Abstract
Background
Nearsightedness (myopia) causes blurry vision when looking at distant objects. Highly nearsighted people are at greater risk of several vision-threatening problems such as retinal detachments, choroidal atrophy, cataracts and glaucoma. Interventions that have been explored to slow the progression of myopia include bifocal spectacles, cycloplegic drops, intraocular pressure-lowering drugs, muscarinic receptor antagonists and contact lenses. The purpose of this review was to systematically assess the effectiveness of strategies to control progression of myopia in children.
Objectives
To assess the effects of several types of interventions, including eye drops, undercorrection of nearsightedness, multifocal spectacles and contact lenses, on the progression of nearsightedness in myopic children younger than 18 years. We compared the interventions of interest with each other, to single vision lenses (SVLs) (spectacles), placebo or no treatment.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2011, Issue 10), MEDLINE (January 1950 to October 2011), EMBASE (January 1980 to October 2011), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to October 2011), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (http://clinicaltrials.gov). There were no date or language restrictions in the electronic searches for trials. The electronic databases were last searched on 11 October 2011. We also searched the reference lists and Science Citation Index for additional, potentially relevant studies.
Selection criteria
We included randomized controlled trials (RCTs) in which participants were treated with spectacles, contact lenses or pharmaceutical agents for the purpose of controlling progression of myopia. We excluded trials where participants were older than 18 years at baseline or participants had less than −0.25 diopters (D) spherical equivalent myopia.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias for each included study. When possible, we analyzed data with the inverse variance method using a fixed-effect or random-effects model, depending on the number of studies and amount of heterogeneity detected.
Main results
We included 23 studies (4696 total participants) in this review, with 17 of these studies included in quantitative analysis. Since we only included RCTs in the review, the studies were generally at low risk of bias for selection bias. Undercorrection of myopia was found to increase myopia progression slightly in two studies; children who were undercorrected progressed on average 0.15 D (95% confidence interval (CI) −0.29 to 0.00) more than the fully corrected SVLs wearers at one year. Rigid gas permeable contact lenses (RGPCLs) were found to have no evidence of effect on myopic eye growth in two studies (no meta-analysis due to heterogeneity between studies). Progressive addition lenses (PALs), reported in four studies, and bifocal spectacles, reported in four studies, were found to yield a small slowing of myopia progression. For seven studies with quantitative data at one year, children wearing multifocal lenses, either PALs or bifocals, progressed on average 0.16 D (95% CI 0.07 to 0.25) less than children wearing SVLs. The largest positive effects for slowing myopia progression were exhibited by anti-muscarinic medications. At one year, children receiving pirenzepine gel (two studies), cyclopentolate eye drops (one study), or atropine eye drops (two studies) showed significantly less myopic progression compared with children receiving placebo (mean differences (MD) 0.31 (95% CI 0.17 to 0.44), 0.34 (95% CI 0.08 to 0.60), and 0.80 (95% CI 0.70 to 0.90), respectively).
Authors’ conclusions
The most likely effective treatment to slow myopia progression thus far is anti-muscarinic topical medication. However, side effects of these medications include light sensitivity and near blur. Also, they are not yet commercially available, so their use is limited and not practical. Further information is required for other methods of myopia control, such as the use of corneal reshaping contact lenses or bifocal soft contact lenses (BSCLs) with a distance center are promising, but currently no published randomized clinical trials exist.
PLAIN LANGUAGE SUMMARY
Interventions to slow progression of nearsightedness in children
Nearsightedness (myopia) causes blurry vision when looking at distant objects. Approximately 33% of the population in the United States is nearsighted, and some Asian countries report that up to 80% of children are nearsighted. Several studies have examined a variety of methods (including eye drops, incomplete correction (known as ‘undercorrection’) of nearsightedness, multifocal lenses and contact lenses) to slow the worsening of nearsightedness.
In this review we included 23 clinical investigations of myopia treatments in children. Two studies investigated undercorrection of myopia; twelve studies investigated multifocal spectacles (progressive addition lenses (PALs) or bifocal spectacles); one study investigated bifocal soft contact lenses (BSCLs); one study investigated novel lenses designed to reduce peripheral hyperopic defocus (peripheral vision farsightedness) (i.e. lenses that help to focus peripheral vision as well as central vision); two studies investigated rigid gas permeable contact lenses (RGPCLs); and six studies investigated pharmaceutical eye drops (five of these studies were of anti-muscarinic medications). There was one study that evaluated both multifocal lenses and pharmaceutical eye drops. In all studies the interventions of interest were compared with each other, single vision lenses (SVLs) (spectacles), single vision soft contact lenses (SVSCLs) or placebo. The follow-up period was at least one year for all studies.
The largest positive effects for slowing myopia progression were exhibited by anti-muscarinic medications (eye drops), but they either cause light sensitivity or blurred near vision, and are not yet available for use. Multifocal spectacles including PALs and bifocal spectacles were found to yield a small slowing of myopia progression. Undercorrection of myopia was found to increase myopia progression slightly, while RGPCLs were found to have no evidence of effect on myopic eye growth.
BACKGROUND
Description of the condition
Myopia, also known as nearsightedness, is a condition where near objects are seen clearly but distant objects appear blurred. In myopia, distant objects are focused in front of the retina instead of on it, as occurs in non-myopic individuals. Myopia occurs because the cornea or lens is too powerful or because the eyeball is longer than normal.
Epidemiology
Myopia is an important cause of reduced vision in populations throughout the world and is one of the five immediate priorities for the ‘Vision 2020’ initiative by the World Health Organization (WHO) (Pararajasegaram 1998). Approximately 2% of the United States population is myopic at school entry (Blum 1959)and about 15% of the people entering high schools are myopic (Sperduto 1983). Racial and ethnic differences in magnitude and prevalence of myopia have been observed (Garner 1999; Lin 1999; Maul 2000; Voo 1998; Zhan 2000), both being greater in Asia than other parts of the world (Lin 1999; Zhan 2000).
Juvenile-onset myopia typically develops at approximately six to eight years of age and progresses at a rate of approximately 0.50 D (diopters) per year through 15 to 16 years (COMET Study; Fulk 2002; Goss 1987; Perrigin 1990). The progression of myopia is typically faster at younger ages (Braun 1996; Goss 1987; Goss 1990; Pärssinen 1989; Saw 2000), but myopia onset, progression, and stabilization vary widely among individuals (Braun 1996; Pärssinen 1989; Saw 2000). Similar proportions of boys and girls are affected by myopia and the degree of myopia is similar between the two genders (Zadnik 2003).
Etiology and risk factors
Several factors have been suggested to have a role in the development of myopia. Many models estimate greater genetic effects than environmental effects for myopia (Chen 1985; Hammond 2001). Children with two myopic parents have greater axial lengths which indicates a higher risk of myopia than children with one or no myopic parents (Zadnik 1994). Environmental influences relate to prolonged reading or near work, which has been associated with increased myopia prevalence (Saw 2001; Young 1969). Myopic individuals exhibit a greater accommodative lag (poor focusing accuracy while looking at near objects) than emmetropes (those who do not require spectacles to see either distant or near objects clearly). This high lag of accommodation leads to blur, which may stimulate myopic eye growth (Gwiazda 1993; Gwiazda 1995). Fewer hours spent outdoors also has been associated with myopia (Jones 2007; Rose 2008).
Presentation and diagnosis
The primary symptom of myopia is blurred distance vision. Children often present to an eye care practitioner after failing a vision screening at school or after a parent or teacher notices the child squinting or having difficulty seeing distant objects.
An eye care practitioner using autorefraction or retinoscopy may confirm the diagnosis of myopia objectively, or it can be confirmed by performing a subjective refraction, which requires responses from the child. In order to diagnose myopia in children, cycloplegic drops can be placed in the child’s eyes, hindering his or her ability to focus the eyes so that an accurate prescription can be determined.
Description of the intervention
Spectacles are often the initial treatment for children with myopia because they provide clear vision with few potential side effects. Spectacles for myopia correction use concave lenses that focus light more posteriorly, resulting in a clear image focused on the retina. Contact lenses are typically a secondary treatment option for children because they require greater dexterity and responsibility to care for them than spectacles. They also bear greater risks than spectacles that range from innocuous redness of the eyes to severe pain and vision loss due to corneal ulcers (Fonn 1988; MacRae 1991; Schein 1989).
Laser refractive surgery, such as Laser In Situ Keratomileusis (LASIK) or Photorefractive Keratectomy (PRK), causes a permanent flattening of the central corneal curvature by removing stromal tissue with a laser (Duffey 2003). Although it is frequently performed in adults (Shortt 2006), children’s eyes are still developing and the myopia continues to change during adolescence, so surgery is not routinely performed in children.
Other forms of myopia correction, such as a lens placed inside the eye and clear rings placed in the cornea, also are not used routinely in children due to children’s potential for myopia progression (Barsam 2010).
How the intervention might work
In terms of slowing myopia progression, multifocal spectacles and undercorrection of myopic refractive error are thought to reduce accommodative error, which may act as a stimulus for increased eye growth. Myopic patients exhibit greater accommodative lag than non-myopic patients (COMET Study; Mutti 2006). Accommodative lag results in light focused behind the retina during near work, which may act as a signal to increase eye growth and result in myopia. If the accommodative error can be reduced with bifocals or undercorrection, then the stimulus for eye growth will be reduced and may slow myopia progression.
Cycloplegic agents were thought to reduce myopic progression by eliminating accommodation, but it has since been shown to be a local retinal effect that slows myopia progression (Troilo 1987). Anti-muscarinic receptor binding may lead to a biochemical change that slows eye growth, but the exact mechanism is unknown.
Why it is important to do this review
Myopia has been reported to reach epidemic proportions in parts of the world (Park 2004). Strategies to control progression of myopia gain importance in the context of the ‘Vision 2020’ initiative by the WHO to eliminate preventable causes of blindness, including risks associated with high myopia, by the year 2020 (Pararajasegaram 1998). Interventions that have been explored for this purpose include bifocal spectacles, cycloplegic drops, intraocular pressure-lowering drugs, muscarinic receptor antagonists and contact lenses. In this review we systematically assessed the effectiveness of strategies to control progression of myopia in children.
OBJECTIVES
The objective of this review was to assess the effects of interventions, including spectacles, contact lenses and pharmaceutical agents, for slowing myopia progression in children.
METHODS
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs).
Types of participants
We included trials in which participants were treated with spectacles, contact lenses, or pharmaceutical agents for controlling progression of myopia. We excluded trials where participants were older than 18 years at the start of the trial. We also excluded trials that included participants with less than −0.25 D spherical equivalent myopia at baseline. (The spherical equivalent is an optical measurement based on a mathematical calculation: the sum of the spherical power plus half the cylindrical power of the lens.)
Types of interventions
We included trials in which any of the following interventions for controlling progression of myopia were compared with single vision lenses (spectacles) or single vision soft contact lenses (SVSCLs) (control treatment), placebo or with each other.
Bifocal soft contact lenses (BSCLs), rigid gas permeable contact lenses (RGPCLs) and corneal reshaping (orthokeratology) contact lenses.
Bifocal lenses (spectacles), progressive addition lenses (PALs) and undercorrection of myopia.
Pharmaceutical agents.
Types of outcome measures
Primary outcomes
The primary outcome for this review was progression of myopia assessed as the mean change in refractive error (spherical equivalent) from baseline for each year of follow-up and measured using any method.
Secondary outcomes
Mean change in axial length, measured by any method.
Mean change in corneal radius of curvature, measured by any method.
We analyzed the secondary outcomes for each year of follow-up when sufficient data were available. We included data as reported by each included study (i.e. data from one eye, from each eye individually or the average of both eyes) and pooled the results, regardless of how the data were analyzed in an individual study.
Adverse effects
We summarized the reported adverse effects related to the interventions as described in the included studies, including but not limited to blurry vision, red eyes, infections and conjunctival reactions.
Economic data
We documented reported cost-analyses and other data on economic outcomes when reported from the included trials.
Quality of life measures
We documented any quality of life information when reported from the included trials.
Follow-up
We reported outcomes for follow-up at one year, two years and as available throughout the study periods. We imposed no restrictions based on the length of follow-up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 10, part of The Cochrane Library, www.thecochranelibrary.com (accessed 11 October 2011), MEDLINE (January 1950 to October 2011), EMBASE (January 1980 to October 2011), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to October 2011), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (http://clinicaltrials.gov). There were no date or language restrictions in the electronic searches for trials. The electronic databases were last searched on 11 October 2011.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5) and ClinicalTrials.gov (Appendix 6).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We used the Science Citation Index (assessed 1 November 2011) to find studies that had cited the identified trials. We contacted the primary investigators of identified trials for details of other potentially relevant trials not identified by the electronic searches, recently completed trials or ongoing trials. We did not conduct manual searches of abstracts of conference proceedings and optometry literature specifically for this review, as these sources are searched by the Cochrane Eyes and Vision Group and listed in CENTRAL.
Data collection and analysis
Selection of studies
Two review authors, including at least one clinician (JJW, DOM, SAC, JDT) and one methodologist (SSV, KL), independently assessed the titles and abstracts of all reports identified by the electronic and manual searches as per the ‘Criteria for considering studies for this review’. The abstracts were classified as (1) definitely include, (2) unsure or (3) definitely exclude. We obtained and assessed the full-text of articles classified as (1) or (2) by at least one review author. After assessing the full-text we reclassified the studies as (A) include, (B) awaiting assessment or (C) exclude. A third review author resolved disagreements. The review authors were unmasked to the report authors, institutions and trial results during this assessment. For studies identified as (A), we included and assessed them further for study design and risk of bias. We contacted the authors of studies classified as (B) for clarification and reassessed these studies as per the inclusion criteria as further information became available. We excluded the studies in (C) and documented the reasons for exclusion in this review.
We initially included Cheng 2010, but after full-text review and data extraction we assessed it to be quasi-randomized and thus ineligible for the review. However, as we initially included the study we did not exclude it post hoc and instead conducted sensitivity analyses for inadequate randomization when applicable.
Data extraction and management
Two review authors independently extracted the data for the primary and secondary outcomes on to paper data collection forms developed by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. We contacted primary investigators for missing data. One review author entered the data into Review Manager 5 (RevMan 2011) and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed the sources of systematic bias in trials according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements between authors through discussion.
We considered the following parameters:
selection bias (random sequence generation, quality of allocation concealment);
performance bias and detection bias (masking of participants, outcome assessors and data analyzers);
attrition bias (completeness of follow-up and intention-to-treat (ITT) analysis);
reporting bias; and
other potential sources of bias (such as funding source).
For attrition bias, we considered whether or not reasons for losses to follow-up were comparable between treatment arms and whether or not all participants were analyzed as randomized. If studies reported that an ITT analysis was performed we assessed whether both a) participants in which no outcome was collected, and b) participants who received only some or none of their allotted treatment were included. We interpreted a true ITT analysis to have been undertaken only when both of these criteria were fulfilled. We classified the risk of bias for each parameter as “low risk of bias”, “unclear risk of bias”, or “high risk of bias”. For example, studies using allocation concealment by centralized randomization and sequential opaque envelopes, (which provided reasonable confidence that the participating eye care providers and patients were not aware of the randomization sequence), were considered to be at low risk of bias. We contacted the authors of trials when additional information was needed to assess risk of bias. If the authors did not respond within an eight-week period we classified the trial based on the available information.
Measures of treatment effect
We reported mean differences (MDs) for continuous outcome measures and risk ratios (RRs) for dichotomous outcomes.
Unit of analysis issues
We included data from one eye, from each eye individually, or the average of both eyes, and pooled the results, regardless of how the data were analyzed. We sought advice from the Cochrane Eyes and Vision Group editorial base for analysis issues involving included trials with multiple treatment groups, cross-over design and cluster randomized designs.
Dealing with missing data
We contacted the authors of trial reports for any missing data. When we did not receive a response within eight weeks, we analyzed the studies based on available information. We will include any new information in future updates of the review.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test and I2 statistic. We considered a P value less than 0.05 as significant for the test of heterogeneity. We assessed the inconsistency of effect estimates across studies using the I2 statistic.
Assessment of reporting biases
We assessed reporting biases based on communications with trial authors regarding any outcomes assessed but not reported.
Data synthesis
If the I2 statistic was greater than 50%, (indicating a substantial degree of heterogeneity), we did not combine the study results in a meta-analysis; instead, we presented a tabulated summary. We examined the funnel plot for other sources of variation between studies when more than three studies were included in the analysis. When there was no substantial statistical or clinical heterogeneity we combined the results of included trials. We used a fixed-effect model for meta-analyses including three or fewer studies and a random-effects model for meta-analyses including four or more studies. We calculated the MD with 95% confidence intervals (CIs) for continuous outcomes. Change-from-baseline outcomes were combined in meta-analyses with studies reporting mean outcomes at annual measurement time points using the generic inverse variance (unstandardized) MD method as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses for types of intervention modalities (i.e. bifocals, progressive addition lenses (PALs) and specific pharmaceutical agents). In the future, if sufficient evidence becomes available, we will also conduct subgroup analyses according to age, degree of myopia at baseline and type of contact lens (soft versus hard).
Sensitivity analysis
We conducted a sensitivity analysis for meta-analyses in which more than three studies were included and when change-from-baseline outcomes were combined in analysis with mean outcomes at annual measurement time points. We combined studies using autorefraction in analysis with subjective refraction or when the analyses included the Cheng 2010 study. In the future, if sufficient evidence becomes available, we will conduct sensitivity analyses to determine the impact of excluding studies with lower methodological quality, unpublished studies and industry-funded studies. We also conducted sensitivity analyses to determine the effect on the primary outcome of inclusion of trials in which refraction was measured after administration of cycloplegic agents.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Characteristics of included studies [ordered by study ID]
| Adler 2006 | ||
| Methods | Study design: RCT Study center: urban private optometric practice in Jerusalem, Israel Number randomized: 62 children Study follow-up: 18 months Exclusions and losses to follow-up: 5 (8%) children who were randomized were excluded from the analyses; 9 (14.5%) were lost to follow-up |
|
| Participants | Age: mean = 10.08 years (range 6 to 15 years) Gender: 34 boys, 14 girls Culture: most children were orthodox Jews who attended school year round and performed a study method of swaying back and forth while learning and reading Inclusion criteria: pediatric patients from study center with early-onset myopia aged 6 to 15 years Exclusion criteria: 1) strabismus; 2) amblyopia; 3) VA < 6/9; 4) spherical equivalent > −6.00 D or < −0.50 D in either eye; 5) astigmatism over 1.50 D in either eye; 6) anisometropia over 1.50 D; 7) a difference between objective and subjective refraction findings of 0.75 D or more; 8) any ocular pathological manifestations; and 9) born prematurely |
|
| Interventions | Undercorrected group (n = 25): blurred by +0.50 D; glasses were to be worn continuously Fully-corrected group (n = 23): glasses were to be worn continuously Note: changes in prescription were made if the subjective refraction had changed by at least 0.50 D for one or both eyes |
|
| Outcomes |
Progression of early-onset myopia
Unit of analysis: the average values of both eyes were used for all results |
|
| Notes | Study dates: enrolment was over an eight-month period Materials: free spectacle lenses were supplied by Einit Optical Clinic Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A coin was tossed to determine the group assignment |
| Allocation concealment (selection bias) | Low risk | The assignment for each participant was determined after enrolment by tossing a coin |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | The optometrist conducting the examination was masked to the treatment group and previous results for each participant |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Unclear risk | The analysis of the results was carried out by the other member of the team only after all the data had been collected |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data | High risk | Of the 62 children recruited, 48 are included in the analysis; 5 were excluded (3 did not wear their glasses continually, 2 were twins born prematurely) and 9 were lost to follow-up |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | |
| Aller 2006 | ||
| Methods | Study design: RCT Study center: 1 Number randomized: 78 children Study follow-up: 1 year Exclusions and losses to follow-up: not reported |
|
| Participants | Age: range 8 to 18 years Gender: included boys and girls Culture: California, US Inclusion criteria: 1) myopia between −0.50 D and −6.00 D; 2) esofixation disparity at 33 cm with distance correction; 3) astigmatism 1.00 D or less; 4) ability to wear SCLs Exclusion criteria: 1) presence of ocular disease preventing wear of contacts; 2) pregnancy or nursing; 3) use of certain medications |
|
| Interventions | (n = 38): wore BSCLs with simultaneous vision design (n = 40): wore SVSCLs |
|
| Outcomes |
Primary outcomes:
Unit of analysis: the average values of both eyes |
|
| Notes | Study dates: start date was October 2003; completed in 2006 Funding source: Vistakon Notes: study was also known as the CONTROL study; trial was registered at www.clinicaltrials.gov (NCT00214487), but has not been published as a full length, peer reviewed article Additional information: study author provided unpublished information via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were coded and randomly assigned to either single vision or bifocal contact lens groups. A matrix was used to help ensure that each group contained roughly equal mixes of sexes, ages, prescription strengths, ethnicities and degrees of esofixation disparity (via email communication with author) |
| Allocation concealment (selection bias) | Unclear risk | Contact lens prescriptions for eligible participants were transmitted to an off-site research assistant for allocation (via email communication with author) |
| Masking of participants (performance bias) | Unclear risk | The packages containing contact lenses were masked so that the participant did not know from the label what he/she was wearing; however, it was not clear whether the two types of contact lenses were different functionally. Information about whether or not the masking worked was not reported |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “Masking was aided by the choice of lenses; both were 58% water, two-week disposable lenses, identical in appearance and supplied in masked packaging” |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Unclear risk | Final sample size was reported, but it was unclear whether the same number of participants were enrolled; exclusion and loss to follow-up data were not reported |
| Selective reporting (reporting bias) | High risk | The complete results for this study are not yet available. Primary investigator’s website cited: ‘Full disclosure of the results is awaiting approval by the sponsor, but some results have already been published in abstract form’ (Aller 2010) |
| Other bias | High risk | A detailed description of the methods was lacking as the study has only been reported in abstract form. Full publications are awaiting approval by the study sponsor |
| ATOM study | ||
| Methods | Study design: RCT, with 2 week run-in period Study center: 1 Number randomized: 400 children Study follow-up: 2 years Exclusions and losses to follow-up: no exclusions; 54 (13.5%) were lost to follow-up |
|
| Participants | Age: mean = 9.2 years (range 6 to 12 years) Gender: 220 boys, 180 girls Culture: Chinese (94%) and Indian children (4%) in Singapore Inclusion criteria: 1) age 6 to 12 years old; 2) myopia with spherical equivalent refractive error between −1.00 D and −6.00 D in each eye as measured by cycloplegic autorefraction; 3) distance vision correctable to logMAR 0.2 or better in both eyes; 4) normal ocular health; 5) good general health with no history of cardiac or significant respiratory diseases; 6) normal binocular function and stereopsis; 7) willingness and ability to tolerate monocular cycloplegia and mydriasis Exclusion criteria: 1) astigmatism greater than −1.50 D by cycloplegic autorefraction; 2) IOP of 21 mmHg or greater; 3) allergies to atropine, cyclopentolate, proparacaine or benzalkonium chloride; 4) previous or current use of contact lenses, bifocals, PALs or other forms of myopia treatment; and 5) amblyopia or manifest strabismus, including intermittent tropia |
|
| Interventions | Atropine (n = 200): one eye randomized to one drop of 1% atropine sulfate nightly; the other eye received nothing Placebo-control (n = 200): one eye randomized to one drop of vehicle nightly; the other eye received nothing Note: all children received single vision photochromatic lenses for the correction of refractive errors |
|
| Outcomes |
Primary efficacy outcome: progression of myopia defined as the change in spherical equivalent refractive error from baseline and measured by cycloplegic autorefraction Secondary efficacy outcome: change in axial length from baseline and measured by A-scan ultrasonography Primary safety outcome: occurrence of adverse events Secondary safety outcomes: best-corrected VA, IOP, slit-lamp biomicroscopy and fundus examination Measurements taken at baseline and annually for 2 years Note: baseline measurements were recorded 2 weeks after treatment began to allow for stabilization of the cycloplegic effect of atropine Unit of analysis: only one eye per patient was randomized to receive treatment (fellow eyes were controls) |
|
| Notes | Study dates: enrolment between April 1999 to September 2000 Materials: vehicle drops prepared by Acon Laboratories; spectacles were SOLA Transitions SVLs Funding source: National Medical Research Council, Singapore Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were allocated to groups based on a computer-generated randomization list |
| Allocation concealment (selection bias) | Low risk | Methods sectionstated “allocatedwith concealment” |
| Masking of participants (performance bias) | Low risk | Study was placebo-controlled and identical appearing bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Use of identical appearing bottles with coded labels, dilation of both pupils before examination |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Unclear risk | “All statistical analyses were based on the intention-to-treat principle” Study authors noted (via personal communication) that there was a typographical error in the publication (54 were lost to follow-up, 34 from the atropine group and 20 from the placebo group), the paper reports that those who did not complete the study were characteristically similar to those that completed the study for each group |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | There was pre-randomization administration of the intervention that may have enhanced or diminished the effect of the intervention during the subsequent, randomized evaluation period |
| Cheng 2010 | ||
| Methods | Study design: RCT Study center: 1 (optometric practice in Mississauga, Ontario, Canada) Number randomized: 150 children Study follow-up: 2 years Exclusions and losses to follow-up: 15 (10%) children who were randomized were excluded from the analyses; 4 (3%) were lost to follow-up |
|
| Participants | Age: mean = 10 years (range 8 to13 years) Gender: 62 boys and 73 girls received treatment Culture: Chinese Canadian children were recruited by reviewing clinical records and mailing invitation letters addressed to their parents, by responding to poster in the practice, or during regular eye examinations Inclusion criteria: 1) Chinese Canadian children who were seen at the practice in the last 9 to 18 months; 2) age 8 to 13 years old; 3) myopia between −1.00 D to −5.50 D; 4) myopia progression equal to or greater than 0.50 D in the preceding year; 5) distance monocular visual acuity of 6/6 or better; 6) near monocular visual acuity of 6/6 or better; 7) stereoacuity of ≤ 40 s of arc at 40 cm; 8) single-vision distance lens wear; and 9) consent of child and parent for study participation Exclusion criteria: 1) astigmatism > 1.50 D; 2) anisometropia > 1.50 D; 3) strabismus; 4) unable to respond to subjective testing; 5) history of systemic or ocular diseases; and 6) history of bifocal lens wear and/or contact lens use |
|
| Interventions | SVLs (n = 50): single-vision distance lenses Bifocal lenses (n = 50): bifocal lenses with +1.50 D near addition Prismatic bifocal lenses (n = 50): prismatic bifocal lenses with +1.50 D addition and a 3-prism diopters base-in prism in the near segment Note: distance prescription changes were made if subjective refraction changed by 0.50 Dor more in either eye |
|
| Outcomes |
Primary outcome: myopic progression defined as the difference between the mean cycloplegic spherical equivalent measured by an automated refractor at the baseline visit and subsequent 6-month visits for 24 months Secondary outcome: eye growth defined as the difference between mean axial lengths measured by ultrasonography at the baseline visit and subsequent 6-month visits for 24 months Measurements taken at baseline and every 6-months for two years Unit of analysis: only data from right eyes were used |
|
| Notes | Study dates: April 2003 to April 2008 Funding source: Essilor International of France Auxiliary data: Parents and/or guardians completed questionnaires relating to vision habits of the enrolled child and the child’s birth parents’ refractive errors. The number of years the children were myopic prior to entering the study were estimated from clinical records. Auxiliary data were used as covariates for regression statistics and to test the hypothesis that bifocal treatment is more effective with a shorter duration of myopia Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Randomization was implemented by putting the subjects’ file numbers on slips of paper and drawing them from a container at random…The first 50 subjects drawn were assigned to the control group; the second 50 were assigned to the bifocal group, and so forth” |
| Allocation concealment (selection bias) | High risk | “The first 50 subjects drawn were assigned to the control group; the second 50 were assigned to the bifocal group, and so forth” |
| Masking of participants (performance bias) | High risk | “The subjects and the investigator were aware of the treatment assignments.” Masking was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
High risk | “The subjects and the investigator were aware of the treatment assignments. Masking was difficult to achieve in a practice-based intervention, particularly when the lens treatments were visually very different.” The primary study investigator dispensed lenses and performed examinations |
| Masking of outcome assessors (detection bias) Secondary outcomes |
High risk | “…the primary and secondary outcome variables were measured by objective methods to minimize possible bias of the unmasked investigator” |
| Masking of data analyzers | Low risk | “The data analyst discerned the study investigated the effect of three types of lenses on ocular refraction, but he was masked to the possible effect of bifocal or prismatic bifocal lens on myopia control” (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | “The analysis of the data followed the intention-to-treat approach, and we used the last progression information (i.e. carry forward) method for subjects lost to follow-up” Although study authors stated that they used intention-to-treat analysis, 15 of the 150 children randomized were not included in the analysis: 9 children randomized to single vision lenses dropped out because their parents wanted them to receive bifocals; and 2 children in the bifocals group and 4 in the prismatic bifocals group were excluded due to adverse reactions following cycloplegia |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | This study was funded by a company that produces the types of lenses being investigated |
| Chung 2002 | ||
| Methods | Study design: RCT Study center: patient care unit at the Department of Optometry, Faculty of Allied Health Science, National University of Malaysia Number randomized: 106 children Study follow-up: 2 years Exclusions and losses to follow-up: no exclusions; 12 (11%) were lost to follow-up |
|
| Participants | Age: mean =11.56 years (range 9 to 14 years) Gender: 39 boys, 55 girls Culture: Malay and Chinese ethnic origin Inclusion criteria: 1) age 9 to 14 years old; 2) myopia with spherical equivalent refractive error of −0.50 D or more in both eyes, with no principle meridian being plano or having any amount of plus power; 3) corrected VA of 6/6 or better in each eye; 4) normal ocular health; and 5) willingness to give written consent Exclusion criteria: 1) more than two diopters of astigmatism in each eye; 2) binocular vision problems, including anisometropia over 2.00 D, problems requiring refractive therapy, strabismus and amblyopia; 3) previous contact lens wear; 4) family was planning to leave the area before the end of the study period |
|
| Interventions | Undercorrected group (n = 47): monocular VA blurred to 6/12 (approximately +0.75 D) in each eye with spectacles Fully-corrected group (n = 47): monocular VA maintained at 6/6 or better in each eye with spectacles Note: In the fully-corrected group, changes in prescription were made if the subjective refraction had changed by at least 0.50 D for one or both eyes. For the undercorrected group, changes in prescription were made to maintain a vision of 6/12 in each eye |
|
| Outcomes |
Progression of early-onset myopia
Measurements taken at baseline and every 6 months for two years Unit of analysis: the average values of both eyes were used for all results |
|
| Notes | Study dates: not reported Funding source: IRPA grant Compliance in wearing glasses was monitored with questionnaires. Compliance was defined as wearing glasses for at least 8 hours a day (40 patients in the undercorrected group versus 41 in the fully-corrected group). Partial compliance was defined as wearing glasses 6 to 8 hours a day (7 patients in the undercorrected group versus 6 in the fully-corrected group) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization based on age, sex, race and refractive error. Treatment and control pairings were made to complete cells based on 3 age categories, 4 refractive error categories, 2 racial groups and 2 gender groups (3 × 4 × 2 × 2 = 48 cells) Patients were designated as subject 1 or subject 2 for each cell based on a predetermined randomization procedure |
| Allocation concealment (selection bias) | Low risk | Once the patients were paired, a coin toss determined which patient was assigned to the treatment or control group. Heads meant subject 1 was allocated to undercorrection and subject 2 received full correction. Tails meant subject 1 was allocated to full correction and subject 2 received undercorrection. A coin toss was performed for each pair |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to performance differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | During all evaluations, the examining optometrist was not aware of the group assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Results were only analyzed after the last reading of the last patient was collected |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | Of the 106 children recruited, 94 completed the study and were included in the analyses; 12 (11.3%) dropped out and were excluded from the analyses |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | |
| CLAMP Study | ||
| Methods | Study design: RCT, with run-in period Study center: 1 (The Ohio State University College of Optometry) Number randomized: 116 children Study follow-up: 3 years Exclusions and losses to follow-up: none |
|
| Participants | Age: mean = 10.7 years (range 8 to 12 years) Gender: 47 boys, 69 girls Culture: Columbus, Ohio, US; 84.5% white (not of Hispanic origin), 8.6% Asian or Pacific Islander, 4.3% Black (not of Hispanic origin) Inclusion criteria: 1) age 8 to 11 years old at time of randomization; 2) myopia with spherical equivalent refractive error between −0.75 D and −4.00 D in each eye, as measured by cycloplegic refraction; 3) corrected VA of 20/20 or better in each eye Exclusion criteria: 1) astigmatism > 1.50 DC in each eye by cycloplegic refraction or > 1.00 DC on manifest refraction; 2) previous or attempted history of contact lens wear; 3) anisometropia > 1.00 D between eyes; 4) eye disease and binocular vision problems; 5) systemic disease that may affect vision or vision development Note: All participants had to successfully complete a run-in period before enrolment into the study in order to exclude those who could not adapt to rigid contact lenses; 32 children did not complete the run-in period and were excluded. Success for the runin period was defined as wearing the lenses at least 40 hours/week and stating that the lenses were “always comfortable” or “usually comfortable” |
|
| Interventions | (n = 59): RGPCLs worn during waking hours for 3 years (n = 57): SCLs worn during waking hours for 3 years Note: prescription changes were made by an unmasked examiner based on participant complaints and improvement in visual acuity |
|
| Outcomes |
Primary outcome: change in cycloplegic autorefraction during 3 years (spherical equivalent) Secondary outcomes:
Measurements taken at baseline and every 6 months for three years Unit of analysis: data analyzed for right eye only |
|
| Notes | Study dates: enrolment 8 July 1998 to 26 February 2000 Funding source: National Eye Institute, National Institutes of Health; Menicon Co, Ltd.; CIBA Vision Corporation; SOLA Optical; and Essilor |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized participants stratified by gender and in treatment blocks of 3. A list of randomized treatment assignments was prepared by an independent person before the beginning of the study |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments from the list were placed in sequentially numbered envelopes that were sealed. Envelopes were drawn from the pool in sequential order according to the participant’s gender |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to material differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Masked examiners conducted the primary outcome procedure and all secondary outcomes except visual acuity. When the masked examiner was in the room, the participants wore only spectacle correction or no correction and was told not to mention any contact lens wear to the masked examiner. No assessment of masking was reported |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | The outcome measures were performed by examiners masked to the mode of correction worn by the participant with the exception of the visual acuity measurements |
| Masking of data analyzers | Unclear risk | Not reported in the paper, but the persons conducting the analyses were not masked to treatment group allocation (JW). Outcome measures were not presented by treatment group until the conclusion of the trial. Data was managed using a dual-entry format |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | All data were analyzed according to the original result of the random assignment and there were no missing data. “We analyzed all data using intention-to-treat methods” |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the study protocol were reported |
| Other bias | Unclear risk | There was pre-randomization administration of the intervention that may have enhanced or diminished the effect of the intervention during the subsequent, randomized evaluation period The study was partially funded by companies that produce the interventions being investigated |
| COMET Study | ||
| Methods | Study design: RCT Study center: multicenter, including (1) a study chair, (2) a coordinating center, (3) four clinical centers and (4) the National Eye Institute, US Number randomized: 469 children Study follow-up: 3 years Exclusions and losses to follow-up: no exclusions; 7 (1.5%) were lost to follow-up |
|
| Participants | Age: mean = 9.3 years (range 6 to 11 years) Gender: 223 boys, 246 girls Culture: four major cities in the US (Birmingham, Alabama: n = 133; Boston, Massachusetts: n =110; Philadelphia, Pennsylvania: n = 108; and Houston, Texas: n = 118) Inclusion criteria: 1) age 6 to 11 years old; 2) myopia with spherical equivalent refractive error between −1.25 D and −4.50 D in both eyes, as measured by cycloplegic autorefraction; 3) astigmatism ≤ 1.50 D; 4) no anisometropia (difference in spherical equivalent < 1.00 D between eyes); 5) best corrected VA of 20/32 or better; 6) no strabismus by cover test for far (4.0 m) and/or near (0.33 m) fixation; 7) willingness to not wear contact lenses for study duration Exclusion criteria: 1) strabismus detected by cover test; 2) any ocular, systemic, or neurodevelopmental conditions that could influence refractive development; 3) chronic medication use that might affect myopia progression or visual acuity; 4) birthweight < 1250g; 5) previous use of bifocals, PALs, or contact lenses; 6) problems with adherence to the protocol or follow-up period |
|
| Interventions | PAL group (n = 235): multifocal lenses (no-line bifocals) with gradual and progressive change toward less negative or more positive power from the distance portion to the near portion of the lens (power +2.00 D); worn during waking hours for 3 years SVL (n = 234): single vision lenses with same focal power throughout the lens area; worn during waking hours for 3 years Note: Prescription changes were made if the subjective refraction had changed by at least 0.50 D for one or both eyes. Smaller prescription changes were made if clinically indicated. Both groups were offered single vision sports glasses to use while participating in sports activities |
|
| Outcomes |
Primary outcome: change in refractive error Magnitude of change in spherical equivalent refractive error relative to baseline measured by cycloplegic autorefraction with 2 drops of 1% tropicamide Secondary outcomes:
Unit of analysis: child-based The average values of both eyes were used if the correlation coefficient was > 0.85 between eyes and the mean difference was not statistically significant; otherwise the eye with more myopic change was used for each child |
|
| Notes | Study dates: enrolment was from September 1997 to September 1998; follow-up was designed for 3 years, but continued for 7 years, including 5 years wearing original lens assignments and 2 years wearing either glasses or contact lenses Funding source: NEI grants, Essilor of America, Marchon Eyewear, Marco Technologies and Welch Allyn Sample of 150 children were followed up at 1 month to evaluate possible lens-induced phoria changes; no problems were detected in either group Compliance in wearing glasses was monitored with separate questionnaires for children and parents (93% compliance in PAL group, 96% compliance in SVL group). Attitude towards wearing glasses and self-esteem were also measured Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization derived by permuted block design with preset block size and stratified by clinical center by the coordinating center |
| Allocation concealment (selection bias) | Low risk | Randomization assignments were allocated by the coordinating center after the eligibility of each participant was verified |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Optometrists responsible for assessing study outcomes were unaware of the lens assignments |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | “The data analysts were not masked to treatment assignment when analyzing the data” (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | “Follow-up data were analyzed by applying an intention-to-treat principle according to the child’s original lens assignment and the last known value of the outcome measures. For the seven children lost to follow-up and thus without data at the third annual visit, progression information from the latest follow-up visit was used” |
| Selective reporting (reporting bias) | Low risk | All outcomes published a priori in the design paper (Hyman 2001) were reported in results papers |
| Other bias | Unclear risk | The study was partially funded by companies that produce the interventions being investigated |
| Edwards 2002 | ||
| Methods | Study design: RCT Study center: 1 (Centre for Myopia Research) Number randomized: 298 children Study follow-up: 2 years Exclusions and losses to follow-up: no exclusions; 44 (15%) were lost to follow-up |
|
| Participants | Age: mean = 9.09 years (range 7 to 10.5 years) Gender: 122 boys, 132 girls Culture: Hong Kong children, recruited through newspaper advertisements Inclusion criteria: 1) age 7 to 10.5 years old; 2) spherical equivalent refractive error between −1.25 D and −4.50 D, as measured under cycloplegia; 3) best corrected VA of 0.00 logMAR or better; 4) no previous use of contact lenses and willingness to not wear contact lenses; 5) willingness to wear glasses constantly; 6) parents acceptance of randomization Exclusion criteria: 1) astigmatism > 1.50 D; 2) anisometropia > 1.50 D in spherical or cylindrical error; 3) any ocular or systemic condition that might affect refractive development; 4) previous use of bifocals or PALs; 5) problems with adherence to the protocol or follow-up period |
|
| Interventions | PAL group (n =138): SOLA MC progressive lenses (add +1.50 D); worn constantly for 2 years SVL (n = 160): SOLA single vision lenses; worn constantly for 2 years Note: prescription changes were made if there was a reduction in aided vision of ≥ 0.10 logMAR units |
|
| Outcomes |
Primary outcomes
Unit of analysis: only data from the right eyes are reported |
|
| Notes | Materials: Lenses provided by Sola (Hong Kong) Ltd Funding source: Centre for Myopia Research (Area of Strategic Development), The Hong Kong Polytechnic University |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined random sequence |
| Allocation concealment (selection bias) | Low risk | The investigator was not aware of group allocation until child was enrolled in study |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | The investigator measuring refractive error was masked to treatment assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | The investigator measuring axial length was masked to treatment assignment |
| Masking of data analyzers | Low risk | The masked and unmasked investigators independently analyzed the data |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | There were no exclusions after randomization. There were 44 patients lost to follow-up: n = 17 in PAL group; n = 27 in SVL group. It was reported that whether or not a patient was retained in the study was not statistically associated with treatment allocation |
| Selective reporting (reporting bias) | Low risk | The results for study outcomes were reported at 2-year follow-up |
| Other bias | Low risk | |
| Fulk 1996 | ||
| Methods | Study design: RCT Study center: 1 (Indian Health Service Hospital, Optometry Department, Tahlequah, Oklahoma, USA) Number randomized: 32 children Study follow-up: 18 months Exclusions and losses to follow-up: no exclusions; 4 (12.5%) were lost to follow-up |
|
| Participants | Age: range 6 to 13 years Gender: included boys and girls (numbers not reported) Culture: children with myopia and nearpoint esophoria identified from medical records and referred by local optometrists Inclusion criteria: 1) at least 0.50 D of myopia in both principal meridians of both eyes; 2) ages 6 to 13.99 years for boys and 6 to 12.99 years for girls; 3) nearpoint esophoria; 4) corrected acuity of at least 20/25 in each eye, distance and near, with SVLs; 5) ability to respond to subjective tests Exclusion criteria: 1) strabismus; 2) astigmatism greater than 2.00 D in either eye; 3) anisometropia greater than 2 D; 4) convergence insufficiency accompanied by symptoms; 5) diabetes or other systemic disease with potential effects on refractive error; 6) ocular disease other than mild inflammation of the adnexa |
|
| Interventions | Bifocals (n = 16): bifocal lenses with +1.25 D addition SVLs (n= 16): single vision lenses Note: prescription changes were made if the spherical equivalent in either eye had changed by 0.50 D |
|
| Outcomes |
Primary outcomes:
Unit of analysis: the average values of both eyes |
|
| Notes | Funding source: Northeastern State University Faculty Research Committee (Tahlequah, Oklahoma, USA) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization process was used with reference to Zelen |
| Allocation concealment (selection bias) | Low risk | “The optician kept envelopes containing the assignment and fitted the appropriate glasses at the end of the base-line examination” |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “A research assistant who did not know what type of glasses the child wore, measured…” However, the success of masking of examiner was not addressed in paper |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | 32 participants enrolled; 4 dropped out; 2 from each treatment group; does not address reason for drop-outs. Analysis is for the 28 remaining participants, so not “intention to treat” in terms of including all enrolled participants for analysis at end; however, equal drop-outs in each arm and patients were analyzed by group they were randomized. It was not stated whether all 28 participants completed all 3 follow-up visits, although it was stated that they completed the study |
| Selective reporting (reporting bias) | Unclear risk | Refractive error outcome was reported. Axial length outcome (in mm) was plotted against myopia progression in D, but mean values by treatment groups were not given |
| Other bias | Low risk | |
| Fulk 2002 | ||
| Methods | Study design: RCT and study of variables that may influence myopia progression in children Study centers: 2 (Tahlequah and Tulsa, Oklahoma, USA) Number randomized: 82 children Study follow-up: 30 months Exclusions and losses to follow-up: no exclusions; 7 (8.5%) were lost to follow-up |
|
| Participants | Age: mean = 10.7 years (range 6 to 12 years) Gender: 43 boys and 39 girls Culture: children with myopia and nearpoint esophoria recruited locally and through clinics operated by the Cherokee Nation: 58% Caucasian, 29% American Indian, 5% Hispanic, 4% African-American, 3% other and 1% Asian/Pacific Islander Inclusion criteria: 1) at least 0.50 D of myopia in both principal meridians of both eyes; 2) ages 6 to 12.99 years for boys and 6 to 11.99 years for girls; 3) nearpoint esophoria; 4) corrected VA of at least 20/25 in each eye at distance and binocularly with SVLs; 5) corrected stereoacuity of at least 40 sec arc with SVLs at 40 cm; 6) assent of child and consent to participate Exclusion criteria: 1) strabismus; 2) astigmatism or anisometropia greater than 2.00 D; 3) diabetes or other systemic disease with potential effects on refractive error; 4) ocular disease other than mild inflammation of the adnexa; 5) known history of allergic reaction to proparacaine or tropicamide; 6) history of use of RGPs; 7) current use of bifocals or use within the last year; 8) high myopia of −6.00 D or more for children younger than 9 year or −8.00 D or more for children 9 years or older; 9) inability to respond to subjective testing or hold fixation sufficiently to allow for study measurements |
|
| Interventions | Bifocals (n = 42): bifocal lenses with +1.50 D add SVLs (n = 40): single vision lenses Note: prescription changes were made if 1) the spherical equivalent in either eye had changed by 0.50 D or 2) any combination of sphere or cylinder change could improve the distance acuity by three letters or more in either eye |
|
| Outcomes |
Primary outcome: change in refractive error (spherical equivalent) (cycloplegic autorefraction) Secondary outcomes:
Measurements taken at baseline and every 6 months for 30 months Unit of analysis: the average values of both eyes |
|
| Notes | Study dates: enrolment between August 20 and October 15, 1996; original follow-up was for 30 months, some children remained for 54 months Funding source: National Eye Institute, National Institutes of Health Notes: study was also know as the Myopia Progression Study |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized permuted block design, with separate number sequences for each of the 2 sites stratified by gender to assign participants in approximately equal allocation to the 2 treatments |
| Allocation concealment (selection bias) | Low risk | “Sealed envelopes containing the treatment assignments were maintained at each site and opened by the optician after a subject was enrolled” |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “A research assistant who did not know what type of glasses the child wore, measured…” However, the success of masking of examiner was not addressed in paper |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | “This is an intention-to-treat analysis, with all subjects being classified according to their original treatment assignment — disregarding the fact that many discontinued that mode of correction during the last year” Seven participants did not complete the study: 6 of 42 randomized to bifocals (2 died, 1 drowned and 1 died in auto accident; 4 were “unwilling”) and 1 of 40 randomized to SVL (participant moved). “In a secondary analysis, estimates of myopia progression were imputed for children who did not complete the study; each subject who left the study prematurely was assumed to have myopia progression equal to that of mean progression observed in the SVL group for the time period for which their data were missing.” This weakened the treatment effect |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported a priori |
| Other bias | Low risk | |
| Hasebe 2008 | ||
| Methods | Study design: randomized cross-over trial Study center: 1 (Okayama University Medical School) Number randomized: 92 children Study follow-up: 3 years Exclusions and losses to follow-up: no exclusions; 6 (6.5%) were lost to follow-up |
|
| Participants | Age: mean = 9.85 years (range 6 to 12 years) Gender: 47 boys, 45 girls Culture: Okayama, Japan Inclusion criteria: 1) age 6 to 12 years old; 2) spherical equivalent refractive error between −1.25 D and −6.00 D in both eyes, as measured by noncycloplegic autorefraction; 3) best corrected VA of 20/20 or better in each eye; 4) no other eye disease; 5) experience wearing spectacles; 6) willingness to wear glasses constantly and attend follow-up visits; 7) acceptance of randomization Exclusion criteria: 1) astigmatism > 1.50 D in both eyes; 2) anisometropia > 1.50 D; 3) manifest strabismus; 4) birthweight < 1250 g; 5) heterotropia or severe ophthalmic disease that may affect refractive development; 6) previous use of PALs or contact lenses |
|
| Interventions | PALs (n = 46): 18 months wearing PALs (add +1.50 D), followed by 18 months wearing SVLs SVLs (n = 46): 18 months wearing SVLs, followed by 18 months wearing PALs (addition + 1.50 D) Note: prescription changes were made if corrected distance visual acuity was less than 20/30 in at least one eye |
|
| Outcomes |
Primary outcome: progression of myopia measured by cycloplegic autorefraction Secondary outcomes:
Measurements taken at baseline and every 6 months for three years Unit of analysis: child-based (mean of both eyes or right eye only) |
|
| Notes | Study dates: enrolled July 2002 to June 2003 Funding source: Japanese Ministry of Education, Culture, Sports, Science and Technology and Megane Tanaka Chain, Ltd |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated to Group 1 or Group 2 by drawing lots. “Participants drew lots (number of 1–80 was described in each card) at initial inspection and participant number was randomly decided” (Hasebe 2002) |
| Allocation concealment (selection bias) | Low risk | Physicians conducting examinations did not know the allocation. Participants drew lots from numbered cards, then the principal investigator and three opticians determined allocation based on the number drawn (Hasebe 2002) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | The examiners collecting data or prescribing spectacles were masked to lens assignment |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Low risk | Methods paper stated that the statistician was masked to the lens assignments (Hasebe 2002) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | “Only six children, two in group 1 and four in group 2, failed to return for the final visit. The reasons for being lost to follow-up or excluded from the analysis included a problem in using cycloplegic eye drops (two children), moving to another prefecture (two children), desire to wear contact lenses (one child), or the occurrence of exotropia (one child)” |
| Selective reporting (reporting bias) | Low risk | All outcomes published a priori in the design paper were reported in results papers |
| Other bias | Unclear risk | Design-specific risk of bias: cross-over trial. Four children dropped out during the second study period The study was partially funded by a company that produces the type of lenses being investigated |
| Houston Study | ||
| Methods | Study design: RCT Study center: 1 (University of Houston, Texas, USA) Number randomized: 207 children Study follow-up: 3 years Exclusions and losses to follow-up: 83 (40%) children were excluded from or dropped out of the study |
|
| Participants | Age: range 6 to 15 years Gender: 58 boys and 66 girls completed the study Culture: children were recruited from patients, from family members of faculty and staff and from the racially diverse Houston community Inclusion criteria: 1) myopia of −0.25 D in one or both eyes; 2) ages 6 to 15 years; 3) best corrected VA of 20/20 or 20/15; 4) normal ocular health; and 5) able to provide informed consent Exclusion criteria: 1) strabismus or amblyopia; 2) contact lens wearers; 3) astigmatism of 2.00 D or more; and 4) particularly high or low gradient AC/A ratios |
|
| Interventions | Bifocals 1: bifocal lenses with +1.00 D addition Bifocals 2: bifocal lenses with +2.00 D addition SVLs Note: prescription changes were made if 1) there was a change of spherical power of 0.50 D or more in one or both eyes or 2) there was an improvement of one line of visual acuity |
|
| Outcomes |
Patient care team outcomes (unmasked):
Unit of analysis: data from the right eyes |
|
| Notes | Study dates: “Subjects were admitted to the study over a period of 20 months, in five ‘accrual groups.’ The first group of subjects entered the study in February, 1981 and completed the study in February, 1984, whereas the last group of subjects entered the study in October, 1982,” and completed the study in October, 1985 Materials: bifocals were executive one-piece lenses in CR-39 plastic (American Optical Corporation); SVLs were polycarbonate lenses (Gentex Corporation) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Placed in treatment group “on basis of a table of random numbers” using block randomization technique |
| Allocation concealment (selection bias) | Low risk | “Neither the investigator nor the subject know the treatment to be assigned at the time the subject is registered for the study” |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
High risk | This study involved a team of masked observers (Evaluation team) and a team of unmasked observers (Patient care team) The results presented in the final analysis are from the unmasked group |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Evaluation team members collecting this data were masked |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | Patients were dismissed for “noncompliance” and some were fitted with contact lenses without letting study personnel know, so they were also dropped. Also, participants who did not return and those who moved were dropped. Out of 207 enrolled 83 (40% dropped out). It was not clear from which treatment groups the drops-outs came. Incomplete data as only 60% remained in study |
| Selective reporting (reporting bias) | High risk | Results were not reported for the evaluation team measurements or for other secondary outcomes outlined in the design paper. The methods paper stated that an evaluation team report would be based on 1) cycloplegicretinoscopy, 2) non-cycloplegic autorefraction and 3) cycloplegic autorefraction performed by masked examiners. However, these were never reported in outcome paper. Also secondary outcomes, including axial length, were never reported in outcome paper |
| Other bias | High risk | One participant was allowed to wear contact lenses when playing basketball Primary outcome measure was not under cycloplegic conditions, was an unmasked subjective refraction, and sometimes student examiners were used |
| Jensen 1991 | ||
| Methods | Study design: RCT Study center: 1 (Odense University Hospital, Denmark) Number randomized: 159 children Study follow-up: 2 years Exclusions and losses to follow-up: 4 (2.5%) children who were randomized were excluded from the analyses; 16 (10%) were lost to follow-up |
|
| Participants | Age: mean = 10.9 years Gender: 87 boys, 72 girls Culture: Medical records of children from schools in Odense, Denmark were screened for myopia; n = 8769. Possible cases of myopia underwent a primary examination; n = 1216. Myopic children with at least −1.0 D in either eye, and in 2nd to 5th grades were examined at the eye clinic; n = 361. Children meeting inclusion/exclusion criteria at the eye exam were mailed invitations to participate in the trial; n = 227 Inclusion criteria: 1) in 2nd to 5th grades at screening; 2) myopia with spherical equivalent refractive error between −1.25 D and −6.00 D in both eyes; 3) normal corrected vision; 4) Danish parents; and 5) affirmative response to mailed invitation for study Exclusion criteria: 1) unilateral myopia; 2) eye disease or general illness, especially heart/lung disease; and 3) experience in pilot study |
|
| Interventions | Bifocals (n = 57): constant wear of bifocals with +2.0 D addition to upper edge of reading segment Timolol (n = 51): one drop of 0.25% timolol maleate in each eye twice daily and constant wear of SVLs for corrected visual acuity ≥ 0.8 Control (n = 51): constant wear of SVLs for corrected visual acuity ≥ 0.8 Note: participants were permitted to wear their own SVLs if corrected visual acuity was >0.8 |
|
| Outcomes |
Primary outcomes:
Measurements taken at baseline and every 6 months for two years Unit of analysis: right eyes and left eyes analyzed separately |
|
| Notes | Study dates: screening January to April 1983; eye clinic exams October 1984 to April 1985 Notes: the children who chose not to participate in the study (n = 44) did not statistically differ from those examined with regard to age and degree of myopia |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization based on age, sex and refractive error. Intervention and control groups were made by completing cells based on 3 age categories, 3 refractive error categories and 2 gender groups (3 × 3 × 2 = 18 cells). Participants were assigned to each cell after baseline examinations |
| Allocation concealment (selection bias) | Low risk | For each cell the children in groups of 3 were allocated to study groups by drawing numbers 1 to 6 for the first assignment The second and third assignments were dependent on the first assignment |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
High risk | Masking was not reported, but there was only one study investigator |
| Masking of outcome assessors (detection bias) Secondary outcomes |
High risk | Primary and secondary outcomes were assessed by the same examiner |
| Masking of data analyzers | Unclear risk | Data were analyzed by the study investigator |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | There were 159 children enrolled: 51 SVLs, 57 bifocals, 51 timolol. At the first year visit 13 (8%) children were excluded or lost to follow-up: one patient given contact lenses was excluded and one patient dropped out from the control group; six patients dropped out from the bifocal group (3 because they could not adapt to the bifocals); and two patients were excluded and three dropped out from the timolol group. At the second year visit an additional seven (4.5%) children were excluded or lost to follow-up: one patient given contact lenses was excluded from the control group; three patients dropped out from the bifocal group because they could not adapt to the bifocals; and three dropped out from the timolol group |
| Selective reporting (reporting bias) | High risk | The methods section of the article described that results would be discussed only if exceptional |
| Other bias | Low risk | |
| Katz 2003 | ||
| Methods | Study design: RCT, with 3 month adaptation period Study center: 1 (Myopia Clinic of the Singapore Eye Research Institute) Number randomized: 564 children (428 children attended initial visit; 383 children completed the adaptation period) Study follow-up: 2 years Exclusions and losses to follow-up: 136 (24%) children who were randomized did not attend the initial visit and 45 (8%) more did not complete the adaptation period; 86 (22%) of the 383 children who completed the adaptation period were lost to follow-up |
|
| Participants | Age: mean = 8.3 years (range 6 to 12 years) Gender: 204 boys, 179 girls Culture: Singaporean children with Chinese ethnicity Inclusion criteria: 1) age 6 to 12 years old; 2) myopia with spherical equivalent refractive error between −1.0 D and −4.0 D; 3) Chinese ethnicity; and 4) provided informed consent Exclusion criteria: 1) astigmatism > 2.0 D; 2) previous contact lens wear; 3) other ocular pathologies Note: all participants were provided a three-month period to adapt to assigned intervention |
|
| Interventions | Contact lenses (n = 158): RGPCLs worn daily for at least 8 hours per day Spectacles (n = 225): SVLs worn daily for at least 8 hours per day Note: prescription changes were made if corrected visual acuity fell below 20/40 |
|
| Outcomes |
Primary outcome: change in refractive error (spherical equivalent) Measured by subjective cycloplegic refraction from post-adaption through 2 years of follow-up Secondary outcomes:
Unit of analysis: only data from the right eyes are reported |
|
| Notes | Materials: Asian Design Lens, Baush and Lomb, Rochester, New York, USA Adherence to treatment was measured from children and parents (agreement was almost 100%) and defined as use of contact lenses or spectacle use for at least 8 hours per day, 7 days per week Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization schedule of block size 6, generated from random number tables in Baltimore, US |
| Allocation concealment (selection bias) | Low risk | Assignments were placed in sealed envelopes with sequential patient numbers |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
High risk | Clinical observers were not masked to treatment group |
| Masking of outcome assessors (detection bias) Secondary outcomes |
High risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | The data analyst was not masked (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Unclear risk | Of the 564 children randomized, 297 (53%) completed the study. There were 86 (22%) children who completed the adaptation period who were lost to follow-up or censored from the study for not attending the final study visit. Statistical comparisons between those lost to follow-up and those who completed the study showed “a higher proportion of girls in the contact lens (59%) than spectacle group (42%) completed the study (P = 0.004)”. It was also reported that “axial length and astigmatism were similar between the treatment groups that completed the study, but the contact lens group that completed the study had 0.3 diopters more myopia at baseline than did those in the spectacle group that completed the study (P = 0.003) |
| Selective reporting (reporting bias) | High risk | Although all outcomes identified in the study methods were reported, it is unclear why some participants were not included in the analyses. There should be 105 RGPCL and 192 SVL wearers examined over 2 years, but only 97 RGPCL and 188 SVL wearers were included in the analyses |
| Other bias | High risk | Unequal loss to follow-up; imbalance in gender, corneal curvature and refractive error at baseline visit (controlled for in analyses); many participants lost from study before being examined for outcomes |
| MIT study | ||
| Methods | Study design: RCT Study center: 1 (National Taiwan University Hospital vision care center) Number randomized: 227 children Study follow-up: 18 months Exclusions and losses to follow-up: 39 (17%) children were excluded or lost to follow-up |
|
| Participants | Age: range 6 to 13 years Gender: 105 boys, 122 girls Culture: school children in Taiwan with an average myopia of −3.27 D Inclusion criteria: 1) age 6 to 13 years old; 2) provided informed consent; 3) willing to wear glasses; and 4) available for follow-up period Exclusion criteria: 1) tropia or amblyopia; and 2) increase of more than 2 D in any eye during the treatment period |
|
| Interventions | SVLs (n = 76): regular SVLs worn all the time and placebo drops PALs (n = 75): multi-focal lenses with the near addition part for reading and placebo drops PALs plus atropine (n = 76): 0.5% atropine instilled once a day at bedtime, in addition to PALs Note: An intervention group of atropine and SVLs was omitted from the study design since difficulty while reading due to the intervention would have induced poor compliance. Prescription changes were made for any child whose refractive error increased more than 0.75 D |
|
| Outcomes |
Primary outcome: myopic progression measured by cycloplegic autorefraction (spherical equivalent) Secondary outcomes:
Measurements taken at baseline and every 3 months over an 18 month period Unit of analysis: data from the right eyes were analyzed |
|
| Notes | Study dates: 1997 to 2000 Materials: HOYALUX3 plastic lenses were used for PALs; polycarbonate plastic lenses were used for SVLs Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification was based on age (younger than 9.5 years or not), sex (boy or girl) and myopic severity (more than −4.0 D for both eyes or not) at the baseline visit. This resulted in the total of 8 strata. Each participant was categorized into one of the strata and then randomized to receive one of the three treatments. A block size of 9 was used to balance the number of patients in the three treatment categories for each stratum (via email communication with study author) |
| Allocation concealment (selection bias) | Low risk | Group assignments were unknown to study personnel when participants were being enrolled by using coded bottles with sealed envelopes (via email communication with study author) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Ophthalmologists were masked and it would be improbable to guess the patients’ treatment by examination of the pupils because the ocular examination was performed only after giving the cycloplegic agent in each patient at each visit (via email communication with study author) |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Ophthalmologists (outcome assessors) were masked (via email communication with study author) |
| Masking of data analyzers | Low risk | Data analysts were masked (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | Attritions were reported by group: atropine, n = 10 (13%); PALs, n = 14 (19%); SVLs, n = 15 (20%). Reasons for attrition were poor follow-up, switching to contact lenses, poor compliance, myopic progression greater than 2.00 D per year (one patient from the PAL group and one from the SVL group) or loss to follow-up without any specified reason |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Low risk | |
| PIR-205 Study | ||
| Methods | Study design: RCT Study centers: 13 (US academic clinics and private practices) Number randomized: 174 children Study follow-up: 1 year (planned), plus 1 year extension Exclusions and losses to follow-up: 27 (15.5%) children who were randomized were excluded from the analyses; 2 (1%) were lost to follow-up |
|
| Participants | Age: mean = 9.9 ± 1.3 years (range 8 to 12 years) Gender: 71 boys, 103 girls Culture: children from US cities of study centers: 73% white, 7% black, 4% Asian, 12% Hispanic, 4% other Inclusion criteria: 1) age 8 to 12 years old; 2) myopia of −0.75 D to −4.00 D; 3) best corrected VA of 20/25 or better; 4) normal pupils; and 5) good general health Exclusion criteria: 1) anisometropia or astigmatism greater than 1.00 D; 2) any manifest tropia; 3) current use of either contact lenses or bifocals; 4) history of ocular surgery, trauma, or chronic ocular disease, including allergic conjunctivitis; 5) diseases requiring long-term or regular intermittent medication; 6) behavioral or neurological disorders that would interfere with the study; 7) participation in any study that involved an investigational drug within one month of enrolment; 8) intolerance or hypersensitivity to topical anesthetics, mydriatics, or components of the formulations; 9) contraindications to antimuscarinic agents; and 10) pregnancy or planned pregnancy |
|
| Interventions | Pirenzepine (n =117): 2% pirenzepine ophthalmic gel applied twice a day Control (n = 57): vehicle-placebo gel applied twice a day | |
| Outcomes |
Primary outcome: change in refractive error measured by cycloplegic autorefraction (spherical equivalent) Secondary outcome: change in axial length measured by A-scan ultrasonography Measurements taken at baseline and every 3 months for 1 year Unit of analysis: the average of both eyes |
|
| Notes | Study dates: March 1, 2000 to February 28, 2002 Funding source: Valley Forge Pharmaceuticals, Inc Notes: study also known as the Collaborative Assessment of Myopia Progression with Pirenzepine (CAMPP) study |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomized using a sponsor-prepared, computer-generated list stratified by site. Randomization was in a 2:1 ratio of pirenzepine to placebo |
| Allocation concealment (selection bias) | Low risk | “Study sites used coded lists to determine which tube of medication to administer to the next enrolled subject. The pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical. Thus study personnel had no way of knowing which tubes had which medications, same for patients” (SC) |
| Masking of participants (performance bias) | Low risk | Study was placebo-controlled and identical bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “Since the pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical, the study personnel had no way of knowing which tubes had which medications” (SC) |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Data analyzers were not masked (personal communication with biostatistician) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | Screened 277 patients and enrolled 174. Attrition was reported: 145 of 174 patients completed the trial. There were significantly more dropouts in pirenzepine arm: 26/117 (26%), compared with placebo arm: 3/57 (5%). Reasons for dropout included occurrence of adverse events, non-adherence and loss to follow-up “Additional methods were used to impute missing values due to patients who discontinued the study. In all 3 methods used (last observation carried forward, visit-to-visit extrapolation using median of respective treatment, and visit-to-visit extrapolation using median of placebo group), the treatment effect was similar to or greater than in the primary analysis method” |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were chosen a priori and all were reported |
| Other bias | Unclear risk | Some study authors were employed by the pharmaceutical company funding the study |
| Pärssinen 1989 | ||
| Methods | Study design: RCT Study center: 1 (outpatient clinic of the Central Hospital of Central Finland) Number randomized: 240 children Study follow-up: 3 years Exclusions and losses to follow-up: 1 (0.4%) child who was randomized was excluded from the analyses; and 2 (0.8%) were lost to follow-up |
|
| Participants | Age: mean = 10.9 years (range 8.8 to 12.8 years) Gender: 119 boys, 121 girls Culture: school children with suspected myopia were referred by school nurses and doctors after routine vision check-ups Inclusion criteria: 1) in 3rd to 5th grades; 2) myopia with spherical equivalent refractive error between −0.25 D and −3.0 D in both eyes and ≥ −0.50 D in the worst eye; and 3) corrected VA of 6/6 or better in both eyes Exclusion criteria: 1) astigmatism > 2.0 D; 2) anisometropia > 2.0 D; 3) manifest strabismus; 4) horizontal phorias more than −10 or +9 Δ or vertical more than 1 Δ; 5) previous use of spectacles for myopia; 6) eye disease or serious general disease; and 7) plans to move out of the area in the near future or the child not wanting to have spectacles |
|
| Interventions | Distant use (n = 80): minus lenses with full correction to be used for distant vision only; advised to read at greatest distance possible Bifocals (n = 80): clear plastic bifocal lenses with +1.75 D addition for continuous use Continuous use (n = 79): minus lenses with full correction for continuous use; advised to only remove spectacles if there was a danger of breaking them Note: prescription changes were made if corrected visual acuity fell below 20/40 |
|
| Outcomes |
Primary outcome: change in spherical equivalent (subjective cycloplegic refraction) Secondary outcomes:
Measurements taken at baseline and annually for three years Unit of analysis: right eyes and left eyes analyzed separately |
|
| Notes | Study dates: enrolment March 1983 to April 1985 Funding source: Academy of Finland Compliance was measured by questionnaires and classified as compliant, partly compliant ornoncompliant |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random, sex-stratified codes |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were sealed in envelopes |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
High risk | The ophthalmologist did not look at the group assignment before the examination, but often, for different reasons, the group was revealed. The 3-year examinations were conducted by two different ophthalmologists, one of whom did not know the group assignments |
| Masking of outcome assessors (detection bias) Secondary outcomes |
High risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Low risk | “After allocation one of the boys in the continuous use group was excluded from comparison with the other treatment groups when we found that his sister has been included previously in a different group. Two children moved from the area, and their refraction values could not be obtained” The remaining participants were analyzed by their original treatment assignments |
| Selective reporting (reporting bias) | Low risk | Results reported for outcomes described in a study design paper |
| Other bias | Low risk | |
| Sankaridurg 2010 | ||
| Methods | Study design: RCT Study center: 1 (Zhongshan Ophthalmic Center, Sun Yet Sen University China) Number randomized: 210 children Study follow-up: 12 months (the study was originally planed to be two years in duration) Exclusions and losses to follow-up at 12-month visit: two children who were randomized were excluded from the analyses; 7 (3.3%) were lost to follow-up |
|
| Participants | Age: mean = 11 years (range 6 to 16 years) Gender: 110 boys, 100 girls Culture: Chinese children in Guangzhou, China Inclusion criteria: 1) age 6 to 16 years; 2) bilaterally myopic (spherical component range from −0.75 D to −3.50 D inclusive) with astigmatism not exceeding −1.50 D and a maximum of 1.00 D of anisometropia; 3) vision correctable to 6/9.5 or better in each eye; 4) ocular findings considered to be normal; 5) willing to wear the study spectacles and adhere to the protocol schedule |
|
| Interventions | Novel spectacle lens type I (n = 50): a rotationally symmetrical design, featured a clear central aperture of 20 mm diameter, a maximum spherical equivalent of+ 1.0 D relative peripheral power achieved 25 mm from its axis Novel spectacle lens type II (n = 60): a rotationally symmetrical design, featured a clear central aperture of 14 mm diameter, a maximum spherical equivalent of+2.00 D relative peripheral power achieved 25mm from its axis Novel spectacle lens type III (n = 50): an asymmetric design, a clear central aperture extended approximately 10 mm either side of center along the horizontal meridian and a similar distance inferiorly, a positive additional peripheral power of 1.9 D 25 mm from the axis in that meridian SVLs (n = 50): a conventional, single-vision design Note: lenses were fitted to spectacle frames that ranged in eye-size from 45 mm to 55 mm with depths from 27 mm to 33 mm |
|
| Outcomes |
Primary outcome: cycloplegic autorefraction assessed with an open-field autorefractor Secondary outcomes: axial length Measurements taken at baseline, 6 months and 12 months Unit of analysis: the average of both eyes |
|
| Notes | Study dates: recruitment October 2007 to January 2009 Funding source: Australian Federal Government; Institute for Eye Research, Sydney, Australia; Vision CRC, Australia Lenses were provided by industry |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomized to a single treatment using the method of randomly permuted blocks of a constant size of 20” The website randomization.com was used |
| Allocation concealment (selection bias) | Low risk | Central allocation by web-based randomization was performed: “The four designs (3 novel and one standard) were coded A, B, C, and D and the randomization scheme generated using the website randomization.com (http://www.randomization.com).” |
| Masking of participants (performance bias) | Unclear risk | Although masking was reported for the participants, the study authors noted that the novel lenses were substantially different from SVLs. For this reason, 10 additional participants were enrolled for group allocated to lens type II |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “Access to the study randomization table was restricted to the study optical dispenser who allocated the randomization and coordinated with the laboratory for the delivery of the spectacles. The dispenser was masked to the lens design. Also, the participants and investigators were masked to the spectacle lenses used in the study” |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | “Access to the study randomization table was restricted to the study optical dispenser who allocated the randomization and coordinated with the laboratory for the delivery of the spectacles. The dispenser was masked to the lens design. Also, the participants and investigators were masked to the spectacle lenses used in the study” |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | Two children who were randomized were excluded from the analyses, and 7 (3.3 %) were lost to follow-up at 12 months (two in type I, one each in type II and control and three in type III). One child in type II withdrew due to adverse event, and one in type III lenses withdrew consent. They were not included in the analysis, and there was no re-inclusion |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Study originally was planned for two years, but stopped early at one year Lenses being investigated were provided by industry |
| Schwartz 1981 | ||
| Methods | Study design: randomized twin study Study center: 1 Number randomized: 52 children (26 twin pairs) Study follow-up: 3 years (planned), extended 6 months Exclusions and losses to follow-up: 2 (4%) children (1 twin pair) who were randomized were excluded from the study; none were lost to follow-up |
|
| Participants | Age: mean = 11.2 years (range 7 to 14 years) Gender: 26 boys (13 twin pairs) and 24 girls (12 twin pairs) completed the study Culture: pairs of monozygotic (MZ) twins identified from the Twin Registry of Eye Examinations from the Washington, D.C. area; all were Caucasian Inclusion criteria: 1) MZ twins with bilateral myopia; 2) ages 7 to 13 years; 3) shared domicile in local area; 4) good general health; 5) vision correctable to 20/20 or better; 6) third degree fusion; 7) no other significant abnormality Exclusion criteria: 1) astigmatism or anisometropia greater than 1.00 D; 2) difference in refraction between co-twins of 1.50 D or more in the more advanced eye |
|
| Interventions | Treatment group (n = 26): combined treatment of bifocal spectacles with 1.25 D addition and two drops of 1% tropicamide ophthalmic solution instilled to each eye nightly Control group (n = 26): standard spectacle correction (SVLs) Note: full cycloplegic correction in the treatment group was sometimes reduced up to 0.50 D when it did not impair vision below 20/20 |
|
| Outcomes |
Primary outcome: change in refractive error (spherical equivalent) (cycloplegic refraction) Secondary outcome: compliance to treatment regimen (child and parent interviews) Measurements taken at baseline and every 6 months for 3 years Unit of analysis: the average values of both eyes |
|
| Notes | Materials: 1% tropicamide (Mydriacyl) ophthalmic solution supplied by Alcon Laboratories Inc | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The assignment of special treatment or control status to members of each twinship was based on a strict randomization protocol” |
| Allocation concealment (selection bias) | Low risk | “Treatment or control status was randomly assigned only after the twin pair and the parents expressed willingness to accept the rigorous requirements and the desire to participate” |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | “All refractions were performed by the author, who was unaware of the treatment or control status of examinees” |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | Of 26 twin pairs enrolled, 25 pairs completed the study and one pair was excluded due to non-compliance after one year |
| Selective reporting (reporting bias) | Low risk | Outcomes chosen a priori and presented in methods and design paper |
| Other bias | Unclear risk | Unclear who reviewed the participants’ activities when they came in for follow-up and how the examiner remained masked at follow-up visits |
| Shih 1999 | ||
| Methods | Study design: RCT Study center: 1 (National Taiwan University Hospital) Number randomized: 200 children Study follow-up: 2 years Exclusions and losses to follow-up: 14 (7%) children who were randomized were excluded from the study; none were lost to follow-up |
|
| Participants | Age: mean = 9.2 years (range 6 to 13 years) Gender: included boys and girls Culture: children recruited from the vision care center at National Taiwan University Hospital Inclusion criteria: 1) age 6 to 13 years old; 2) myopia with refractive error between −0.50 D and −6.75 D Exclusion criteria: 1) amblyopia or tropia; 2) astigmatism −2.00 D or greater; and 3) anisometropia−2.00 Dor greater |
|
| Interventions | Atropine 0.5% (n = 50): one drop of 0.5% atropine nightly; advised to wear bifocal spectacles Atropine 0.25% (n = 50): one drop of 0.25% atropine nightly; advised to wear slightly undercorrected spectacles Atropine 0.1% (n = 50): one drop of 0.1% atropine nightly; advised to wear fully corrective spectacles Control (n = 50): one drop of 0.5% tropicamide nightly Note: all children were advised to wear sunglasses with UV protection in bright light |
|
| Outcomes |
Primary outcome: change in refractive error measured by cycloplegic autorefraction (spherical equivalent) Measurements taken at baseline and every 3 months for 2 years Unit of analysis: the average values of both eyes |
|
| Notes | Study dates: 1994 Funding source: Department of Health grant (Taiwan) Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization: each participant was categorized into one of the strata and then randomized to receive one of the four treatments. A block size of 12 was used to balance the number of patients in the four treatment categories for each stratum (via email communication with study author) |
| Allocation concealment (selection bias) | Low risk | Group assignments were unknown to study personnel when participants were being enrolled by using coded bottles with sealed envelopes (via email communication with study author) |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to visual and functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | For all study personnel, physicians, examiner and data analysts were all masked to treatment assignment (via email communication with study author) |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Unclear risk | N/A (study did not measure secondary outcomes of this review) |
| Masking of data analyzers | Low risk | For all study personnel, physicians, examiner and data analysts were all masked to treatment assignment (via email communication with study author) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | There were 14 (7%) children excluded from the study: 9 from the 0.5% atropine group (4 had no patience for eye drops, 2 for photophobia, 2 from fear of drops, 1 for allergic blepharitis); 3 from the 0.25% atropine group (no patience for eye drops); 1 from the 0.1% atropine group (no patience for eye drops); and 1 from the 0.5% tropicamide group (no patience for eye drops) |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | High risk | Children were advised to wear differing types of spectacle lenses depending on the concentration of atropine received |
| Tan 2005 | ||
| Methods | Study design: RCT Study centers: 7 (academic centers and clinical practices in Singapore, Hong Kong, and Thailand) Number randomized: 353 children Study follow-up: 1 year Exclusions and losses to follow-up: 55 (16%) children who were randomized were dropped from the analyses |
|
| Participants | Age: mean = 8.7 years (range 6 to 13 years) Gender: 177 boys, 176 girls Culture: 99.4% Asian Inclusion criteria: 1) age 6 to 12 years old; 2) myopia of −0.75 D and −4.00 D; 3) good general health; 4) round pupils; 5) refractive to light; and 6) best corrected VA of 20/25 or better in each eye Exclusion criteria: 1) astigmatism greater than 1.00 D; 2) anisometropia greater than 1.00 D; 3) strabismus; 4) current use of either contact lenses or bifocals; 5) history of ocular surgery, trauma, or chronic ocular disease, including allergic conjunctivitis; 6) previous use of atropine for myopia; 7) diseases requiring long-term or regular intermittent medication; 8) behavioral or neurological disorders that would interfere with the study; 9) participation in any study that involved an investigational drug within one month of enrolment; 10) intolerance or hypersensitivity to topical anesthetics, mydriatics, or components of the formulations; 11) contraindications to antimuscarinic agents; 12) and pregnancy or planned pregnancy |
|
| Interventions | Gel/gel (n = 142): 2% pirenzepine ophthalmic gel applied twice a day Placebo/gel (n = 140): 2% pirenzepine ophthalmic gel applied once a day and placebo gel applied once a day Placebo/placebo (n = 71): vehicle-placebo gel applied twice a day |
|
| Outcomes |
Primary outcome: change in refractive error measured by cycloplegic autorefraction (spherical equivalent) Secondary outcome: change in axial length measured by A-scan ultrasonography Measurements taken at baseline and every 3 months for 1 year Unit of analysis: the average of both eyes |
|
| Notes | Study dates: November 2000 to July 2002 Funding source: Valley Forge Pharmaceuticals, Inc. and Novartis Ophthalmics AG |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomized using a sponsor-prepared, computer-generated list stratified by site. Randomisation was in a 2: 2:1 ratio of pirenzepine twice daily, pirenzepine once daily, and placebo |
| Allocation concealment (selection bias) | Low risk | Study sites used coded lists to determine which tubes of medication to administer to the next enrolled participant. The pirenzepine gels and placebo gels were packaged in identical tubes for morning and night applications and the gels themselves appeared identical. All morning tubes had yellow labels and all evening tubes had blue labels. Thus study personnel had no way of knowing which tubes had which medications, same for patients |
| Masking of participants (performance bias) | Low risk | Study was placebo-controlled and identical appearing bottles with coded labels were distributed |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Since the pirenzepine gels and placebo gels were packaged in identical tubes and the gels themselves appeared identical, the study personnel had no way of knowing which tubes had which medications. The study reported that “no treatment code was unmasked for any subject during the study” |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | Data analyzers were not masked (personal communication with biostatistician) |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | There were 55 (16%) children who were dropped from the study after randomization: 25 (18%) were gel/gel, 21 (15%) were placebo/gel, and 9 (13%) were placebo/placebo (this difference was not statistically different). Of these 55 patients, 31 discontinued the study because of adverse events (20 gel/gel and 11 placebo/gel, none in placebo); 5 were not adherent to study medication regimen; and 1 was dropped for inadequate efficacy (progression of myopia) “We considered statistical methods to impute missing values due to patients who discontinued treatment. However, as the proportion of patients not completing the study was similar across treatment groups, any correction would apply similarly to all groups, and thus we did not conduct these analyses” |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were chosen a priori |
| Other bias | Unclear risk | Some study authors were employed by the pharmaceutical company funding the study |
| Yang 2009 | ||
| Methods | Study design: RCT Study center: 1 (Guangzhou city, China) Number randomized: 178 children Study follow-up: 2 years Exclusions and losses to follow-up: no exclusions; 29 (16%) were lost to follow-up |
|
| Participants | Age: range 7 to 13 years Gender: 94 boys, 84 girls Culture: urban children from Guangzhou city, China Inclusion criteria: 1) age 7 to 13 years old; 2) myopia with spherical equivalent refractive error between −0.50 D and −3.00 D in both eyes, as measured under cycloplegia; 3) astigmatism ≤ 1.50 D; 4) no anisometropia (difference in spherical equivalent ≤ 1.00 D between eyes); 5) best corrected VA of 6/6 or better; 6) no strabismus; 7) normal IOP; 8) willingness to wear glasses constantly for study duration; 9) and understanding of random assignment and willingness to not use other medications Exclusion criteria: 1) any ocular or systemic conditions known to influence refractive development; 2) use of medications that might affect refractive development; 3) moderately or highly myopic (< −3.00 D) parents; 4) birthweight ≤ 1250 g; and 5) previous use of bifocals, PALs or contact lenses |
|
| Interventions | PAL group (n = 89): multifocal lenses with +1.50 D near addition worn constantly SVL group (n = 89): single vision lenses worn constantly Note: prescription changes were made if the subjective refraction had changed by at least 0.50 D for one or both eyes or if clinically indicated |
|
| Outcomes |
Primary outcome: progression of myopia Change in spherical equivalent refractive error relative to baseline measured by cycloplegic autorefraction with 0.5% tropicamide + 0.5% phenylephrine hydrochloride Secondary outcomes:
Measurements taken at baseline and every 6 months for two years Unit of analysis: not reported |
|
| Notes | Study dates: enrolment was from July 2004 to March 2005 Funding source: National Natural Science Grant, China Materials: lenses provided by Sola (China) Ltd Compliance in wearing glasses was monitored with separate questionnaires for children and parents (87% overall compliance) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Enrolled subjects were assigned randomly to either the SV group or PAL group” |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Masking of participants (performance bias) | High risk | Masking of participants was not applicable due to functional differences between the interventions studied |
| Masking of outcome assessors (detection bias) Primary outcome |
Low risk | Masked investigators were unaware of allocation groups during evaluations; although an unmasked investigator was available if clinical consultations were needed |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Low risk | Primary and secondary outcomes were assessed by the same examiners |
| Masking of data analyzers | Unclear risk | “All statistical analysis was carried out independently” |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
Unclear risk | 29 (16%) patients dropped out of the study: 15 in the PAL group and 14 in the SVL group. Statistical analyses comparing the retained participants to those lost to follow-up showed that drop outs had significantly worse myopia than those who remained in the study at baseline (P = 0.01) |
| Selective reporting (reporting bias) | Low risk | Results were reported for outcomes described in the methods section of the paper |
| Other bias | Unclear risk | Unit of analysis (i.e. average value of both eyes or right eye only) was not reported |
| Yen 1989 | ||
| Methods | Study design: RCT Study center: 1 (Refraction Clinic, Veterans General Hospital, Taipei, Taiwan) Number randomized: 247 children Study follow-up: 1 year Exclusions and losses to follow-up: 151 (61%) children were excluded or lost to follow-up |
|
| Participants | Age: mean = 9 years (range 6 to 14 years) Gender: 118 boys, 129 girls Culture: children with simple myopia randomly selected from clinic records Inclusion criteria: 1) age 6 to 14 years old; and 2) myopia with refractive error between −0.5 D and −4.0 D Exclusion criteria: 1) amblyopia or tropia; and 2) cylinder refraction greater than 1.0 D |
|
| Interventions | Atropine: 1% atropine drops every other night; bifocal spectacles prescribed 2 weeks after treatment began Cyclopentolate: 1% cyclopentolate drops every night; single vision spectacles prescribed if necessary Saline control: normal saline eye drops every night; single vision spectacles prescribed if necessary |
|
| Outcomes |
Primary outcome: change in refractive error measured by cycloplegic refraction (spherical equivalent) Secondary outcomes: changes in vision, fundoscopy, and IOP Measurements taken at baseline and every 3 months for 1 year Note: baseline for atropine group was measured 2 weeks after treatment began Unit of analysis: right eyes only |
|
| Notes | Study dates: enrolment from July 1, 1985 to October 31, 1986 Additional data: study author provided unpublished data via email correspondence |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The ophthalmic examinations were done by 3 doctors. Each doctor got a random table. The patients were assigned to three groups according to the sequence on the random table (via email communication with study author) |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done using a random numbers table, but it was not clear whether or not the allocation sequence was concealed |
| Masking of participants (performance bias) | High risk | Masking of participants was not reported for the pharmaceutical agents; however, one group received bifocal spectacles rather than single vision spectacles which therefore was not masked |
| Masking of outcome assessors (detection bias) Primary outcome |
Unclear risk | No details given |
| Masking of outcome assessors (detection bias) Secondary outcomes |
Unclear risk | “To avoid deviation during retinoscopy all examinations were done by three doctors” |
| Masking of data analyzers | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) Incomplete outcome(s) data |
High risk | “Patients who used the eye drops continuously for one year received another complete ophthalmologic examination…96 such patients were collected for evaluation, 32 in each group”. Patients who discontinued using the eye drops or did not use them consistently were excluded from the study. Of the 247 children randomized in the study 151 (61%) were not included in the analyses. The 96 patients analyzed included the first subset of children who followed the treatment protocol and were examined at one year (via email communication with study author) |
| Selective reporting (reporting bias) | High risk | Not all outcomes described in the methods section were reported (changes in vision, funduscopyandIOP) |
| Other bias | High risk | “For statistical significance, each group should include at least 30 samples. Our aim was to evaluate the results after using the medication for 1 year. So, when we felt we had enough numbers of patients who had continuously used the eye drops for 1 year, we decided to analyze the data. It happened to be 32 in each group” (via email communication with study author) |
AC/A: accommodative convergence (in prism diopters) to the stimulus/accommodation ratio
BSCL: bifocal soft contact lens
cm: centimeters
D: diopters
DC: diopter cylinder
IOP: intraocular pressure
logMAR: logarithm of the Minimum Angle of Resolution
mmHg: millimeters of Mercury
N/A: not applicable
PAL: progressive addition lens
RCT: randomized controlled trial
RGPCL: rigid gas permeable contact lens
SCL: soft contact lens
SVL: single vision lens
SVSCL: single vision soft contact lens
VA: visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1966 | Not randomized: case report |
| ACHIEVE study | Not intended to control progression of myopia: glasses vs. contacts for self-esteem in school children |
| Aller 2008 | Interventional twin case series: included only one pair of twins, one randomized to wear bifocal SCLs and the other to wear SVSCLs for one year Both wore BSCLs for the second year |
| Andreo 1990 | Not randomized; not intended to control progression of myopia; participants older than 18 included |
| Baldwin 1969 | Not randomized: patients selected treatment assignment |
| Baltimore myopia project | Not intervention of interest: vision training for myopia |
| Baronet 1979 | Not randomized: retrospective review of patients treated with atropine at a medical practice with no comparison group |
| Bedrossian 1979 | Not randomized: method of allocation is not specified. Cross-over study of atropine in one eye for one year, with the fellow eye serving as the control, then alternated treatment group after each year for four years |
| Berkeley OK Study | Not population of interest: participants were 21 to 28 years old |
| Bier 1988 | Not randomized: sequential assignment to groups |
| Brodstein 1984 | Not randomized: “The lack of randomization permits a possibility for bias” |
| Chou 1997 | Not randomized: allocation by parental decision |
| Dumbleton 1999 | Not an intervention pre-specified in protocol: lenses with different oxygen permeability |
| Dyer 1979 | Not randomized: case-control study |
| Ebri 2007 | Not intended to control progression of myopia: cycloplegic effect and pupillary dilation outcomes, as well as cost-effectiveness; follow-up was 3 days |
| Filip 2000 | Not population of interest: myopia progression in adult myopes |
| Gimbel 1973 | Not randomized: patients compared to historical cohort |
| Goss 1984 | Not randomized: treatment group included patients with overcorrection; controls included random patients selected retrospectively |
| Grosvenor 1991 | Not randomized: historical control group |
| Horner 1999 | Not intended to control progression of myopia: compared soft spherical contact lenses to spectacles; SCLs are not expected to slow myopia progression. In fact, the study was conducted because they believed the SCLs may increase myopia progression |
| Hosaka 1982 | Not randomized: interventional case series of children aged 6 to 14 years treated with labetalol ophthalmic solution |
| Hosaka 1988 | Not randomized: interventional case series |
| Huffman 2002 | Not intended to control progression of myopia: aspheric vs. spheric lenses; outcome was to decrease spherical aberration; included adults |
| Kao 1988 | Not randomized: children were enrolled in two separate series of patients |
| Keller 1996 | Not randomized: all children wore RGPCLs |
| Kennedy 1995 | Not randomized: treatment was atropine; controls were patients matched using medical records |
| Khoo 1999 | Allocation method not clear, randomization not specified; compared RGPs to spectacles over 3 years |
| Lakkis 2006 | Not intended to control progression of myopia: two-week randomized cross-over trial to evaluate visual performance and satisfaction of clear and photochromic spectacle lenses in children aged 10 to 15 years wearing fully corrected spectacles |
| Leung 1999 | Not randomized: quasi-randomized (odd & even case numbers to determine the two groups) |
| Li 2005 | Not randomized: experimental group received progressive multifocal lenses, control group wore common glasses; participants were 6 to 23 years old |
| Liang 2008 | Not intervention of interest: RCT comparing atropine eyedrops alone with a combined treatment of atropine and stimulation of the auricular acupoints in school-aged children with myopia |
| Lu 2010 | Not randomized: case-control study comparing myopic children treated with seasonal doses of atropine with non-myopic children |
| Mandell 1959 | Not randomized: historical cohort, included adults |
| Meythaler 1971 | Not randomized: interventional cases series (70 eyes from 8–35 years of age persons were checked); 3 groups based on age, youngest group was 8 to 19 years old |
| Neetens 1985 | Not randomized: control group consisted of participants that could not use bifocals |
| Nesterov 1990 | Not randomized: compared a group using cycloplegics and ocular hypotensives to a reference group for progression of myopia |
| Oakley 1975 | Not randomized: control group consisted of children (or parents) who refused bifocals |
| Parker 1958 | Not randomized: compared author’s practice to other practices |
| Perrigin 1990 | Not randomized: treatment was silicone lenses; control was a historical cohort |
| Pirenzepine 2003 | Not randomized: review of Pirenzepine studies and mechanism of action |
| Pritchard 1999 | Not intended to control progression of myopia: extended wear for low Dk vs. high Dk lenses in adults |
| Rah 2002 | Not randomized; overnight orthokeratology in adults (LOOK study) |
| Rainey 2000 | Not an intervention pre-specified in protocol: vision therapy vs. control |
| Ritchey 2005 | Not population of interest: included adults aged 18 and older (COLM study) |
| Sankaridurg 2003 | Not intended to control progression of myopia: RCT to compare adverse events for SCLs vs. SVLs (spectacles); participants were 16 to 35 years old |
| Savoliuk 1968 | Not randomized: compared groups using SVLs continuously or for distance-use only with no spectacles |
| Shimmyo 2003 | Allocation method not clear, randomization not specified: Atropine vs. control for 2 years |
| Soni 2006 | Not randomized; included adults |
| Stone 1976 | Not intended to control progression of myopia: authors state that “the research team is not purposely attempting to flatten the cornea in order to arrest the myopia” |
| Sun 2007 | Not randomized: case-control study of spectacle users vs. controls |
| Syniuta 2001 | Not randomized: intervention group included patients whose parents requested treatment for myopic progression, control group comprised the next myopic child by alphabetical order after study child’s record number |
| Takano 1964 | Not randomized: cohort study comparing treatment with Mydrine (tropicamide + phenylephrine) eye drops with or without Neosynesin (phenylephrine) eye drops, included boys and girls with myopia ages 7 to 19 years, follow-up was 20 days |
| Toki 1960 | Not randomized: cohort study of patients receiving 5% Neosynesin (phenylephrine) eye drops, included boys and girls with myopia ages 7 to 21 years, follow-up was 14 to 28 days |
| Tokoro 1964 | Not randomized: non-randomized study of treatment with Mydrine (tropicamide + phenylephrine) eye drops + 5% Neosynesin (phenylephrine) eye drops + low frequency electro stimulus in children ages 7 to 15 years, included children with hyperopia |
| Tokoro 1965 | Not randomized: retrospective cohort comparing full correction spectacles to under correction (< −1 D) spectacles or full correction in case of need in children ages 7 to 14 years, included children with hyperopia |
| Xiao 2009 | Not randomized: observational study of two groups of children who wore RGPCLs or spectacles |
| Yamada 2004 | Not randomized: review article with some cohort data on children with high myopia |
| Yamaji 1967 | Not randomized: observation of children treated with Mydrine-M; no control group |
| Yi 2011 | Not an intervention pre-specified in protocol: RCT of children, intervention was less than 30 hours of near- and middle-vision activities per week and more than 14 hours of outdoor activities per week compared with controls |
| Young 1992 | Not intended to control progression of myopia: comparison of overnight lenses for 12 months in adults only |
| Zeng 2009 | Not intended to control progression of myopia: RCT to evaluate visual performance and satisfaction of ready-made spectacles with custom spectacles in Chinese school-aged children with uncorrected refractive error |
BSCL: Bifocal soft contact lens
COLM: Comparison of Overnight Lens Modalities
Dk: oxygen permeability
LOOK: Lenses and Overnight Orthokeratology
SCL: soft contact lens
SVL: single vision lens
RCT: randomized controlled trial
RGPCL: rigid gas permeable contact lens
Characteristics of studies awaiting assessment [ordered by study ID]
| Anstice 2011 | |
| Methods | Study name: Effect of Dual-Focus Soft Contact Lens Wear on Axial Myopia Progression in Children Study design: paired-eye RCT with cross-over Study center: 1 Number randomized: 40 children Study follow-up: 20 months (10 months for each period) Exclusions and loss to follow-up: no exclusions; 5 (12.5%) and 6 (15.0%) were lost to follow-up in 10-month and 20-month visit, respectively |
| Participants | Country: New Zealand Age: mean 13.4 ± 0.85 years Gender: 11 boys, 29 girls Inclusion criteria: 1) 11 to 14 years old at recruitment; 2) spherical equivalent between −1.25 and −4.50 D in the least myopic eye as determined by non-cycloplegic subjective refraction; 3) myopia progression ≥0.50 D in the previous 12 months, established from previous records or estimated from the current spectacle prescription; 4) best corrected spectacle visual acuity of Snellen 6/6 or better in each eye; 5) prepared to wear contact lenses for at least 8 hours per day during the study Exclusion criteria: history of 1) astigmatism ≥1.25 D; 2) anisometropia ≥1.00 D; 3) strabismus at distance or near as assessed by cover test; 4) ocular or systemic pathology likely to affect refractive development or successful contact lens wear; 5) a birth weight of ≤1250 g |
| Interventions | Group 1 (n=21): 10 months wearing Dual-Focus (DF) lens in the dominant eye and single vision distance (SVD) lens in the contralateral eye, followed by 10 months wearing of the swapped lens assignment Group 2 (n= 19): 10 months wearing DF lens in the non-dominant eye and SVD lens in the contralateral eye, followed by 10 months wearing of the swapped lens assignment |
| Outcomes |
Primary outcomes: change in spherical equivalent refraction measured by cycloplegic autorefraction Secondary outcomes: change in axial eye length measured by partial coherence interferometry Measurements taken at baseline and every 5 months for 20 months Unit of analysis: the eye |
| Notes | Study dates: not reported Publication language: English Purpose of this study was to test the efficacy of an experimental Dual-Focus soft contact lens in reducing myopia progression |
| ATOM 2 | |
| Methods | Study name: Atropine for the treatment of childhood myopia 2 Study design: Phase I was an RCT; Phase II was an open-label study Study center: 1 Number randomized: 400 children Study follow-up: 24 months for Phase I Exclusions and loss to follow-up: one participant in the 0.5% excluded due to missing second year visit; 21 (13%), 14 (9%) and 9 (11%) were lost to follow-up in the 0.5%, 0.1% and 0.01% groups respectively |
| Participants | Age: mean = 9 years (range 6 to 12 years) Gender: 211 boys, 189 girls Culture: ethnic Chinese origin Inclusion criteria: 1) age 6 to 12 years old; 2) refractive error of spherical equivalent −2.00 D or worse in each eye; 3) astigmatism of less than 1.50 D; 4) documented myopia progression of at least spherical equivalent −0.50 D over the last 12 months as determined or suggested by refractive records or change in lens power Exclusion criteria: 1) ocular pathology (e.g., amblyopia or strabismus); 2) previous use of atropine or pirenzepine; 3) allergy to atropine; 4) systemic ill health (e.g., cardiac or respiratory illness) |
| Interventions | Three concentrations of atropine eye drops to be applied once nightly in both eyes: |
| 1. 0.5% atropine (n=161); 2. 0.1% atropine (n=155); or 3. 0.01 % atropine (n=84) |
|
| Outcomes |
Primary outcome: myopia progression at two years (spherical equivalent refraction determined by cycloplegic autorefraction) Secondary outcomes:
Measurements taken at baseline, two weeks, and 4, 8, 12, 16, 20 and 24 months Note: baseline for myopia progression and axial length was measured 2 weeks after treatment began Unit of analysis: the individual (data for both eyes pooled for each person) |
| Notes | Study dates: not reported Funding source: National Medical Research Council and SingHealth (Singapore) Additional data: information assessed from Clinicaltrials.gov website on 10 April 2009 Purpose of this study was to find an optimal dose of atropine for preventing the rapid progression of myopia in children by comparing the efficacy, safety and functional impact of binocular treatment with 0.5%, 0.1% and 0.01% atropine and to develop a treatment regimen for the routine management of childhood myopia |
| COMET 2 | |
| Methods | Study name: Progressive-Addition Lenses versus Single-Vision Lenses for Slowing Progression of Myopia in Children with High Accommodative Lag and Near Esophoria (also called the COMET 2 study) Study design: RCT Study centers: 8, including 7 optometry colleges and schools and 1 community-based ophthalmology practice Number randomized: 118 children Study follow-up: 3 years Exclusions and loss to follow-up: no exclusions; 8 (7%) were lost to follow-up |
| Participants | Country: USA Age: mean 10.1 ± 1.1 years Gender: 54 boys, 64 girls |
| Inclusion criteria: 1) 8 to <12 years of age; 2) refractive error determined by cycloplegic autorefraction which meets all of the following: spherical equivalent −0.50 to −3.00 D in both eyes; astigmatism ≤1.5 D in both eyes; anisometropia ≤ 1.00 D difference between eyes in spherical equivalent; 3) visual acuity at least 20/20 with best subjective refraction in both eyes; 4) accommodative response at near (33 cm) is less than 2.0 D by non-cycloplegic autorefraction; 5) near esophoria (≥ 2.0 PD) present by alternate prism and cover test (APCT) at near using best refractive correction determined from non-cycloplegic subjective refraction | |
| Exclusion criteria: 1) history of strabismus; 2) current or prior use of PALs, bifocals, or contact lenses in either eye (prior or current use of SVLs was permitted) | |
| Interventions | PAL group (n=59): Varilux Ellipse progressive addition lenses with a +2.00 D near addition; worn during all waking hours for three years |
| SVL group (n=59): standard single vision lenses (spectacles); worn during all waking hours for three years Notes: the distance correction was changed if the endpoint of the noncycloplegic subjective refraction differed from the current prescription by 0.50 D or more in spherical equivalent. Prescription changes could be made for smaller differences at investigator discretion if the new prescription improved the subject’s visual acuity by at least one line over that in their current correction |
|
| Outcomes |
Primary outcomes: change in spherical equivalent refractive error in diopters from baseline to the three year visit measured by cycloplegic autorefraction Secondary outcomes: main axis astigmatism (J0, dioptric power of a Jackson cross cylinder with axis at 0°) and oblique astigmatism (J45, dioptric power of a Jackson cross cylinder with axis at 45°) by using the power vector approach Measurements taken at baseline and every six months for three years Unit of analysis: child-based (the median for each eye was averaged to obtain the spherical equivalent used for analysis) |
| Notes | Study dates: enrollment from April 2005 to March 2007 Publication language: English Purpose of this study was to determine whether progressive-addition lenses (PALs) relative to single-vision lenses (SVLs) slowed the progression of low myopia in children with high accommodative lag and near esophoria Study information also assessed from Clinicaltrials.gov website on 1 November 2011 |
BCVA: best corrected visual acuity
D: diopters
g: grams
PAL: progressive addition lens (spectacles)
RCT: randomized controlled trial
SVL: soft vision lens (spectacles)
Characteristics of ongoing studies [ordered by study ID]
| Berntsen 2011 | |
| Trial name or title | Study of theories about myopia progression (STAMP) |
| Methods | Study design: randomized clinical trial Study center: 1 (The Ohio State University College of Optometry) Number randomized: 85 children Study follow-up: 24 months |
| Participants | Age: mean = 9.8 years (range 6 to 11 years) Gender: 41 boys, 44 girls Culture: Ohio, USA: 20% Black, 68% White, 7% Asian and 5% other Inclusion criteria: 1) 6 to 11 years of age; 2) ≥1.30 D accommodative lag (4 D stimulus) without correction for lens effectivity; 3) esophoria at near if more than −2.25 D spherical equivalent; 4) at least −0.75 D myopia in each meridian measured with cycloplegic autorefraction but not more than −4.50 D in each meridian in each eye; 5) astigmatism ≤ 2.00 DC in each eye; 6) anisometropia ≤2.00 D; 7) best-corrected VA of at least 20/32 logMAR equivalent; 8) birth weight ≥ 1250 g by parental report Exclusion criteria: 1) strabismus; 2) history of contact lens wear; 3) history of previous bifocal wear; and 4) diabetes mellitus |
| Interventions | PALs (n = 42): PALs with a + 2.00 D addition SVLs (n = 43) Note: children were randomly assigned to wear either PALs or SVLs for the first year of the study, and all children wore SVLs for the second year of the study |
| Outcomes |
Primary outcome: Central spherical equivalent refractive error Secondary outcomes:
Unit of analysis: not specifically reported |
| Starting date | Study dates: enrolment December 2006 to May 2008 |
| Contact information | David A. Berntsen, OD, PhD, FAAO University of Houston College of Optometry 505JDavisArmisteadBldg Houston, TX 77204-2020 e-mail: dberntsen@optometry.uh.edu |
| Notes | Design and baseline data were published Funding source: The Ohio State University and National Eye Institute |
AC/A: accommodative convergence (in prism diopters) to the stimulus/accommodation ratio
D: diopters
DC: diopter cylinder
g: grams
logMAR: logarithm of the Minimum Angle of Resolution
PAL: progressive addition lens (spectacles)
SVL: soft vision lens (spectacles)
VA: visual acuity
Results of the search
We conducted an electronic literature search as of 11 October 2011 and identified 1310 unique records of journal articles and conference abstracts. After screening the titles and abstracts, we ob-tained full-text copies of 138 records for further review. Of those, we included 75 records (23 studies) and excluded 60 records (43 studies); one record was for an ongoing study and two records are awaiting classification. We handsearched the reference lists of included studies and used the Science Citation Index (1 November 2011) to identify additional studies. From the manual search, we identified 38 additional records not identified by the electronic search. Of these 38 records, there were 14 additional records to studies already included, two additional records to studies already excluded, 20 records that were excluded (from 18 studies), one record for a study already awaiting assessment, and one record for an ongoing study. Thus overall, we reviewed 176 potentially relevant full-text articles or conference abstracts of which we included 89 records (from 23 studies), excluded 82 records (from 61 studies), identified four records awaiting classification (from three studies), and identified one ongoing study (Figure 1).
Figure 1.
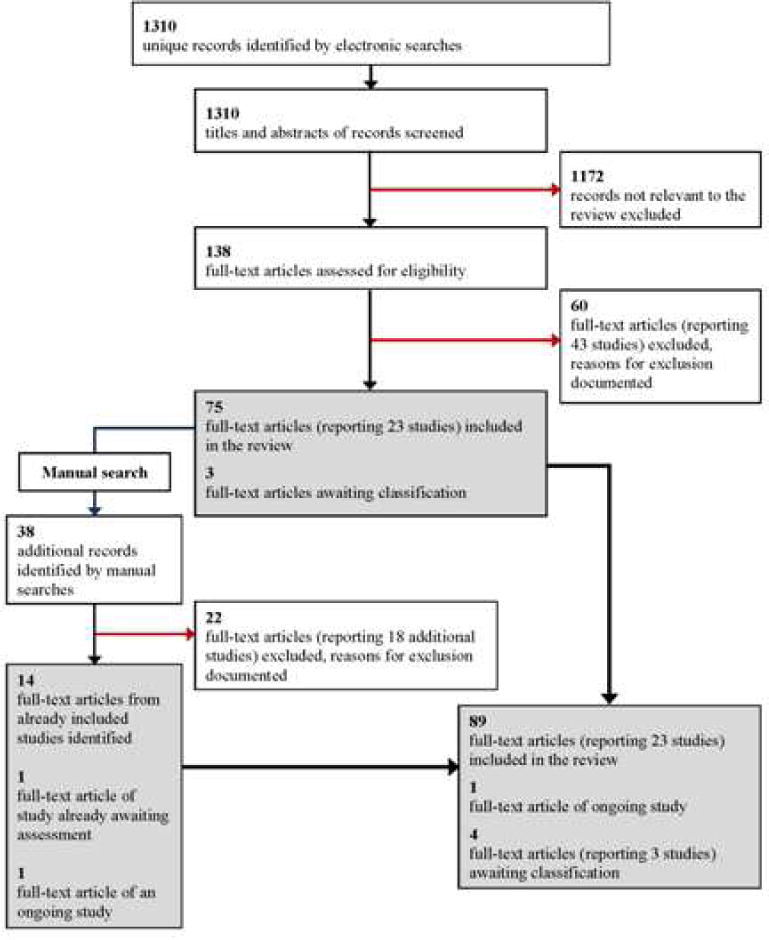
Results from searching for studies for inclusion in review.
Included studies
We included 23 RCTs in this review. The studies evaluated varying interventions, including spectacles, contact lenses and pharmaceutical agents (Table 1). The age range of children participating in the included studies was six to 18 years and no child had myopia less than −0.25 D. The primary outcome of all included studies was the progression of myopia, measured as the change in refractive error (Table 2; Table 3; Table 4). Twenty-one studies (91%) measured refraction under cycloplegia, of which 16 used autorefraction. Only one study has not been published as a full report in a peer reviewed journal (Aller 2006). Three studies were funded primarily by industry (Aller 2006; PIR-205 Study; Tan 2005) and four studies were funded partially by industry (Cheng 2010; CLAMP Study; COMET Study; Hasebe 2008).
Table 1.
Interventions of included studies
| STUDY | Spectacles | Contact lenses | Pharmaceutical agents | Com- bina- tion of inter- ven- tions |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Un- der- cor- rected SVLs a |
Multi-focal lenses | Fully-cor- rected SVLs |
RGP c |
Soft bifo- cal lenses |
SVSd | Atropine | Piren- zip- ine |
Ref- er- ence group |
|||||||
| Bi- focal lenses 1 |
Bi- focal lenses 2 |
PALs b |
Dis- tance use only |
Con- tinu- ous use |
1 | 2 | 3 | ||||||||
| Adler 2006; 2 study arms | X | X | |||||||||||||
| Chung 2002; 2 study arms | X | X | |||||||||||||
| Fulk 1996; 2 study arms | +1.25 | X | |||||||||||||
| Fulk 2002; 2 study arms | +1.50 | X | |||||||||||||
| Jensen 1991; 3 study arms | +2.00 | X | Timolol + SVLs | ||||||||||||
| Houston Study; 3 study arms | +1.00 | +2.00 | X | ||||||||||||
| Cheng 2010; 3 study arms | +1.50 | +1.50 prism | X | ||||||||||||
| Pärssin, 1989; 3 study arms | +1.75 | X | X | ||||||||||||
| COMET Study; 2 study arms | +2.00 | X | |||||||||||||
| Edwards 2002; 2 study arms | +1.50 | X | |||||||||||||
| Yang 2009; 2 study arms | +1.50 | X | |||||||||||||
| Hasebe 2008; 2 study armse | +1.50 | X | |||||||||||||
| Sankari 2010; 4 study arms | X* | ||||||||||||||
| Katz 2003; 2 study arms | X | X | |||||||||||||
| CLAMP Study; 2 study arms | X | X | |||||||||||||
| Aller 2006; 2 study arms | X | X | |||||||||||||
| MIT study; 3 study arms | Plus placebo drops | Plus placebo drops | Atropine + PALs | ||||||||||||
| ATOM study; 2 study arms | 1% | Placebo drops | |||||||||||||
| Shih 1999; 4 study arms | 0.1% | 0.25% | 0.5% | 0.5% tropicamide | |||||||||||
| Yen 1989; 3 study arms | 1% + bifocals | Saline + SVLs | Cyclopentolate + SVLs | ||||||||||||
| PIR-205 Study; 2 study arms | 2% gel | Placebo gel | |||||||||||||
| Tan 2005; 3 study arms | 2% gelx2 | Placebo gelx2 | 2% gel + placebo gel | ||||||||||||
| Schwar 1981; 2 study arms | X | Tropicamide + bifocals | |||||||||||||
SVLs: single vision lenses.
PALs: progressive addition lenses.
RGP: rigid gas permeable.
SVS: single vision soft lenses.
cross-over trial.
study included four groups; three groups received spectacles with novel lens designs and the fourth group received SVLs. The novel lenses were designed to reduce peripheral hyperopic defocus.
Table 2.
Outcomes reported by studies of spectacle interventions*
| Outcomes | Interventions studied | |||
|---|---|---|---|---|
| Undercorrected lenses: 2 studies | Bifocal lenses: 6 studies | Progressive addition lenses: 5 studies | Novel lenses to minimize peripheral hyper-opic defocus: 1 study | |
| Primary outcome: change in refractive error | Analysis 1.1 | Analysis 2.1; Analysis 2.2; Analysis 2.3 | Analysis 2.1; Analysis 2.2; Analysis 2.3 | Analysis 3.1 |
| Secondary outcome: change in axial length | Analysis 1.2 | Analysis 2.4; Analysis 2.5; Analysis 2.6 | Analysis 2.4; Analysis 2.5; Analysis 2.6 | Analysis 3.2 |
| Secondary outcome: change in corneal radius of curvature | Not measured by Adler 2006 and reported as non-significant by Chung 2002 | Not reported | Analysis 2.7 | Not reported |
| Adverse effects | Two participants who were under-corrected complained of blurred vision (Adler 2006) | None reported | None reported | Participants reported blurred side vision, visual distortion, dizziness, headaches and falls (Sankaridurg 2010) |
Compared with fully-corrected single vision lenses.
Table 3.
Outcomes reported by studies of contact lens interventions*
| Outcomes | Interventions studied | |
|---|---|---|
| Soft bifocal contact lenses: 1 study | Rigid gas permeable contact lenses: 2 studies | |
| Primary outcome: change in refractive error | Analysis 4.1 | Analysis 5.1 |
| Secondary outcome: change in axial length | Analysis 4.2 | Analysis 5.2 |
| Secondary outcome: change in corneal radius of curvature | Not reported | Analysis 5.3 |
| Adverse effects | Not reported | Not reported |
Compared with fully-corrected single vision lenses or soft contact lenses.
Table 4.
Outcomes reported by studies of pharmaceutical interventions*
| Outcomes | Interventions studied | ||||
|---|---|---|---|---|---|
| Pirenzepine: 2 studies | Atropine: 2 studies | Cyclopentolate: 1 study | Timolol: 1 study | Tropicamide (plus bifocals): 1 study | |
| Primary outcome: change in refractive error | Analysis 6.1; Analysis 6.2 | Analysis 6.1; Analysis 6.2 | Analysis 6.1 | Analysis 7.1 | Control twins showed more progression in myopia than their cotwins who received tropicamide and bifocals, but this difference was not statistically significant (Schwartz 1981) |
| Secondary outcome: change in axial length | Analysis 6.3 | Analysis 6.3; Analysis 6.4 | Not reported | Not reported | Not reported |
| Secondary outcome: change in corneal radius of curvature | Not reported | Not reported | Not reported | Not reported | Not reported |
Compared with placebo or no drops.
Undercorrected versus fully-corrected spectacles
Two studies compared the use of undercorrected spectacles with fully-corrected spectacles. In one study, 62 children in Israel aged six to 15 years old were randomized to receive spectacles blurred by +0.50 D or spectacles with full correction (Adler 2006). In the second study, 106 Malay and Chinese children were evenly randomized to receive spectacles undercorrected by approximately +0.75 D or fully-corrected spectacles (Chung 2002). The study periods were 18 months and two years, respectively.
Multi-focal versus single vision lenses (spectacles)
Twelve studies included in the review compared multi-focal spectacles with single vision lenses (SVLs) (spectacles) for slowing progression of myopia in children. Five of these studies directly compared bifocal spectacles to SVLs for slowing progression of myopia in children. One study, conducted in Tahlequah, Oklahoma, USA, randomized 32 children to receive either bifocals with +1.25 D addition or SVLs (Fulk 1996). The children were six to 13 years old and were followed for 18 months. Following this pilot study, the study authors initiated a larger study with slight modifications to the study design (Fulk 2002). For their second study, the study authors added another study center in Tulsa, Oklahoma, USA, enrolled 82 children aged six to 12 years, changed the addition for the bifocal lenses to +1.50 D, and extended the follow-up period to 30 months.
The third study comparing bifocals to SVLs was the Houston Myopia Control Study (Houston Study). The 207 children enrolled in the study (ages six to 15 years) were randomized to one of three treatment groups and followed for three years. The treatment groups included two intervention groups that received bifocals with either +1.00 D or +2.00 D addition, and a standard treatment group that received SVLs.
A study from central Finland enrolled myopic schoolchildren referred by local doctors and nurses after routine vision check-ups (Pärssinen 1989). In all, 240 children with a mean age of 10.9 years were randomized to one of three treatment groups and followed for three years. The first intervention group, the distant-use group, received minus lenses with full correction and were advised to use them for distance vision only and to read at the greatest distance possible. The second intervention group, the bifocal group, received clear plastic bifocal lenses with +1.75 D addition for continuous use. The third group was the reference group and received minus lenses with full correction for continuous use. The fifth study investigated the effect of bifocal lenses (+1.50 D) with or without a 3-prism diopters base-in prism in the near segment with single vision distance lenses for slowing the progression of myopia in Chinese Canadian children (aged eight to 13 years) (Cheng 2010). One hundred and fifty children were enrolled in the two-year study.
Four included studies directly compared the use of progressive addition lenses (PALs) to SVLs. The Correction of Myopia Evaluation Trial (COMET) was a three year, multi-center trial conducted in four major US cities (COMET Study). In all, 469 children aged six to 11 years were randomized to receive either multifocal lenses (no-line bifocals) with gradual and progressive changes of power or SVLs with the same focal power throughout the lens area. Studies of 298 children from seven to 10.5 years of age and 178 children from seven to 13 years of age were completed in Hong Kong (Edwards 2002) and China (Yang 2009), respectively. The children in both studies, randomized to receive PALs or SVLs, were followed up for two years. A Japanese cross-over trial followed up children aged six to 12 years for 18 months after randomization to PALS or SVLs (Hasebe 2008). After 18 months, each child was switched to receive the alternate type of lens and followed up for another 18 months.
The Myopia Intervention Trial (MIT) included 227 Taiwanese children and investigated SVLs, PALs, and PALs in combination with atropine drops for controlling the progression of myopia (MIT study). The children, between six to 13 years of age, were randomized to one of three treatment groups and followed up for 18 months: 1) SVLs and eyedrop placebo; 2) PALs and eyedrop placebo; and 3) PALs and 0.5% atropine instilled once a day at bedtime.
A three-arm trial including interventions of bifocals, timolol maleate and SVLs was completed in Odense, Denmark (Jensen 1991). For two years, 159 schoolchildren with a mean age of 10.9 years were followed up after being randomized to one of three treatment groups. The bifocal group received bifocal lenses with +2.0 D addition for constant wear. The timolol group received one drop of 0.25% timolol maleate (an IOP reducing beta-blocker) in each eye twice daily in addition to SVLs for constant wear. The control group received only SVLs for constant wear.
In a study of 26 twin pairs, the combined use of bifocal lenses and tropicamide ophthalmic solution for controlling myopia progression was compared to the use of SVLs over a three and a half year period (Schwartz 1981). This Washington D.C. area study included monozygotic twin pairs between the ages of seven and 14 with similar myopia. For each twin pair, one twin was randomized to receive combined treatment of bifocal spectacles with +1.25 D addition and two drops of 1% tropicamide ophthalmic solution (a short-acting cycloplegic) instilled into each eye nightly; the other twin received standard spectacle correction.
A study of 78 children from eight to 18 years of age compared bifocal soft contact lenses (BSCLs) to SVSCLs for controlling myopia progression (Aller 2006). The children, from California, USA, were randomized to wear BSCLs or SVSCLs every day for one year.
Spectacles to reduce peripheral hyperopic defocus versus single vision lenses (spectacles)
One study compared three novel lenses designed to reduce peripheral hyperopic defocus (peripheral vision farsightedness) with SVLs in 210 Chinese children aged six to 16 years (Sankaridurg 2010). The novel lenses corrected for central vision as SVLs do, as well as peripheral vision using novel lens designs that had (1) a symmetrical, clear central aperture (20 mm) with increasing peripheral power to +1 D; (2) a symmetrical, clear central aperture (14mm) with increasing peripheral powerto+2D;or(3) an asym-metrical, clear central aperture with increasing peripheral power to +1.9 D. The study was planned for two years of follow-up, but was terminated at year one due to observing lower than expected progression in myopia among all study participants.
Rigid gas permeable contact lenses versus single vision lenses
Two studies included in the review compared rigid gas permeable contact lenses (RGPCLs) to either SVSCLs or spectacles (SVLs). The Contact Lens and Myopia Progression (CLAMP) study was a three year trial to compare RGPCLs to SVSCLs for controlling myopia in school-aged children (CLAMP Study). All the participants had to complete a run-in period successfully prior to enrolment to exclude those that could not adapt to rigid contact lenses. After the run-in period, 116 children aged eight to 12 were randomized to RGPCL or SVSCL treatment groups. A study of 564 children in Singapore, aged six to 12 years, compared the use of RGPCLs to SVL spectacles for controlling myopia over a two-year period (Katz 2003). After a three-month adaptation period, 383 participants remained in the study.
Pharmaceutical agents versus control
The use of topical ophthalmic solutions for the control of myopia progression was investigated in six studies. The Myopia Intervention Trial mentioned previously evaluated SVLs, PALs, and PALs in combination with atropine drops for controlling the progression of myopia (MIT study). The Atropine in the Treatment of Myopia (ATOM) Study enrolled 400 Singaporean children aged six to 12 years (ATOM study). Once each child was randomized to a treatment group, one eye of each child was randomized to receive treatment and the other eye served as a natural control. The atropine group applied one drop of 1% atropine sulfate nightly to the appropriate eye and the placebo group applied one drop of vehicle nightly to the appropriate eye. Follow-up for this study was two years.
Another study, completed in Taiwan, investigated the effectiveness of lower concentrations of atropine for controlling the progression of myopia in children aged six to 13 years (Shih 1999). Two hundred children were randomized to one of three atropine groups or to a control group: 1) daily drop of 0.5% atropine and advised to wear bifocal spectacles; 2) daily drop of 0.25% atropine and advised to wear slightly undercorrected SVLs; 3) daily drop of 0.1% atropine and advised to wear fully corrective SVLs; and 4) daily drop of 0.5% tropicamide. A three-arm trial of 247 children from Taiwan compared the use of topical eye drops for one year in controlling myopia progression (Yen 1989). The children aged six to 14 were randomized to one of three treatment groups: 1) 1% atropine drops every other night and bifocal spectacles prescribed after 2 weeks of treatment; 2) 1% cyclopentolate drops every night and SVLs prescribed if necessary; and 3) normal saline eye drops every night and SVLs prescribed if necessary.
Two studies compared the use of pirenzepine gel (an anti-muscarinic) to placebo gel for the control of myopia progression. The first study was a multi-center US study that enrolled 174 children eight to 12 years old, and followed them up for one year (PIR-205 Study). Children were randomized in a 2:1 ratio to apply either 2% pirenzepine ophthalmic gel or placebo gel twice a day. An additional year of follow-up was continued for children who completed the first year.
The final study was a three-arm, multi-center trial from Singapore, Hong Kong and Thailand (Tan 2005). For one year, 353 children aged six to 13 years were randomly treated with either 1) 2% pirenzepine gel applied twice daily (gel/gel); 2) placebo once daily and 2% pirenzepine gel once daily (placebo/gel); or 3) placebo gel twice daily (placebo/placebo).
Excluded studies
We excluded 61 studies from this review after full-text assessment. The complete list of studies and reasons for exclusion are shown in the Characteristics of excluded studies table. Our reasons for exclusion are based on four categories: (1) the study was not randomized, 43 studies; (2) the study interventions were not intended to control myopia progression, 10 studies; (3) the study interventions were not in the scope of this review, five studies; and (4) the study population was not eligible for this review, three studies. We excluded two RCTs from this review comparing SVSCLs with spectacles in myopic children and adolescents (ACHIEVE study; Horner 1999). We excluded these studies because SVSCLs and spectacles are not meant to control the progression of myopia. The purpose of the ACHIEVE study was to compare the effects of contact lens wear with spectacle wear on the children’s self-perception.
Risk of bias in included studies
Allocation
This review was limited to RCTs only. Twenty (87%) of the 23 included studies described the randomization procedure used to allocate patients to treatment groups and we judged them as having adequate sequence generation (Figure 2). Methods employed for adequate sequence generation included block randomization schemes, computer-generated randomization lists, independently prepared randomization lists or tables and flipping coins or drawing lots. Of these studies, we graded 19 to also have adequate allocation concealment. Methods considered to be at low risks of bias for this domain included using sequentially numbered sealed envelopes, calling a centralized coordinating center, or allocating the participants to treatment groups after being enrolled in the study. One study which used random number tables did not report whether or not allocation was concealed (Yen 1989).
Figure 2.
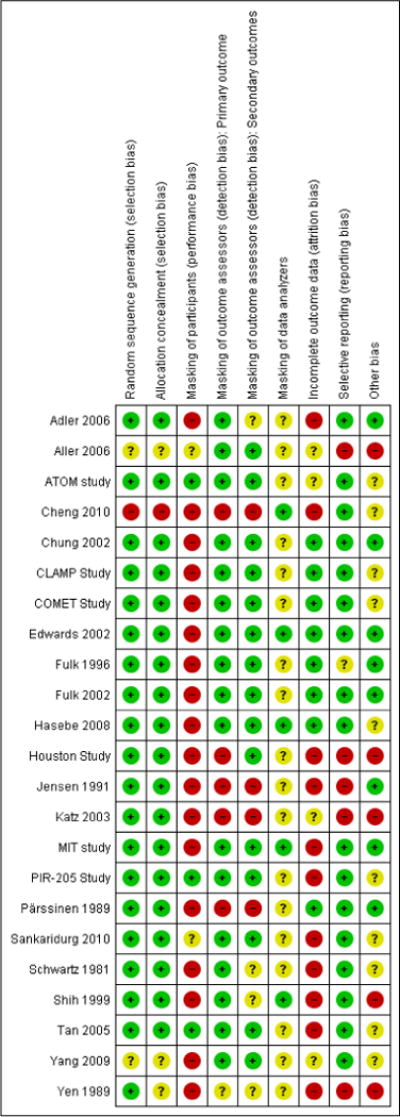
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
We judged one study as having inadequate sequence generation and allocation concealment (Cheng 2010). Group assignments for this study were determined by selecting pieces of paper with patient numbers written on them from a container. The first 50 numbers pulled out were assigned to the control group, the second 50 to the bifocal group and the remaining 50 to the bifocal plus prism group. Since participants did not have equal chances of being assigned to all treatment groups once the first 50 numbers were drawn, we considered this method of sequence generation to be inadequate. Since treatment assignments were consecutive, there was inadequate allocation concealment as well.
The remaining two studies we considered to have unclear risks of bias for sequence generation and allocation concealment were Aller 2006 and Yang 2009. These studies stated that patients were randomized, but did not report further details on how randomization was implemented or whether concealment of allocation was done.
Masking (performance bias and detection bias)
We assessed the use of masking (blinding), for three types of roles: outcome assessors, study participants and data analysts. Furthermore, we considered separately the masking of outcome assessors for primary (change in refractive error) and secondary (changes in axial length and corneal radius of curvature) outcomes. Adequate methods of masking outcome assessors involved having the patients examined by an investigator unaware of treatment assignments. This method was implemented for spectacle or contact lens studies by having the patients remove contact lenses and spectacles prior to being examined or distributing SVLs for all patients to wear during office visits. Use of coded, identical packaging was considered adequate masking for pharmaceutical studies. Overall, masking of primary outcome assessors was done for 17 (74%) of the 23 included studies (Figure 2). Of these 17 studies that masked primary outcome assessors, 14 of the studies were masked similarly for the secondary outcome assessors and three of the studies did not measure secondary outcomes related to this review.
We judged five included studies as not masking primary outcome assessors adequately. In a three-armed study comparing bifocal lenses or timolol with SVLs there was only one study investigator, who therefore could not be masked to treatment assignments (Jensen 1991). Refractive errors for this study were measured by cycloplegic autorefraction. In another three-armed trial comparing bifocals or distance-use spectacles with continuous-use spectacles, it was reported that the examining ophthalmologist did not look at the group assignment before the examination, but often, for different reasons, the group assignments were revealed (Pärssinen 1989). However, the three-year follow-up examinations were conducted by two different ophthalmologists, one of whom did not know the group assignments. Refractive errors for this study were measured by subjective cycloplegic refraction. The Houston Myopia Control Study included a team of masked observers (evaluation team) and a team of unmasked observers (patient care team) to measure outcomes in a trial of bifocal versus SVLs (Houston Study). Since the results presented in the final analysis of the primary outcome were from the non-masked group, we judged the study as having inadequate masking of primary outcome assessors. Refractive errors for this study’s results were measured by subjective noncycloplegic refraction. There were two included studies that did not attempt to mask primary outcome assessors, one measured refractive errors by cycloplegic autorefraction (Cheng 2010) and the other measured refractive error by subjective cycloplegic refraction (Katz 2003). With the exception of the Houston Study, secondary outcome assessors were the same as the primary outcome assessors. Data for secondary outcomes in the Houston Study were collected by the masked evaluation team and, therefore we considered them to have a low risk of bias.
One study did not report masking of outcome assessors and we judged it to have an unclear risk of bias (Yen 1989). This used cycloplegic refraction, but did not specify whether it was autorefraction or subjective refraction.
Due to the interventions under investigation, masking of participants was not feasible for many of the studies included in this review. Participants from 18 (78%) of the 23 included studies could not be masked because of significant physical (e.g. contact lenses versus spectacles), functional (e.g. multifocal lenses versus SVLs), or performance (e.g. undercorrected versus fully-corrected spectacles) differences between the study interventions. The three studies evaluating pharmaceutical agents exclusively did mask participants adequately by distributing identically packaged, coded bottles (ATOM study; PIR-205 Study; Tan 2005). One study reported masking participants, but we judged it as having an unclear risk of bias because it was not clear whether the two types of contact lenses under study, BSCLs versus SVSCLs, performed differently when used by the patients (Aller 2006). A second study also reported masking participants, but we judged it as having an unclear risk of bias because it was not clear whether the novel lens designs were noticeably different to participants when compared with the control SVLs (Sankaridurg 2010).
The final assessments for masking applied to the study data analysts. How data were handled and whether or not data analysts were masked to treatment groups was not reported in 10 (43%) of the 23 included studies. Two studies explicitly stated that masked investigators analyzed the data independently (Edwards 2002; Hasebe 2008). Additionally, study authors contacted for clarification replied that data analysts were masked for Cheng 2010, MIT study and Shih 1999. Although three studies stated that data were analyzed independently after the conclusion of the trial, we considered masking of data analysts to be unclear since the treatment assignments may have been accessible in the data (Adler 2006; Chung 2002; Yang 2009). One study could not be masked because there was only one investigator involved (Jensen 1991). Study authors of four studies informed us that data analysts were not masked (via email communications) (CLAMP Study; Katz 2003; PIR-205 Study; Tan 2005). We assessed studies in which data analysts were not masked or masking of data analysts was not reported, to have an unclear risk of bias for this parameter.
Incomplete outcome data
Attrition rates reported by the included studies varied from 0% to 61%. There were three studies that followed the intention-to-treat (ITT) analysis as defined by this review: 1) participants were analyzed in the intervention groups to which they were randomized, regardless of the intervention they actually received; and 2) all randomized participants were included in the analysis, even participants in which no outcome was collected. One study had follow-up data for all participants at the final follow-up visit (CLAMP Study) and two used statistical methods to account for all randomized patients by imputing values for missing data (COMET Study; Fulk 2002). The COMET Study used the last-observation-carried-forward method to impute data for the seven (1.5%) children who did not complete the study. The Fulk 2002 study imputed missing data for seven (8.5%) children that did not complete the study by assuming that they had “myopia progression equal to that of mean progression observed in the SVL group for the time period for which their data were missing.”
There were seven studies that analyzed participants in the intervention groups to which they were randomized, but did not include all randomized participants in the analysis due to attrition. In none of the studies were patients excluded from the analysis due to noncompliance, switching intervention groups or failure to adhere to treatment protocols. In four of these studies, outcome data were missing in both intervention groups, but drop-outs were balanced across groups and participants who dropped out were similar to those who remained (Chung 2002; Edwards 2002; Fulk 1996; Hasebe 2008). The attrition rate for each of these studies was between 6.5% and 15%. For these considerations we judged these four studies as having a low risk of bias due to minimal amounts of incomplete outcome data. The other three studies had an unclear risk of attrition bias due to unbalanced drop-out rates between treatment groups or because there were statistically significant differences between participants who dropped out compared with those who remained in the study (ATOM study; Katz 2003; Yang 2009).
We assessed one additional study to be at low risk of bias due to incomplete outcome data (Pärssinen 1989). In this study, one child who was randomized was excluded from the study because he was subsequently found to be ineligible (his sister had been included previously in a different group). Two other children moved from the area (less than 1% of the study population) and were excluded from the analyses due to missing data. The remaining participants were analyzed by their original treatment assignments. One study published only as an abstract reported the number of patients included in the analyses, but it was not clear whether this number represented the total number who were initially enrolled in the study and randomized to treatment (Aller 2006).
We judged the remaining 11 studies to have a high risk of bias due to incomplete outcome data. The percentage of missing data ranged from 4% to 61%. In all of these studies, a proportion of participants were excluded after randomization for not adhering to treatment protocol, having adverse events or withdrawing consent. In a study evaluating undercorrection with full-correction spectacles, participants were excluded for not wearing spectacles continuously (Adler 2006). A study comparing bifocal lenses with SVLs excluded patients from the analysis who were randomized to receive SVLs, but dropped out because their parents wanted them to receive bifocals (Cheng 2010). In another study evaluating bifocals and SVLs, noncompliant patients were dismissed from the study as were patients who were fitted with contact lenses without informing study personnel (Houston Study). One study excluded two participants for withdrawing due to having an adverse event or withdrawing consent, as well as seven participants who were lost to follow-up (Sankaridurg 2010). The other seven studies evaluated a pharmaceutical agent in at least one treatment arm (seven of the eight pharmaceutical studies included in this review). In five of these studies, participants were excluded for not using the eye drops or gel, or for not using them consistently (MIT study; PIR-205 Study; Shih 1999; Tan 2005; Yen 1989). Another study evaluating timolol plus SVLs versus bifocals or SVLs, excluded patients for switching to contact lenses or because they could not adapt to the bifocal lenses (Jensen 1991). In the last study, a co-twin study in which one twin received bifocal spectacles and 1% tropicamide ophthalmic solution and the other twin received SVLs, one twin pair was excluded from the study for noncompliance (Schwartz 1981).
In addition to excluding participants for non-adherence, two studies reported an imbalance in drop-out rates (PIR-205 Study; Tan 2005). The PIR-205 Study reported that there were significantly more drop-outs in the pirenzepine arm compared with the placebo arm, although three additional analytical methods used to impute missing values for those who discontinued the study found similar or more beneficial treatment effects for pirenzepine compared with analysis censoring the drop-outs. The Tan 2005 study also reported more drop-outs in the pirenzepine treated groups than the placebo only group. Although the difference was not statistically different, all the patients who dropped out because of adverse events received pirenzipine.
Two studies excluded participants for inefficacy of treatment(MIT study; Tan 2005). In the MIT study, two children were excluded from the study for having myopic progression greater than 2.00 D per year. One child was from the SVL group and one child was from the PAL group (none were from the atropine plus PAL group). One child was dropped from the placebo group of the Tan 2005 study for inadequate efficacy.
Finally, the study with the highest percentage of missing data enrolled 247 children, but data were missing for 151 (61%) children (Yen 1989). Reasons for missing data were not reported. The study authors stated that “patients who used the eye drops continuously for one year received another complete ophthalmologic examination” and “96 such patients were collected for evaluation, 32 in each group”. It was not clear whether the 96 patients analyzed included all the children who were examined at one year or a subset of those examined.
Selective reporting
All the studies included in this review reported outcomes related to the progression of myopia with at least one year of follow-up. Study specific outcomes used to measure progression of myopia included mean changes in refractive error, mean rate changes in refractive errors and mean differences (MDs) in refractive errors. We assessed seventeen (74%) studies to have a low risk of bias for selective reporting: eight studies reported results for study outcomes defined a priori (i.e. in a design and methods publication, baseline report or clinical trial registry); and nine studies reported results for the outcomes described in the methods section of each paper (Figure 2).
One study had an unclear risk of bias due to inadequate reporting for one of the two outcomes measured (Fulk 1996). For this study, the refractive error outcome was reported by treatment assignment, however, the axial length outcome was presented only as it correlated to myopia progression and results by treatment groups were not given.
We considered five studies to have a high risk of reporting bias. For one study only published as an abstract (Aller 2006), the study author’s website stated: “Full disclosure of the results is awaiting approval by the sponsor, but some results have already been published in abstract form” (Aller 2010). The results published in the abstract did not include all the study outcomes listed in the clinicaltrials.gov registry. Another study stated in its methods section that results would be discussed only if exceptional (Jensen 1991). There was one study in which not all outcomes described in the methods section were reported (Yen 1989) and one study in which all outcomes identified in the study methods were reported, but the number of participants included in the analyses were not consistent between outcomes (Katz 2003). In the final study, results were not reported for evaluation team (masked observers) measurements or for other secondary outcomes outlined in the design paper (Houston Study). The methods paper stated that an evaluation team report would be based on 1) cycloplegic retinoscopy, 2) non-cycloplegic autorefraction and 3) cycloplegic autorefraction performed by masked examiners. However, findings from masked examinations were not reported in the outcomes paper, and instead results were reported from the patient team (unmasked observers). Also secondary outcomes, such as change in axial length, were not reported.
Other potential sources of bias
We assessed eight (35%) studies to be free of other potential sources of bias, ten (43%) studies to have an unclear risk of bias and five (22%) studies to be at high risk of bias (Figure 2).
One study was a cross-over trial and we assessed this as having an unclear risk of bias since carry-over effects were not investigated and some participants who completed the first period dropped out during the second period (Hasebe 2008). Thus, we only used data from the first period to estimate treatment effects.
One study reported having imbalances between treatment groups at baseline in gender, corneal curvature and refractive error (Katz 2003). This study also had unequal losses to follow-up between treatment groups and by gender. In addition to these considerations and due to the 32% of participants who dropped out of the study between randomization and the end of the adaptation period, we judged this study to have a high risk of bias. Two other studies incorporated a pre-randomization administration of an intervention in their study designs (ATOM study; CLAMP Study). Run-in periods may enhance or diminish the effect of a subsequent, randomized, intervention; thus we assessed these studies as having an unclear risk of bias.
The CLAMP Study, along with seven other studies, was fully or partially funded by companies with financial interests in at least one of the interventions being studied. Of the eight included studies funded by industry, we considered seven to have an unclear risk of bias (Cheng 2010;CLAMP Study; COMET Study; Hasebe 2008; PIR-205 Study; Sankaridurg 2010; Tan 2005). We judged the eighth study to have a high risk of bias due to the author’s note that “full disclosure of the results is awaiting approval by the sponsor” (Aller 2006; Aller 2010).
There were two additional studies with unclear risk of bias for other sources. One study reported that the study investigator was masked; however, there was only one study investigator and it was not reported who reviewed the participants’ activities when they came in for follow-up or how the examiner remained masked at follow-up visits (Schwartz 1981). The other study did not report the unit of analysis for the results, such as the average of both eyes, the right eye only and the worse eye, etc. (Yang 2009).
We judged the remaining three studies to be at high risk of other sources of bias (Houston Study; Shih 1999; Yen 1989). In the Houston Study, outcomes were assessed by masked and unmasked personnel. Although the study protocol described presenting primary outcome data collected by the masked investigators, results were only published for data measured by unmasked subjective refraction without cycloplegic and sometimes measured by student examiners. In the Shih 1999 study, participants in different treatment groups were advised to wear differing types of spectacle lenses depending on the concentration of atropine received. The rationale for recommending different types of spectacles for different atropine doses (bifocals in the 0.5% group; undercorrected lenses in the 0.25% group; and fully corrective lenses in the 0.1% group) was not explained. In the Yen 1989 study it was unknown why equal numbers of participants dropped out of each group or how the equal numbers of participants per group were selected for analysis (“96 such patients were collected for evaluation, 32 in each group”).
Effects of interventions
We compared several interventions to SVL (spectacles) or SCLs in order to determine which treatments may best slow the progression of myopia in children. We pooled results for prespecified outcomes when appropriate; otherwise we reported study-specific results. For the primary outcome of this review, progression of myopia assessed as the mean change in refractive error (spherical equivalent) from baseline for each year of follow-up, negative mean differences (MDs) represented faster progression of myopia in the treatment group compared with progression in the control group. Thus, point estimates to the left of null on the forest plots favor the control group for this outcome. For axial length, negative MDs represent less axial elongation for treatment group participants compared with control group participants (point estimates to the left of null on the forest plots favor the treatment group for this outcome). The unit of analysis reported by each study is shown in Table 5.
Table 5.
Unit of analysis for included studies
| Unit of analysis | Studies reporting each type of unit of analysis |
|---|---|
| The average of both eyes | 10 studies: Adler 2006; Aller 2006; Chung 2002; Fulk 1996; Fulk 2002; PIR-205 Study; Sankaridurg 2010; Schwartz 1981; Shih 1999; Tan 2005 |
| Right eye only | 7 studies: Cheng 2010; CLAMP Study; Edwards 2002; Houston Study; Katz 2003; MIT study; Yen 1989 |
| The average of both eyes or one eye only* | 2 studies: COMET Study; Hasebe 2008 |
| Right and left eyes reported separately | 2 studies: Jensen 1991; Pärssinen 1989 |
| Eye randomized and treated | 1 study: ATOM study |
| Not reported | 1 study: Yang 2009 |
The average values of both eyes were used if the correlation coefficient was > 0.85 between eyes and the mean difference (MD) was not statistically significant; otherwise the eye with more myopic change was used for each child (COMET Study). The mean of both eyes or right eye only (Hasebe 2008).
Undercorrected versus fully-corrected spectacles
Two studies with a total of 142 participants compared spectacles that undercorrected myopia by approximately −0.50 to −0.75 D, with SVLs that fully corrected myopia (Adler 2006; Chung 2002).
Change in refractive error (Analysis 1.1)
After one year, 72 children who were undercorrected progressed, on average, −0.15 D (95% CI −0.29 to 0.00) more than the 70 SVLs wearers (Figure 3). At two years, Chung 2002 reported faster progression of myopia from baseline for the undercorrection group compared with the full correction group (MD −0.23 D, 95% CI −0.50 to 0.04).
Figure 3.
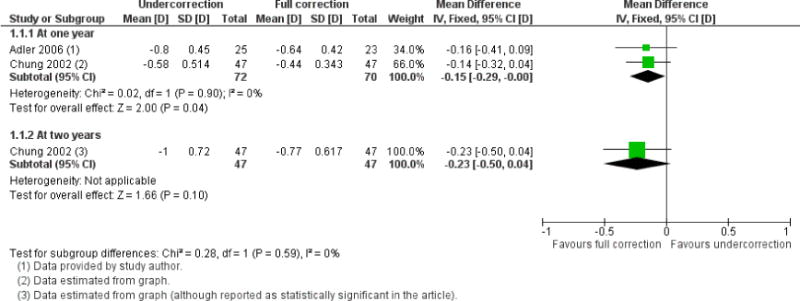
Forest plot of comparison: 1 Undercorrection vs. Full correction spectacles, outcome: 1.2 Change in refractive error from baseline (1 year).
Change in axial length (Analysis 1.2)
Changes in axial length were measured by Chung 2002. The undercorrected group showed greater axial elongation than the fully-corrected group atone year (MD 0.05 mm, 95% CI −0.01 to 0.11) and at two years (MD 0.06 mm, 95% CI −0.04 to 0.16).
Change in corneal radius of curvature
Changes in corneal radius of curvature were not measured by Adler 2006 and reported to be statistically non-significant during the two-year study by Chung 2002.
Adverse effects
Two participants who were undercorrected complained of blurred vision in the study by Adler 2006. No other adverse effects were reported by either Adler 2006 or Chung 2002.
Multifocal spectacles versus single vision lens spectacles
There were six studies that compared bifocal lenses (Cheng 2010; Fulk 1996; Fulk 2002; Houston Study; Jensen 1991; Pärssinen 1989) and five studies that compared progressive addition lenses (PALs) (COMET Study; Edwards 2002; Hasebe 2008; MIT study; Yang 2009) to SVLs to slow the progression of myopia in children. Eight studies were included in quantitative analysis and three studies did not provide adequate data for meta-analysis: one study did not report data for each year of follow-up (Hasebe 2008) and two studies reported outcomes as rates of change per year based on varying follow-up times (Fulk 1996; Houston Study). Of the eight studies that we analyzed quantitatively, six studies reported mean changes from baseline and two reported final values (Edwards 2002; MIT study). Since the studies were randomized with no significant imbalances in potential confounders between groups at baseline, we pooled MDs based on changes from baseline with MDs based on final measurements, given the assumption that these measures address the same underlying intervention effects. With the exception of Pärssinen 1989, which measured refractive error by subjective cycloplegic refraction, the studies included in the analysis used cycloplegic autorefraction for refraction measurements. We included Cheng 2010 in the review following full-text assessment, but subsequently classified it as not being adequately randomized.
Change in refractive error (Analysis 2.1; Analysis 2.2; Analysis 2.3)
At one year follow-up, the average progression was 0.16 D slower (95% CI 0.07 to 0.25) for 633 multifocal (+1.50 to +2.00 near addition) spectacle wearers than for 633 SVL wearers in seven studies (Figure 4). The effect, from a meta-analysis of only three to four trials in each comparison, was similar among PALs wearers (MD 0.17 D, 95% CI 0.10 to 0.24) and bifocal lens wearers (MD 0.16 D, 95% CI 0.01 to 0.32). One study with quantitative data did not report data at one year (Yang 2009). Excluding from the analysis the two studies with MDs based on final values did not influence the result significantly (MD 0.16 D, 95% CI 0.06 to 0.26). Excluding Pärssinen 1989, which used subjective refraction, from the analysis did not influence the result significantly (MD 0.19 D, 95% CI0.10 to 0.27). Excluding Cheng 2010, which was not randomized adequately, from the analysis did not influence the overall result significantly (MD 0.14 D, 95% CI 0.08 to 0.19); however, when excluding Cheng 2010 from the analysis, the I2 for the bifocal subgroup was reduced from74% to 0%.
Figure 4.
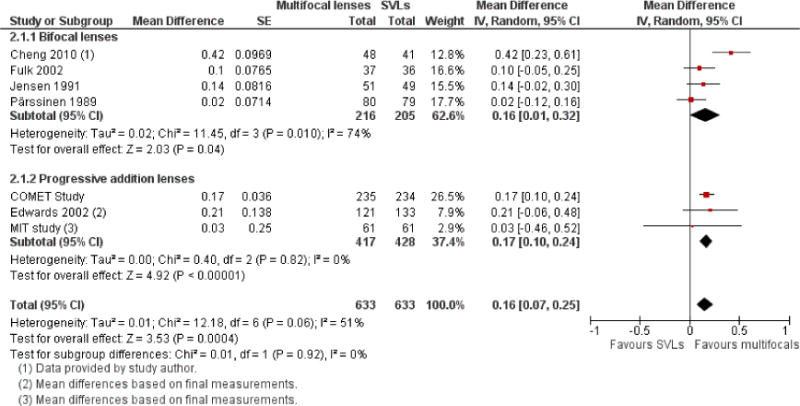
Forest plot of comparison: 2 Multifocal lenses vs. Single vision lenses, outcome: 2.1 Change in refractive error from baseline (1 year).
Seven of the eight studies with quantitative data followed up participants for at least two years, of which four evaluated bifocal lenses and three evaluated PALs (Figure 5). Due to the amount of statistical heterogeneity (I2 = 62%), we did not include these studies in a meta-analysis. Qualitatively, at two years, four studies favored multifocal lenses over SVLs (Cheng 2010; COMET Study; Fulk 2002; Yang 2009); a further two studies favored multifocal lenses over SVLs, although the effect was not statistically significant (Edwards 2002; Jensen 1991); and one study favored SVLs over multifocal lenses, although the effect was not statistically significant (Pärssinen 1989).
Figure 5.
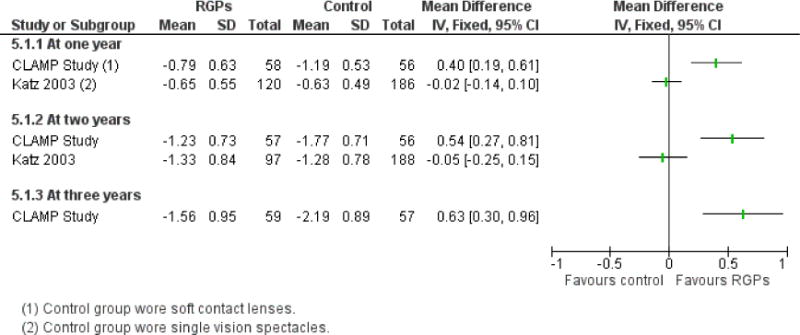
Forest plot of comparison: 5 Rigid gas permeable contact lenses vs. Control, outcome: 5.1 Change in refractive error from baseline.
Two of the eight studies with quantitative data followed up participants for three years (COMET Study; Pärssinen 1989). We did not combine these studies in a meta-analysis due to statistical heterogeneity (I2 = 81.9%). The COMET Study reported a significant MD of 0.19 D (95% CI 0.04 to 0.34) for PAL wearers compared with SVL wearers. Pärssinen 1989 reported a non-significant MD in the opposite direction for bifocal wearers compared with SVL wearers (MD −0.19, 95% CI −0.47 to 0.09).
Three studies not included in the meta-analyses showed mixed effects of multifocal lenses for slowing myopia progression. In a cross-over study of +1.50 PALs versus SVLs, children wearing PALs during the first 18-month treatment period showed significantly less progression than children wearing SVLs (MD 0.31 D, 95% CI 0.11 to 0.51); however, no difference was observed between groups for the second 18-month period (MD 0.02 D, 95% CI −0.17 to 0.21) (Hasebe 2008). In an 18-month study of 14 children assigned to wear +1.25 bifocal lenses and 14 children assigned to wear SVLs, bifocal wearers progressed −0.39 D/year and SVL wearers progressed −0.57 D/year (P = 0.26) (Fulk 1996). The authors noted that during the first year of the study the rate of progression was equal between groups, but during the last six months of the study the SVL group progressed more rapidly than the bifocal group. In a three-arm trial of +1.00 bifocals, +2.00 bifocals, and SVLs, no significant differences were observed between groups after three years of follow-up (Houston Study). The reported average change in refraction error per year during the three-year study for +1.00 bifocals was −0.36 D/year (n = 41), +2.00 bifocals was −0.32 D/year (n = 44) and SVLs was −0.34 D/year (n = 39).
Change in axial length (Analysis 2.4; Analysis 2.5; Analysis 2.6)
Five studies reported axial length outcomes, three of which reported results for one year follow-up (Cheng 2010; COMET Study; Edwards 2002). At one year the summary MD was −0.07 mm (95% CI −0.09 to −0.04) for 404 PAL wearers compared to 408 SVL wears. This was similar to the summary results after two years of follow-up(MD −0.05mm, 95%CI −0.10to −0.01) for two of these studies (COMET Study; Edwards 2002); Cheng 2010 did not report results for two years. After three years of follow-up participants in the COMET Study wearing PALs continued to have less axial elongation compared with participants wearing SVLs (MD −0.11 mm, 95% CI −0.17 to −0.05). Cheng 2010 and Edwards 2002 did not follow up participants at three years.
The three studies that did not report one year data showed treatment effects in the same direction as the studies included in the meta-analysis, although results were not significant in two studies. In a three-arm trial of PALs with or without atropine compared with SVLs, a pair-wise comparison showed participants who wore PALs without atropine had on average 0.10 mm (95% CI 0.00 to 0.20) less axial elongation compared with participants who wore SVLs at 18-months follow-up (MIT study). In the cross-over trial by Hasebe 2008, axial length was not measured at baseline; however, there was no significant difference in axial length between groups after the first 18-month study period (MD −0.08 mm, 95% CI −0.41 to 0.25) and no significant change in axial length was reported between groups after the second 18-month study period (MD −0.01 mm, 95% CI −0.09 to 0.07). In the third study, changes in axial length were not significantly different between bifocal wearers and SVL wearers after 30 months of follow-up (MD −0.09 mm, 95% CI −0.24 to 0.06) (Fulk 2002).
Change in corneal radius of curvature (Analysis 2.7)
Changes in corneal radius of curvature outcomes were reported in three studies. Two studies only stated that differences were not significantly different between treatment and control groups (Edwards 2002; Hasebe 2008). In the COMET Study, neither horizontal measurements nor vertical measurements differed between groups after three years of follow-up (MD 0.00 D, 95% CI −0.15 to 0.15 and MD 0.00 D, 95% CI −0.14 to 0.14, respectively).
Novel lens spectacles versus single vision lens spectacles
One study, which included 210 participants, compared three novel lens designs with SVLs (Sankaridurg 2010). Each of the four study groups included 50 participants, with 10 additional participants allocated to the most radical lens type (novel lens type II) in anticipation of higher attrition in this group. The study originally was planned for two years, but was terminated after the first year due to observing slower progression of myopia than expected for all participants.
Change in refractive error (Analysis 3.1)
Refractive error was measured by cycloplegic autorefraction. At one year, there were no significant differences in myopia progression between novel lens types, either compared with each other or with SVLs.
Change in axial length (Analysis 3.2)
At one year, there were no significant differences in axial length between novel lens types, either compared with each other or with SVLs.
Change in corneal radius of curvature
Corneal radius of curvature was not assessed by Sankaridurg 2010.
Adverse effects
Telephone questionnaires were conducted at one week post-distribution of lenses. At this time 2/50 participants in the type I group, 2/60 participants in the type II group, 5/50 participants in the type III group and 1/50 participants in the SVL group reported noticing blurred side vision. Three participants reported visual distortion, one in the type I group and two in the SVL group. Two participants in the type II group experienced dizziness; for one participant the dizziness resolved after one month, for the other, the dizziness was accompanied by headaches causing the participant to withdraw from the study. Two falls were reported during the study period, both occurred in the type II lens group during the first weeks of the study.
Bifocal soft contact lenses versus single vision soft contact lenses
One study compared bifocal soft contact lenses (BSCLs) with single vision soft contact lenses (SVSCLs) (Aller 2006). There were 38 participants in the BSCL group and 40 in the SVSCL group.
Change in refractive error (Analysis 4.1)
At one year, myopia in the BSCL group progressed significantly slower than in the SVSCL group (MD 0.56 D, 95% CI 0.38 to 0.74). Refractive error was measured by cycloplegic autorefraction.
Change in axial length (Analysis 4.2)
At one year, axial elongation in the BSCL group was significantly less than in the SVSCL group (MD −0.19 mm, 95% CI −0.26 to −0.12).
Change in corneal radius of curvature
Corneal radius of curvature was not assessed by Aller 2006.
Rigid gas permeable contact lenses versus spectacles or soft contact lenses
Two studies investigated the use of rigid gas permeable contact lenses (RGPCLs) in slowing the progression of myopia in children. RGPCLs were compared with soft contact lenses (SCLs) in one study (CLAMP Study) and with SVLs in the other (Katz 2003). The CLAMP Study followed up participants for three years and the Katz 2003 study followed up participants for two years.
Change in refractive error (Analysis 5.1)
Data from the CLAMP Study suggest the use of RGPs to slow the progression of myopia in children compared with SCLs. At one (MD 0.40 D, 95% CI 0.19 to 0.61), two (MD 0.54 D, 95% CI 0.27 to 0.81) and three (MD 0.63 D, 95% CI 0.30 to 0.96) years follow-up, participants wearing RGPs had significantly less progression of myopia compared with participants wearing SCLs (Figure 5). After one and two years of follow-up, no difference in myopia progression was observed between RGP wearers and SVL wearers in the Katz 2003 study (MD −0.02, 95% CI −0.14 to 0.10 and MD −0.05 D, 95% CI −0.25 to 0.15, respectively). Data were not pooled for these studies due to statistical heterogeneity (I2 = 91% at one year and 92% at two years).
Change in axial length (Analysis 5.2)
At one year, meta-analysis of the two studies showed that axial elongation was 0.02 mm (95% CI −0.05 to 0.10) greater for the 176 RGP wearers than the 239 control participants. After two years, it was 0.03 mm greater (95% CI −0.05 to 0.12) for the 154 RGP wearers than the 240 control participants who were followed-up by the two studies. After three years, it was 0.05 mm greater (95% CI −0.12 to 0.22) for the 59 RGP wearers than the 57 SCL participants in the CLAMP Study.
Change in corneal radius of curvature (Analysis 5.3)
Data from the CLAMP Study suggest that the use of RGPs may prevent increases in the corneal radius of curvature compared with SCLs. At one, two and three years follow-up, the MD between participants wearing RGPs and participants wearing SCLs was −0.24 D (95% CI −0.43 to −0.05), −0.38 D (95% CI −0.56 to −0.20) and −0.26 D (95% CI −0.48 to −0.04), respectively. After one year follow-up, the Katz 2003 study also suggested that RGPs may be beneficial compared with SCLs (MD −0.08 D, 95% CI −0.14 to −0.01); however, these results were not statistically significant at two years follow-up (MD −0.06 D, 95% CI −0.14 to 0.02). Data were not pooled for these studies due to statistical heterogeneity (I2 = 60% for year one results and 90% for year two results).
Anti-muscarinic agents versus placebo
Five studies compared topical anti-muscarinic agents with placebo for slowing the progression of myopia in children. Two studies evaluated 2% pirenzepine gel (PIR-205 Study; Tan 2005); two studies evaluated an atropine ophthalmic solution, one at 0.5% (MIT study) and one at 1% (ATOM study); and one study evaluated 1% cyclopentolate ophthalmic solution (Yen 1989). Study groups included in these analyses from the MIT study were also provided with PALs. With the exception of Yen 1989, which measured refractive error by subjective cycloplegic refraction, the studies included in the analysis used cycloplegic autorefraction for refraction measurements.
Change in refractive error (Analysis 6.1; Analysis 6.2)
Due to statistical heterogeneity (I2 = 90%), we did not combine results for all anti-muscarinic agents and instead pooled the subgroups separately. At one year follow-up, the average progression was 0.31 D slower (95% CI 0.17 to 0.44) for participants treated with pirenzepine, 0.80 D slower (95% CI 0.70 to 0.90) for participants treated with atropine and 0.34 D slower (95% CI 0.08 to 0.60) for participants treated with cyclopentolate (Figure 6). The difference in progression between groups continued among participants in the two studies with two years of follow-up: MD 0.41 D (95% CI 0.13 to 0.69) for pirenzepine (PIR-205 Study) and MD 0.92 D (95% CI 0.75 to 1.09) for atropine (ATOM study).
Figure 6.
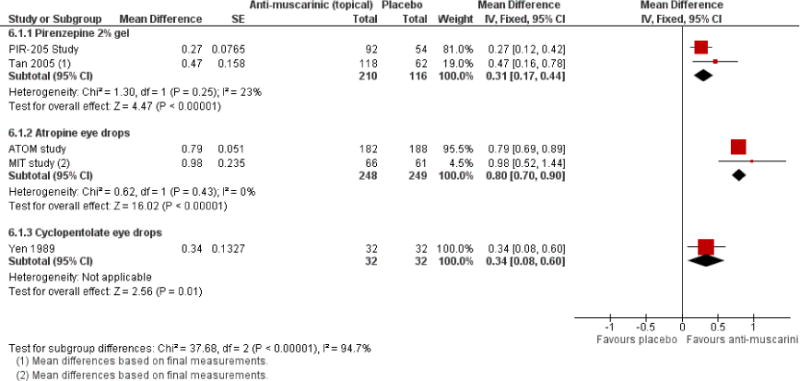
Forest plot of comparison: 6 Anti-muscarinic agents vs. Placebo, outcome: 6.1 Change in refractive error from baseline (1 year).
Change in axial length (Analysis 6.3; Analysis 6.4)
Three studies reported axial length outcomes; however we did not pool results due to statistical heterogeneity (I2 = 96%). At one year follow-up the ATOM study reported significantly less axial elongation for participants assigned to atropine compared with participants assigned to placebo (MD −0.34 mm, 95% CI −0.40 to −0.28). This effect persisted at the end of two years (MD −0.40 mm, 95% CI −0.48 to −0.32). Tan 2005 reported that after one year, the mean increase in axial length was greatest in the placebo/placebo treated group (0.33 mm) compared with the placebo/gel (0.30 mm) and gel/gel (0.20 mm) groups. Although the standard deviations (DSs) for mean changes in axial length were only shown in a graph, the paper reported that there was a statistically significant treatment effect at one year (repeated-measures analysis of variance, P = 0.008). No significant changes in axial length were observed at one year in the PIR-205 Study (MD −0.04 mm, 95% CI −0.15 to 0.07).
Change in corneal radius of curvature
Corneal radius of curvature outcomes were not assessed by studies comparing topical anti-muscarinic agents with placebo.
Adverse effects
Both of the studies evaluating pirenzepine documented ocular and systemic adverse events during the trials (Table 6). Both studies used a significance level of P < 0.15 for reporting adverse events. The three most frequent systemic adverse events reported were headache, common cold and flu syndrome in the PIR-205 Study and increased cough, respiratory infection and rhinitis in Tan 2005. In the PIR-205 Study, events of common cold, rhinitis and sinusitis differed statistically between groups (P < 0.15) and occurred more frequently in the placebo group than the pirenzepine group. In the Tan 2005 study, there were more complaints of abdominal pain in the gel/gel group than the placebo/placebo group (P = 0.065) and more incidents of rash in the placebo/gel group than the placebo/placebo group (P = 0.104). The three most frequent ocular adverse events reported by both studies were symptoms of decreased accommodation, papillae/follicles and medication residue on the eyelids or eyelashes. Six ocular adverse events differed significantly (P < 0.15) between groups in the PIR-205 Study: symptoms of decreased accommodation, papillae/follicles, decreases in visual acuity, eye discomfort and mydriasis occurred more frequently in the pirenzepine treated group and medication residue on the eyelids or eyelashes occurred more frequently in the placebo group. Four ocular adverse events differed significantly (P < 0.15) between groups in Tan 2005: symptoms of decreased accommodation, papillae/follicles and decreases in visual acuity occurred more frequently in the gel/gel and placebo/gel groups, and itchy eyes occurred more frequently in the placebo/gel group compared with the placebo group.
Table 6.
Adverse effects reported by studies of pharmaceutical interventions
| Study | Interventions Studied | Details |
|---|---|---|
| PIR-205 Study | Pirenzepine gel vs. placebo gel | Reported 6 ocular adverse events with P ≤ 0.15
|
| Tan 2005 | Pirenzepine gel and placebo gel
|
Reported 4 ocular adverse events with P ≤ 0.15 (compared to PLC/PLC)
|
| ATOM study | Atropine 1% vs. placebo eye drops | No serious adverse events reported, but reasons for withdrawal among atropine users included: allergic or hypersensitivity reactions or discomfort (4.5%), glare (1.5%), blurred near vision (1%) and logistical difficulties (3.5%) |
| Yen 1989 | Atropine 1% + bifocals vs. cyclopentolate + SVLs vs. placebo + SVLs | All atropine users reported photophobia; most reported that they stopped gym classes and did not like going outdoors. No other systemic or ocular complications were observed |
| Shih 1999 | Atropine 0.5%, 0.25%, 0.1% and tropicamide 0.5% | Three events reported in the atropine 0.5% group: two patients complained of photophobia, one with allergic blepharitis |
PIR: Pirenzepine gel
PLC: Placebo gel
SVLs: single vision lenses
vs: versus
Four studies included in this review evaluated atropine in at least one study arm (ATOM study; MIT study; Shih 1999; Yen 1989); however, only two studies compared atropine with placebo directly (ATOM study; MIT study) and three studies reported adverse effects. In the ATOM study, no serious adverse events were reported, although the four most common reasons for study withdrawal in the atropine group were allergic or hypersensitivity reactions or discomfort (4.5%), logistical difficulties (3.5%), glare (1.5%) and blurred near vision (1%). There were no instances of decreased visual acuity, intraocular pressure changes over 5.5 mmHg, or lenticular, optic disc, or macular changes reported. Yen 1989 reported that all patients in the atropine (plus bifocal lenses) group had photophobia, which was not reported in the cyclopentolate (plus SVLs) or placebo (plus SVLs) groups. Shih 1999 reported three adverse events, all of which occurred in the highest dose atropine group (0.5%): two participants complained of photophobia and one participant had allergic blepharitis.
Timolol drops versus no drops
One study compared 0.25% timolol drops with no drops for slowing the progression of myopia in children (Jensen 1991). Participants in both groups wore SVLs. Refractive error was measured by cycloplegic autorefraction.
Change in refractive error (Analysis 7.1)
There were no statistically significant differences in myopia progression for 46 participants who used timolol compared with 49 participants who did not, at one year (MD −0.05 D, 95% CI −0.21 to 0.11) and at two years (MD −0.04 D, 95% CI −0.30 to 0.22).
Change in axial length
Axial length was not measured by Jensen 1991.
Change in corneal radius of curvature
Corneal radius of curvature was not measured by Jensen 1991.
Other comparisons of interventions
Bifocal spectacles versus SVLs with timolol drops (Analysis 8.1)
In a three-arm trial of +2.00 bifocal lenses, 0.25% timolol drops plus SVLs, and SVLs (Jensen 1991), a pair-wise comparison of bifocal and SVL plus timolol groups suggested use of bifocals slowed the progression of myopia more effectively than SVLs plus timolol drops at one year (MD 0.19 D, 95% CI 0.06 to 0.32) and two years (MD 0.23 D, 95% CI 0.00 to 0.46). Neither intervention when compared with the SVL only group were statistically significant for this study (see Analysis 2.1; Analysis 2.2; Analysis 7.1).
Tropicamide plus bifocal spectacles versus SVLs
In a co-twin study, one twin from each twin pair was randomized to receive either 1% tropicamide once per day and +1.25 bifocals or SVLs. Follow-up was for 3.5 years (Schwartz 1981). No numerical results were presented in the paper. The study authors stated that control twins showed more progression in myopia than their co-twins who received tropicamide and bifocals, but that this difference was not statistically significant.
Atropine plus multifocal spectacles versus placebo plus SVLs (Analysis 9.1; Analysis 9.2)
Two studies compared atropine drops plus multifocal lenses with placebo drops plus SVLs to slow the progression of myopia in children. The MIT study used 0.5% atropine plus PALs and Yen 1989 used 1% atropine plus bifocal lenses. At one year, both studies showed less progression among atropine plus multifocal lens users compared to placebo plus SVL users (summary MD 0.78 D, 95% CI 0.54 to 1.02). At the end of the 18-month MIT study, participants in the atropine plus multifocal lens group had significantly less axial elongation compared with participants in the placebo plus SVL group (MD −0.37 mm, 95% CI −0.47 to −0.27).
Atropine plus bifocal spectacles versus cyclopentolate plus SVLs (Analysis 10.1)
One study compared 1% atropine drops plus bifocal lenses with 1% cyclopentolate drops plus SVLs (Yen 1989). At one year, participants in the atropine plus bifocal lens group had significantly less myopia progression compared with participants in the cyclopentolate plus SVL group (MD 0.36 D, 95% CI 0.11 to 0.61).
Atropine versus tropicamide (Analysis 11.1; Analysis 11.2)
One study compared three doses of atropine with tropicamide (Shih 1999). In the four-arm trial participants were assigned to receive 0.5% atropine drops plus bifocals, 0.25% atropine drops plus slightly undercorrected lenses, 0.1% atropine drops plus fully corrected SVLs, or 0.5% tropicamide drops (control group). At one year follow-up, myopia progression was significantly slowed for each atropine group compared with the tropicamide group, with the highest atropine dose showing the least progression (MD 0.78, 95% CI 0.49 to 1.07 for 0.1% atropine; MD 0.81, 95% CI 0.57 to 1.05 for 0.25% atropine; and MD 1.01, 95% CI 0.74 to 1.28 for 0.5% atropine). This effect was also observed at two years follow-up for each atropine group compared with the tropicamide group (MD 1.95, 95% CI 1.60 to 2.30 for 0.1% atropine; MD 1.98, 95% CI 1.68 to 2.28 for 0.25% atropine; and MD 2.42, 95% CI 2.16 to 2.68 for 0.5% atropine).
DISCUSSION
Summary of main results
Our findings suggest that there is limited evidence favoring full correction of myopia compared with undercorrection. Trials have shown a clinically insignificant benefit with progressive addition lenses (PALs) compared with single vision lenses (SVLs). The effects for axial length and corneal curvature did not support this small beneficial effect for refractive error comparing PALs with SVLs. Evidence supporting the benefit of bifocal lenses compared with SVLs is limited and inconsistent across heterogeneous trials. A single study, at high risk of bias, provided evidence of slower progression of refractive error when using bifocal soft contact lenses (BSCLs) compared with single vision soft contact lenses (SVSCLs). The evidence from this single trial should not be considered conclusive. The evidence regarding the beneficial effect of rigid gas permeable contact lenses (RGPCLs) is conflicting and may be related to the ethnicity of participants and/or the comparator intervention in the included trials. For example, Asian children are more likely to be myopic and their myopia progresses faster than Caucasian children (Lin 1999; Zhan 2000), so any myopia control agent may be more or less effective for Asian children than Caucasian children because the cause of their myopia may be different. A statistically significant effect of 0.63 D (95% CI 0.30 to 0.96) favoring RGPCLs was observed in a trial conducted among children comprised predominantly of Caucasian ethnicity. The comparator was soft contact lenses (SCLs). In a trial of children with Chinese ethnicity, compared with SVLs, we observed a very small, statistically non-significant beneficial effect.
We found consistent evidence favoring anti-muscarinic drugs compared with placebo for reducing the progression of myopia and elongation of the axial length in children with myopia. Atropine resulted in an effect with larger magnitude than pirenzepine or cyclopentolate. There was no trial directly comparing the three different anti-muscarinic drugs. These drugs also were associated with frequent side effects, such as sensitivity to light and blur while reading, which may lead to approximately 15% of children quitting the therapy (ATOM study). One study directly compared atropine versus tropicamide, although the concentration of atropine (0.1% and 0.25%) was much lower than that used in other trials, and found a statistically significant beneficial effect with atropine. Evidence from one trial each suggests that using atropine in addition to either bifocal (at 1% concentration) or PALs (at 0.05% concentration) resulted in slower progression of myopia and lesser elongation of axial length compared with SVLs without the use of atropine.
In summary, we found consistent evidence of a meaningful benefit with using anti-muscarinic drugs for slowing progression of myopia in children. Neither the optimal dose of anti-muscarinic drugs nor the additional value of using anti-muscarinic drugs along with spectacles or contact lenses has been adequately answered by available evidence. The evidence regarding beneficial effects of the other interventions included in this review is neither consistent nor confirmatory.
Overall completeness and applicability of evidence
Several interventions have been investigated by more than one reporting source (journal publication, conference abstract, trial registry, etc.) and they provided sufficient evidence to determine the applicability of the treatment for slowing myopia progression. However, reporting of results was inconsistent among studies, so grouping of findings was difficult. Anti-muscarinic pharmaceutical agents hold the most promise for slowing myopia progression in children, but most investigations reported in the literature lacked complete data to include in the analysis.
The included trials have been conducted across populations of diverse ethnic and geographic locations. The effects that we observed for anti-muscarinic drugs was consistent across studies conducted in Caucasian populations as well as Asian populations.
Quality of the evidence
This review was limited to RCTs, eliminating the chance of treatment selection bias based on participants’ desires for a specific correction. However, not all biases were completely eliminated. For example, participants could not be masked with regard to treatment when they were assigned randomly to spectacles versus contact lenses or to one of two types of contact lenses. Although it is unlikely the participants could influence the outcome of myopic eye growth, they may have been more likely to end participation in a study if they received a treatment that did not interest them, which could potentially increase the risk of bias.
The primary outcome for myopia progression studies typically has been change in refractive error over time; however, as new methods of assessing myopia have become available, the primary outcome has switched to axial growth of the eye. During this transition, both methods have been measured and reported, which may add confusion if the two methods provide differing information. For example, the RGPCL trial by Walline and colleagues found that RGPCLs significantly slowed myopia progression, but they did not slow axial eye growth (CLAMP Study). The change in the primary outcome of myopia control studies therefore could lead to a reporting bias, depending on how the study authors reported the data.
Although most studies used masked examiners to measure primary outcomes, several trials lacked masked examiners or did not report masking in the study, so the risk of examiner bias still exists. The vast majority of studies either did not mask the person analyzing the study data or they did not report whether or not the data were masked. It is important for statisticians to make decisions based solely on available data that should not include treatment allocation in order to reduce or eliminate the potential for reporting bias.
Overall, with the improvement of trials following the CONSORT Statement for RCTs (Schulz 2010), the potential for bias has been largely addressed, but many studies still lack the required rigor of reporting that is necessary to allow the reader to assess the risk of bias in individual trials.
Potential biases in the review process
We reduced the risk of bias during the review process by utilizing a thorough literature search and by not limiting studies that were reviewed based on language or dates. Two review authors, including at least one clinician (JJW, DOM, SAC, JDT) and one methodologist (SSV, KL), independently assessed the search results for eligibility and extracted data. There is little reason to believe that investigations would have been missed by the search methods unless the results of the study were never reported.
Agreements and disagreements with other studies or reviews
Saw 2002a, Saw 2002b and Gwiazda 2009 did not include a systematic and comprehensive literature search and any meta-analyses. The conclusions in these three reviews are consistent with our observations in this systematic review. Other treatments, such as undercorrection of myopia, multifocal spectacles and RGPCLs do not slow the growth of the eye in a clinically meaningful manner (slowing the growth of the eye by 50% or more).
AUTHORS’ CONCLUSIONS
Implications for practice
Based on available evidence, the most effective method of slowing myopia progression is with anti-muscarinic topical medications, but the side effects and limited availability make them a little-used option for myopia control. Further investigations of myopia control must be conducted in order to find a treatment that is clinically meaningful and beneficial and with few adverse effects. The leading potential candidates at this time are corneal reshaping and BSCLs, but much more evidence must be presented in order to determine their abilities to slow myopia progression.
None of the interventions studied have slowed myopia progression in a clinically meaningful manner, with the exception of anti-muscarinic pharmaceutical agents. However, anti-muscarinic pharmaceutical agents either have significant side effects, such as mydriasis and cycloplegia, or they are not commercially available at this time.
Implications for research
Until recently, there have been few RCTs conducted to investigate myopia control. The reporting of results from RCTs was extremely variable. Investigators must compare results to previous investigations and report findings according to the CONSORT statement in order to maximize the potential for combining results of a variety of studies. Future investigators should consider findings from this systematic review in determining the comparisons that should be examined and the patient populations to be studied. We have not found conclusive evidence of the effects of most interventions included in this review, despite our consistent findings for the effects of anti-muscarinic drugs. For example, there is limited evidence on an optimal dose of anti-muscarinic drugs for use in children. The evidence that we examined was limited in several ways including the potential for bias. Future trials should be designed and reported considering the potential for application of novel analytical methods such as multiple treatment meta-analyses. The added value of using anti-muscarinic drugs along with spectacles or contact lenses and the effects of other combinations of interventions in slowing the progression of myopia in children need to be clarified. If future investigators find a clinically and statistically significant treatment effect, they should determine whether the effect continues to be sustained after the treatment is discontinued and attempt to determine the true mechanism of the treatment effect.
Acknowledgments
Iris Gordon, Trials Search Co-ordinator for the Cochrane Eyes and Vision Group, designed and conducted the electronic searches for this version of the review. We acknowledge Milan Mathew’s contribution to the original published protocol as well as the earlier contributions made by Karla Zadnik and Mark A. Bullimore. Drs. Barbara Hawkins, Tianjing Li, and Ann Ervin provided statistical guidance and general comments throughout the process of this review. We would also like to acknowledge Satoshi Hasebe (Okayama University Medical School), Akiko Miki (Johns Hopkins School of Medicine) and Sueko Matsumura (Johns Hopkins School of Public Health) for their assistance with screening Japanese language articles. Sueko Matsumura also contributed to contacting study authors and assisting with data collection.
We acknowledge gratefully the following study authors for providing additional study information and/or data: Aller TA, Cheng D, Chua WH, Gwiazda J, Hasebe S, Katz J, Millodot M, Shih YF and Yen MY.
Richard Wormald (Co-ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
SOURCES OF SUPPORT
Internal sources
Ohio State University, College of Optometry, USA.
Johns Hopkins University, USA.
External sources
National Eye Institute, National Institutes of Health, Contract N01-EY-2-1003, USA.
National Eye Institute, National Institutes of Health, Grant K23-EY00383, USA.
National Eye Institute, National Institutes of Health, Grant U10-08893, USA.
Research to Prevent Blindness, USA.
APPENDICES
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Myopia
#2 myop*
#3 short near sight*
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Eyeglasses
#6 spectacles or glasses
#7 MeSH descriptor Contact Lenses
#8 contact next lens*
#9 MeSH descriptor Mydriatics
#10 mydriat*
#11 MeSH descriptor Muscarinic Antagonists
#12 muscarinic next antagonist*
#13 anti next muscarinic
#14 MeSH descriptor Cholinergic Antagonists
#15 cholinergic next antagonist*
#16 anti next cholinergic
#17 MeSH descriptor Timolol
#18 timolol*
#19 MeSH descriptor Atropine
#20 atropine*
#21 MeSH descriptor Cyclopentolate
#22 cyclopentolate*
#23 MeSH descriptor Phenylephrine
#24 phenylephrine*
#25 MeSH descriptor Pirenzepine
#26 pirenzepine*
#27 MeSH descriptor Tropicamide
#28 tropicamide*
#29 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR # 20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28)
#30 MeSH descriptor Refractive Errors
#31 refract*
#32 MeSH descriptor Accommodation, Ocular
#33 MeSH descriptor Visual Acuity
#34 accommodat* or acuity
#35 progress* or slow* or retard* or funct*
#36 (#30 OR #31 OR #32 OR #33 OR #34 OR #35)
#37 (#4 AND #29 AND #36)
Appendix 2. MEDLINE (OVID) search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp myopia/
myop$.tw.
((short or near) adj3 sight$).tw.
or/13–15
exp eyeglasses/
(spectacles or glasses).tw.
exp contact lenses/
(contact adj2 lens$).tw.
exp mydriatics/
mydriat$.tw.
exp muscarinic antagonists/
(muscarinic adj2 antagonist$).tw.
(anti adj1 muscarinic).tw.
exp cholinergic antagonists/
(cholinergic adj2 antagonist$).tw.
(anti adj1 cholinergic).tw.
exp timolol/
timolol$.tw.
exp atropine/
atropine$.tw.
exp cyclopentolate/
cyclopentolate$.tw.
exp phenylephrine/
phenylephrine$.tw.
exp pirenzepine/
pirenzepine$.tw.
exp tropicamide/
tropicamide$.tw.
or/17–40
exp refractive errors/
refract$.tw.
exp accommodation, ocular/
exp visual acuity/
(accommodat$ or acuity).tw.
(progress$ or slow$ or retard$ or funct$).tw.
or/42–47
16 and 41 and 48
12 and 49
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OVID) search strategy
exp randomized controlled trial/
exp randomisation/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp myopia/
myop$.tw.
((short or near) adj3 sight$).tw.
or/33–35
exp spectacles/
(spectacles or glasses).tw.
exp contact lens/
(contact adj2 lens$).tw.
exp mydriatic agent/
mydriat$.tw.
exp Muscarinic Receptor Blocking Agent/
(muscarinic adj2 antagonist$).tw.
(anti adj1 muscarinic).tw.
exp Cholinergic Receptor Blocking Agent/
(cholinergic adj2 antagonist$).tw.
(anti adj1 cholinergic).tw.
exp timolol/
timolol$.tw.
exp atropine/
atropine$.tw.
exp cyclopentolate/
cyclopentolate$.tw.
exp phenylephrine/
phenylephrine$.tw.
exp pirenzepine/
pirenzepine$.tw.
exp tropicamide/
tropicamide$.tw.
or/37–60
exp refraction error/
refract$.tw.
exp accommodation/
exp visual acuity/
(accommodat$ or acuity).tw.
(progress$ or slow$ or retard$ or funct$).tw.
or/32–67
36 and 61 and 68
32 and 69
Appendix 4. LILACS search terms
myop$ and spectacle$ or glasses or lens$ or mydriatic$ or muscarin$ or cholinergic and refract$ or accommodat$ or acuity or progress$ or slow$ or retard$ or funct$
Appendix 5. metaRegister of Controlled Trials search strategy
(progress or slow or retard) and myopia
Appendix 6. ClinicalTrials.gov search strategy
(Progress OR Slow OR Retard OR Function) AND Myopia
DATA AND ANALYSES
Comparison 1. Undercorrection vs. Full correction spectacles
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At one year | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | −0.15 [−0.29, −0.00] |
| 1.2 At two years | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | −0.23 [−0.50, 0.04] |
| 2 Change in axial length from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At two years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Multifocal lenses vs. Single vision lenses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 7 | 1266 | Mean Difference (Random, 95% CI) | 0.16 [0.07, 0.25] |
| 1.1 Bifocal lenses | 4 | 421 | Mean Difference (Random, 95% CI) | 0.16 [0.01, 0.32] |
| 1.2 Progressive addition lenses | 3 | 845 | Mean Difference (Random, 95% CI) | 0.17 [0.10, 0.24] |
| 2 Change in refractive error from baseline (2 years) | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Bifocal lenses | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Progressive addition lenses | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in refractive error from baseline (3 years) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Bifocal lenses | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Progressive addition lenses | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in axial length from baseline (1 year) | 3 | 812 | Mean Difference (Random, 95% CI) | −0.07 [−0.09, −0.04] |
| 5 Change in axial length from baseline (2 years) | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | −0.05 [−0.10, −0.01] |
| 6 Change in axial length from baseline (3 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Change in corneal radius of curvature from baseline-Horizontal (3 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At three years, horizontal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At three years, vertical | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Novel lens spectacles vs. Single vision lenses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Type I | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Type II | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Type III | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Type I | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Type II | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Type III | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Bifocal soft contact lenses vs. Single vision soft contact lenses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Rigid gas permeable contact lenses vs. Control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At one year | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At two years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in axial length from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At one year | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [−0.05, 0.10] |
| 2.2 At two years | 2 | 394 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [−0.05, 0.12] |
| 2.3 At three years | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [−0.12, 0.22] |
| 3 Change in corneal radius of curvature from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At one year | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At two years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 At three years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Anti-muscarinic agents vs. Placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 5 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 Pirenzepine 2% gel | 2 | 326 | Mean Difference (Fixed, 95% CI) | 0.31 [0.17, 0.44] |
| 1.2 Atropine eye drops | 2 | 497 | Mean Difference (Fixed, 95% CI) | 0.80 [0.70, 0.90] |
| 1.3 Cyclopentolate eye drops | 1 | 64 | Mean Difference (Fixed, 95% CI) | 0.34 [0.08, 0.60] |
| 2 Change in refractive error from baseline (2 years) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pirenzepine 2% gel | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Atropine eye drops | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in axial length from baseline (1 year) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Pirenzepine 2% gel | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Atropine eye drops | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in axial length from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Timolol 0.25% eye drops vs. No eye drops
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At two years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Bifocal spectacles vs. Single vision lenses + Timolol
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At two years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Atropine + Multifocal lenses vs. Placebo + Single vision lenses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 2 | 191 | Mean Difference (Fixed, 95% CI) | 0.78 [0.54, 1.02] |
| 2 Change in axial length from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Atropine + Multifocal lenses vs. Cyclopentolate + Single vision lenses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. Atropine vs. Tropicamide
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in refractive error from baseline (1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Atropine 0.5% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Atropine 0.25% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Atropine 0.1% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in refractive error from baseline (2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Atropine 0.5% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Atropine 0.25% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Atropine 0.1% | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 1.1.
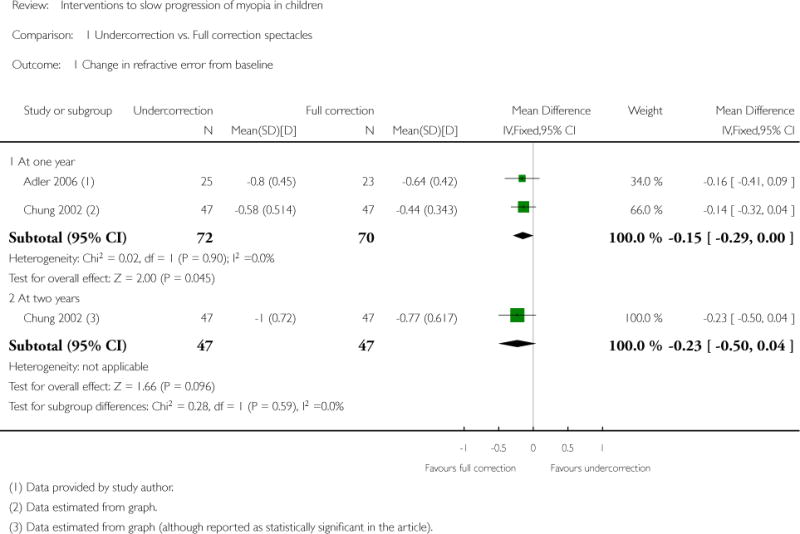
Comparison 1 Undercorrection vs. Full correction spectacles, Outcome 1 Change in refractive error from baseline.
Analysis 1.2.
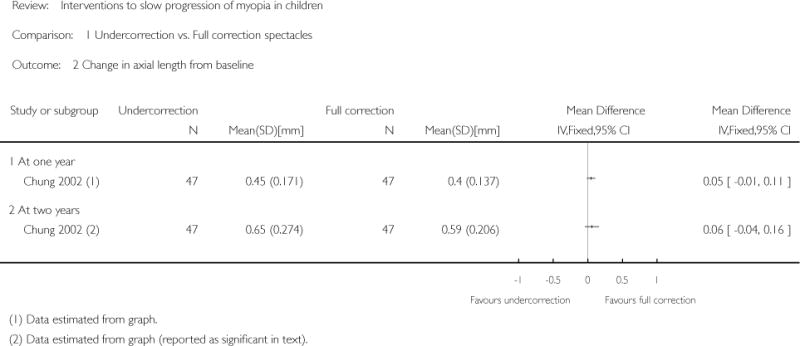
Comparison 1 Undercorrection vs. Full correction spectacles, Outcome 2 Change in axial length from baseline.
Analysis 2.1.
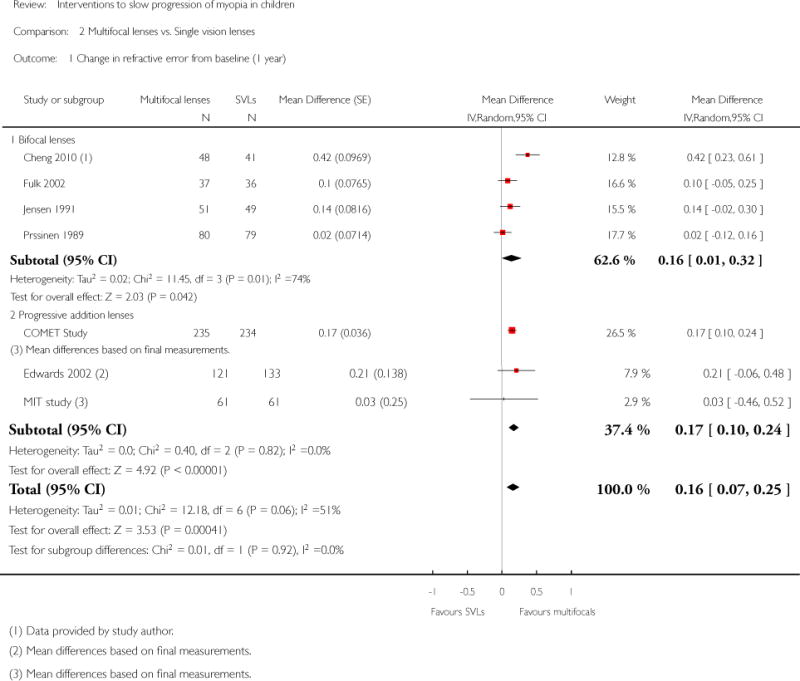
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 2.2.
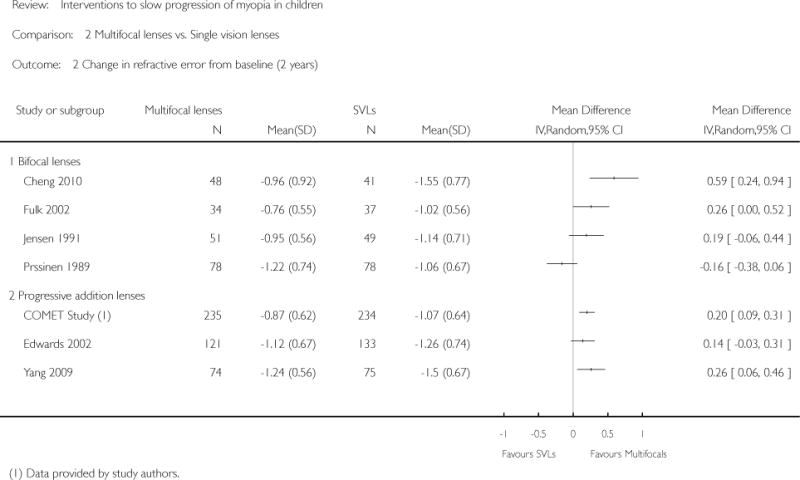
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 2 Change in refractive error from baseline (2 years).
Analysis 2.3.
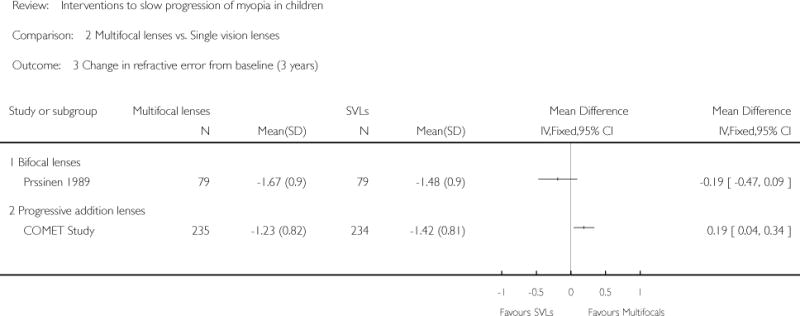
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 3 Change in refractive error from baseline (3 years).
Analysis 2.4.
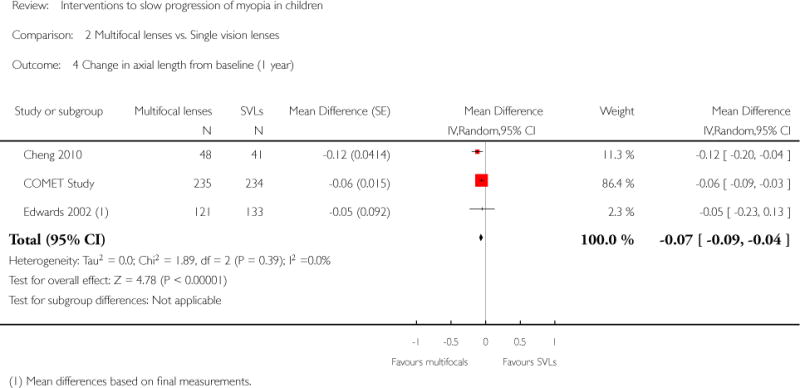
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 4 Change in axial length from baseline (1 year).
Analysis 2.5.
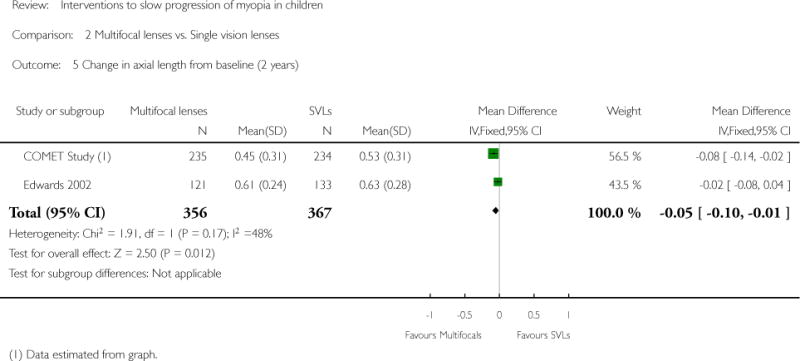
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 5 Change in axial length from baseline (2 years).
Analysis 2.6.
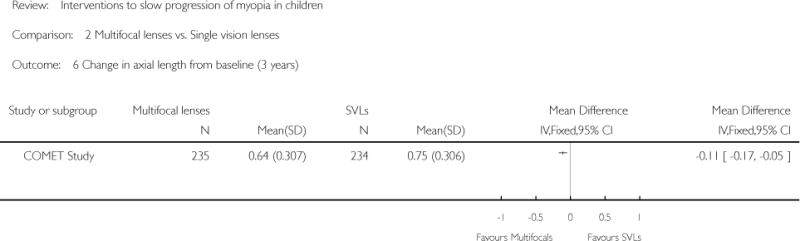
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 6 Change in axial length from baseline (3 years).
Analysis 2.7.
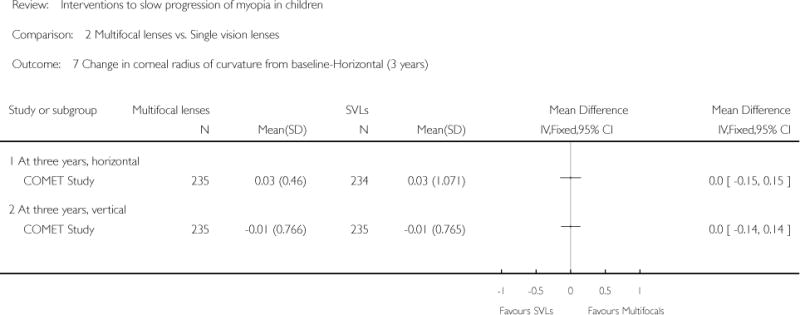
Comparison 2 Multifocal lenses vs. Single vision lenses, Outcome 7 Change in corneal radius of curvature from baseline-Horizontal (3 years).
Analysis 3.1.
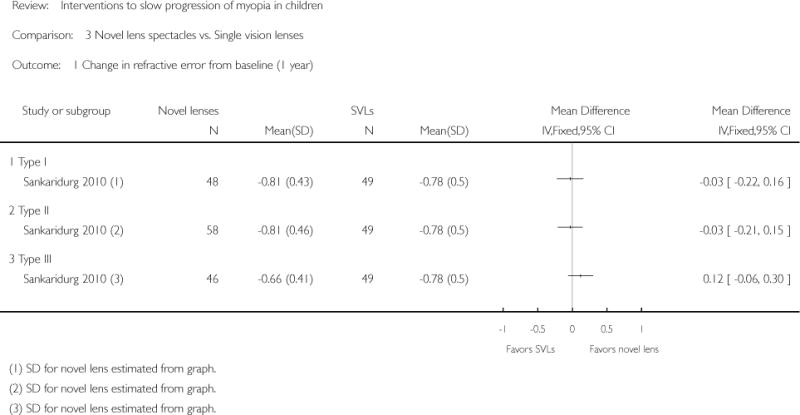
Comparison 3 Novel lens spectacles vs. Single vision lenses, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 3.2.
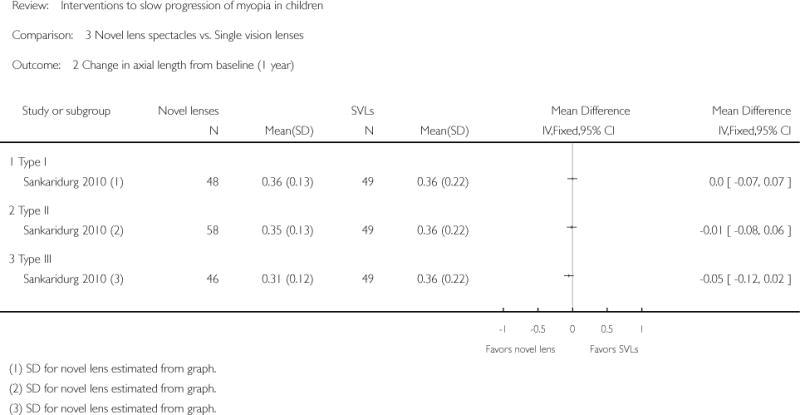
Comparison 3 Novel lens spectacles vs. Single vision lenses, Outcome 2 Change in axial length from baseline (1 year).
Analysis 4.1.
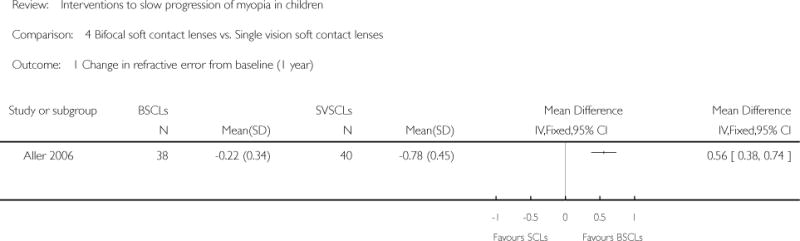
Comparison 4 Bifocal soft contact lenses vs. Single vision soft contact lenses, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 4.2.
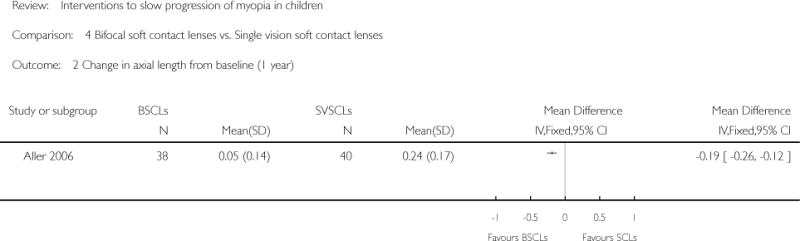
Comparison 4 Bifocal soft contact lenses vs. Single vision soft contact lenses, Outcome 2 Change in axial length from baseline (1 year).
Analysis 5.1.
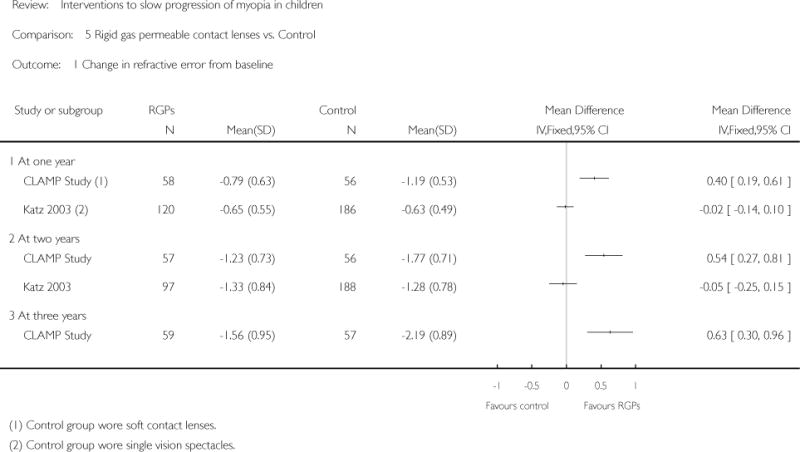
Comparison 5 Rigid gas permeable contact lenses vs. Control, Outcome 1 Change in refractive error from baseline.
Analysis 5.2.
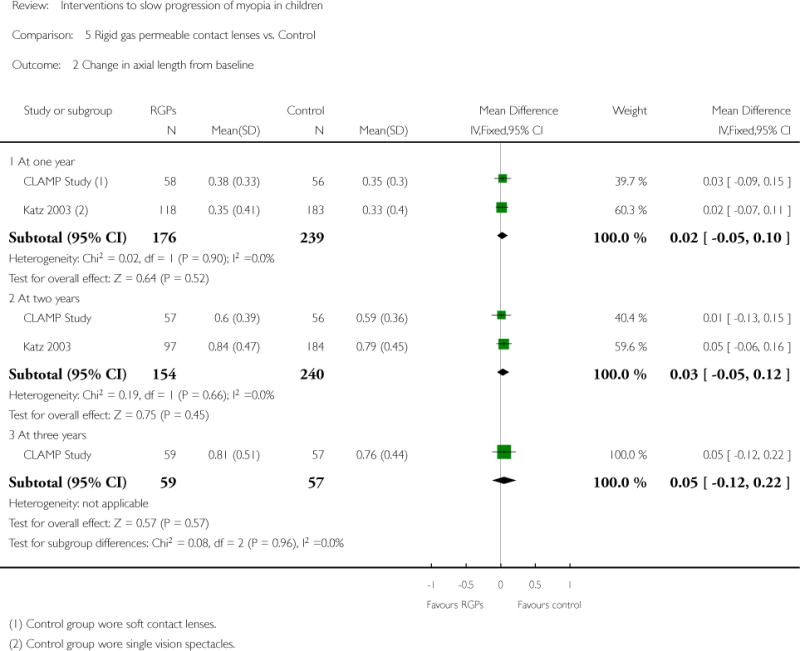
Comparison 5 Rigid gas permeable contact lenses vs. Control, Outcome 2 Change in axial length from baseline.
Analysis 5.3.
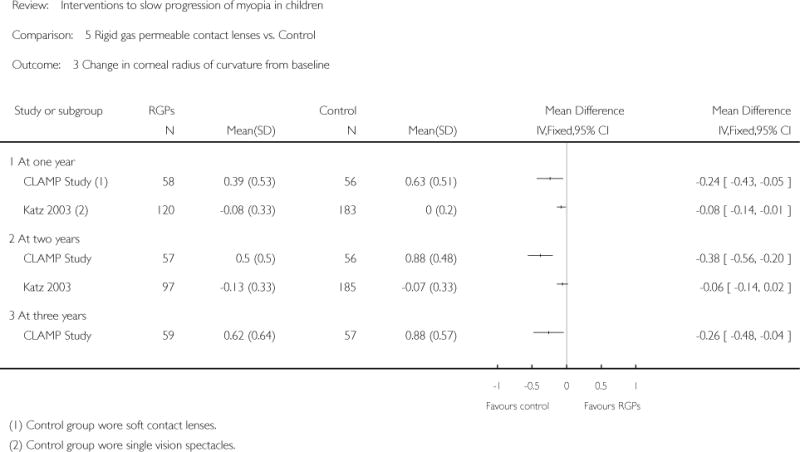
Comparison 5 Rigid gas permeable contact lenses vs. Control, Outcome 3 Change in corneal radius of curvature from baseline.
Analysis 6.1.
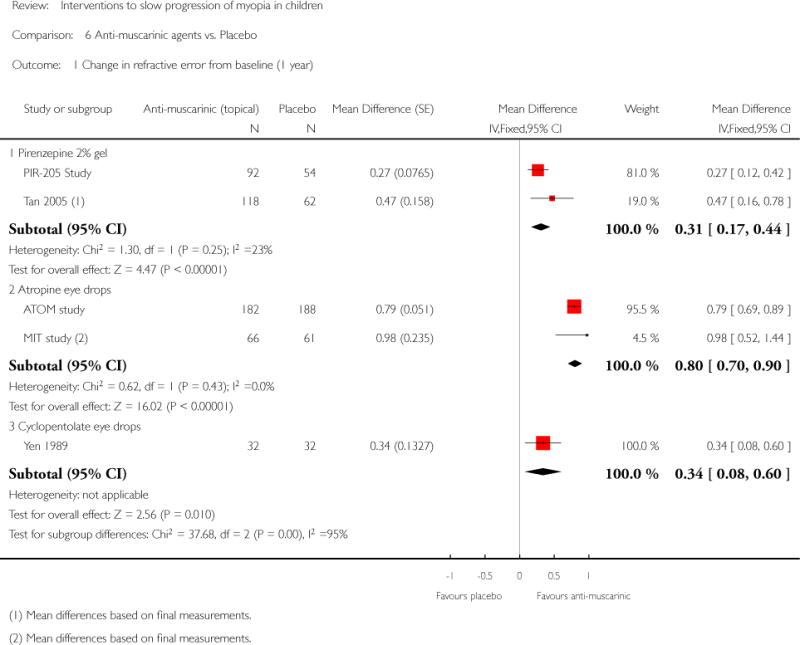
Comparison 6 Anti-muscarinic agents vs. Placebo, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 6.2.
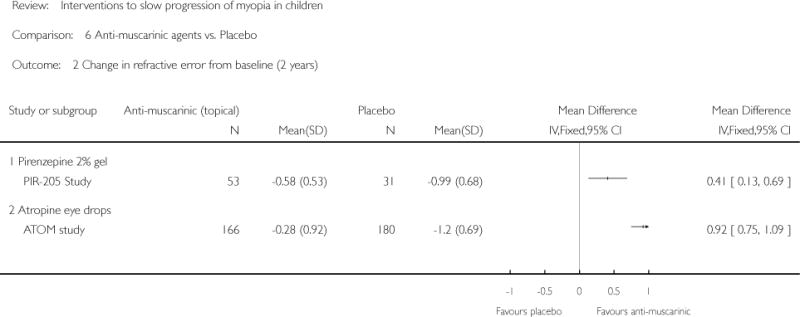
Comparison 6 Anti-muscarinic agents vs. Placebo, Outcome 2 Change in refractive error from baseline (2 years).
Analysis 6.3.
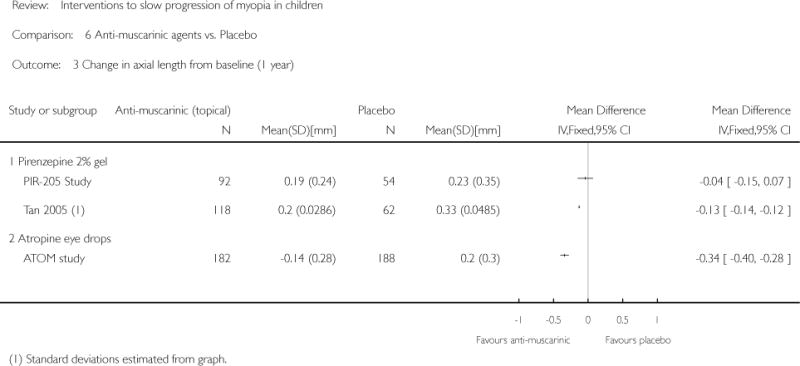
Comparison 6 Anti-muscarinic agents vs. Placebo, Outcome 3 Change in axial length from baseline (1 year).
Analysis 6.4.
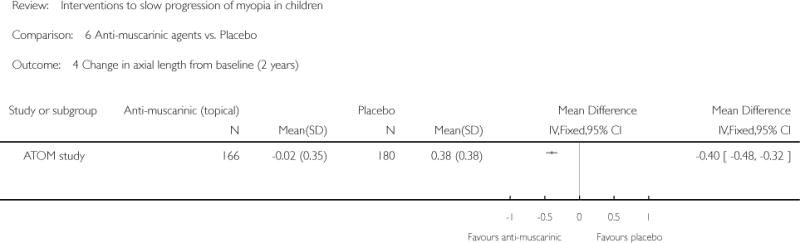
Comparison 6 Anti-muscarinic agents vs. Placebo, Outcome 4 Change in axial length from baseline (2 years).
Analysis 7.1.
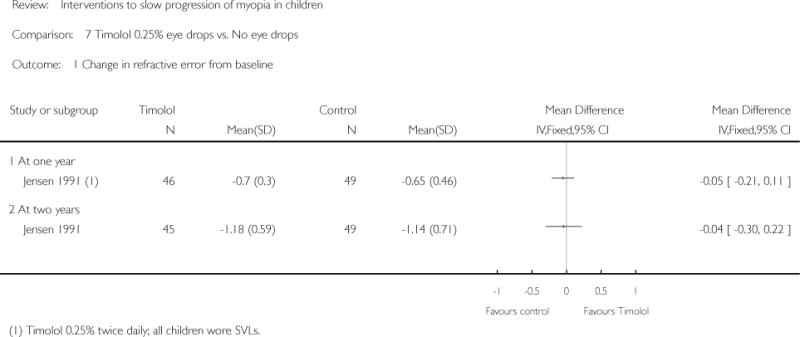
Comparison 7 Timolol 0.25% eye drops vs. No eye drops, Outcome 1 Change in refractive error from baseline.
Analysis 8.1.
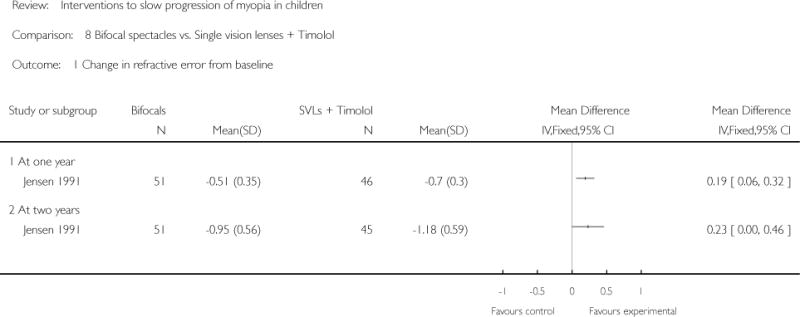
Comparison 8 Bifocal spectacles vs. Single vision lenses + Timolol, Outcome 1 Change in refractive error from baseline.
Analysis 9.1.
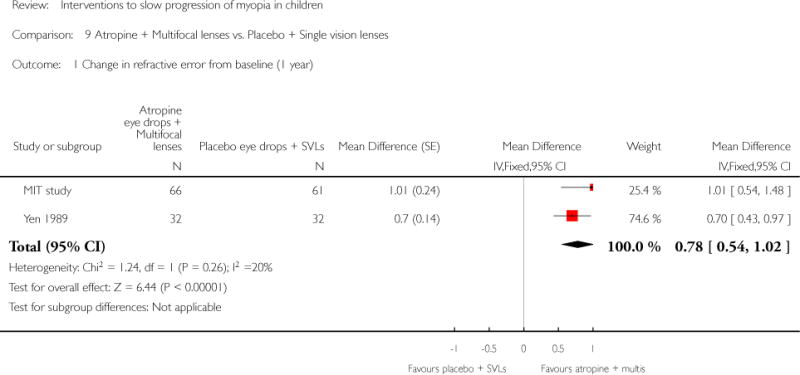
Comparison 9 Atropine + Multifocal lenses vs. Placebo + Single vision lenses, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 9.2.
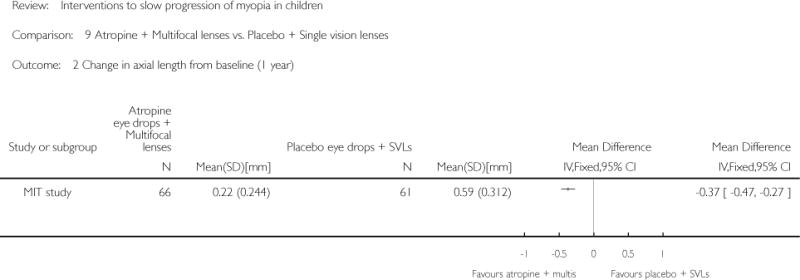
Comparison 9 Atropine + Multifocal lenses vs. Placebo + Single vision lenses, Outcome 2 Change in axial length from baseline (1 year).
Analysis 10.1.
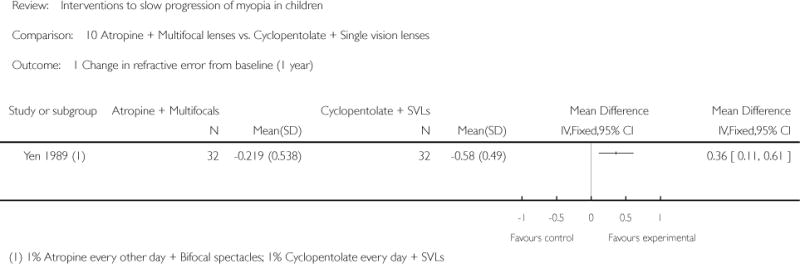
Comparison 10 Atropine + Multifocal lenses vs. Cyclopentolate + Single vision lenses, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 11.1.
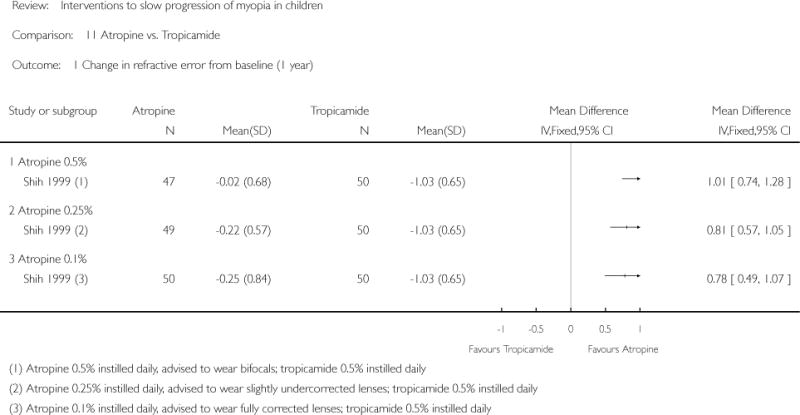
Comparison 11 Atropine vs. Tropicamide, Outcome 1 Change in refractive error from baseline (1 year).
Analysis 11.2.
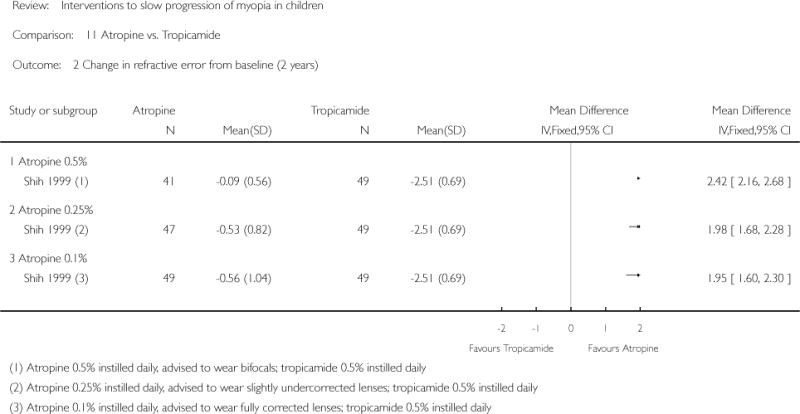
Comparison 11 Atropine vs. Tropicamide, Outcome 2 Change in refractive error from baseline (2 years).
Footnotes
- Conceiving the review: JJW, K Zadnik, DOM
- Designing the review: DOM, JJW, K Zadnik
- Co-ordinating the review: KL, SSV, JJW
- Data collection for the review
-
◦Designing electronic search strategies: CEVG editorial base
-
◦Undertaking manual searches: KL, SSV, JJW
-
◦Screening search results: JJW, KL, SSV, SAC, DOM, JDT
-
◦Organising retrieval of papers: KL, SSV, JJW
-
◦Screening retrieved papers against inclusion criteria: JJW, KL, SSV, SAC, DOM, JDT
-
◦Appraising quality of papers: JJW, KL, SSV, SAC, DOM, JDT
-
◦Extracting data from papers: JJW, KL, SSV, SAC, DOM, JDT
-
◦Writing to authors of papers for additional information: JJW, SSV, KL
-
◦Providing additional data about papers: JJW, SSV
-
◦Obtaining and screening data on unpublished studies: JJW, SSV, KL
-
◦
- Data management for the review
-
◦Entering data into RevMan: JJW, SSV, KL
-
◦Analysis of data: JJW, KL, SSV, SAC, DOM, JDT
-
◦
- Interpretation of data
-
◦Providing a methodological perspective: JJW, KL, SSV, SAC, DOM, JDT
-
◦Providing a clinical perspective: JJW, DOM, SAC, JDT
-
◦Providing a policy perspective: JJW, DOM, SAC, JDT
-
◦Providing a consumer perspective: JJW, DOM, SAC, JDT, SSV, KL
-
◦
- Writing the review: JJW, KL, SSV, SAC, DOM, JDT
- Providing general advice on the review: JJW, JDT, DOM, SAC, K Zadnik
- Securing funding for the review: JJW, DOM, CEVG@US
- Performing previous work that was the foundation of the current study: JJW, DOM, SAC, JDT, K Zadnik, MA Bullimore, M Mathew
DECLARATIONS OF INTEREST
Jeffrey J Walline, OD, PhD was the Principal Investigator of the Contact Lens and Myopia Progression (CLAMP) Study which was an RCT to examine the effects of rigid gas permeable contact lenses (RGPCLs) on myopia progression in children. Susan Cotter, OD, MS was the Principal Investigator on a trial evaluating pirenzepine ophthalmic gel for slowing myopia progression in children. Both studies were included in this review.
Jeffrey J. Walline received research funding, consulted for companies, received honoraria from companies, and has pending grants with companies, all relating to myopia and/or myopia progression. Susan Cotter’s institution received grant funding for participation in the following NIH/NEI-funded multicenter study related to myopia: Correction of Myopia Evaluation Trial — 2 (COMET-2).
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We modified the interventions under review compared with the published protocol. Single vision soft contact lenses (SVSCLs) are considered a control intervention and we did not study them as an active treatment intervention for the purposes of this review. We did not include studies that compared SVSCLs with single vision lenses (SVLs) (spectacles) in this review.
NOTES
This protocol was previously published as ‘Contact lenses for reducing myopia progression in children’ by Walline J, Mathew M, Twelker JD.
References to studies included in this review
*Indicates the major publication for the study
- Adler 2006 {published data only}.Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clinical and Experimental Optometry. 2006;89(5):315–21. doi: 10.1111/j.1444-0938.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Aller 2006 {published data only}.Aller TA. Design of a prospective clinical trial of the use of bifocal soft contact lenses to control myopia progression (CONTROL). 10th International Myopia Conference; 2004. [Google Scholar]
- *.Aller TA, Wildsoet C. Results of a one-year prospective clinical trial (CONTROL) of the use of bifocal soft contact lenses to control myopia progression. Ophthalmic and Physiological Optics. 2006;26(Suppl 1):8–9. [Google Scholar]
- ATOM study {published data only}.Chia A, Chua WH, Tan D. Effect of topical atropine on astigmatism. British Journal of Ophthalmology. 2009;93(6):799–802. doi: 10.1136/bjo.2008.147421. [DOI] [PubMed] [Google Scholar]
- *.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–91. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Chua WH, Tan D, Balakrishnan V, Chan YH, ATOM Study Group Progression of childhood myopia following cessation of atropine treatment. Investigative Ophthalmology and Visual Science. 2005;46 ARVO E-abstract 4625. [Google Scholar]
- Luu CD, Lau AM, Koh AH, Tan D. Multifocal electroretinogram in children on atropine treatment for myopia. British Journal of Ophthalmology. 2005;89(2):151–3. doi: 10.1136/bjo.2004.045526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572–9. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Cheng 2010 {published data only}.Cheng D, Schmid KL, Woo GC, Drobe B. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Archives of Ophthalmology. 2010;128(1):12–9. doi: 10.1001/archophthalmol.2009.332. [DOI] [PubMed] [Google Scholar]
- Chung 2002 {published data only}.Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Research. 2002;42(22):2555–9. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- CLAMP Study {published data only}.Jones-Jordan LA, Walline JJ, Mutti DO, Rah MJ, Nichols KK, Nichols JJ, et al. Gas permeable and soft contact lens wear in children. Optometry and Vision Science. 2010;87(6):414–20. doi: 10.1097/OPX.0b013e3181dc9a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Walline JJ, Jones LA, Mutti DO, Zadnik K. A randomized trial of the effects of rigid contact lenses on myopia progression. Archives of Ophthalmology. 2004;122(12):1760–6. doi: 10.1001/archopht.122.12.1760. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Mutti DO, Zadnik K. The contact lens and myopia progression (CLAMP) study. Ophthalmic and Physiological Optics. 2006;26:29. doi: 10.1097/00006324-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Mutti DO, Zadnik K. Use of a run-in period to decrease loss to follow-up in the Contact Lens and Myopia Progression (CLAMP) study. Controlled Clinical Trials. 2003;24(6):711–8. doi: 10.1016/s0197-2456(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Mutti DO, Jones LA, Rah MJ, Nichols KK, Watson R, et al. The contact lens and myopia progression (CLAMP) study: design and baseline data. Optometry and Vision Science. 2001;78(4):223–33. doi: 10.1097/00006324-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Mutti DO, Jones LA, Zadnik K. Baseline characteristics of the contact lens and myopia progression (CLAMP) study. American Academy of Optometry. 2000:213. [Google Scholar]
- Walline JJ, Zadnik K. Child and parent agreement on report of contact lens wearing time and comfort. American Academy of Optometry. 1999:158. [Google Scholar]
- Walline JJ, Zadnik K. Retention of subjects in clinical trials: The contact lens and myopia progression (CLAMP) example. American Academy of Optometry. 1997:89. [Google Scholar]
- COMET Study {published data only}.Comet Study Group. The design of the correction of myopia evaluation trial. American Academy of Optometry. 1997:130. [Google Scholar]
- Dias L, Hyman L, Manny RE, Fern K, COMET Group Evaluating the self-esteem of myopic children over a three-year period: The COMET Experience. Optometry and Vision Science. 2005;82(4):338–47. doi: 10.1097/01.opx.0000159365.16184.bf. [DOI] [PubMed] [Google Scholar]
- Dias L, Manny R, Hyman L, Fern K, COMET Group Ocular factors and self perception in myopic children. Investigative Ophthalmology and Visual Science. 2001;42 ARVO Abstract 2101. [Google Scholar]
- Dias L, Schoenfeld E, Thomas J, Baldwin C, Mcleod J, Smith J, et al. Reasons for high retention in pediatric clinical trials: Comparison of participant and staff responses in the Correction of Myopia Evaluation Trial. Clinical Trials. 2005;2(5):443–52. doi: 10.1191/1740774505cn113oa. [DOI] [PubMed] [Google Scholar]
- Dong L, Thorn F, Gwiazda J, Hyman L, Norton T, the COMET Group Use of the Gompertz function to describe the course of myopia progression and stabilization in the correction of myopia evaluation trial (COMET) Optometry and Vision Science. 2006;83 E-abstract 065421. [Google Scholar]
- Dong LM, Hyman L, Manny RE, Thomas J, Dias L, McLeod J, et al. Evaluating masking in a randomized, double-masked clinical trial in children with myopia. Optometry and Vision Science. 2006;83(1):46–52. doi: 10.1097/01.opx.0000195564.38235.39. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Dong LM, Everett D, Norton T, COMET Group Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) Cohort. Ophthalmic Epidemiology. 2007;14(4):230–7. doi: 10.1080/01658100701486459. [DOI] [PubMed] [Google Scholar]
- *.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Investigative Ophthalmology and Visual Science. 2003;44(4):1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Marsh-Tootle WL, Hyman L, Hussein M, Norton TT, COMET Group Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET) Investigative Ophthalmology and Visual Science. 2002;43(2):314–21. [PubMed] [Google Scholar]
- Gwiazda JE, Hyman L, Norton T, Hussein M, Marsh-Tootle W, COMET Group Baseline accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investigative Ophthalmology and Visual Science. 2004;45 doi: 10.1167/iovs.03-1306. ARVO E-abstract 2740. [DOI] [PubMed] [Google Scholar]
- Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, COMET Group Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investigative Ophthalmology & Visual Science. 2004;45(7):2143–51. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- Hyman L, Gwiazda J. The Correction of Myopia Evaluation Trial: Lessons from the study design. Annals of the Academy of Medicine, Singapore. 2004;33(1):44–8. [PubMed] [Google Scholar]
- Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Archives of Ophthalmology. 2005;123(7):977–87. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, Hussein M, COMET Group The Correction of Myopia Evaluation Trial (COMET): design and general baseline characteristics. Controlled Clinical Trials. 2001;22(5):573–92. doi: 10.1016/s0197-2456(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, COMET Group The Correction of Myopia Evaluation Trial (COMET): design and baseline characteristics. Investigative Ophthalmology and Visual Science. 1999;40:S754. [Google Scholar]
- Hyman L, Hussein M, Gwiazda J, COMET group Validity of cycloplegic autorefraction and axial length measurements in a clinical trial. Investigative Ophthalmology and Visual Science. 1998;39:4348. [Google Scholar]
- Kowalski PM, Wang Y, Owens RE, Bolden J, Smith JB, Hyman L. Adaptability of myopic children to progressive addition lenses with a modified fitting protocol in the Correction of Myopia Evaluation Trial (COMET) Optometry and Vision Science. 2005;82(4):328–37. doi: 10.1097/01.opx.0000159360.88324.01. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Hyman L, Gwiazda JE, Manny R, Dong LM, COMET Group Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Investigative Ophthalmology and Visual Science. 2007;48(2):562–70. doi: 10.1167/iovs.06-0408. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Manny R, Hussein M. Variability of the ocular component measurements in children using a-scan ultrasonography. Optometry and Vision Science. 2004;81(1):35–43. doi: 10.1097/00006324-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Manny R, Hussein M, COMET group Reliability of ocular components measured by A-scan in children. Optometry and Vision Science. 2000;77:S286. [Google Scholar]
- Manny RE, Deng L, Crossnoe C, Gwiazda J. IOP, myopic progression and axial length in a COMET subgroup. Optometry and Vision Science. 2008;85(2):97–105. doi: 10.1097/OPX.0b013e3181622633. [DOI] [PubMed] [Google Scholar]
- Manny RE, Hussein M, Gwiazda J, Marsh-Tootle W, COMET Group Repeatability of ETDRS visual acuity in children. Investigative Ophthalmology and Visual Science. 2003;44(8):3294–300. doi: 10.1167/iovs.02-1199. [DOI] [PubMed] [Google Scholar]
- Manny RE, Hussein M, Scheiman M, Kurtz D, Niemann K, COMET Group Tropicamide (1%): an effective cycloplegic agent for myopic children. Investigative Ophthalmology and Visual Science. 2001;42(8):1728–35. [PubMed] [Google Scholar]
- Marsh-Tootle W, Gwiazda J, Hyman L, COMET group Refractive phoria and axial measures at baseline in children enrolled in the Correction of Myopia Evaluation Trial (COMET) Investigative Ophthalmology and Visual Science. 1999;40:3992. [Google Scholar]
- Marsh-Tootle WL, Li MD, Weise KK, Fern KD, Gwiazda J, Norton T, et al. Myopia progression in children wearing spectacles vs. switching to contact lenses. Optometry and Vision Science. 2009;86(6):741–7. doi: 10.1097/OPX.0b013e3181a6a250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards 2002 {published data only}.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Investigative Ophthalmology and Visual Science. 2002;43(9):2852–8. [PubMed] [Google Scholar]
- Fulk 1996 {published data only}.Fulk GW, Cyert LA. Can bifocals slow myopia progression? Journal of the American Optometric Association. 1996;67(12):749–54. [PubMed] [Google Scholar]
- Fulk 2002 {published data only}*.Fulk GW, Cyert LA, Parker DE. A randomized clinical trial of bifocal glasses for myopic children with esophoria: results after 54 months. Optometry. 2002;73(8):470–6. [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optometry and Vision Science. 2000;77(8):395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. Baseline characteristics in the Myopia Progression Study, a clinical trial of bifocals to slow myopia progression. Optometry and Vision Science. 1998;75(7):485–92. doi: 10.1097/00006324-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. Seasonal variation in myopia progression and ocular elongation. Optometry and Vision Science. 2002;79(1):46–51. doi: 10.1097/00006324-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE, West RW. The effect of changing from glasses to soft contact lenses on myopia progression in adolescents. Ophthalmic and Physiological Optics. 2003;23(1):71–7. doi: 10.1046/j.1475-1313.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- Hasebe 2008 {published data only}.Hasebe S. A clinical trial to evaluate progressive addition lenses on myopia control. Study design. Japanese Journal of Vision Science. 2002;23:63–8. [Google Scholar]
- Hasebe S, Nakatsuka C, Hamasaki I, Ohtsuki H. Downward deviation of progressive addition lenses in a myopia control trial. Ophthalmic and Physiological Optics. 2005;25(4):310–4. doi: 10.1111/j.1475-1313.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Nonaka F, Nakatsuka C, Ohtsuki H. Myopia control trial with progressive addition lenses in Japanese schoolchildren: baseline measures of refraction, accommodation, and heterophoria. Japanese Journal of Ophthalmology. 2005;49(1):23–30. doi: 10.1007/s10384-004-0131-6. [DOI] [PubMed] [Google Scholar]
- *.Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Investigative Ophthalmology and Visual Science. 2008;49(7):2781–9. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- Nakatsuka C. Assessment of downward deviation of progressive addition lenses in a myopia control study. Investigative Ophthalmology and Visual Science. 2004;45 ARVO E-abstract 2732. [Google Scholar]
- Suemaru J, Hasebe S, Ohtsuki H. Visual symptoms and compliance with spectacle wear in myopic children: double-masked comparison between progressive addition lenses and single vision lenses. Acta Medica Okayama. 2008;62(2):109–17. doi: 10.18926/AMO/30955. [DOI] [PubMed] [Google Scholar]
- Houston Study {published data only}.Grosvenor T, Maslovitz B, Perrigin DM, Perrigin J. The Houston myopia control study: a preliminary report by the patient care team. Journal of the American Optometric Association. 1985;56(8):636–43. [PubMed] [Google Scholar]
- *.Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: a randomized clinical trial. Part II. Final report by the patient care team. American Journal of Optometry and Physiological Optics. 1987;64(7):482–98. [PubMed] [Google Scholar]
- Young FA, Leary GA, Grosvenor T, Maslovitz B, Perrigin DM, Perrigin J, et al. Houston myopia control study: a randomized clinical trial. Part I. Background and design of the study. American Journal of Optometry and Physiological Optics. 1985;62(9):605–13. [PubMed] [Google Scholar]
- Jensen 1991 {published data only}*.Jensen H. Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmologica. 1991;200(Supplement):1–79. [PubMed] [Google Scholar]
- Jensen H. Timolol maleate in the control of myopia. A preliminary report. Acta Ophthalmologica. 1988;185(Supplement):128–9. doi: 10.1111/j.1755-3768.1988.tb02686.x. [DOI] [PubMed] [Google Scholar]
- Katz 2003 {published data only}.Katz J, Schein OD, Levy B, Cruiscullo T, Saw SM, Rajan U, et al. A randomized trial of rigid gas permeable contact lenses to reduce progression of children’s myopia. American Journal of Ophthalmology. 2003;136(1):82–90. doi: 10.1016/s0002-9394(03)00106-5. [DOI] [PubMed] [Google Scholar]
- MIT study {published data only}.Hsiao CK, Chen CJ, Shih YF, Lin LL, Hung PT, Yao CL, et al. Design and statistical analysis for the Myopia Intervention Trial in Taiwan. Myopia Updates II, 7th International Conference on Myopia; Taipei, Taiwan. Nov–Dec, 1998; Springer-Verlag; 2000. pp. 161–4. [Google Scholar]
- Hsiao CK, Tsai MY, Chen HM. Inference of nested variance components in a longitudinal myopia intervention trial. Statistics in Medicine. 2005;24(21):3251–67. doi: 10.1002/sim.2211. [DOI] [PubMed] [Google Scholar]
- *.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmologica Scandinavica. 2001;79(3):233–6. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- PIR-205 Study {published data only}.Bartlett JD, Niemann K, Houde B, Allred T, Edmondson MJ, Crockett RS. A tolerability study of pirenzepine ophthalmic gel in myopic children. Journal of Ocular Pharmacology and Therapeutics. 2003;19(3):271–9. doi: 10.1089/108076803321908392. [DOI] [PubMed] [Google Scholar]
- Chu R, Cotter S, Kwon S, PIR-205 Investigator Group Pirenzepine 2% ophthalmic gel retards myopia progression in 8–12 year old children. American Academy of Optometry. 2003:170. [Google Scholar]
- Cotter SA, Chu RH, Kwon S. Pirenzepine 2% ophthalmic gel retards myopia progression in 8- to 12-year-old children. Optometry. 2003;74:382–3. [Google Scholar]
- Kwon S, Cotter S, Chu R, Flores Y. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: year two. American Academy of Optometry. 2001:144. [Google Scholar]
- Kwon S, Cotter S, Flores Y. Collaborative assessment of myopia progression with pirenzepine (CAMP) study: recruitment underway for fda (pir-205) clinical trial. American Academy of Optometry. 2000:99. [Google Scholar]
- *.Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Ophthalmology. 2004;122(11):1667–74. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, et al. Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Journal of AAPOS. 2008;12(4):332–9. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter SA, Miller JM, Scher CA, Crockett RS, US Pirenzepine, Study Group Pirenzepine 2% ophthalmic gel retards myopic progression in 8–12 year old children over two years. Investigative Ophthalmology and Visual Science. 2004;45 ARVO E-abstract 2733. [Google Scholar]
- Pärssinen 1989 {published data only}.Hemminki E, Pärssinen O. Prevention of myopic progress by glasses. Study design and the first-year results of a randomized trial among schoolchildren. American Journal of Optometry and Physiological Optics. 1987;64(8):611–6. doi: 10.1097/00006324-198708000-00008. [DOI] [PubMed] [Google Scholar]
- Pärssinen O. Astigmatism and school myopia. Acta Ophthalmologica. 1991;69(6):786–90. doi: 10.1111/j.1755-3768.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Pärssinen O. Corneal refraction and topography in school myopia. CLAO Journal. 1993;19(1):69–72. doi: 10.1097/00140068-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Pärssinen O, Hemminki E. Spectacle-use, bifocals and prevention of myopic progression. The two-years results of a randomized trial among schoolchildren. Acta Ophthalmologica. 1988;185(Supplement):156–61. doi: 10.1111/j.1755-3768.1988.tb02698.x. [DOI] [PubMed] [Google Scholar]
- *.Pärssinen O, Hemminki E, Klemetti A. Effect of spectacle use and accommodation on myopic progression: final results of a three-year randomised clinical trial among schoolchildren. British Journal of Ophthalmology. 1989;73(7):547–51. doi: 10.1136/bjo.73.7.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Investigative Ophthalmology and Visual Science. 1993;34(9):2794–802. [PubMed] [Google Scholar]
- Pärssinen TO. Long-term connection of myopic progression with different background variables. Myopia Updates II: Proceedings of the 7th International Conference on Myopia; Taipei, Taiwan. 1998; Springer-Verlag; 2000. pp. 21–3. [Google Scholar]
- Pärssinen TO. Progression of school myopia from its onset at the mean age of 10.9 Years: 13-year follow-up study. Myopia Updates II: Proceedings of the 7th International Conference on Myopia; Taipei, Taiwan. 1998; Springer-Verlag; 2000. pp. 25–8. [Google Scholar]
- Sankaridurg 2010 {published data only}.Sankaridurg P, Donovan L, Varnas S, Ho A, Chen X, Martinez A, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optometry and Vision Science. 2010;87(9):631–41. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz 1981 {published data only}.Schwartz JT. A monozygotic co-twin control study of a treatment for myopia. Acta Geneticae Medicae et Gemellologiae. 1976;25:133–6. doi: 10.1017/s0001566000013994. [DOI] [PubMed] [Google Scholar]
- Schwartz JT. Results of a monozygotic co-twin control study on a treatment for myopia. Acta Geneticae Medicae et Gemellologiae. 1980;29(1):30. doi: 10.1017/s0001566000013994. [DOI] [PubMed] [Google Scholar]
- *.Schwartz JT. Results of a monozygotic co-twin control study on a treatment for myopia. Progress in Clinical & Biological Research. 1981;Pt C:249–58. [PubMed] [Google Scholar]
- Shih 1999 {published data only}.Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics. 1999;15(1):85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- Tan 2005 {published data only}.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS, Asian Pirenzepine Study Group One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112(1):84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Yang 2009 {published data only}.Yang Z, Lan W, Ge J, Liu W, Chen X, Chen L, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic and Physiological Optics. 2009;29(1):41–8. doi: 10.1111/j.1475-1313.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- Yen 1989 {published data only}.Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Annals of Ophthalmology. 1989;21(5):180–2. 187. [PubMed] [Google Scholar]
References to studies excluded from this review
- Abraham 1966 {published data only}.Abraham SV. Control of myopia with tropicamide. Journal of Pediatric Ophthalmology. 1966;3:10–22. [Google Scholar]
- ACHIEVE study {published data only}.Jones-Jordan LA, Chitkara M, Coffey B, Jackson JM, Manny RE, Rah MJ, et al. A comparison of spectacle and contact lens wearing times in the ACHIEVE Study. Clinical and Experimental Optometry. 2010;93(3):157–63. doi: 10.1111/j.1444-0938.2010.00480.x. [DOI] [PubMed] [Google Scholar]
- Rah MJ, Walline JJ, Jones-Jordan LA, Sinnott LT, Jackson JM, ACHIEVE Study Group Vision specific quality of life of pediatric contact lens wearers. Optometry and Vision Science. 2010;87(8):560–6. doi: 10.1097/OPX.0b013e3181e6a1c8. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Chitkara M, Coffey B, Jackson JM, ACHIEVE Study Group The Adolescent and Child Health Initiative to encourage vision empowerment (ACHIEVE) study. American Academy of Optometry. 2004 doi: 10.1097/01.opx.0000195566.94572.eb. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Chitkara M, Coffey B, Jackson JM, ACHIEVE Study Group The Adolescent and Child Health initiative to encourage vision empowerment (Achieve) study design and baseline data. Optometry and Vision Science. 2006;83(1):37–45. doi: 10.1097/01.opx.0000195566.94572.eb. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott L, Chitkara M, Coffey B, ACHIEVE Study Group Randomized trial of the effect of contact lens wear on self-perception in children. Optometry and Vision Science. 2009;86(3):222–32. doi: 10.1097/OPX.0b013e3181971985. [DOI] [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott L, Manny RE, Gaume A, ACHIEVE Study Group A randomized trial of the effect of soft contact lenses on myopia progression in children. Investigative Ophthalmology and Visual Science. 2008;49(11):4702–6. doi: 10.1167/iovs.08-2067. [DOI] [PubMed] [Google Scholar]
- Aller 2008 {published data only}.Aller TA, Wildsoet C. Bifocal soft contact lenses as a possible myopia control treatment: a case report involving identical twins. Clinical and Experimental Optometry. 2008;91(4):394–9. doi: 10.1111/j.1444-0938.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- Andreo 1990 {published data only}.Andreo LK. Long-term effects of hydrophilic contact lenses on myopia. Annals of Ophthalmology. 1990;22(6):224–7. [PubMed] [Google Scholar]
- Baldwin 1969 {published data only}.Baldwin WR, West D, Jolley J, Reid W. Effects of contact lenses on refractive corneal and axial length changes in young myopes. American Journal of Optometry and Archives of American Academy of Optometry. 1969;46(12):903–11. doi: 10.1097/00006324-196912000-00002. [DOI] [PubMed] [Google Scholar]
- Baltimore myopia project {published data only}.Betts AE. An evaluation of the Baltimore myopia control project part A. Experimental procedures. Journal of the American Optometric Association. 1947;18:481–5. [Google Scholar]
- Ewalt HW., Jr The Baltimore myopia control project. Journal of the American Optometric Association. 1946;17:167–85. [Google Scholar]
- Baronet 1979 {published data only}.Baronet Lecaillon-Thiobon. Longitudinal study of developing myopia and effects of treatment with atropine and Difrarel E [Étude longitudinale des myopies évolutives et effets du traitement par atropine et Difrarel E] Bulletin des Societes d Ophtalmologie de France. 1979;79(4–5):417–21. [PubMed] [Google Scholar]
- Bedrossian 1979 {published data only}.Bedrossian RH. The effect of atropine on myopia. Annals of Ophthalmology. 1971;3(8):891–7. [PubMed] [Google Scholar]
- Bedrossian RH. The effect of atropine on myopia. Ophthalmology. 1979;86(5):713–9. doi: 10.1016/s0161-6420(79)35455-0. [DOI] [PubMed] [Google Scholar]
- Bedrossian RH. XX Concilium Ophthalmologicum Germania. Amsterdam: Excerpta Medica Foundation; 1966. Treatment of progressive myopia with atropine; pp. 612–7. (International Congress Series No. 146). [Google Scholar]
- Berkeley OK Study {published data only}.Brand RJ, Polse KA, Schwalbe JS. The Berkeley Orthokeratology Study, Part I: General conduct of the study. American Journal of Optometry and Physiological Optics. 1983;60(3):175–86. doi: 10.1097/00006324-198303000-00005. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ. Contact lens effects on ametropia: a current example of the clinical trial. American Journal of Optometry and Physiological Optics. 1981;58(4):281–8. [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Schwalbe JS, Vastine DW, Keener RJ. The Berkeley Orthokeratology Study, Part II: Efficacy and duration. American Journal of Optometry and Physiological Optics. 1983;60(3):187–98. doi: 10.1097/00006324-198303000-00006. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Vastine DW, Schwalbe JS. Corneal change accompanying orthokeratology. Plastic or elastic? Results of a randomized controlled clinical trial. Archives of Ophthalmology. 1983;101(12):1873–8. doi: 10.1001/archopht.1983.01040020875008. [DOI] [PubMed] [Google Scholar]
- Bier 1988 {published data only}.Bier N, Lowther G. Myopia Control Study: effect of different contact lens refractive corrections on the progression of myopia. Optometry Today. 1988;28:38–40. [Google Scholar]
- Brodstein 1984 {published data only}.Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals: a long-term prospective study. Ophthalmology. 1984;91(11):1373–9. doi: 10.1016/s0161-6420(84)34138-0. [DOI] [PubMed] [Google Scholar]
- Chou 1997 {published data only}.Chou AC, Shih YF, Ho TC, Lin LL. The effectiveness of 0.5% atropine in controlling high myopia in children. Journal of Ocular Pharmacology and Therapeutics. 1997;13(1):61–7. doi: 10.1089/jop.1997.13.61. [DOI] [PubMed] [Google Scholar]
- Dumbleton 1999 {published data only}.Dumbleton K, Richter D, Fonn D, Chalmers R. Refractive and keratometric changes following extended wear. American Academy of Optometry. 1998:170. [Google Scholar]
- *.Dumbleton KA, Chalmers RL, Richter DB, Fonn D. Changes in myopic refractive error with nine months’ extended wear of hydrogel lenses with high and low oxygen permeability. Optometry and Vision Science. 1999;76(12):845–9. doi: 10.1097/00006324-199912000-00020. [DOI] [PubMed] [Google Scholar]
- Dyer 1979 {published data only}.Dyer JA. Role of cycloplegics in progressive myopia. Ophthalmology. 1979;86(5):692–4. doi: 10.1016/s0161-6420(79)35459-8. [DOI] [PubMed] [Google Scholar]
- Ebri 2007 {published data only}.Ebri A, Kuper H, Wedner S. Cost-effectiveness of cycloplegic agents: Results of a randomized controlled trial in Nigerian children. Investigative Ophthalmology and Visual Science. 2007;48(3):1025–31. doi: 10.1167/iovs.06-0604. [DOI] [PubMed] [Google Scholar]
- Filip 2000 {published data only}.Filip M, Stefaniu I, Stefan C. Changes in myopic refractive errors after 9 months of extensive wear of hydrogel lenses with high oxygen permeability and compared with those with low permeability. Oftalmologia. 2000;51(2):35–40. [PubMed] [Google Scholar]
- Gimbel 1973 {published data only}.Gimbel HV. The control of myopia with atropine. Canadian Journal of Ophthalmology. 1973;8(4):527–32. [PubMed] [Google Scholar]
- Goss 1984 {published data only}.Goss DA. Overcorrection as a means of slowing myopic progression. American Journal of Optometry and Physiological Optics. 1984;61(2):85–93. doi: 10.1097/00006324-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Grosvenor 1991 {published data only}.Grosvenor T, Perrigin D, Perrigin J, Quintero S. Do rigid gas-permeable contact lenses control the progress of myopia? Contact Lens and Spectacles. 1991;6(7):29–35. [Google Scholar]
- Horner 1999 {published data only}.Horner DG, Salmon TO, Soni PS. Junior high age children’s myopia progression in soft lenses vs. spectacles. American Academy of Optometry. 1994:78. [Google Scholar]
- Horner DG, Salmon TO, Soni PS. Junior high age children’s myopia progression in soft lenses vs. spectacles. American Academy of Optometry. 1995:96. [Google Scholar]
- Horner DG, Soni PS, Ross J. Junior high age children’s myopia progresses equally in soft lenses and spectacles. Investigative Ophthalmology and Visual Science. 1994;35 Abstract number 735. [Google Scholar]
- Horner DG, Soni PS, Salmon TO, Swartz TS. Myopia progression in adolescent wearers of soft contact lenses and spectacles. Optometry and Vision Science. 1999;76(7):474–9. doi: 10.1097/00006324-199907000-00023. [DOI] [PubMed] [Google Scholar]
- Terry RL, Soni PS, Horner DG. Spectacles, contact lenses, and children’s self-concepts: a longitudinal study. Optometry and Vision Science. 1997;74(12):1044–8. [PubMed] [Google Scholar]
- Hosaka 1982 {published data only}.Hosaka A, Abiko Y, Teranishi C. Topical use of labetalol in the treatment of pseudomyopia. Proceedings from the 2nd International Conference on Myopia; San Francisco. 1978; New York: Myopia International Research Foundation Proceedings; 1982. pp. 339–52. [Google Scholar]
- Hosaka 1988 {published data only}.Hosaka A. Myopia prevention and therapy. The role of pharmaceutical agents. Japanese studies. Acta Ophthalmologica. 1988;185(Supplement):130–1. doi: 10.1111/j.1755-3768.1988.tb02687.x. [DOI] [PubMed] [Google Scholar]
- Huffman 2002 {published data only}.Huffman KD, Ross S, Pack L, Salmon T, Hoenes R. Visual and optical performance of frequency 55 aspheric vs. spheric contact lenses. American Academy of Optometry. 2002 [Google Scholar]
- Kao 1988 {published data only}.Kao SC, Lu HY, Liu JH. Atropine effect on school myopia. A preliminary report. Acta Ophthalmologica. 1988;185(Supplementum):132–3. doi: 10.1111/j.1755-3768.1988.tb02688.x. [DOI] [PubMed] [Google Scholar]
- Keller 1996 {published data only}.Keller J. Myopia control with RGPs in children. Contact Lens and Spectacles. 1996;11(12):45–8. [Google Scholar]
- Kennedy 1995 {published data only}.Kennedy RH. Progression of myopia. Transactions of the American Ophthalmological Society. 1995;93:755–800. [PMC free article] [PubMed] [Google Scholar]
- Khoo 1999 {published data only}.Khoo CY, Chong J, Rajan U. A 3-year study on the effect of RGP contact lenses on myopic children. Singapore Medical Journal. 1999;40(4):230–7. [PubMed] [Google Scholar]
- Lakkis 2006 {published data only}.Lakkis C, Weidemann K. Evaluation of the performance of photochromic spectacle lenses in children and adolescents aged 10 to 15 years. Clinical and Experimental Optometry. 2006;89(4):246–52. doi: 10.1111/j.1444-0938.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- Leung 1999 {published data only}.Brown B, Edwards MH. Is esophoria a factor in slowing of myopia by progressive lenses? Author’s response. Optometry and Vision Science. 2003;80(3):199. doi: 10.1097/00006324-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Brown B, Edwards MH, Leung JT. Is esophoria a factor in slowing of myopia by progressive lenses? Optometry and Vision Science. 2002;79(10):638–42. doi: 10.1097/00006324-200210000-00009. [DOI] [PubMed] [Google Scholar]
- *.Leung JT, Brown B. Progression of myopia in Hong Kong Chinese schoolchildren is slowed by wearing progressive lenses. Optometry and Vision Science. 1999;76(6):346–54. doi: 10.1097/00006324-199906000-00013. [DOI] [PubMed] [Google Scholar]
- Li 2005 {published data only}.Li JP, Wang W. Effect of progressive multifocal lenses for juvenile myopia in 876 cases. International Journal of Ophthalmology. 2005;5(3):599–601. [Google Scholar]
- Liang 2008 {published data only}.Liang CK, Ho TY, Li TC, Hsu WM, Li TM, Lee YC, et al. A combined therapy using stimulating auricular acupoints enhances lower-level atropine eyedrops when used for myopia control in school-aged children evaluated by a pilot randomized controlled clinical trial. Complementary Therapies in Medicine. 2008;16(6):305–10. doi: 10.1016/j.ctim.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Lu 2010 {published data only}.Lu PC, Chen JC. Retarding progression of myopia with seasonal modification of topical atropine. Journal of Ophthalmic and Vision Research. 2010;5(2):75–81. [PMC free article] [PubMed] [Google Scholar]
- Mandell 1959 {published data only}.Mandell RB. Myopia control with bifocal correction. American Journal of Optometry and Archives of American Academy of Optometry. 1959;36:652–8. doi: 10.1097/00006324-195912000-00005. [DOI] [PubMed] [Google Scholar]
- Meythaler 1971 {published data only}.Meythaler H, Ruppert W. The myopic and miotic effect of pilocarpin and glaucostat [Vergleichende Untersuchungen über den myopisierenden und miotischen Effekt von Pilocarpin und Aceclydine (Glaucostat)] Albrecht Von Graefe’s Archive for Clinical and Experimental Ophthalmology. 1971;181(3):234–45. doi: 10.1007/BF02390254. [DOI] [PubMed] [Google Scholar]
- Neetens 1985 {published data only}.Neetens A, Evens P. The use of bifocals as alternative in the management of low grade myopia. Bulletin de la Societe Belge d Ophtalmologie. 1985;214:79–85. [PubMed] [Google Scholar]
- Nesterov 1990 {published data only}.Nesterov AP, Svirin AV, Lapochkin VI. Drug therapy of progressive myopia. Vestnik Oftalmologii. 1990;106(2):25–8. [PubMed] [Google Scholar]
- Oakley 1975 {published data only}.Oakley KH, Young FA. Bifocal control of myopia. American Journal of Optometry and Physiological Optics. 1975;52(11):758–64. doi: 10.1097/00006324-197511000-00005. [DOI] [PubMed] [Google Scholar]
- Parker 1958 {published data only}.Parker MW. Protective-corrective program for young myopes. Optometry Weekly. 1958;49:681–3. [Google Scholar]
- Perrigin 1990 {published data only}.Grosvenor T, Perrigin J, Perrigin D, Quintero S. Use of silicone-acrylate contact lenses for the control of myopia: Results after two years of lens wear. Optometry and Vision Science. 1989;66(1):41–7. doi: 10.1097/00006324-198901000-00013. [DOI] [PubMed] [Google Scholar]
- *.Perrigin J, Perrigin D, Quintero S, Grosvenor T. Silicone-acrylate contact lenses for myopia control: 3-year results. Optometry and Vision Science. 1990;67(10):764–9. doi: 10.1097/00006324-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Pirenzepine 2003 {published data only}.Pirenzepine for the treatment of myopia [Schärfer sehen mit Pirenzepin] Deutsche Apotheker Zeitung. 2003;143(12):54. [Google Scholar]
- Pritchard 1999 {published data only}.Pritchard N, Fonn D. Myopia associated with extended wear of low-oxygen-transmissible hydrogel lenses. American Academy of Optometry. 1999:169. [Google Scholar]
- Rah 2002 {published data only}.Rah MJ, Jackson JM, Jones LA, Marsden HJ, Bailey MD, Barr JT. Overnight orthokeratology: preliminary results of the Lenses and Overnight Orthokeratology (LOOK) study. Optometry and Vision Science. 2002;79(9):598–605. doi: 10.1097/00006324-200209000-00011. [DOI] [PubMed] [Google Scholar]
- Rainey 2000 {published data only} Rainey BB, Goss DA. The effect of vision therapy on myopia progression. American Academy of Optometry. 2000:283. [Google Scholar]
- Ritchey 2005 {published data only}.Ritchey ER, Barr JT, Mitchell GL. The comparison of overnight lens modalities (COLM) study. Eye and Contact Lens. 2005;31(2):70–5. doi: 10.1097/01.icl.0000146323.18919.13. [DOI] [PubMed] [Google Scholar]
- Sankaridurg 2003 {published data only}.Sankaridurg PR, Sweeney DF, Holden BA, Naduvilath T, Velala I, Gora R, et al. Comparison of adverse events with daily disposable hydrogels and spectacle wear: results from a 12-month prospective clinical trial. Ophthalmology. 2003;110(12):2327–34. doi: 10.1016/S0161-6420(03)00795-4. [DOI] [PubMed] [Google Scholar]
- Savoliuk 1968 {published data only}.Savoliuk MM. Optical correction and progressive myopia. Vestnik Oftalmologii. 1968;81(1):82–3. [PubMed] [Google Scholar]
- Shimmyo 2003 {published data only}.Shimmyo M, Rho DS. Retardation of myopic progression and axial growth in human children by muscarinic inhibitor. American Academy of Ophthalmology. 2003:143–4. [Google Scholar]
- Soni 2006 {published data only}.Soni PS, Nguyen TT. Overnight orthokeratology experience with XO material. Eye & Contact Lens. 2006;32(1):39–45. doi: 10.1097/01.icl.0000172285.44187.c4. [DOI] [PubMed] [Google Scholar]
- Stone 1976 {published data only}.Stone J. Contact lens wear in the young myope. British Journal of Physiological Optics. 1973;28:90–134. [Google Scholar]
- Stone J. The possible influence of contact lenses on myopia. British Journal of Physiological Optics. 1976;31(3):89–114. [PubMed] [Google Scholar]
- Sun 2007 {published data only}.Sun J, Li Y. Study on near-distance reading addition controlling the aggravating of adolescent myopia. Chinese Ophthalmic Research. 2007;25(6):462–4. [Google Scholar]
- Syniuta 2001 {published data only}.Syniuta LA, Isenberg SJ. Atropine and bifocals can slow the progression of myopia in children. Binocular Vision and Strabismus Quarterly. 2001;16(3):203–8. [PubMed] [Google Scholar]
- Takano 1964 {published data only}.Takano J. Treatment of myopia by the installation of tropicamide. Japanese Journal of Clinical Ophthalmology. 1964;18:45–50. [PubMed] [Google Scholar]
- Toki 1960 {published data only}.Toki T. Treatment of myopia with local use of Neosynephrine hydrochloride. Japanese Journal of Clinical Optometry. 1960;14:248–52. [Google Scholar]
- Tokoro 1964 {published data only}.Tokoro T, Kabe S. Treatment of myopia and changes in optical components. I. Topical application of Neosynephrine and N-ethyln9gamma-picolyl)-tropamide. Nippon Ganka Gakkai Zasshi. 1964;68(13):1958–61. [PubMed] [Google Scholar]
- Tokoro 1965 {published data only}.Tokoro T, Kabe S. Treatment of the myopia and the changes in optical components. Report II. Full-or under-correction of myopia by glasses. Nippon Ganka Gakkai Zasshi. 1965;69(2):140–4. [PubMed] [Google Scholar]
- Xiao 2009 {published data only}.Xiao ZG, Tao LJ, Guo Y, Wang P. Effect of rigid gas permeable contact lenses in controlling the progress of high myopia in children. International Journal of Ophthalmology. 2009;9(5):991–3. [Google Scholar]
- Yamada 2004 {published data only}.Yamada Y. Myopia in primary school children. Japanese Journal of Clinical Ophthalmology. 2004;58(2):125–9. [Google Scholar]
- Yamaji 1967 {published data only}.Yamaji R, Sakiyama A, Yoshihara M, Furuta I, Nakamura R. Clinical study of the effect of tropic acid amide on the visual acuity and refraction in myopic children. Part VII. Nippon Ganka Kiyo – Folia Ophthalmologica Japonica – Bulletin of Japanese Ophthalmology. 1967;18(3):333–45. [PubMed] [Google Scholar]
- *.Yamaji R, Yoshihara M, Sakiyama A, Ishikawa K, Furuta I. Group therapy with tropic acid amide (Mydrin M) against pseudo-myopia in primary school children. Bulletin of the Osaka Medical School. 1967;13(1):42–51. [PubMed] [Google Scholar]
- Yi 2011 {published data only}.Yi JH, Li RR. Influence of near-work and outdoor activities on myopia progression in school children. Chinese Journal of Contemporary Pediatrics. 2011;13(1):32–5. [PubMed] [Google Scholar]
- Young 1992 {published data only}.Young G, Port M. Rigid gas-permeable extended wear: a comparative clinical study. Optometry and Vision Science. 1992;69(3):214–26. [PubMed] [Google Scholar]
- Zeng 2009 {published data only}.Zeng Y, Keay L, He M, Mai J, Munoz B, Brady C, et al. A randomized, clinical trial evaluating ready-made and custom spectacles delivered via a school-based screening program in China. Ophthalmology. 2009;116(10):1839–45. doi: 10.1016/j.ophtha.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Anstice 2011 {published data only}.Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118(6):1152–61. doi: 10.1016/j.ophtha.2010.10.035. [DOI] [PubMed] [Google Scholar]
- ATOM 2 {published data only}.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2011 doi: 10.1016/j.ophtha.2011.07.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chua WH. Establishing an optimal dose of atropine for slowing progression of childhood myopia. Ophthalmic and Physiological Optics. 2006;26:29. [Google Scholar]
- COMET 2 {published data only}.Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Investigative Ophthalmology and Visual Science. 2011;52(5):2749–57. doi: 10.1167/iovs.10-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Berntsen 2011 {published data only}.Berntsen DA, Mutti DO, Zadnik K. Study of theories about myopia progression (STAMP) design and baseline data. Optometry and Vision Science. 2011;87(11):823–32. doi: 10.1097/OPX.0b013e3181f6f776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Aller 2010.Aller TA. Nearsightedness research. www.draller.com/sightresearch (accessed 26 August 2010)
- Barsam 2010.Barsam A, Allan BDS. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database of Systematic Reviews. 2010;(Issue 5) doi: 10.1002/14651858.CD007679.pub2. [DOI] [PubMed] [Google Scholar]
- Blum 1959.Blum H, Bettman J. Vision Screening for Elementary Schools: The Orinda Study. Berkeley: University of California Press; 1959. [Google Scholar]
- Braun 1996.Braun CI, Freidlin V, Sperduto RD, Milton RC, Strahlman ER. The progression of myopia in school age children: data from the Columbia Medical Plan. Ophthalmic Epidemiology. 1996;3(1):13–21. doi: 10.3109/09286589609071597. [DOI] [PubMed] [Google Scholar]
- Chen 1985.Chen CJ, Cohen BH, Diamond EL. Genetic and environmental effects on the development of myopia in Chinese twin children. Ophthalmic Paediatrics and Genetics. 1985;6(1–2):353–9. [PubMed] [Google Scholar]
- Deeks 2011.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Chapter 9: Analysing data and undertaking meta-analyses. Available from www.cochrane-handbook.org. [Google Scholar]
- Duffey 2003.Duffey RJ, Leaming D. US trends in refractive surgery: 2002 ISRS survey. Journal of Refractive Surgery. 2003;19(3):357–63. doi: 10.3928/1081-597X-20030501-14. [DOI] [PubMed] [Google Scholar]
- Fonn 1988.Fonn D, Holden BA. Rigid gas-permeable vs. hydrogel contact lenses for extended wear. American Journal of Optometry and Physiological Optics. 1988;65(7):536–44. doi: 10.1097/00006324-198807000-00003. [DOI] [PubMed] [Google Scholar]
- Garner 1999.Garner LF, Owens H, Kinnear RF, Frith MJ. Prevalence of myopia in Sherpa and Tibetan children in Nepal. Optometry and Vision Science. 1999;76(5):282–5. doi: 10.1097/00006324-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Glanville 2006.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Goss 1987.Goss DA. Cessation age of childhood myopia progression. Ophthalmic and Physiological Optics. 1987;7(2):195–7. doi: 10.1111/j.1475-1313.1987.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Goss 1990.Goss DA. Variables related to the rate of childhood myopia progression. Optometry and Vision Science. 1990;67(8):631–6. doi: 10.1097/00006324-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Gwiazda 1993.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology and Visual Science. 1993;34(3):690–4. [PubMed] [Google Scholar]
- Gwiazda 1995.Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Research. 1995;35(9):1299–304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- Gwiazda 2009.Gwiazda J. Treatment options for myopia. Optometry and Vision Science. 2009;86(6):624–8. doi: 10.1097/OPX.0b013e3181a6a225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond 2001.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Investigative Ophthalmology and Visual Science. 2001;42(6):1232–6. [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Chapter 8: Assessing risk of bias in included studies. Available from www.cochrane-handbook.org. [Google Scholar]
- Jones 2007.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Investigative Ophthalmology and Visual Science. 2007;48:3524–32. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin 1999.Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, et al. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optometry and Vision Science. 1999;76(5):275–81. doi: 10.1097/00006324-199905000-00013. [DOI] [PubMed] [Google Scholar]
- MacRae 1991.MacRae S, Herman C, Stulting RD, Lippman R, Whipple D, Cohen E, et al. Corneal ulcer and adverse reaction rates in premarket contact lens studies. American Journal of Ophthalmology. 1991;111(4):457–65. doi: 10.1016/s0002-9394(14)72381-5. [DOI] [PubMed] [Google Scholar]
- Maul 2000.Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. American Journal of Ophthalmology. 2000;129(4):445–54. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- Mutti 2006.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, et al. Accommodative lag before and after the onset of myopia. Investigative Ophthalmology and Visual Science. 2006;47(3):837–46. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- Pararajasegaram 1998.Pararajasegaram R. Vision 2020 – the right to sight: from strategies to action. American Journal of Ophthalmology. 1999;128(3):359–60. doi: 10.1016/s0002-9394(99)00251-2. [DOI] [PubMed] [Google Scholar]
- Park 2004.Park DJ, Congdon NG. Evidence for an “epidemic” of myopia. Annals of the Academy of Medicine, Singapore. 2004;33(1):21–6. [PubMed] [Google Scholar]
- RevMan 2011.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- Rose 2008.Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Saw 2000.Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Factors related to the progression of myopia in Singaporean children. Optometry and Vision Science. 2000;77(10):549–54. doi: 10.1097/00006324-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Saw 2001.Saw SM, Wu HM, Seet B, Wong TY, Yap E, Chia KS, et al. Academic achievement, close up work parameters, and myopia in Singapore military conscripts. British Journal of Ophthalmology. 2001;85(7):855–60. doi: 10.1136/bjo.85.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw 2002a.Saw SM, Gazzard G, Au Eong KG, Tan DT. Myopia: attempts to arrest progression. British Journal of Ophthalmology. 2002;86(11):1306–11. doi: 10.1136/bjo.86.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw 2002b.Saw SM, Shih-Yen EC, Koh A, Tan D. Interventions to retard myopia progression in children: an evidence-based update. Ophthalmology. 2002;109(3):415–21. doi: 10.1016/s0161-6420(01)00972-1. [DOI] [PubMed] [Google Scholar]
- Schein 1989.Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. New England Journal of Medicine. 1989;321(12):773–8. doi: 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- Schulz 2010.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortt 2006.Shortt AJ, Allan BDS. Photorefractive keratectomy (PRK) versus laser-assisted in-situ keratomileusis (LASIK) for myopia. Cochrane Database of Systematic Reviews. 2006;(Issue 2) doi: 10.1002/14651858.CD005135.pub2. [DOI] [PubMed] [Google Scholar]
- Sperduto 1983.Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Archives of Ophthalmology. 1983;101(3):405–7. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- Troilo 1987.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Current Eye Research. 1987;6(8):993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Voo 1998.Voo I, Lee DA, Oelrich FO. Prevalences of ocular conditions among Hispanic, white, Asian, and black immigrant students examined by the UCLA Mobile Eye Clinic. Journal of the American Optometric Association. 1998;69(4):255–61. [PubMed] [Google Scholar]
- Young 1969.Young FA, Leary GA, Baldwin WR, West DC, Box RA, Harris E, et al. The transmission of refractive errors within eskimo families. American Journal of Optometry and Archives of American Academy of Optometry. 1969;46(9):676–85. doi: 10.1097/00006324-196909000-00005. [DOI] [PubMed] [Google Scholar]
- Zadnik 1994.Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children’s eye size. JAMA. 1994;271(17):1323–7. [PubMed] [Google Scholar]
- Zadnik 2003.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in schoolchildren as a function of age and gender. Optometry and Vision Science. 2003;80(3):226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Zhan 2000.Zhan MZ, Saw SM, Hong RZ, Fu ZF, Yang H, Shui YB, et al. Refractive errors in Singapore and Xiamen, China — A comparative study in school children aged 6 to 7 years. Optometry and Vision Science. 2000;77(6):302–8. doi: 10.1097/00006324-200006000-00010. [DOI] [PubMed] [Google Scholar]


