophthalmology. 1991:105.
Luchs 2008 {published data only}
- Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Advances in Therapy 2008;25(9):858‐70. [DOI] [PubMed] [Google Scholar]
Macsai 2008 {published data only}
- Macsai MS. The role of omega‐3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Transactions of the American Ophthalmological Society 2008;106:336‐56. [PMC free article] [PubMed] [Google Scholar]
Matsumoto 2006 {published data only}
- Matsumoto Y, Dogru M, Goto E, Ishida R, Kojima T, Onguchi T, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea 2006;25(6):644‐50. [DOI] [PubMed] [Google Scholar]
More 1968 {published data only}
- More BM. Penotrane in blepharitis. A double‐blind controlled trial. British Journal of Ophthalmology 1968;52(5):383‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mori 2003 {published data only}
- Mori A, Shimazaki J, Shimmura S, Fujishima H, Oguchi Y, Tsubota K. Disposable eyelid‐warming device for the treatment of meibomian gland dysfunction. Japanese Journal of Ophthalmology 2003;47(6):578‐86. [DOI] [PubMed] [Google Scholar]
Nelson 1990 {published data only}
- Nelson ME, Midgley G, Blatchford NR. Ketoconazole in the treatment of blepharitis. Eye 1990;4(Pt 1):151‐9. [DOI] [PubMed] [Google Scholar]
Nguyen 1990 {published data only}
- Nguyen HL, Jenevein SS, McCulley JP, Abshire R. Evaluation of the efficacy and safety of ciprofloxacin vs. tobrex in the treatment of blepharitis. Investigative Ophthalmology and Visual Science 1990;31(Suppl):483. [Google Scholar]
Olson 2003 {published data only}
- Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens 2003;29(2):96‐9. [DOI] [PubMed] [Google Scholar]
Perry 2006 {published data only}
- Perry HD, Doshi‐Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea 2006;25(2):171‐5. [DOI] [PubMed] [Google Scholar]
Pinna 2007 {published data only}
- Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma‐linolenic acid on meibomian gland dysfunction. Cornea 2007;26(3):260‐4. [DOI] [PubMed] [Google Scholar]
Rubin 2006 {published data only}
- Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior blepharitis. Journal of Ocular Pharmacology and Therapeutics 2006;22(1):47‐53. [DOI] [PubMed] [Google Scholar]
Seal 1995 {published data only}
- Seal DV, Ficker LA, Wright P, Menday P, Hagen KB, Troski M. Placebo‐controlled trial of fusidic acid gel and oxytetracycline for recurrent blepharitis and rosacea. American Academy of Ophthalmology. 1994:114. [DOI] [PMC free article] [PubMed]
- Seal DV, Wright P, Ficker L, Hagan K, Troski M, Menday P. Placebo controlled trial of fusidic acid gel and oxytetracycline for recurrent blepharitis and rosacea. British Journal of Ophthalmology 1995;79(1):42‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shulman 1982 {published data only}
- Shulman J, Koreman N, Hirshman M, Samson C, Trochelmann L. A double‐blind, comparative clinical trial of a new steroid, anti‐infective ophthalmic ointment for chronic staphylococcal blepharoconjunctivitis. Journal of Ocular Therapy and Surgery 1982;1(3):192‐7. [Google Scholar]
Sore 2002 {published data only}
- Sore G, et al. Ocular safety and efficacy of two lotions applied on ocular blepharitis (Abstract). 20th World Congress of Dermatology. 2002:P0466.
- Sore G, Rougier A, Richard A, Péricoi M. Ocular tolerance and efficiency of two solutions applied to non‐infectious blepharitis. European Journal of Dermatology 2002;12(4):LXII‐LXIV. [PubMed] [Google Scholar]
Wasserman 1989 {published data only}
- Wasserman EL. Blepharitis and the collagen eye patch. Annals of Ophthalmology 1989;21(4):124‐8. [PubMed] [Google Scholar]
White 2008 {published data only}
- White EM, Macy JI, Bateman KM, Comstock TL. Comparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Current Medical Research and Opinion 2008;24(1):287‐96. [DOI] [PubMed] [Google Scholar]
Wong 1956 {published data only}
- Wong AS, Fasanella RM, Haley LD, Marshall DL, Krehl WA. Selenium (selsun) in the treatment of marginal blepharitis. Archives of Ophthalmology 1956;55(2):246‐53. [DOI] [PubMed] [Google Scholar]
Yalçin 2002 {published data only}
- Yalçin E, Altin F, Cinhüseyinoglue F, Arslan MO. N‐acetylcysteine in chronic blepharitis. Cornea 2002;21(2):164‐8. [DOI] [PubMed] [Google Scholar]
Yoo 2005 {published data only}
- Yoo SE, Lee DC, Chang MH. The effect of low‐dose doxycycline therapy in chronic meibomian gland dysfunction. Korean Journal of Ophthalmology 2005;19(4):258‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adenis 1995 {published data only}
- Adenis JP, Colin J, Verin P, Saint‐Blancat P, Malet F. Ciprofloxacin ophthalmic solution versus rifamycin ophthalmic solution for the treatment of conjunctivitis and blepharitis. European Journal of Ophthalmology 1995;5(2):82‐7. [DOI] [PubMed] [Google Scholar]
Adenis 1996b {published data only}
- Adenis JP, Brasseur G, Demailly P, Malet F, Verin P, Saint‐Blancat P, et al. Comparative evaluation of efficacy and safety of ciprofloxacin and norfloxacin ophthalmic solutions. European Journal of Ophthalmology 1996;6(3):287‐92. [DOI] [PubMed] [Google Scholar]
Asano‐Kato 2003 {published data only}
- Asano‐Kato N, Fukagawa K, Takano Y, Kawakita T, Tsubota K, Fujishima H, et al. Treatment of atopic blepharitis by controlling eyelid skin water retention ability with ceramide gel application. British Journal of Ophthalmology 2003;87(3):362‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahn 1954 {published data only}
- Bahn GC. Treatment of seborrheic blepharitis. Southern Medical Journal 1954;47(8):749‐53. [DOI] [PubMed] [Google Scholar]
Barnhorst 1996 {published data only}
- Barnhorst DA Jr, Foster JA, Chern KC, Meisler DM. The efficacy of topical metronidazole in the treatment of ocular rosacea. Ophthalmology 1996;103(11):1880‐3. [DOI] [PubMed] [Google Scholar]
Bartholomew 1982 {published data only}
- Bartholomew RS, Reid BJ, Cheesbrough MJ, Macdonald M, Galloway NR. Oxytetracycline in the treatment of ocular rosacea: a double‐blind trial. British Journal of Ophthalmology 1982;66(6):386‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blackie 2008 {published data only}
- Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optometry and Vision Science 2008;85(8):675‐83. [DOI] [PubMed] [Google Scholar]
Breakey 1969 {published data only}
- Breakey AS. Comparative study of two corticosteroid antimicrobial ophthalmic preparations. Eye, Ear, Nose and Throat Monthly 1969;48(11):632‐5. [PubMed] [Google Scholar]
Bron 1991 {published data only}
- Bron AJ, Leber G, Rizk SN, Baig H, Elkington AR, Kirkby GR, et al. Ofloxacin compared with chloramphenicol in the management of external ocular infection. British Journal of Ophthalmology 1991;75(11):675‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnside 1966 {published data only}
- Burnside RM. Triamcinolone acetonide formula for ophthalmologic conditions. Texas Medicine 1966;62(2):42‐6. [PubMed] [Google Scholar]
Cagle 1981 {published data only}
- Cagle G, Davis S, Rosenthal A, Smith J. Topical tobramycin and gentamicin sulfate in the treatment of ocular infections: multicenter study. Current Eye Research 1981‐1982;1(9):523‐34. [DOI] [PubMed] [Google Scholar]
Chisari 2003 {published data only}
- Chisari G, Sanfilippo M, Reibaldi M. Treatment of bacterial conjunctivitis with topical ciprofloxacin and norfloxacin: a comparative study [Trattamento delle congiuntiviti batteriche con ciprofloxacina e norfloxacina per uso topico: studio comparativo]. Infezioni in Medicina 2003;11(1):25‐30. [PubMed] [Google Scholar]
Cohen 1954 {published data only}
- Cohen LB. Use of selsun in blepharitis marginalis. American Journal of Ophthalmology 1954;38(4):560‐2. [DOI] [PubMed] [Google Scholar]
Filho 2011 {published data only}
- Filho PA, Hazarbassanov RM, Grisolia AB, Pazos HB, Kaiserman I, Gomes JA. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. British Journal of Ophthalmology 2011;95(6):893‐5. [DOI] [PubMed] [Google Scholar]
Foulks 1988 {published data only}
- Foulks GN, Austin R, Knowlton G. Clinical comparison of topical solutions containing trimethoprim in treating ocular surface bacterial infections. Journal of Ocular Pharmacology 1988;4(2):111‐5. [DOI] [PubMed] [Google Scholar]
Fox 1973 {published data only}
- Fox SL. External ocular infections: a randomized double‐blind study of a new ophthalmic anti‐infective. Medical Digest 1973;19:37‐44. [Google Scholar]
Friedlaender 1998 {published data only}
- Friedlaender MH. Twice‐a‐day versus four‐times‐a‐day ofloxacin treatment of external ocular infection. CLAO Journal 1998;24(1):48‐51. [PubMed] [Google Scholar]
Frucht‐Pery 1989 {published data only}
- Frucht‐Pery J, Chayet AS, Feldman ST, Lin S, Brown SI. The effect of doxycycline on ocular rosacea. American Journal of Ophthalmology 1989;107(4):434‐5. [DOI] [PubMed] [Google Scholar]
Frucht‐Pery 1993 {published data only}
- Frucht‐Pery J, Sagi E, Hemo I, Ever‐Hadani P. Efficacy of doxycycline and tetracycline in ocular rosacea. American Journal of Ophthalmology 1993;116(1):88‐92. [DOI] [PubMed] [Google Scholar]
Gordon 1970 {published data only}
- Gordon DM. Gentamycine sulfate in external eye infections. American Journal of Ophthalmology 1970;69(2):300‐6. [DOI] [PubMed] [Google Scholar]
Gwon 1992a {published data only}
- Gwon A. Ofloxacin vs tobramycin for the treatment of external ocular infection. Ofloxacin Study Group II. Archives of Ophthalmology 1992;110(9):1234‐7. [DOI] [PubMed] [Google Scholar]
Gwon 1992b {published data only}
- Gwon A. Topical ofloxacin compared with gentamicin in the treatment of external ocular infection. Ofloxacin Study Group. British Journal of Ophthalmology 1992;76(12):714‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobson 1988 {published data only}
- Jacobson JA, Call NB, Kasworm EM, Dirks MS, Turner RB. Safety and efficacy of topical norfloxacin versus tobramycin in the treatment of external ocular infections. Antimicrobial Agents and Chemotherapy 1988;32(12):1820‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kastl 1987 {published data only}
- Kastl PR, Ali Z, Mather F. Placebo‐controlled, double‐blind evaluation of the efficacy and safety of yellow mercuric oxide in suppression of eyelid infections. Annals of Ophthalmology 1987;19(10):376‐9. [PubMed] [Google Scholar]
Kitano 1998 {published data only}
- Kitano S. Norfloxacin controlled study (multicenter phase III clinical trial on norfloxacin ophthalmic solution: well controlled study). Folia Ophthalmologica Japonica 1998;39:1151‐9. [Google Scholar]
Korb 1994 {published data only}
- Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian gland dysfunction. Advances in Experimental Medicine and Biology 1994;350:293‐8. [DOI] [PubMed] [Google Scholar]
Lamberts 1984 {published data only}
- Lamberts DW, Buka T, Knowlton GM. Clinical evaluation of trimethoprim‐containing ophthalmic solutions in humans. American Journal of Ophthalmology 1984;98(1):11‐6. [DOI] [PubMed] [Google Scholar]
Leibowitz 1981 {published data only}
- Leibowitz HM, Hyndiuk RA, Smolin GR, Nozik RA, Hunter GJ, Cagle GD, et al. Tobramycin in external eye disease: a double‐masked study vs. gentamicin. Current Eye Research 1981;1(5):259‐66. [DOI] [PubMed] [Google Scholar]
Lin 2004 {published data only}
- Lin Z. Observation on therapeutic effect of squamous blepharitis treated with liquefacient nitrogen cryotherapy. Eye Science 2004;20(2):77‐9. [PubMed] [Google Scholar]
Maxwell 1964 {published data only}
- Maxwell E, Aijian KM. Maxitrol suspension and ointment: clinical evaluation of two new antibiotic‐steroid preparations. Current Therapeutic Research, Clinical and Experimental 1964;6:130‐3. [PubMed] [Google Scholar]
Miller 1992a {published data only}
- Miller IM, Vogel R, Cook TJ, Wittreich J. Topically administered norfloxacin compared with topically administered gentamicin for the treatment of external ocular bacterial infections. The Worldwide Norfloxacin Ophthalmic Study Group. American Journal of Ophthalmology 1992;113(6):638‐44. [DOI] [PubMed] [Google Scholar]
Miller 1992b {published data only}
- Miller IM, Wittreich JM, Cook T, Vogel R. The safety and efficacy of topical norfloxacin compared with chloramphenicol for the treatment of external ocular bacterial infections. The Norfloxacin‐Chloramphenicol Ophthalmic Study Group. Eye 1992;6(Pt 1):111‐4. [DOI] [PubMed] [Google Scholar]
Mitsui 1986 {published data only}
- Mitsui Y, Sakuragi S, Tamura O, Abe M, Watanabe I, Ueno M, et al. Effect of ofloxacin ophthalmic solution in the treatment of external bacterial infections of the eye. Folia Ophthalmologica Japonica 1986;37:1115‐40. [Google Scholar]
Nozik 1985 {published data only}
- Nozik RA, Smolin G, Knowlton G, Austin R. Trimethoprim‐polymyxin B ophthalmic solution in treatment of surface ocular bacterial infections. Annals of Ophthalmology 1985;17(12):746‐8. [PubMed] [Google Scholar]
Olson 1969 {published data only}
- Olson CL. Bacterial flora of the conjunctiva and lid margin. Effects of parenteral trisulfapyramidines and topical tetracycline and erythromycin. Archives of Ophthalmology 1969;82(2):197‐202. [DOI] [PubMed] [Google Scholar]
Pecori Giraldi 1990 {published data only}
- Pecori Giraldi J, Grechi G, Anania A, Filadoro P, Miglionini RA. [La ciprofloxacina per via topica nel trattamento delle affezioni ocular esterne: sua efficacia e tollerabilita]. Proceedings II Congress of Ocular Pharmacology, Catania. 1990.
Pettinger 2005 {published data only}
- Pettinger D. Sodium bicarbonate in the treatment of blepharitis. Clinical and Experimental Ophthalmology 2005;33(4):448. [DOI] [PubMed] [Google Scholar]
Portellinha 1983 {published data only}
- Portellinha WM, Cai S, Belfort R Jr. Baby shampoo x placebo, on chronic blepharitis. Arquivos Brasileiros de Oftalmologia 1983;46(5):134‐7. [Google Scholar]
Power 1993 {published data only}
- Power WJ, Collum LMT, Easty DL, Bloom PA, Laidlaw DAH, Libert J, et al. Evaluation of efficacy and safety of ciprofloxacin ophthalmic solution versus chloramphenicol. European Journal of Ophthalmology 1993;3(2):77‐82. [DOI] [PubMed] [Google Scholar]
Rhee 2007 {published data only}
- Rhee SS, Mah FS. Comparison of tobramycin 0.3%/dexamethasone 0.1% and tobramycin 0.3%/loteprednol 0.5% in the management of blepharo‐keratoconjunctivitis. Advances in Therapy 2007;24(1):60‐7. [DOI] [PubMed] [Google Scholar]
Sawa 1997 {published data only}
- Sawa M, Masuda K, Usui M, Komemushi S. Efficacy of 0.1% bromfenac sodium ophthalmic solution for ocular surface inflammation: a double‐masked controlled trial. Folia Ophthalmologica Japonica 1997;48(5):717‐24. [Google Scholar]
Schechter 2009 {published data only}
- Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Advances in Therapy 2009;26(6):651‐9. [DOI] [PubMed] [Google Scholar]
Shulman 1996 {published data only}
- Shulman DG, Sargent JB, Stewart RH, Mester U. Comparative evaluation of the short‐term bactericidal potential of a steroid‐antibiotic combination versus steroid in the treatment of chronic bacterial blepharitis and conjunctivitis. European Journal of Ophthalmology 1996;6(4):361‐7. [DOI] [PubMed] [Google Scholar]
Souchier 2008 {published data only}
- Souchier M, Joffre C, Beynat J, Grégoire S, Acar N, Bretillon L, et al. Changes in meibomian fatty acids and ocular surface in patients with meibomian gland dysfunction after minocycline treatment. Investigative Ophthalmology and Vision Science 2008:ARVO E‐ abstract 2391. [DOI] [PubMed] [Google Scholar]
- Souchier M, Joffre C, Grégoire S, Bretillon L, Muselier A, Acar N, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. British Journal of Ophthalmology 2008;92(6):819‐22. [DOI] [PubMed] [Google Scholar]
Torkildsen 2011 {published data only}
- Torkildsen GL, Cockrum P, Meier E, Hammonds WM, Silverstein B, Silverstein S. Evaluation of clinical efficacy and safety of tobramycin/dexamethasone ophthalmic suspension 0.3/0.05 compared to azithromycin ophthalmic solution 1 in the treatment of moderate to severe acute blepharitis/blepharoconjunctivitis. Current Medical Research and Opinion 2011;27(1):171‐8. [DOI] [PubMed] [Google Scholar]
Tovilla 1992 {published data only}
- Tovilla JL, Avila F, Quintero J, Rhenals R. Comparative clinical study of norfloxacin 0.3% and chloramphenicol 0.5% ophthalmic solutions in the treatment of acute bacterial conjunctivitis, blepharitis, and blepharoconjunctivitis. Current Therapeutic Research, Clinical and Experimental 1992;52(1):64‐9. [Google Scholar]
Watson 2010 {published data only}
- Watson SL, Daniels JT, Geerling G, Dart JK. Clinical trials of therapeutic ocular surface medium for moderate to severe dry eye. Cornea 2010;29(11):1241‐6. [DOI] [PubMed] [Google Scholar]
Wilson 1982 {published data only}
- Wilson LA, Weinstein AJ, Wood TO, Lindsey C, Davis S. Treatment of external eye infections: a double‐masked trial of tobramycin and gentamicin. Journal of Ocular Therapy and Surgery 1982;1(6):364‐7. [Google Scholar]
Wojtowicz 2011 {published data only}
- Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double‐masked, placebo‐controlled clinical trial of an omega‐3 supplement for dry eye. Cornea 2011;30(3):308‐14. [DOI] [PubMed] [Google Scholar]
Yactayo‐Miranda 2009 {published data only}
- Yactayo‐Miranda Y, He L, Weimann S, Kreutzer T, Kampik A, Mino de Kaspar H. Efficacy of 0.5% levofloxacin therapy against aerobic‐anaerobic bacterial flora in chronic‐blepharoconjunctivitis patients: a prospective semi‐randomized study. Investigative Ophthalmology and Visual Science 2008:ARVO E‐abstract 840. [Google Scholar]
- Yactayo‐Miranda Y, Ta CN, He L, Kreutzer TC, Nentwich MM, Kampik A, et al. A prospective study determining the efficacy of topical 0.5% levofloxacin on bacterial flora of patients with chronic blepharoconjunctivitis. Graefe's Archive for Clinical and Experimental Ophthalmology 2009;247(7):993‐8. [DOI] [PubMed] [Google Scholar]
Zhao 2010 {published data only}
- Zhao JY, Lu B, Sun Q, Wang YD, Zhang TS. Clinical evaluation of pranoprofen eye drops for controlling ocular inflammation in dry eye syndrome. International Journal of Ophthalmology 2010;10(3):492‐4. [Google Scholar]
References to studies awaiting assessment
John 2008 {published data only}
- John T, Shah AA. Use of azithromycin ophthalmic solution in the treatment of chronic mixed anterior blepharitis. Annals of Ophthalmology 2008;40(2):68‐74. [PubMed] [Google Scholar]
Additional references
AAO 2008
- American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern® Guidelines. Blepharitis. San Francisco, CA: American Academy of Ophthalmology; 2008. http://www.aao.org/ppp (accessed 3 April 2012).
Aronowicz 2006
- Aronowicz JD, Shine WE, Oral D, Vargas JM, McCulley JP. Short term oral minocycline treatment of meibomianitis. British Journal of Ophthalmology 2006;90(7):856‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowman 1987
- Bowman RW, Dougherty JM, McCulley JP. Chronic blepharitis and dry eyes. International Ophthalmology Clinics 1987;27(1):27‐35. [DOI] [PubMed] [Google Scholar]
Czepita 2007
- Czepita D, Kuzna‐Grygiel W, Czepita M, Grobelny A. Demodex folliculorum and Demodex brevis as a cause of chronic marginal blepharitis. Annales Academiae Medicae Stetinensis 2007;53(1):63‐7. [PubMed] [Google Scholar]
Dougherty 1984
- Dougherty JM, McCulley JP. Comparative bacteriology of chronic blepharitis. British Journal of Ophthalmology 1984;68(8):524‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougherty 1986a
- Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Investigative Ophthalmology and Visual Science 1986;27(1):52‐6. [PubMed] [Google Scholar]
Dougherty 1986b
- Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Investigative Ophthalmology and Visual Science 1986;27(4):486‐91. [PubMed] [Google Scholar]
Dougherty 1991a
- Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Investigative Ophthalmology and Visual Science 1991;32(11):2970‐5. [PubMed] [Google Scholar]
Dougherty 1991b
- Dougherty JM, Osgood JK, McCulley JP. The role of wax and sterol ester fatty acids in chronic blepharitis. Investigative Ophthalmology and Visual Science 1991;32(6):1932‐7. [PubMed] [Google Scholar]
Driver 1996
- Driver PJ, Lemp MA. Meibomian gland dysfunction. Survey of Ophthalmology 1996;40(5):343‐7. [DOI] [PubMed] [Google Scholar]
Driver 2005
- Driver PJ, Lemp MA. Seborrhea and meibomian gland dysfunction. In: Krachmer JH, Mannis MJ, Holland EJ editor(s). Cornea. 2nd Edition. Philadelphia: Elsevier Mosby, 2005:485‐91. [Google Scholar]
Eliason 2005
- Eliason JA. Blepharitis: overview and classification. In: Krachmer JH, Mannis MJ, Holland EJ editor(s). Cornea. 2nd Edition. Philadelphia: Elsevier Mosby, 2005:481‐4. [Google Scholar]
Ficker 1991
- Ficker L, Ramakrishnan M, Seal D, Wright P. Role of cell‐mediated immunity to staphylococci in blepharitis. American Journal of Ophthalmology 1991;111(4):473‐9. [DOI] [PubMed] [Google Scholar]
Geerling 2011
- Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, et al. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative Ophthalmology and Visual Science 2011;52(4):2050‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Groden 1991
- Groden LR, Murphy B, Rodnite J, Genvert GI. Lid flora in blepharitis. Cornea 1991;10(1):50‐3. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huber‐Spitzy 1991
- Huber‐Spitzy V, Baumgartner I, Bohler‐Sommeregger K, Grabner G. Blepharitis ‐ a diagnostic and therapeutic challenge. A report on 407 consecutive cases. Graefe's Archive for Clinical and Experimental Ophthalmology 1991;229(3):244‐7. [DOI] [PubMed] [Google Scholar]
Inspire 2010
- Press release: Inspire announces results from AZASITE(R) phase 2 blepharitis trials and plans to continue clinical development in anterior and posterior blepharitis. Inspire Pharmaceuticals, Inc. http://ir.inspirepharm.com/phoenix.zhtml?c=120779&p=irol‐newsArticle_print&ID=1402141&highlight= (accessed 7 March 2011).
Jackson 2008
- Jackson WB. Blepharitis: current strategies for diagnosis and management. Canadian Journal of Ophthalmology 2008;43(2):170‐9. [DOI] [PubMed] [Google Scholar]
Jester 1989a
- Jester JV, Nicolaides N, Kiss‐Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Investigative Ophthalmology and Visual Science 1989;30(5):936‐45. [PubMed] [Google Scholar]
Jester 1989b
- Jester JV, Nicolaides N, Smith RE. Meibomian gland dysfunction. I. Keratin protein expression in normal human and rabbit meibomian glands. Investigative Ophthalmology and Visual Science 1989;30(5):927‐35. [PubMed] [Google Scholar]
Kernan 1999
- Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. Journal of Clinical Epidemiology 1999;52(1):19‐26. [DOI] [PubMed] [Google Scholar]
Lemp 2009
- Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey‐based perspective on prevalence and treatment. Ocular Surface 2009;7(Suppl 2):S1‐14. [DOI] [PubMed] [Google Scholar]
Mathers 1993
- Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology 1993;100(3):347‐51. [DOI] [PubMed] [Google Scholar]
McCulley 1982
- McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology 1982;89(10):1173‐80. [DOI] [PubMed] [Google Scholar]
McCulley 1984
- McCulley JP. Blepharoconjunctivitis. International Ophthalmology Clinics 1984;24(2):65‐77. [DOI] [PubMed] [Google Scholar]
McCulley 1985
- McCulley JP, Dougherty JM. Blepharitis associated with acne rosacea and seborrheic dermatitis. International Ophthalmology Clinics 1985;25(1):159‐72. [DOI] [PubMed] [Google Scholar]
McCulley 2000
- McCulley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea 2000;19(5):650‐8. [DOI] [PubMed] [Google Scholar]
NDTI 1982
- National disease and therapeutic index (NDTI). IMS America 1982.
Nelson 2011
- Nelson JD, Shimazaki J, Benitez‐del‐Castillo JM, Craig JP, McCulley JP, Den S, et al. The International Workshop on Meibomian Gland Dysfunction: report of the definition and classification subcommittee. Investigative Ophthalmology and Visual Science 2011;52(4):1930‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Probst 2005
- Probst LE. Bacterial eyelid infections. In: Krachmer JH, Mannis MJ, Holland EJ editor(s). Cornea. 2nd Edition. Philadelphia: Elsevier Mosby, 2005:495‐500. [Google Scholar]
Raskin 1992
- Raskin EM, Speaker MG, Laibson PR. Blepharitis. Infectious Disease Clinics of North America 1992;6(4):777‐87. [PubMed] [Google Scholar]
Review Manager 2011 [Computer program]
- The Nordic Cochrane Centre,The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration, 2011.
Rolando 1985
- Rolando M, Refojo MF, Kenyon KR. Tear water evaporation and eye surface diseases. Ophthalmologica 1985;190(3):147‐9. [DOI] [PubMed] [Google Scholar]
Seal 1990
- Seal D, Ficker L, Ramakrishnan M, Wright P. Role of staphylococcal toxin production in blepharitis. Ophthalmology 1990;97(12):1684‐8. [DOI] [PubMed] [Google Scholar]
Shimazaki 1995
- Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Archives of Ophthalmology 1995;113(10):1266‐70. [DOI] [PubMed] [Google Scholar]
Shine 1991
- Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Investigative Ophthalmology and Visual Science 1991;32(8):2272‐80. [PubMed] [Google Scholar]
Shine 2003
- Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Experimental Eye Research 2003;76(4):417‐20. [DOI] [PubMed] [Google Scholar]
Valenton 1973
- Valenton MJ, Okumoto M. Toxin‐producing strains of Staphylococcus epidermidis (albus). Isolates from patients with staphylococcic blepharoconjunctivitis. Archives of Ophthalmology 1973;89(3):186‐9. [DOI] [PubMed] [Google Scholar]
Viswalingham 2005
- Viswalingham M, Rauz S, Morlet N, Dart JK. Blepharokeratoconjunctivitis in children: diagnosis and treatment. British Journal of Ophthalmology 2005;89(4):400‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woods 1995
- Woods KL. Mega‐trials and management of acute myocardial infarction. Lancet 1995;346(8975):611‐4. [DOI] [PubMed] [Google Scholar]


