Abstract
Background
The enzyme NADPH–P450 oxidoreductase (POR) is the main electron donor to all microsomal CYPs. The possible contribution of common POR variants to inter- and intra-individual variability in drug metabolism is of great pharmacogenetic interest.
Aim
To search for POR polymorphic alleles and estimate their frequencies in a Jewish population.
Materials & methods
We analyzed the POR gene in 301 Ashkenazi and Moroccan Jews.
Results
A total of 30 POR SNPs were identified, nine in the noncoding regions and 21 in the protein-coding regions (ten synonymous, 11 missense). Six of these missense variants are previously undescribed (S102P, V164M, V191M, D344N, E398A and D648N).
Conclusion
The data collected in this study on missense POR SNPs, interpreted in light of the crystallographic structure of human POR, indicate that some POR missense variants may be potential biomarkers for future POR pharmacogenetic screening.
Keywords: allele frequencies, CYP oxidoreductase, haplotype, Jewish, populations, pharmacogenetics, POR
The enzyme NADPH–P450 oxidoreductase (POR, EC 1.6.2.4.) is a membrane-bound microsomal protein that contains both flavin adenine dinucleotide (FAD) and flavin mono-nucleotide (FMN) moieties. The principal function of POR is to transfer electrons from reduced NADPH to all known microsomal (type II) CYP enzymes [1]. By interacting with microsom-ally localized cytochrome P450 enzymes, POR participates in xenobiotic and drug metabolism, and in steroidogenesis.
Human POR is encoded by a single gene, POR, which is located on the long arm of chromosome 7 [2]. POR contains 15 protein-coding exons (exons 1–15) and one noncoding exon (exon 1U) [3]. Human POR is a 79 kDa protein containing 680 amino acid residues. The crystallographic structure of rat POR revealed that the molecule is composed of four structural domains: (from the N- to C-termini) the FMN-binding domain, the connecting domain, and the FAD- and NADPH-binding domains [4]. A sequence of 25 amino acids in the N-terminal of the protein determines the microsomal localization of the protein and its linkage to the membrane [5]. The recently published structure of human POR confirmed that these features are conserved [6]. The enzyme functions as a monomer in solution and requires the hydrophobic N-terminus for interaction with CYPs [5].
In recent years, investigation of POR has become an issue of great interest and a large number of new POR genetic variants have been described [7–15,101]. Phenotypic manifestations of POR variation cover a wide spectrum of developmental outcomes. The POR knockout of the Por gene in mice leads to embryonic lethality [16]. It was found that Por deletion does not block early embryonic development, but has profound impact on progression past midgestation [16]. Defects in the development of Por-deleted mice may be related to the adverse effects of elevated retinoids caused by defective function of the POR-dependent retinoic acid-metabolizing CYP26 enzymes [17]. On the other hand, liver-specific deletion of the Por gene yields morphologically and reproductively normal mice that accumulate hepatic lipids and exhibit impaired hepatic drug metabolism [18]. Notably, silencing of POR by siRNA in primary human hepatocytes induced upregulation of CYP3A4 but did not affect CYP1A2 or CYP2D6 expression [19]. Thus, levels of POR expression may be linked to those of certain CYP enzymes [19]. In humans, POR mutations are less severe than total gene deletion, since they provide some degree of residual POR activity. Disorders associated with POR mutations, referred to cumulatively as ‘POR deficiency’ (OMIM #201750), comprising a number of divergent steroidogenic anomalies (e.g., ambiguous genitalia, female virilization and decreased male masculinization) are often accompanied by several craniofacial and skeletal malformations (e.g., brachycephaly, bowed femora, radioulnar and radiohumeral synostoses) characteristic of Antley–Bixler syndrome [10,11,20]. In addition to the developmental defects associated with reductase deficiency, the involvement of POR in drug metabolism has recently also made POR the subject of intense investigation. It is known that CYPs from families CYP1–CYP3 mediate 80–90% of all phase I-dependent metabolism of drugs used clinically [21,22]. Therefore, it is reasonable to assume that discontinuity in the electron flow from POR to CYPs, caused by POR polymorphisms, would have deleterious effects on drug oxygenation. A number of sequence variants found in POR give rise to the question of whether such variants might contribute to interindividual variations in drug response (both drug safety and efficacy). Ideally, all patients would be genotyped for relevant genetic markers before initiation of drug therapy. As an interim research step to supplement individual genotyping, population studies might be performed for identifying relative allele frequencies. To date, four POR pharmacogenetic studies have been carried out, gathering allelic frequency data from four ethnic groups (Caucasian, African–American, Hispanic and Asian) [12–14,23]. Many areas of the world remain under-represented in current clinical genetics and pharmacogenetics research [24,25], which contributes to our limited knowledge of POR polymorphisms. Here we present POR allele frequencies of two additional ethnic groups, Ashkenazi Jewish (AJ) and Moroccan Jewish (MJ) and compare our results with data from other studies.
Materials & methods
Samples
Anonymous DNA samples were obtained from the collection of The National Laboratory for Genetics of Israeli Populations (NLGIP; [102]) at Tel Aviv University (Tel Aviv, Israel). All samples in the study group (301 individuals) were from healthy, unrelated adult donors who have self-identified their ethnicity as AJ (35 males and 130 females) or as MJ (57 males and 79 females). Notably, women were more likely to donate blood samples to the NLGIP biobank compared with men and this fact is reflected by their higher representation in these DNA samples [26].
DNA analysis
Two approaches for POR ana lysis were used. A new-generation amplicon high-resolution melting (HRM) method was implemented. It has been demonstrated that the HRM method is a useful screening tool for detecting DNA variations [27]. When the project was initiated, the HRM method was optimized only for the POR exons 1, 2, 5 and 6. POR exons 1U, 3, 4 and 7–15 were sequenced directly with a forward primer. Identified DNA variations were confirmed by independent sequencing of the second PCR product from both directions. Using the HRM method, amplicons that resulted in an altered melting curve compared with controls were subjected to direct sequencing from both directions.
Specific primers in the intronic regions flanking the 16 exons were designed to avoid the known polymorphisms and minimize undesirable base pairing interactions. Exon pairs 8 and 9, 12 and 13, and 14 and 15 were amplified as single PCR products, named E89, E1213 and E1415, respectively. All 13 sets of primers and the sizes of the specific PCR products are listed in Table 1.
Table 1.
Primers used for POR genotyping.
| Primer F (5′–3′) | Primer R (5′–3′) | Length of PCR product | |
|---|---|---|---|
| E1U | cgtaccaagagcgcaaat | gagataccgagccctaacc | 493 |
| E1 | accctctgctgacatctgct | caccccaaaatgctacaagg | 347 |
| E2 | ctgtaggggaaatgggaagg | acatcctctatgcggtgacg | 385 |
| E3 | agaagggactcaaagccaggaa | aaggcaacttccgaggacg | 396 |
| E4 | tcccacgacactcagacatcc | attctcgtagtgctggggtctg | 453 |
| E5 | gcccagtgttccttgcagtg | ctctgtgttggaggtgcgtgt | 407 |
| E6 | ccttcctgatgctctgggttt | gtggcagagtgagtccttggct | 407 |
| E7 | gctcccctgcttcttgtcgt | ctcagtacaaactgggcgagtg | 428 |
| E89 | gagattccctgtgctttgtgc | cctaagcagaagctcaaccca | 524 |
| E10 | gccttgtttccagcaccag | tcctaagagacacgggggtga | 415 |
| E11 | cgcaagatggcctcctcct | ccttgcactctgcctgctgt | 354 |
| E1213 | tgcagaacgggacttggg | agcctcatgcccaccttcgt | 605 |
| E1415 | gagcagtcccacaaggtgaga | gccaaacacacccaggagactac | 515 |
F: Forward; R: Reverse.
HRM
PCR was performed using a 1× concentration of the saturating DNA dye LCGreen® Plus (Idaho Technology, UT, USA), 1× Plain PP Combi Master Mix for E1 and E2 or 1× PP Master Mix for E5 and E6 (both from Top-Bio, Prague, Czech Republic), 0.1 μM forward and 0.1 μM reverse primer, 4% (E1 and E2) and 8% (E5 and E6) dimethyl sulfoxide, 25 ng of DNA template, and PCR-grade water were adjusted for a 10-μl final PCR reaction volume. The reaction mix was overlaid with 15 μl of mineral oil (Sigma-Aldrich, MO, USA). All PCR reactions were performed in duplicate using a DNA Engine Dyad Cycler (Bio-Rad, MA, USA). The thermal cycling profiles were as follows: initial denaturation at 94°C (2 min), amplification: 40 cycles of denaturation at 94°C (30 s), annealing at 58°C (E1 and E2) and 62°C (E5 and E6; 30 s), extension at 72°C (40 s), final extension at 72°C (5 min). The PCR products were incubated for an additional 30 s at 94°C followed by rapid cooling to 20°C. After amplification, melting acquisition was performed on the Light Scanner (Idaho Technology), in which DNA samples were melted by gradually increasing the temperature from 55 to 98°C at a rate of 0.1°C/s.
HRM analysis
Upon completion of the run, data were analyzed using the LightScanner Software version 1.5. The melting curves were normalized and the temperatures shifted (80–90°C for the lower temperature range and 85–95°C for the upper temperature range) to allow samples to be directly compared. Samples were then automatically clustered into groups and the melting curves and the plots inspected. The sensitivity level was set at 1. Differences were considered as significant if the replicates fell outside the range of the variations seen in the wild-type samples.
DNA sequencing
PCR was performed using a 1× PP Master Mix (Top-Bio), 0.1 μM forward and 0.1 μM reverse primer, 4% (E4, E5, E6, E7, E89, E1213 and E1415) and 8% (E1U, E1, E2, E3, E10 and E11) dimethyl sulfoxide, 25 ng of DNA template and PCR-grade water adjusted for a 12.5-μl final PCR reaction volume. Thermal cycling profiles were as follows: initial denaturation at 94°C (2 min), amplification: 33 cycles of denaturation at 94°C (30 s), annealing at 55°C (E1 and E2), 58°C (E1U) and 64°C (all other exons; 30 s), extension at 72°C (40 s) and final extension at 72°C (5 min). PCR reactions for all exons were carried out using a DNA Engine Dyad® Cycler. Post-PCR clean-up was performed by adding 0.5 U of shrimp alkaline phosphatase and 10 U of exonuclease I (both from Fermentas, Burlington, Canada) to 2 μl of the PCR product and the final volume of the reaction was adjusted with PCR-grade water to 10 μl. The reaction mixture was subsequently incubated in a DNA Engine Dyad Cycler for 15 min at 37°C and then for 20 min at 80°C. Sequence analysis of amplified DNA was performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, CA, USA). The amplicons were sequenced on the automatic sequencer ABI 3130xl Genetic Analyzer (Applied Biosystems).
Statistical method
Statistical ana lysis was performed by using the STATISTICA 10 software (StatSoft, Czech Republic) and Pearson's c2 test.
Haplotyping studies
The linkage disequilibrium (LD) and haplo-type block estimation analyses were performed using the Genotype Resolution and Block Identification using Likelihood (GERBIL) algorithm [28] implemented in Genotype Visualization and Algorithmic Tool (GEVALT) version 2 software [29].
Results
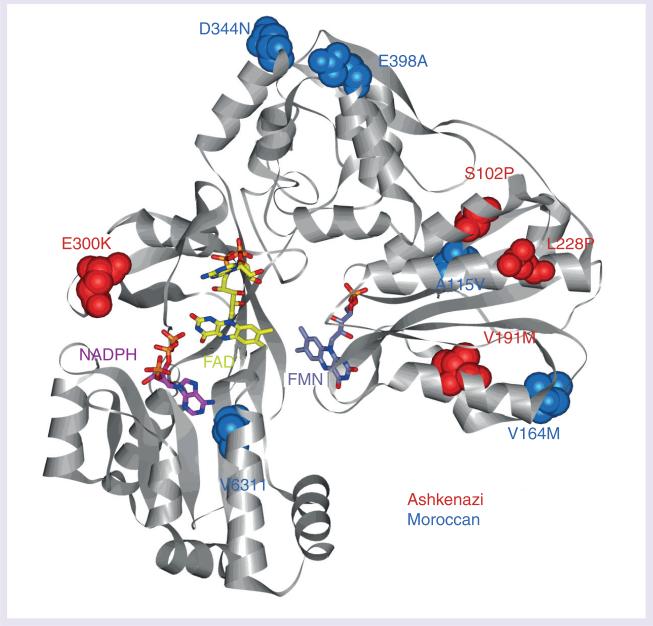
To identify POR gene variations in the AJ and MJ populations, we sequenced first untranslated exon 1U, 15 coding exons and approximately 20 bp of the intronic sequence flanking these exons in 301 individuals. A total of 30 POR SNP genetic variations (Table 2) were identified, nine of which were found in the noncoding regions (introns and exon 1U) and 21 in the protein-coding regions (exons). From the 21 variations found in exons, ten were synonymous and 11 were missense SNPs. Missense variants found in the cohort are summarized in Figure 1.
Table 2.
Genetic variations in the POR gene identified in the cohort.
| Location | Genomic position NG_008930.1 | Coding position NM_000941.2 | Amino acid change NP_000932.3 | SNP ID | Allele frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | Hart et al. [12] | Huang et al. [13] | Gomes et al. [23] | Saito et al. [14] | |||||||||
| MJ | AJ | AA | CA | AS | ME | ||||||||
| Exon 1U | 5003A>C | −80A>C | 0.000 | 0.003 | – | – | – | – | – | – | – | ||
| Exon 1U | 5036A>C | −47A>C | rs3823884 | 0.221 | 0.224 | – | 0.812 | 0.266 | 0.311 | 0.377 | – | 0.255 | |
| Exon 1U | 5050G>T | −33G>T** | rs72553977 | 0.018 | 0.000 | – | 0.000 | 0.000 | 0.000 | 0.012 | – | – | |
| Exon 1U | 5078G>A | −5G>A | 0.000 | 0.006 | – | – | – | – | – | – | – | ||
| Exon 1 | 43906A>G | 15A>G* | Gly5=† | rs10262966 | 0.037 | 0.012 | 0.045 | 0.282 | 0.014 | 0.006 | 0.008 | 0.007 | 0.011 |
| Exon 1 | 43978G>A | 87G>A | Thr29=† | rs41295381 | 0.011 | 0.003 | 0.010 | 0.028 | 0.002 | 0.003 | 0.000 | – | – |
| Intron 1 | 62302T>C | 189-10T>C | 0.004 | 0.003 | – | – | – | – | – | – | – | ||
| Exon 3 | 69416T>C | 304T>C | Ser102Pro‡ | 0.000 | 0.003 | – | – | – | – | – | – | – | |
| Exon 3 | 69456C>T | 344C>T | Ala115Val§ | 0.004 | 0.000 | – | – | – | – | – | 0.000 | – | |
| Exon 4 | 70258A>G | 387A>G | Pro129=† | rs1135612 | 0.360 | 0.318 | 0.212 | 0.083 | 0.268 | 0.419 | 0.420 | 0.260 | 0.440 |
| Exon 4 | 70300C>T | 429C>T* | Tyr143=§ | 0.015 | 0.000 | – | – | – | – | – | – | – | |
| Exon 4 | 70361G>A | 490G>A | Val164Met§ | 0.004 | 0.000 | – | – | – | – | – | – | – | |
| Exon 5 | 71001G>A | 571G>A | Val191Met‡ | 0.000 | 0.003 | – | – | – | – | – | – | – | |
| Intron 5 | 71411C>G | 642-5C>G | 0.007 | 0.009 | – | 0.005 | 0.004 | 0.000 | 0.000 | – | – | ||
| Exon 6 | 71457C>T | 683C>T | Pro228Leu‡ | rs17853284 | 0.000 | 0.003 | – | 0.000 | 0.006 | 0.000 | 0.000 | 0.003 | 0.002 |
| Exon 6 | 71461C>T | 687C>T | Ala229=‡ | rs72557906 | 0.000 | 0.003 | – | 0.002 | 0.000 | 0.003 | 0.000 | – | – |
| Exon 7 | 72135C>T | 744C>T | Tyr248=§ | 0.004 | 0.000 | – | – | – | – | – | – | – | |
| Exon 8 | 73486G>A | 898G>A | Glu300Lys‡ | rs11540674 | 0.000 | 0.006 | – | – | – | – | – | 0.000 | – |
| Exon 9 | 73718C>T | 1029C>T | Ala343=§ | rs72557942 | 0.004 | 0.000 | – | 0.000 | 0.000 | 0.003 | 0.000 | – | – |
| Exon 9 | 73719G>A | 1030G>A | Asp344Asn§ | 0.004 | 0.000 | – | – | – | – | – | – | – | |
| Intron 9 | 74663C>G | 1067-13C>G | rs4732516 | 0.938 | 0.991 | 0.953 | 0.609 | 0.959 | 0.870 | 0.869 | – | 0.132 | |
| Exon 10 | 74802A>C | 1193A>C | Glu398Ala§ | 0.004 | 0.000 | – | – | – | – | – | – | – | |
| Exon 10 | 74809G>A | 1200G>A | Ser400=§ | 0.004 | 0.000 | – | – | – | – | – | – | – | |
| Intron 10 | 74869C>T | 1248+12C>T | rs2286822 | 0.393 | 0.385 | 0.359 | 0.208 | 0.319 | 0.360 | 0.407 | 0.293 | 0.389 | |
| Intron 10 | 74877G>A | 1248+20G>A | rs2286823 | 0.393 | 0.382 | 0.374 | 0.194 | 0.317 | 0.360 | 0.407 | 0.297 | 0.389 | |
| Exon 12 | 75534T>C | 1455T>C** | Ala485=† | rs2228104 | 0.952 | 0.991 | 0.923 | 0.675 | 0.967 | 0.881 | 0.868 | 0.990 | 0.134 |
| Exon 12 | 75587C>T | 1508C>T** | Ala503Val† | rs1057868 | 0.206 | 0.294 | 0.219 | 0.191 | 0.264 | 0.367 | 0.310 | 0.303 | 0.434 |
| Exon 13 | 75868G>A | 1716G>A | Ser572=† | rs1057870 | 0.335 | 0.306 | 0.309 | 0.182 | 0.378 | 0.138 | 0.132 | 0.363 | 0.028 |
| Exon 14 | 76133G>A | 1891G>A | Val631Ile§ | rs145782750 | 0.004 | 0.000 | – | – | – | – | – | 0.007 | – |
| Exon 15 | 76279G>A | 1942G>A | Asp648Asn‡ | 0.000 | 0.003 | – | – | – | – | – | – | – | |
Genomic positions refer to the POR sequence NG_008930.1 and coding positions refer to the cDNA NM_000941.2. The sign (+) represents nucleotides downstream from the last base translated in the genomic sequence and the sign (-) represents nucleotides upstream from the first base translated in the genomic sequence. The amino acid positions refer to the GenBank accession number NP_000932.3. Bold text indicates a new nonsynonymous amino acid change found in this study Frequency data were compiled from studies Hart et al. [12], Huang et al. [13], Gomes et al. [23] and Saito et al. [14].
p < 0.05
p < 0.02 in Pearson's correlation between allele frequencies of AJ and MJ groups.
Variant found in both groups.
Variant found in AJ.
Variant found in MJ.
=: Silent mutation; AA: African–American; AJ: Ashkenazi Jewish; AS: Chinese–American; CA: Caucasian–American; ME: Mexican–American; MJ: Moroccan–Jewish.
Figure 1. Missense variants found in the Jewish population.
The structure of human POR [19] is shown, gray ribbons indicate the peptide backbone. FAD (yellow) and FMN (blue–gray) cofactors and NADP(H) coenzyme (only 2’,5’-ADP of NADP+ was structurally resolved, pink) are shown in stick configuration. Amino acid residues corresponding to Ashkenazi Jewish variants (red) and Moroccan Jewish variants (blue) are shown in space-filling configuration (see inset labels). FAD: Flavin adenine dinucleotide; FMN: Flavin mononucleotide.
In this study, we have identified 12 new genetic variations, three of which were found in noncoding regions, while the other nine were found in exons (Figure 1). From the novel exonic variants, three silent mutations Y143Y, Y248Y and S400S (according to the GeneBank accession number NP_00932.3) were present in four, one and one heterozygous MJ individuals, respectively, but were not found in the AJ group. Six of the newly described POR variants changed the encoded amino acid (S102P, V164M, V191M, D344N, E398A and D648N). Each of the above missense SNPs were found on a single allele; V164M, D344N and E398A in the MJ population and S102P, V191M and D648N in the AJ population. In one MJ sample, the double mutation (V164M and E398A) has been identified.
No significant differences in the allele frequencies of known variants described in the present study or the allele frequencies obtained in previous studies were found. Eight of the 30 identified SNP variants had allele frequencies greater than 10% in both MJ and AJ groups. From the remaining SNPs, one variant exhibited an allele frequency greater than 1% in both AJ and MJ groups, while three variants exceeded 1% only in the MJ group (rs72553977, T29T and Y143Y). The common variant A503V (POR*28) was present at an allele frequency of 20.6% in the MJ group and 29.4% in the AJ group. Although the difference between the AJ and MJ groups in A503 frequencies is statistically significant (p < 0.02), it still fell into the range of allele frequencies found in previous studies (Table 2). Three other nonsynonymous amino acid variants found in the current study (A115V, P228L and V631I) were present on just one allele. In the sample with the P228L variant, the heterozygous variant A229A was also found. The variant E300K was reported in two AJ samples, but not in the MJ population.
We reported four genetic variations in the first untranslated exon (Table 2). Two of them (rs3823884 and rs72553977) are known polymorphisms described in a previous study [13]. Two genetic variations are new (5003A>C and 5078G>T according to the NG_008930.1), not previously reported. Interestingly, both undescribed genetic variations were found only in the AJ population, while polymorphism rs72553977 was found only in the MJ population. Distribution of the allele rs3823884 is similar to the frequency of this allele in the Caucasian population [13].
Twenty and 22 polymorphic markers were used for LD and haplotype block identification analyses in AJ and MJ, respectively. As depicted in Supplementary Figure 1 (see online at: www.futuremedicine.com/doi/suppl/10.2217/pgs.12.21) we detected a similar pattern of considerable LD across the POR region in both AJ and MJ populations. We identified four and six haplotype blocks in AJ and MJ populations, respectively, in both cases with indication of high values of multiallelic D’ between the blocks (Supplementary FIGURE 2). In both populations, most of the haplotype combinations corresponded to the reference sequence or its variants without amino acid changes (POR*1) – over 60% both in AJ and MJ. POR*28 (A503V)-containing haplotypes were present in approximately 20% of MJ and 28% of AJ individuals. The remaining haplotype block combinations were present in low frequencies.
Discussion
Pharmacogenetics determine correlations between individual DNA sequence variations and variability in drug response [30]. It is often mentioned in connection with adverse drug reactions (ADRs), which represent a major public health concern [31]. There is evidence that drug therapy based on an individual's genetic makeup may result in a clinically significant reduction in adverse outcomes [32]. According to this study, 56% of drugs implicated in ADRs are metabolized by phase I enzymes, of which 86% are POR-dependent CYPs. In light of these findings, knowledge of the effects of POR variation on CYP activity has a potential for predicting ADRs and developing diagnostic tools for improving the safety and efficacy of CYP-metabolized drugs.
This information could provide the basis to yield more efficient and safer drug therapies. Until recently, variability in phase I biotrans-formation of drugs and other xenobiotics was attributed almost exclusively to polymorphisms in CYP genes, the drug and xenobiotic metabolic consequences of which have been well characterized [33]. Since POR is the unique electron donor to all microsomal CYPs, the contribution of common POR variants to inter- and intra-individual variability is of great pharmacogenetic interest.
Four recent studies have investigated the role of POR gene variants in relation to pharmacogenetics [12–14,23]. The present study confirmed several of the already reported common POR SNPs (>10%) and their allelic frequencies in MJ and AJ populations were found to be consistent with the allele frequencies obtained in the previous reports in other ethnic populations including African–Americans, Caucasian–Americans, Chinese–Americans, and Mexican–Americans (see Table 2). Notably, although genetic studies have shown that different Jewish ethnic groups including Ashkenazi Jews share distinct common Middle-Eastern origins, each Jewish group shows evidence of gene flow from local populations [34,35]. Thus, it was of interest to study POR mutations in both Ashkenazi and Moroccan Jews, despite these two ethnic groups having been separated from one another for approximately only two millennia.
All but two of the common variants identified in the study samples have little or no measurable effect on the POR activity [23,36], which suggests that they do not contribute to the differences in drug-metabolizing phenotypes. The common A503V variant, previously investigated in recombinant assays, showed only modest influence on the 17α-hydroxylase enzymatic activity, but did not modify function of either CYP1A2 and CYP2C19 [36] or 21-hydroxylase activity [37]. Further investigation of this variant in microsomal studies by Hart et al. [12] and by Gomes et al. [23] did not reveal any significant association between the A503V POR variant and altered drug-metabolizing CYP activity. Thus, the authors concluded that its role as a pharmacogenetic marker appears to be negligible. On the contrary, in two recent studies the A503V variant was found to reduce CYP3A4 activity to 61–77% of wild-type with midazolam and testosterone [38] and CYP2D6 activity to 53–62% with dextromethorphan and bufuralol [39]. As indicated, the impact of A503V on CYP activities may vary greatly depending on the CYP enzyme and the substrate metabolized, but it seems that in particular cases it could be used to explain differences in drug metabolism. Another common intronic variant (rs22868232) found here has been identified as an effector of CYP drug-oxidation activity [23]. In the POR functional ana lysis of Hart et al., an intronic polymorphism rs41301427 was also found to be associated with a decrease in POR function [12]. In the present study, we have examined only exonic and flanking intronic regions of the POR gene, but the importance of screening POR noncoding regions is clear. Among the MJ and AJ populations analyzed here, none of the known POR amino acid changes or frameshift mutations (T142A, Q153R, Y181D, N185K, M263V, A287P, R457H, Y459H, V492E, G539R, L565P, C569Y, L577R, Y578C, V608F, R616X, delF646, delE217, I444fsX449, L612W620delinsR, 1363delC, 697–698ins-GAAC and delP399_E401) associated with POR deficiency were found [8–11,15,40]. In this study, we have detected 11 amino acid substituted variants. Five (A115V, P228L, E300K, A503V, V631I) are already known and described in the CYP database [101]. The amino acid variant E300K reported by the SNP consortium [103] was found in our study in two heterozygous samples. According to Agrawal et al. E300K has practically no effect on CYP activity, but diverse outcomes can be expected with different protein partners [36]. The variant A115V was first described by Huang et al. [11] and recombinant expression and ana lysis of the A115V-mutant activity showed complete loss of function with drug-metabolizing CYPs, whereas the activity for the steroid-metabolizing CYPs was not dramatically affected [36,41]. These diametrically opposed results with different CYPs are interesting since this residue is located in the hydrophobic core of the FMN domain, which from structural considerations can accommodate the Val side chain [6]. This mutation would not be predicted to affect function. Also, two other POR variants, P228L (first described on the BioVentures Website [104]) and V631I (identified by Huang et al. [11]), exhibit significantly decreased support of CYP1A2- and CYP2C19-mediated activities, but do not appear to substantially affect steroid-metabolizing CYPs [41]. omparatively low activities of these two variants for several CYPs were determined by Gomes et al. [23]. Neither A115V, P228L nor V631I was associated with POR deficiency. In the previous report, all three variants were considered rare and not ordinarily present in the common population [23]. Indeed, in our present study they were observed only once each (on one allele). Considering the number of samples in the performed studies (99 [12], 842 [13], 150 [23], 235 [14] and 301 [present study]), it is probable that they exist at higher frequency among the greater population. A possible higher frequency of the mentioned variants, together with the fact that they play an important role in the activity of the drug-metabolizing CYPs, led us to hypothesize that they could represent a potential biomarker for future POR pharmacogenetic screening, at least in the Jewish populations studied here. For further investigation, allele frequency data from a larger population will inevitably be required. Six of the 11 exonic POR nonsynonymous polymorphisms are new, and are reported here for the first time. The structure of human POR (Figure 1) allows us to localize the new genetic variants and to predict their impact on POR activity [6]. Residue S102 is situated in the middle of helix B of the FMN-binding domain. Since proline residues are known to be helix breakers, the S102P mutation would introduce a kink in helix B. In addition, the OH group of S102 makes a hydrogen bond with the carbonyl oxygen of G112 of the neighboring β-strand that would not be possible in the S102P variant. Replacement of S102 with a proline would result in destabilization of the entire FMN domain, thereby producing functional consequences. V164 is a surface-exposed residue in close proximity to residues that were previously shown to act in membrane binding and orientation of the POR [42]. Variant V164M may therefore affect the association of POR with the microsomal membrane. V191 is located in the middle of a helix that forms part of the FMN-binding site with side-chain atoms facing inward [9]. Valine to methionine substitution at residue 191 may thus influence binding of the FMN. Moreover, the previously described mutations Y181D and N185K that lie close to the residue V191 drastically compromise POR activity [8,11,43]. The additional three variants (D344N, E398A and D648N) change amino acids in the surface-exposed residues. D344 is a solvent-exposed surface residue located at the top of the connecting domain on a short random coil between a β-sheet and an α-helix. E398 is also a solvent-exposed surface residue facing outward from the connecting domain but is located on a sharp turn between two a-helices. D648 is a solvent-exposed surface residue located on an α-helix that forms part of the adenine binding site within the NADPH pocket, but is located at the opposite end of the helix. Variants D344N, E398A and D648N all occur in the connecting domain and change the charge of the residue involved. However, because they are directed toward the surface in a region unlikely to interact with CYPs, these charge changes probably will have no effect on POR function. Recent studies also outlined the question of the role of genetic variations in the first untranslated exon and in POR promoter regions [44,45]. Sequencing of 274 bp of the human POR promoter revealed three common polymorphisms (−208, −173 and −152) [13]. A transcriptional study by Tee et al. showed that one of these polymorphisms (−152) might play a role in steroid bio-synthesis and drug metabolism [45]. Soneda et al. described two deletions encompassing exon 1U in two patients with POR deficiency and showed a pivotal role of the evolutionally conserved SP1 binding sites in the POR transcription initiation site [44]. Moreover, approximately 12% of patients with POR deficiency are known to have one apparently normal POR allele [46] indicating that the second mutation should be somewhere in the unstudied regions of the POR gene. Thus, the importance of exploring noncoding regions of POR is clear. In our study, we focused on the exon 1U and found four genetic changes within this exon, two of which are previously undescribed. Future studies are planned which will also investigate promoter regions.
Our results confirm both the strong LD pattern across the POR region as well as the most prevalent POR*1 and POR*28 haplo-types as reported previously in Caucasian [23] and Japanese [14] subjects. The apparent differences in the inferred haplotype block structure in AJ and MJ populations from the previously published studies may result from utilization of rather distinct algorithms and the relatively limited number of frequent alleles in the AJ and MJ datasets. Actually, given the incomplete coverage of the promoter variants in the study by Gomes et al. [23] and the limited historical recombinations between the blocks in the current study, the differences may well be reconcilable if both sets are analyzed in a unified fashion.
Several new findings have been highlighted in recent POR functional studies, representing important implications for further research. A number of studies have confirmed that the effects of different POR mutations depend on both the particular CYP and the substrate assayed [36,39,40,47]. For that reason, the residual activity of a particular CYP for a particular drug may not be predictive for every CYP, or even other drugs metabolized by the same CYP enzyme, or other POR-dependent enzymes. Thus, numerous electron acceptors must be tested separately with each POR mutant for assessing the effect of each POR variation on drug metabolism.
Much remains to be clarified about the effects of POR mutations on the mechanism of POR action. In some cases, mechanisms that alter protein activity are quite well understood [40,48,49]. For example, deleterious mutants Y459H and V492E result in loss of POR function due to diminished affinity of the mutant protein for the essential FAD cofactor [48]. Mutations affecting the FMN-binding domain, N185K and Y181D, compromise binding of the FMN moiety and therefore ablate nearly all POR activity [8,11,43]. By contrast, residue L577 participates in maintaining the NADPH-binding domain structure with the L577R variant retaining 27–46% of wild-type activity, depending on the tested substrate [40]. Finally, solvent-exposed mutations such as Q153R [11], which could alter the distribution of surface charge of the electron-donating FMN domain, result in different effects on POR activities with different substrates [36]. Some authors hypothesized that differences in the activity of the POR variants may be due to conformational changes in POR [36,50]. Mutations important for FMN and FAD binding may thus be expected to result in deleterious POR activity impairment with all CYPs, while mutations changing the distribution of electronic charge may have variable effects on POR interactions, depending on specific CYP enzymes and drugs tested. All of these conjectures can be tested by examination of the structures of these mutant proteins, provided crystallization is possible. If there are no conformational changes, it may not be possible to obtain data by this method, however, indirect inferences may be made utilizing binding assays and protein interaction measurements.
Most functional investigations of POR–CYP interactions were performed in vitro. Gomes et al. [23] and Hart et al. [12] examined expression and function of POR in human liver micro-somes, an approach that offers an advantage over reconstituted recombinant systems in that the complexity and dynamics of the endoplasmic reticulum are better represented. Lately, several in vivo studies exploring the influence of POR variants on CYP-catalyzed drug metabolism has been carried out. Oneda et al. investigated the activity of the A503V variant in humans and observed a 1.6-fold enhancement of drug clearance in vivo over that observed in wild-type carriers [50]. This result differs from outcomes observed in vitro [23,36,41]. The discrepancy can be explained by the fact that both CYP3A4 and CYP3A5 contribute to midazolam biotrans-formation [51], thus the rate of CYP3A4 participation in midazolam metabolism cannot be clearly established in vivo. Another study examining the A503V variant addressed the question of tacrolimus metabolism in a cohort of renal allograft recipients [52]. They observed that patients expressing CYP3A5*1 and carrying at least one A503V allele required elevated tacrolimus doses, especially in first days after the transplantation.
A recent study investigated an effect of the major POR mutant A287P in an adult patient with POR deficiency and her mother; both individuals exhibited subnormal activities of several CYPs [53] confirming the data obtained in in vitro assays [36,39,41]. Although in vitro assays may not simulate some aspects of the natural environment of the endoplasmic reticulum, they remain useful tools for comparison of residual activities among individual CYP enzymes. At the very least, it is important that in vitro assays mimic the membrane milieu and stoichiometry of the in vivo system as closely as possible [54].
Conclusion
In summary, our results from AJ and MJ populations, along with those from other recent pharmacogenetic reports [12–14,23] and comparisons to our structural studies, suggest that the human POR gene is highly polymorphic and that certain variants correlate with significant differences in residual CYP enzyme activities. Nevertheless, nonsynonymous variants associated with POR deficiency are uncommon in the normal population, indicating that these variants are rare and unlikely to represent genetic factors contributing to the large interindividual variability of drug-metabolizing phenotypes. The continued study of POR polymorphisms promises to be an exciting research area in pharmacogenetics.
Supplementary Material
Executive summary.
Background
■ Recently, the possible contribution of common POR sequence variants to inter- and intra-individual variability in drug metabolism has become of great pharmacogenetic interest.
Materials & methods
■ In order to search for polymorphic alleles in the POR gene, we first sequenced untranslated exon 1U and 15 coding exons and approximately 20 bp of the intronic sequence flanking these exons in the POR gene in 301 healthy unrelated individuals from Ashkenazi and Moroccan Jewish populations.
A new-generation amplicon high-resolution melting method and DNA sequencing was implemented for the analysis of the POR gene.
Results
■ Thirty POR SNPs were identified, nine in the noncoding regions and 21 in the protein-coding regions (ten synonymous, 11 missense). Six of these missense variants were previously undescribed (S102P, V164M, V191M, D344N, E398A and D648N).
Conclusion
■ The linkage disequilibrium pattern of POR was similar between the Ashkenazi and Moroccan Jewish populations and the most prevalent haplotypes corresponded to those previously identified in Caucasians and Asians.
Future perspective
■ The data collected in this study along with examination and comparisons to structural parameters indicate that some of the POR missense variants may be potential biomarkers for future POR pharmacogenetics screening.
Acknowledgements
The authors thank J-J Kim from Medical College of Wisconsin (WI, USA) for helpful discussions regarding potential structural consequences of mutations. The authors thank the anonymous donors of the NLGIP biobank at Tel Aviv University (Tel Aviv, Israel), whose altruism and trust in biomedical research have made this study possible.
Supported by grant GACR P301/10/1426 from Czech Granting Agency and the US NIH GM81568 to BSS Masters, who is the Robert A Welch Distinguished Professor in Chemistry (AQ0012) at the University of Texas Health Science Center at San Antonio (TX, USA).
Footnotes
Disclaimer
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate insti tutional review board approval or have followed the princi ples outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Masters BS. The journey from NADPH-cytochrome P450 oxidoreductase to nitric oxide synthases. Biochem. Biophys. Res. Commun. 2005;338(1):507–519. doi: 10.1016/j.bbrc.2005.09.165. [DOI] [PubMed] [Google Scholar]
- 2.Shephard EA, Phillips IR, Santisteban I, et al. Isolation of a human cytochrome P-450 reductase cDNA clone and localization of the corresponding gene to chromosome 7q11.2. Ann. Hum. Genet. 1989;53(Pt 4):291–301. doi: 10.1111/j.1469-1809.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 3.Scott RR, Gomes LG, Huang N, Van Vliet G, Miller WL. Apparent manifesting heterozygosity in P450 oxidoreductase deficiency and its effect on coexisting 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2007;92(6):2318–2322. doi: 10.1210/jc.2006-2345. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc. Natl Acad. Sci. USA. 1997;94(16):8411–8416. doi: 10.1073/pnas.94.16.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black SD, French JS, Williams CH, Jr, Coon MJ. Role of a hydrophobic polypeptide in the N-terminal region of NADPH-cytochrome P-450 reductase in complex formation with P-450LM. Biochem. Biophys. Res. Commun. 1979;91(4):1528–1535. doi: 10.1016/0006-291x(79)91238-5. [DOI] [PubMed] [Google Scholar]
- 6■■.Xia C, Panda SP, Marohnic CC, Martasek P, Masters BS, Kim JJ. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc. Natl Acad. Sci. USA. 2011;108(33):13486–13491. doi: 10.1073/pnas.1106632108. [The atomic structure of human POR is presented, as well as structures of two naturally occurring missense mutations, V492E and R457H.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi M, Tachibana K, Asakura Y, Yamamoto T, Hanaki K, Oka A. Compound heterozygous mutations of cytochrome P450 oxidoreductase gene (POR) in two patients with Antley–Bixler syndrome. Am. J. Med. Genet. A. 2004;128A(4):333–339. doi: 10.1002/ajmg.a.30169. [DOI] [PubMed] [Google Scholar]
- 8■■.Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [Molecular pathogenesis of the described form of congenital adrenal hyperplasia is caused by mutations in the POR gene.] [DOI] [PubMed] [Google Scholar]
- 9■■.Fluck CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley–Bixler syndrome. Nat. Genet. 2004;36(3):228–230. doi: 10.1038/ng1300. [Initial report of POR deficiency in four individuals resulting in disordered steroidogenesis with and without Antley–Bixler Syndrome and segregation of this genetic defect from FGFR deficiency.] [DOI] [PubMed] [Google Scholar]
- 10.Fukami M, Horikawa R, Nagai T, et al. Cytochrome P450 oxidoreductase gene mutations and Antley–Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J. Clin. Endocrinol. Metab. 2005;90(1):414–426. doi: 10.1210/jc.2004-0810. [DOI] [PubMed] [Google Scholar]
- 11.Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in P450 oxidoreductase in patients with Antley–Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005;76(5):729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart SN, Wang S, Nakamoto K, Wesselman C, Li Y, Zhong XB. Genetic polymorphisms in cytochrome P450 oxidoreductase influence microsomal P450-catalyzed drug metabolism. Pharmacogenet. Genomics. 2008;18(1):11–24. doi: 10.1097/FPC.0b013e3282f2f121. [DOI] [PubMed] [Google Scholar]
- 13■■.Huang N, Agrawal V, Giacomini KM, Miller WL. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc. Natl Acad. Sci. USA. 2008;105(5):1733–1738. doi: 10.1073/pnas.0711621105. [The analysis of the POR gene in a population of 842 healthy unrelated individuals in four ethnic groups: 218 African–Americans, 260 Caucasian–Americans, 179 Chinese–Americans and 185 Mexican–Americans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14■■.Saito Y, Yamamoto N, Katori N, et al. Genetic polymorphisms and haplotypes of POR, encoding cytochrome P450 oxidoreductase, in a Japanese population. Drug Metab. Pharmacokinet. 2011;26(1):107–116. doi: 10.2133/dmpk.dmpk-10-sc-096. [Analyzes genetic variations and the haplotype structures of the POR gene in 235 Japanese subjects.] [DOI] [PubMed] [Google Scholar]
- 15.Fluck CE, Mallet D, Hofer G, et al. Deletion of P399_E401 in NADPH cytochrome P450 oxidoreductase results in partial mixed oxidase deficiency. Biochem. Biophys. Res. Commun. 2011;412(4):572–577. doi: 10.1016/j.bbrc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Shen AL, O'Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J. Biol. Chem. 2002;277(8):6536–6541. doi: 10.1074/jbc.M111408200. [DOI] [PubMed] [Google Scholar]
- 17.Ribes V, Otto DM, Dickmann L, et al. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev. Biol. 2007;303(1):66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CJ, Otto DM, Carrie D, et al. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J. Biol. Chem. 2003;278(15):13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- 19.Feidt DM, Klein K, Nussler A, Zanger UM. RNA-interference approach to study functions of NADPH: cytochrome P450 oxidoreductase in human hepatocytes. Chem. Biodivers. 2009;6(11):2084–2091. doi: 10.1002/cbdv.200900135. [DOI] [PubMed] [Google Scholar]
- 20.Antley R, Bixler D. Trapezoidocephaly, midfacial hypoplasia and cartilage abnormalities with multiple synostoses and skeletal fractures. Birth Defects Orig. Artic. Ser. 1975;11(2):397–401. [PubMed] [Google Scholar]
- 21.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin. Pharmacokinet. 1997;32(3):210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 23.Gomes AM, Winter S, Klein K, et al. Pharmacogenomics of human liver cytochrome P450 oxidoreductase: multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics. 2009;10(4):579–599. doi: 10.2217/pgs.09.7. [DOI] [PubMed] [Google Scholar]
- 24.Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25(11):489–494. doi: 10.1016/j.tig.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Gurwitz D, Lunshof JE. Personalized pharmacotherapy: genotypes, biomarkers, and beyond. Clin. Pharmacol. Ther. 2009;85(2):142. doi: 10.1038/clpt.2008.228. [DOI] [PubMed] [Google Scholar]
- 26.Gurwitz D, Kimchy O, Bonne-Tamir B. Martinus Nijhoff. The Netherlands; Leiden: 2003. The Israeli DNA and cell line collection: a human diversity repository. In: Populations and Genetics. Legal and Socio-Ethical Perspectives. [Google Scholar]
- 27.Ulbrichova-Douderova D, Martasek P. Detection of DNA variations in the future science group polymorphic hydroxymethylbilane synthase gene by high-resolution melting ana lysis. Anal. Biochem. 2009;395(1):41–48. doi: 10.1016/j.ab.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel G, Shamir R. GERBIL: genotype resolution and block identification using likelihood. Proc. Natl Acad. Sci. USA. 2005;102(1):158–162. doi: 10.1073/pnas.0404730102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidovich O, Kimmel G, Shamir R. GEVALT: an integrated software tool for genotype ana lysis. BMC Bioinformatics. 2007;8:36. doi: 10.1186/1471-2105-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichelbaum M, Ingelman-Sundberg M, Evans WE. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006;57:119–137. doi: 10.1146/annurev.med.56.082103.104724. [DOI] [PubMed] [Google Scholar]
- 31.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock J, Lesko LJ. Pharmacogenetics – tailoring treatment for the outliers. N. Engl. J. Med. 2009;360(8):811–813. doi: 10.1056/NEJMe0810630. [DOI] [PubMed] [Google Scholar]
- 33.Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008;392(6):1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 34.Behar DM, Metspalu E, Kivisild T, et al. Counting the founders: the matrilineal genetic ancestry of the Jewish diaspora. PLoS ONE. 2008;3(4):e2062. doi: 10.1371/journal.pone.0002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behar DM, Yunusbayev B, Metspalu M, et al. The genome-wide structure of the Jewish people. Nature. 2010;466(7303):238–242. doi: 10.1038/nature09103. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet. Genomics. 2008;18(7):569–576. doi: 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- 37.Gomes LG, Huang N, Agrawal V, Mendonca BB, Bachega TA, Miller WL. The common P450 oxidoreductase variant A503V is not a modifier gene for 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2008;93(7):2913–2916. doi: 10.1210/jc.2008-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38■■.Agrawal V, Choi JH, Giacomini KM, Miller WL. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet. Genomics. 2010;20(10):611–618. doi: 10.1097/FPC.0b013e32833e0cb5. [The functional effects of POR gene variants on CYP enzyme activity depend on the electron recipient specific characteristics.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandee D, Morrissey K, Agrawal V, et al. Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet. Genomics. 2010;20(11):677–686. doi: 10.1097/FPC.0b013e32833f4f9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahakitrungruang T, Huang N, Tee MK. Clinical, genetic, and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J. Clin. Endocrinol. Metab. 2009;94(12):4992–5000. doi: 10.1210/jc.2009-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fluck CE, Mullis PE, Pandey AV. Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism. Biochem. Biophys. Res. Commun. 2010;401(1):149–153. doi: 10.1016/j.bbrc.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Mast N, Liao WL, Pikuleva IA, Turko IV. Combined use of mass spectrometry and heterologous expression for identification of membrane-interacting peptides in cytochrome P450 46A1 and NADPH-cytochrome P450 oxidoreductase. Arch. Biochem. Biophys. 2009;483(1):81–89. doi: 10.1016/j.abb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolo C, Fluck CE, Mullis PE, Pandey AV. Restoration of mutant cytochrome P450 reductase activity by external flavin. Mol. Cell. Endocrinol. 2010;321(2):245–252. doi: 10.1016/j.mce.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Soneda S, Yazawa T, Fukami M, et al. Proximal promoter of the cytochrome P450 oxidoreductase gene: identification of microdeletions involving the untranslated exon 1 and critical function of the SP1 binding sites. J. Clin. Endocrinol. Metab. 2011;96(11):E1881–E1887. doi: 10.1210/jc.2011-1337. [DOI] [PubMed] [Google Scholar]
- 45.Tee MK, Huang N, Damm I, Miller WL. Transcriptional regulation of the human P450 oxidoreductase gene: hormonal regulation and influence of promoter polymorphisms. Mol. Endocrinol. 2011;25(5):715–731. doi: 10.1210/me.2010-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott RR, Miller WL. Genetic and clinical features of P450 oxidoreductase deficiency. Horm. Res. 2008;69(5):266–275. doi: 10.1159/000114857. [DOI] [PubMed] [Google Scholar]
- 47.Dhir V, Ivison HE, Krone N, et al. Differential inhibition of CYP17A1 and CYP21A2 activities by the P450 oxidoreductase mutant A287P. Mol. Endocrinol. 2007;21(8):1958–1968. doi: 10.1210/me.2007-0066. [DOI] [PubMed] [Google Scholar]
- 48■.Marohnic CC, Panda SP, Martasek P, Masters BS. Diminished FAD binding in the Y459H and V492E Antley–Bixler syndrome mutants of human cytochrome P450 reductase. J. Biol. Chem. 2006;281(47):35975–35982. doi: 10.1074/jbc.M607095200. [Discusses the restoration, by external flavin addition, of diminished POR activity due to selected mutations.] [DOI] [PubMed] [Google Scholar]
- 49.Marohnic C, Panda SP, McCammon K, Rueff J, Masters BS, Kranendonk M. Human cytochrome P450 oxidoreductase deficiency caused by the Y181D mutation: molecular consequences and rescue of defect. Drug Metab. Dispos. 2010;38(2):332–340. doi: 10.1124/dmd.109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oneda B, Crettol S, Sirot EJ, Bochud M, Ansermot N, Eap CB. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenet. Genomics. 2009;19(11):877–883. doi: 10.1097/FPC.0b013e32833225e7. [DOI] [PubMed] [Google Scholar]
- 51.Patki KC, Von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes P450: role of CYP3A4 and CYP3A5. Drug Metab. Dispos. 2003;31(7):938–944. doi: 10.1124/dmd.31.7.938. [DOI] [PubMed] [Google Scholar]
- 52■.De Jonge H, Metalidis C, Naesens M, Lambrechts D, Kuypers DR. The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics. 2011;12(9):1281–1291. doi: 10.2217/pgs.11.77. [Demonstrates that the POR*28 SNP is associated with additional increases in tacrolimus dose requirements in patients carrying a CYP3A5*1 allele.] [DOI] [PubMed] [Google Scholar]
- 53.Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur. J. Endocrinol. 2010;163(6):919–924. doi: 10.1530/EJE-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moutinho D, Marohnic CC, Panda SP, Rueff J, Masters BS, Kranendonk M. Altered human CYP3A4 activity caused by Antley–Bixler syndrome-related variants of NADPH-cytochrome P450 oxidoreductase measured in a robust in vitro system. Drug Metab. Dispos. 2012 doi: 10.1124/dmd.111.042820. doi:10.1124/dmd.111.042820 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Human Cytochrome P450 (CYP) Allele Nomenclature Committee. www.cypalleles.ki.se/por.htm.
- 102.The National Laboratory for Genetics of the Israeli Population at Tel Aviv University. www.tau.ac.il/medicine/NLGIP/nlgip.htm.
- 103.The SNP Consortium. www.ncbi.nlm.nih.gov/projects/SNP.
- 104.BioVentures. www.bioventures.com/products/dmg/por.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.