Abstract
Polybrominated diphenyl ethers (PBDEs) are flame retardant chemicals used in consumer products. They are common contaminants in human serum and associated with adverse health effects. Our objectives were to characterize PBDE serum concentrations in a New England cohort and assess temporal variability of this exposure biomarker over a one-year period. We collected three repeated measurements at six-month intervals from 52 office workers from the greater Boston (MA, United States) area from 2010 to 2011. The intraclass correlation coefficient for BDEs 28, 47, 99, 100, and 153 ranged from 0.87 to 0.99, indicating that a single serum measurement can reliably estimate exposure over a one-year period. This was true for both lipid adjusted and nonlipid adjusted concentrations. The kappa statistics, quantifying the level of agreement of categorical exposure classification, based on medians, tertiles, or quartiles ranged from 0.67 to 0.90. Some congeners showed nonsignificant increases from sampling round 1 (winter) to round 2 (summer) and significant decreases from round 2 to round 3 (winter). This study highlights the high reliability of a single serum PBDE measurement for use in human epidemiologic studies.
Introduction
Polybrominated diphenyl ethers (PBDEs) are additive flame retardant chemicals used since the 1970s in commercial and household products. The technical formulation PentaBDE is composed of PBDE congeners containing three to six bromines, primarily BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153. It was used in furniture containing polyurethane foam to meet fire standards such as California Technical Bulletin 117. BDE-153 also occurs in the OctaBDE technical formulation used in electronics. Of the worldwide production, 95% of the PentaBDE produced was consumed in North America,1 where concentrations of several PentaBDE congeners in the environment and people are approximately an order of magnitude higher than those reported in Europe or Asia.2−5
Because of their persistence, lipophilicity, ability to bioaccumulate, and concerns regarding adverse effects on human health, production of PentaBDE and OctaBDE is now prohibited by the Stockholm Convention, an international treaty that governs persistent organic pollutants.6 The main U.S. chemical manufacturers withdrew PentaBDE and OctaBDE from production in 2004.7 Despite the current restrictions, human exposure is still occurring due to the release of these flame retardant chemicals from existing products and through contaminated foods.8,9 In the United States, incidental ingestion of dust and diet are the dominant routes of human exposure.10,11 Unlike the highly brominated BDE-183 and BDE-209 that have half-lives on the order of weeks to months,12 the half-lives of the major PentaBDE congeners have not been directly measured in humans, but have been estimated to be on the order of years.13 These half-life estimates are uncertain because the calculations assumed steady state conditions and compared body burdens with uncertain exposure estimates.
Toxicological studies have demonstrated that PBDEs, particularly of the PentaBDE formulations, adversely affect endocrine homeostasis14 and neurodevelopment,15 and have reproductive effects.16 Recently, epidemiological studies conducted in the United States have linked PBDE exposure to adverse effects on neurodevelopment,17−19 and altered thyroid and reproductive hormone levels.20−22
Exposure assessment is a critical element of environmental epidemiology. Exposure measurement error occurs when a study participant is assigned an exposure that is different from their true exposure over the biologically relevant time period. Such error, even if independent of outcome, can lead to biased effect estimates. It may even completely remove a true association between an exposure and outcome of interest.23 With continuous exposures, epidemiologists typically use either a continuous exposure measure (e.g., serum concentration) or place participants into categories (e.g., low, medium, or high). Kappa statistics quantify the amount of agreement between an initial exposure categorization and an exposure categorization at a later point in time (e.g., did a participant in the high exposure category remain in the high exposure category later). Intraclass correlation coefficients (ICCs) evaluate continuous exposure measures. If the amount of variability over time within subjects is small compared to variability between subjects, the ICC will be close to 1 and the exposure metric is considered reliable. For example, a high ICC indicates that highly exposed people tend to remain high relative to other people. Both of these analyses require a cohort with repeated exposure measures. There are currently no studies of this kind that have evaluated the potential amount of PBDE exposure misclassification in epidemiological studies.
Our objectives were to characterize PBDE serum concentrations in a New England cohort and assess temporal variability of this exposure biomarker over a one-year period. Additionally, we assessed demographic characteristics and serum lipid concentrations as predictors of serum PBDE concentrations.
Experimental Section
Study Design and Population
We recruited a convenience sample of 52 adults living and working in the greater Boston (MA, United States) metropolitan area from winter 2010 to summer 2011 to participate in the Flame Retardant Exposure Study (FlaRE Study). Eligible subjects had to be healthy, nonsmoking adults over the age of 18, working in an office environment at least 20 h a week, and planning to reside in the greater Boston metropolitan area for the study duration. Participants were excluded for having a prior diagnosis of thyroid or male reproductive disease or if they were pregnant. The City of Boston requires that furniture in public spaces meet certain fire codes.24
We conducted three sampling rounds: Round 1 (1/13/10–4/15/10), Round 2 (6/3/10–9/15/10), and Round 3 (1/31/11–4/27/11). Serum samples were provided by 49 of 51 (96%) participants in Round 1, 50 of 52 (96%) participants in Round 2, and 42 of 52 (81%) participants in Round 3. One participant was added in Round 2. The missing blood samples were due to the following reasons: phlebotomist was unable to conduct venipuncture, participant declined, participant moved out of study area, or participant could no longer be contacted. All blood samples were nonfasting.
During each sampling visit, study personnel administered a questionnaire designed to collect basic demographic and health information. The Boston University Medical Center Institutional Review Board approved the study protocol and all subjects gave written informed consent prior to participation. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
Blood Samples
A trained phlebotomist collected 30 mL of blood from each participant during each sampling round. Blood samples were processed on the day of collection and serum samples were stored at −80 °C in amber glass vials until analysis. To eliminate potential issues with interassay variability, serum samples collected from all three rounds were analyzed at one time following Round 3. Serum samples were analyzed for lipids (total triglycerides, total cholesterol) and 11 PBDE congeners (BDE-17, BDE-28, BDE-47, BDE-66, BDE-85, BDE-99, BDE-100, BDE-153, BDE-154, BDE-183, BDE-209) at the CDC using established methods.25 Final analytic determination of the PBDE congeners was performed by gas chromatography isotope dilution high-resolution mass spectrometry using a MAT95XP (ThermoFinnigan MAT, Bremen, Germany) instrument. Samples were randomized and analyzed with quality control (QC) (n = 3) and blank samples (n = 3) in each batch of 24 unknowns. The coefficient of variation of included QC samples was less than 10%. All concentration data were reported as background subtracted, where correction was made based on the median amount present in blank samples. Limits of detection (LOD) were calculated as the highest of two methods: (i) 3 times the standard deviation of the method blank samples and (ii) as the lowest point in the calibration curve having a signal-to-noise ratio greater than 3 (primarily for analytes with low to no detectable method blank concentration).
Statistical Analysis
For measurements below the LOD, we substituted 1/2 LOD. PBDE congeners were log-normally distributed, as identified by histograms and Shapiro–Wilks tests, and thus log-transformed. We calculated round-specific geometric means (GM) and geometric standard deviations (GSD) for congeners detected in >50% of the samples. ΣPBDEs is defined here as the sum of BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance is reported at the 0.05 level.
We calculated kappa statistics to assess the amount of agreement between exposure categories in Round 1 and Round 3, the two winter time points. We used three exposure classification schemes: median (low, high), tertile (low, medium, high) and quartile (low, medium, high, very high). Kappa statistics range from 0 to 1, with 1 indicating perfect agreement between two observations. These analyses were restricted to participants who provided a blood sample in both sampling rounds (participants = 40, serum samples = 80). Contingency tables were constructed to display the level of agreement between each subject’s initial and final exposure category. We report the weighted kappa, instead of the simple kappa, when assessing agreement for tertiles and quartiles.26
We used the following general linear model with a random intercept to estimate the between- and within-subject variance components associated with PBDE congener levels over the study period:
| 1 |
where Yij represents the natural logarithm of the PBDE congener level of the ith participant on the jth round of sampling, β0 is the fixed effect intercept, bi is the random intercept of the ith individual, and εij is the random error. To determine how serum lipids affect the variance components, we added a predictor variable, LIPIDij, the lipid level of the ith participant on the jth sampling round:
| 2 |
We estimated the intraclass correlation coefficient (ICC) to assess reliability of serum PBDE congener concentrations using the following formula:
| 3 |
where σ2B is the between-subject variance and σ2W is the within-subject variance.
We also determined whether (i) average biomarker levels increased or decreased by study round or (ii) if congener concentrations were associated with predictor variables obtained from questionnaires. Rather than standardizing PBDE measurements to lipids, we adjusted for lipid as a covariate in regression models,27 allowing us to estimate the effect of this variable. To evaluate the fixed effects of time and covariates, we used the following model:
| 4 |
where Yij, β0, bi, and εij are defined as earlier. TIME2 and TIME3 are indicator variables: β1 is the average difference of the log(PBDE) measurement from Round 1 to Round 2; β2 is the average difference of the log(PBDE) measurement from Round 1 to Round 3. AGE is the age of the ith participant at the initial sampling round (categorized as ≥37 or <37 years old); SEX is the gender of the ith participant; BMI is the body mass index of the ith participant at the initial sampling round (categorized as ≥25 mg/kg2 or <25 mg/kg2). We exponentiated the beta estimates to obtain a percent change in PBDE congener concentration per unit change in predictor variable.
We did not have completely balanced data; however, we considered the reasons for missing serum data not related to PBDE level and classified these data as missing completely at random (MCAR). As general linear models are robust to MCAR and missing at random (MAR) patterns of missing data, inferences reported from our regression models are likely unbiased;28 we therefore did not impute missing serum data. Sensitivity analyses were performed using only individuals with complete serum data.
Results
The study population consisted of 27 males and 25 females. The median age was 37 years old, 88% were white, 98% had a college degree, and 64% had BMI < 25 kg/m2 (Table 1). Twenty-three participants lived in the City of Boston and 30 lived in surrounding suburbs. Few participants moved residences during the study: three moved from Round 1 to Round 2, and two moved from Round 2 to Round 3. Baseline self-reported health status ranged from good to excellent (not shown). Forty-one participants provided a serum sample in all three sampling rounds, nine provided a sample in two rounds, and two provided a single serum sample. One serum sample was contaminated during field collection and removed from analysis, leaving 40 participants with three serum samples. After exclusions, we had 142 valid serum PBDE measurements from 52 participants.
Table 1. Baseline Characteristics of 52 Adults from the FlaRE Cohort.
| characteristic | n (%) |
|---|---|
| age | |
| 20–39 years | 29 (56) |
| 40–59 years | 19 (36) |
| ≥ 60 years | 4 (8) |
| sex | |
| female | 25 (48) |
| male | 27 (52) |
| race/ethnicity | |
| white | 46 (88) |
| other | 6 (12) |
| education | |
| college graduate | 51 (98) |
| < college graduate | 1 (2) |
| BMI (kg/m2) | |
| < 25 | 33 (63) |
| 25–29.9 | 17 (33) |
| ≥ 30 | 2 (4) |
Table 2 presents the round-specific geometric means (GM), geometric standard deviations (GSD), and ranges for PBDE congeners that were detected at >50%: BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153. Both lipid-standardized and wet weight serum concentrations are shown. BDE-47 had the highest serum concentration followed by BDE-153; both were detected in 100% of serum samples. BDE-99, BDE-100, and BDE-28 had detection frequencies of 92%, 89%, and 68%, respectively. Detection rates for BDE-17, BDE-66, BDE-85, BDE-154, BDE-183, and BDE-209 ranged from 1% to 22% and were not further analyzed. Supporting Information (SI) Table 1 presents detection rates, LODs, and ranges for all analyzed congeners.
Table 2. Selected Polybrominated Diphenyl Ethers, Lipids, and Their Geometric Means (GM), Geometric Standard Deviation (GSD), and Range by Sampling Rounda.
| round
1 (n = 49) |
round 2 (n = 50) |
round 3 (n = 43) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| analyte | GM (GSD) | range | GM (GSD) | range | GM (GSD) | range | |||
| serum (ng/g lipid) | |||||||||
| ΣPBDE | 20.2 | (2.2) | 2.3–290 | 21.0 | (2.5) | 2.7–294 | 16.9 | (2.3) | 2.4–211 |
| BDE-28 | 0.6 | (2.3) | 0.15–4.2 | 0.6 | (2.2) | 0.15–5.1 | 0.5 | (2.2) | 0.15–3.6 |
| BDE-47 | 9.6 | (2.8) | 0.6–151 | 9.9 | (2.7) | 1.3–149.0 | 7.9 | (2.6) | 0.9–98.9 |
| BDE-99 | 1.8 | (2.9) | 0.2–43.5 | 1.9 | (3.0) | 0.2–34.1 | 1.7 | (2.5) | 0.2–20.1 |
| BDE-100 | 1.8 | (3.4) | 0.2–42.4 | 1.9 | (3.3) | 0.2–44.1 | 1.4 | (3.0) | 0.15–35.2 |
| BDE-153 | 6.4 | (3.2) | 0.6–96.7 | 6.7 | (3.1) | 0.7–94.7 | 5.4 | (3.1) | 0.25–55.2 |
| serum (pg/g wet weight) | |||||||||
| BDE-28 | 3.5 | (2.4) | 1.3–29.1 | 3.7 | (2.4) | 1.3–30.4 | 3.1 | (2.3) | 1.3–24.5 |
| BDE-47 | 57 | (2.9) | 3.9–986 | 59 | (2.8) | 7.3–1000 | 48 | (2.7) | 5.5–707 |
| BDE-99 | 11 | (3.0) | 1.3–285 | 11 | (3.2) | 1.3–229 | 10 | (2.6) | 1.3–144 |
| BDE-100 | 11 | (3.5) | 1.3–278 | 11 | (3.4) | 1.3–297 | 8 | (3.1) | 1.3–252 |
| BDE-153 | 38 | (3.2) | 3.9–695 | 40 | (3.1) | 4.5–584 | 36 | (2.7) | 5.3–395 |
| serum lipids (mg/dL) | |||||||||
| cholesterolb | 190 | (33) | 120–290 | 190 | (33) | 110–360 | 190 | (45) | 92–330 |
| triglyceridesb | 130 | (70) | 50–340 | 130 | (71) | 42–340 | 140 | (65) | 45–290 |
| total lipidsb | 620 | (110) | 450–860 | 620 | (150) | 370–1100 | 640 | (140) | 330–1000 |
Participants = 52, serum samples = 142. Selected congeners were detected in serum no less than 65% of the samples. Additional congeners that were analyzed but detected infrequently (BDE-17, BDE-66, BDE-85, BDE-154, BDE-183, BDE-209) are presented in Supporting Information (SI) Table 1.
Means and standard deviation presented.
Reliability of Serum PBDE Measures over One Year
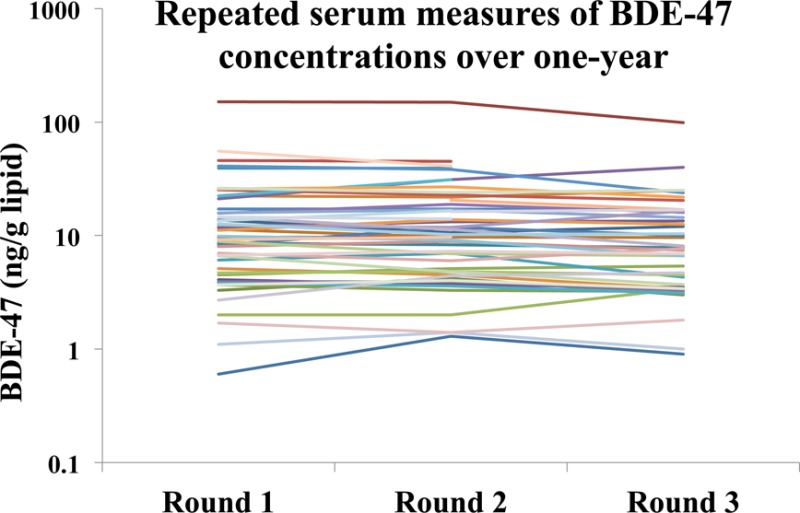
Table 3 presents the estimated ICCs for the serum PBDE congeners calculated using eqs 1 (unadjusted), 2 (adjusted for lipid), or 4 (adjusted for lipid and other covariates). Variance components are shown in SI Table 2. The ICC estimates were very high, particularly for ΣPBDEs, BDE-47, and BDE-153, ranging from 0.96 to 0.99. As a sensitivity analysis for missing data, we calculated ICCs using eq 1 for the subset of individuals that contributed three serum measurements (n = 40) and the results were similar (not shown). See SI Figure 1 for a graph of individual data for BDE-47 and SI Figure 2 for a simple correlation analysis for this congener.
Table 3. Intraclass Correlation Coefficients (ICCs) and Kappa Statistics (κ) for Repeated Serum Markers for Selected PBDEsa.
| estimate
(95% CI) |
||||||
|---|---|---|---|---|---|---|
| parameter | ∑PBDE | BDE-28 | BDE-47 | BDE-99 | BDE-100 | BDE-153 |
| ICCNULLb | 0.96 (0.95 to 0.98) | 0.87 (0.80 to 0.91) | 0.96 (0.95 to 0.98) | 0.91 (0.86 to 0.94) | 0.92 (0.88 to 0.95) | 0.98 (0.97 to 0.99) |
| ICCLIPIDc | 0.97 (0.95 to 0.98) | 0.89 (0.82 to 0.93) | 0.97 (0.95 to 0.98) | 0.91 (0.86 to 0.94) | 0.93 (0.88 to 0.95) | 0.99 (0.98 to 0.99) |
| ICCFULLd | 0.97 (0.95 to 0.98) | 0.89 (0.82 to 0.93) | 0.97 (0.95 to 0.98) | 0.91 (0.86 to 0.94) | 0.93 (0.88 to 0.96) | 0.98 (0.97 to 0.99) |
| median classification | ||||||
| κ | 0.80 (0.61 to 0.99) | 0.80 (0.62 to 0.99) | 0.80 (0.61 to 0.99) | 0.80 (0.61 to 0.99) | 0.85 (0.69 to 1.0) | 0.90 (0.77 to 1.0) |
| tertile classification | ||||||
| κweighted | 0.83 (0.71 to 0.96) | 0.75 (0.60 to 0.90) | 0.67 (0.50 to 0.84) | 0.72 (0.57 to 0.88) | 0.78 (0.64 to 0.92) | 0.81 (0.66–0.96) |
| quartile classification | ||||||
| κweighted | 0.82 (0.71 to 0.93) | 0.78 (0.66 to 0.90) | 0.84 (0.73 to 0.95) | 0.76 (0.64 to 0.88) | 0.82 (0.71 to 0.93) | 0.90 (0.81 to 0.98) |
participants = 52, serum samples = 142.
Equation (1): Yij = β0 + bi + εij.
Equation (2): Yij = β0 + β1LIPID + bi + εij.
Equation (3): Yij = β0 + β1TIME2 + β2TIME3 + β3AGEi + β4SEXi + β5BMIi + β6LIPIDij + bi + εij.
Table 3 also presents the kappa statistics quantifying the agreement between exposure categorization in Round 1 and Round 3 (approximately one year apart). Kappa statistics between 0.61 and 0.80 are considered in substantial agreement and kappa statistics >0.80 are considered in almost perfect agreement.26 For example, the kappa statistics for BDE-47, based on exposure categorization by median (e.g., low, high), tertile (e.g., low, medium, high), or quartile (e.g., low, medium, high, very high), were 0.80, 0.67, and 0.84, respectively. SI Figure 3 presents the contingency tables associated with the kappa statistics for BDE-47. ΣPBDEs had kappa statistics that ranged from 0.80 to 0.83. We also calculated kappa statistics for each congener comparing Round 1 and Round 2 (6 months apart) and the results were similar (SI Table 3).
Predictors of Serum PBDE Measures (Sampling Round, Lipids, Demographic Variables)
Table 4 presents parameter estimates and p-values for regression models (eq 4) predicting PBDE levels as a function of sampling round, serum lipids, age, sex, and BMI. Exponentiating the beta coefficients, we find that BDE-47 and BDE-100 levels significantly decreased by 7.9% (p = 0.029) and 13.9% (p = 0.024), respectively, from Round 2 to Round 3. While there were no other significant temporal changes, all congeners except BDE-99 had negative slopes from Round 2 to Round 3 and positive trends from Round 1 to Round 2. We also ran regression analysis using PBDEs standardized to lipids and our results were similar to the presented model with lipid as a covariate (not shown). Using Akaike Information Criterion (AIC) as a guide, we found that evaluating time as a categorical variable (instead of continuous in months, e.g. j = month) provided the better fit, though results were similar in both models (not shown). We also analyzed these data using a model with continuous time adding a covariate for season (summer vs winter), and did not find any significant trends (not shown). Results were similar when analyzed using only individuals that contributed three serum measurements (not shown).
Table 4. General Linear Models for Repeated Measures Assessing PentaBDE Serum Measurements from 2010–2011 by Demographic Characteristic (Fixed Effects)a.
| ln(pg/g) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∑PBDE |
BDE-28 |
BDE-47 |
BDE-99 |
BDE-100 |
BDE-153 |
|||||||
| parameter | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p |
| time (overall) | 0.22 | 0.33 | 0.077 | 0.88 | 0.077 | 0.31 | ||||||
| time 1 | ref | ref | ref | ref | ref | ref | ||||||
| time 2 | 0.029 (0.032) | 0.37 | 0.048 (0.053) | 0.37 | 0.017 (0.035) | 0.61 | 0.020 (0.063) | 0.75 | 0.076 (0.063) | 0.23 | 0.044 (0.029) | 0.13 |
| time 3 | –0.030 (0.034) | 0.39 | –0.034 (0.057) | 0.55 | –0.064 (0.037) | 0.09 | 0.034 (0.068) | 0.61 | –0.077 (0.067) | 0.26 | 0.028 (0.31) | 0.37 |
| time 2 vs 3 | –0.059 (0.034) | 0.08 | –0.082 (0.056) | 0.15 | –0.082 (0.037) | 0.03 | 0.014 (0.067) | 0.83 | –0.15 (0.067) | 0.02 | –0.017 (0.030) | 0.59 |
| lipid | 0.0016 (0.0003) | <0.001 | 0.0016 (0.0004) | <0.001 | 0.0015 (0.0003) | <0.001 | 0.0014 (0.0005) | 0.005 | 0.0021 (0.0005) | <0.001 | 0.0017 (0.0003) | <0.001 |
| sex: female | ref | ref | ref | ref | ref | ref | ||||||
| male | 0.13 (0.27) | 0.62 | –0.16 (0.21) | 0.45 | –0.12 (0.27) | 0.68 | –0.07 (0.29) | 0.81 | 0.0089 (0.34) | 0.98 | 0.61 (0.32) | 0.061 |
| age: <37 yo | ref | ref | ref | ref | ref | ref | ||||||
| ≥37 yo | –0.069 (0.26) | 0.79 | 0.39 (0.21) | 0.07 | 0.25 (0.28) | 0.37 | 0.34 (0.29) | 0.23 | 0.15 (0.33) | 0.65 | –0.53 (0.31) | 0.09 |
| BMI: <25 kg/m2 | ref | ref | ref | ref | ref | ref | ||||||
| ≥25 kg/m2 | 0.26 (0.28) | 0.35 | 0.29 (0.22) | 0.21 | 0.44 (0.29) | 0.14 | 0.32 (0.30) | 0.30 | 0.41 (0.35) | 0.25 | –0.081 (0.33) | 0.81 |
Participants = 52, serum samples = 142. Abbreviations: (pg/mL) picogram per milliliter serum; (ref) reference group; (SE) standard error; (yo) years old. Beta-coefficients are regression coefficients for log-transformed regression outcome data. Exp(β) yields the multiplicative increase in PBDE per unit change in predictor, e.g., exp(−0.082) = 0.921 = 7.9% decrease.
Serum lipids were highly significant predictors of all the PBDE congeners. For example, BDE-47 increased 0.15% (p < 0.0001) per one-unit increase of total lipids (mg/dL), while controlling for age, sex, time, and BMI. Because PBDEs are lipophilic, we would expect that as lipid levels increase we would see an increase in PBDE levels, which our results confirm.29 For example, if total serum lipids increase one standard deviation above the mean in Round 1 (18%), our model predicts that ΣPBDEs should increase by about 19%. Additionally, we calculated ICCs for the lipid measurements using eqs 1 and 2 with the dependent variable Yij representing the lipid level of the ith participant on the jth sampling round. The ICCs were 0.80, 0.86, and 0.65 for total lipids, total cholesterol, and triglycerides, respectively (data not shown).
Although not statistically significant, sex and age were suggestive predictors for BDE-153. Such associations have been previously observed.4,30,31 On average, men had 84% (p = 0.06) higher BDE-153 levels than women, after controlling for other covariates. Levels of BDE-153 were 41% lower (p = 0.09) in the older participants than the younger participants. Interestingly, for all other congeners, this trend appears to be reversed: levels of BDE-28, BDE-47, BDE-99, and BDE-100 were nonsignificantly higher on average in the older than the younger participants. BMI was not a significant predictor of any PBDE congener. We ran univariate models analyzing each fixed effect independently and found that the univariate and multivariate results were similar.
Discussion
Reliability of Serum PBDE Measures over One Year
Many epidemiologic studies of PBDEs assess exposure using a single biological sample per person. We found ICCs of greater than 0.90 for BDE-47, BDE-99, BDE-100, BDE-153, and ΣPBDE. BDE-28 had a slightly lower ICC of ≥0.87. A high ICC indicates that differences in PBDE serum concentration between subjects are much greater than the variability within subjects over the study period.32 As a result, individuals with high exposure levels tend to stay high compared to individuals with low exposures. The high ICCs we report signify that a single serum measurement of BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153 is likely to provide a reliable biomarker of exposure over a one-year period.
Although an ICC is very useful for analyzing continuous measures, epidemiologists often categorize exposure data as well. We placed participants into exposure categories based on their PBDE serum concentration in Round 1 and Round 3. The kappa statistics were mostly ≥0.80, meaning excellent agreement between time points.26 For example, the ΣPBDE exposure metric had a kappa statistic of 0.83 for categorization by tertiles, indicating almost all participants remained in their respective exposure category (e.g., low, medium, high) after a one-year period.
These data indicate minimal exposure measurement error for epidemiological studies that use a single serum sample to estimate U.S. adult exposure over a year. This information is important for the design and interpretation of epidemiologic studies of the health effects of PentaBDE congeners. Caution is needed in extrapolating to other populations (e.g., infants) or for much longer time periods. Additional research is needed on the time variability of BDE-183 and BDE-209.
Serum PBDE Concentrations
The serum concentrations of BDE-28, BDE-47, BDE-99, and BDE-100 in our study (collected in 2010–2011) were lower than those collected in 2009 from the pilot study to this investigation. Watkins et al. reported GM serum concentrations of BDE-28, BDE-47, BDE-99, and BDE-100 that were approximately 1.5–2 times greater than our Round 1 values collected one year later (p < 0.01).33 For example, Watkins et al. reported GM (GSD) serum levels of BDE-47 of 14.2 (3.0) ng/g lipid, significantly different from our Round 1 BDE-47 serum levels of 9.6 (2.8) ng/g lipid (p < 0.001). This population was demographically and geographically similar to our study and utilized the same analytical laboratory. On the other hand, the levels of BDE-153 in our study were similar to those reported by Watkins et al., 5.0 ng/g lipid.33 It is unclear whether the differences are due to chance variation, some unknown dissimilarity between the two populations, or time trends.
Predictors of Serum PBDE Measures
We observed nonsignificant increases in all PBDE congener concentrations from Round 1 (winter) to Round 2 (summer). In contrast, we found significant decreases of BDE-47 and BDE-100 from Round 2 (summer) to Round 3 (winter). BDE-47, the predominant congener in human serum, and BDE-100 decreased on average 7.9% and 13.9%, respectively, after controlling for age, sex, lipid, and BMI. BDE-28 and -153 also decreased while BDE-99 increased; these changes were not significant. These results occurred in the fully adjusted model (Table 3) as well as the wet weight and lipid standardized PBDE models (Table 2).
The reason for these changes by round is unclear. There is typically a seasonal increase in mean serum cholesterol over the winter months compared to the summer,34,35 but average serum lipid concentrations in our population did not differ by Round (Table 2). Because we only have one summer season, we have limited ability to determine if variations of serum concentration are due to factors that may change seasonally, e.g., activity patterns, diet, body weight. However, the alterations in PentaBDE serum concentrations we observed were not the result of a change in blood lipid levels. Changes in the volume of distribution—primarily adipose tissue for these lipophilic compounds—may affect concentrations in both adipose tissue and serum.36 We could not evaluate this effect as we only had baseline BMI data.
A decrease of some congeners may be consistent with the hypothesis that exposure has declined since the discontinuation of PentaBDE and OctaBDE manufacturing in the mid-2000s. For example, if the half-life of a compound were two years and exposure ceased, one would predict a decrease of 16% over six months; presence of declining but nonzero exposure would reduce observed elimination rates. Furthermore, as stated earlier, the human half-lives of the major PentaBDE congeners are uncertain.
However, it is unclear if PBDE serum levels have declined in the United States since 2004. A recent study using data from the National Health and Examination Survey (NHANES)—designed to be a nationally representative sample of the U.S. civilian, noninstitutionalized population—reported that “PBDE levels overall were lower in 2007–08 than in NHANES 2003–04, however most comparisons were not significantly different.”37 It is difficult to compare our serum concentrations to those based on NHANES because of statistical, geographic, and demographic differences between these studies. The only previous study of Americans using repeated serum measures reported no significant changes in ΣPBDE or individual PentaBDE congeners from 2001 to 2003 to 2004–2005. However, the percent contribution of BDE-153 to ΣPBDE increased while the percent contribution of BDE-47 to ΣPBDE significantly decreased.38,39 A study comparing two demographically similar populations sampled in 2008–2009 and 2011–2012 found that BDE-47, BDE-99, and BDE-100 decreased more than BDE-153.40 Outside of the United States, a retrospective Swedish study comparing pooled breast milk samples reported decreasing concentration of BDE-47 beginning in the late 1990s, while BDE-153 appeared to level off.3,41
There are a few possible explanations for congener-specific temporal patterns in our study, if real. Potential decreases may be partly explained by differences in elimination rates. The median half-life of BDE-153 is indirectly estimated to be 7.4 years compared to 1.4, 1.8, and 3.0 years for BDE-47, BDE-100, and BDE-28, respectively.12 However, the estimated half-life of BDE-99 is 0.8 years, but there were no significant decreases for this congener in our study. Unfortunately, we were unable to reliably estimate half-lives for PBDE congeners with our data.42
The different temporal patterns for congeners may also be partly due to differences in exposure sources. In the United States, both dust from the indoor environment and consumption of food were shown to be important pathways for adult exposure to PBDEs.10 Exposure routes may vary by congener, however. Johnson et al. reported strong correlations between paired household dust and adult serum samples for BDEs 47, 99, and 100, but not for BDE-153.43 In a study of young children, Stapleton et al. reported association among serum concentrations, hand-wipes, and dust for BDEs 47, 99, 100, and 153; only BDE-153 serum concentrations were strongly associated with duration of breastfeeding.44 In a study using NHANES data, BDE-153 serum concentrations were more strongly associated with reported consumption of red meat and total daily fat intake than were serum levels of BDE-28, BDE-47, BDE-99, and BDE-100.11
A major strength of our study is the use of three serum samples from a longitudinal study of a relatively homogeneous population. With similar working conditions and demographic characteristics, the study population is likely to be similar with respect to unknown confounders. A limitation of our study is that our serum collection was not completely balanced; participants contributed one, two, or three serum samples. However, as we had a high retention rate (>80%), and study dropout was classified as MCAR, this limitation was unlikely to have biased our results. Our study sample size (52 participants, 142 serum samples) limited the amount of variables we could evaluate simultaneously in regression models and likely reduced our power to detect statistically significant associations. Participants self-reported height and weight at the beginning of the study, potentially introducing error into the estimate of BMI. With only one estimate of BMI, we cannot examine changes in weight (or adipose tissue) as a determinant of PBDE serum concentrations We used a convenience sample of office workers in the Boston area that were mostly white, not overweight, and highly educated, and we cannot be certain our results can be generalized to the U.S. population.
Our study demonstrates that a single PBDE serum measurement of BDE-28, BDE-47, BDE-99, BDE-100, and BDE-153 reliably estimates a participant’s blood concentration over a one-year period. This result held for both lipid adjusted and nonlipid adjusted serum concentrations. Our analysis thus supports PBDE epidemiology studies that use single serum samples. We also observed decreases of serum concentrations of some PBDE congeners that were not explained by changes in serum lipid. Future studies should investigate changes in serum levels of PBDEs in the U.S. population and the possible implications of sampling season on PBDE serum concentrations.
Acknowledgments
We thank the study participants and Jennifer Ames, Kimberly Burke, Erin Collins, Ashley Miller, Steve Nicholson, and Brittany Weldon for their assistance in sample collection. This work was supported by grants from the National Institute of Environmental Health Sciences: R01ES015829 and T32ES014562. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supporting Information Available
Three tables (T1, T2, and T3) and three figures (F1, F2, and F3) as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org/.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Birnbaum L. S.; Staskal D. F. Brominated flame retardants: Cause for concern?. Environ. Health Perspect. 2004, 11219–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites R. A. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ. Sci. Technol. 2004, 384945–956. [DOI] [PubMed] [Google Scholar]
- Meironyte D.; Noren K.; Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxicol Environ. Health A 1999, 586329–341. [DOI] [PubMed] [Google Scholar]
- Sjödin A.; Jones R. S.; Park A.; Zhang Y.; Hodge C.; DiPietro E.; McClure C.; Turner W.; Needham L. L.; Patterson D. G. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Health Technol. 2008, 4241377–1384. [DOI] [PubMed] [Google Scholar]
- Sjödin A.; Jones R. S.; Focant J. F.; Lapeza C.; Wang R. Y.; McGahee E. E.; Zhang Y. L.; Turner W. E.; Slazyk B.; Needham L. L.; Patterson D. G. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ. Health Perspect. 2004, 1126654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Environmental Programme: Stockholm Convention on Persistent Organic Pollutants, 2001. 2256 UNTS 119:40 ILM 532: http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx.
- U.S. Environmental Protection Agency. Toxicological Review of 2,2′,4,4′-tetrabromodiphenyl ether (CAS No. 5436-43-1); Integrated Risk Information Services: Washington, DC, 2008; www.epa.gov/iris/toxreviews/1010tr.pdf. [Google Scholar]
- Betts K. S. Unwelcome guest: PBDEs in indoor dust. Environ. Health Perspect. 2008, 1165A202–A208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A.; Papke O.; Harris T. R.; Tung K. C.; Musumba A.; Olson J.; Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006, 114101515–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.; Herrmann T.; Paepke O.; Tickner J.; Hale R.; Harvey E.; La Guardia M.; McClean M. D.; Webster T. F. Human exposure to PBDEs: Associations of PBDE body burdens with food consumption and house dust concentrations. Environ. Sci. Technol. 2007, 4151584–1589. [DOI] [PubMed] [Google Scholar]
- Fraser A. J.; Webster T. F.; McClean M. D. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ. Health Perspect. 2009, 117101520–1525 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D.; Scheringer M.; von Goetz N.; Hungerbuhler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ. Sci. Technol. 2011, 4562391–2397 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- Thuresson K.; Hoglund P.; Hagmar L.; Sjödin A.; Bergman A.; Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 2006, 1142176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T. E.; Laws S. C.; Crofton K. M.; Hedge J. M.; Ferrell J. M.; Cooper R. L. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol. Sci. 2004, 781144–155. [DOI] [PubMed] [Google Scholar]
- Eriksson P.; Jakobsson E.; Fredriksson A. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment?. Environ. Health Perspect. 2001, 1099903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven L. T.; van de Kuil T.; Verhoef A.; Leonards P. E.; Slob W.; Canton R. F.; Germer S.; Hamers T.; Visser T. J.; Litens S.; Hakansson H.; Fery Y.; Schrenk D.; van den Berg M.; Piersma A. H.; Vos J. G.; Schrenk D.; van den Berg M.; Piersma A. H.; Vos J. G. A 28-day oral dose toxicity study enhanced to detect endocrine effects of a purified technical pentabromodiphenyl ether (PentaBDE) mixture in Wistar rats. Toxicology 2008, 2451–2109–122 10.1016/j.tox.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Herbstman J. B.; Sjödin A.; Kurzon M.; Lederman S. A.; Jones R. S.; Rauh V.; Needham L. L.; Tang D.; Niedzwiecki M.; Wang R. Y.; Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010, 1185712–719 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B.; Chevrier J.; Rauch S. A.; Kogut K.; Harley K. G.; Johnson C.; Trujillo C.; Sjödin A.; Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 2013, 1212257–262 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K.; Adgent M.; Goldman B. D.; Sjödin A.; Daniels J. L. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ. Health Perspect. 2012, 120101438–1442 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M. E.; Persky V. W.; Imm P.; Knobeloch L.; Chatterton R.; Anderson H. A. Hormone disruption by PBDEs in adult male sport fish consumers. Environ. Health Perspect. 2008, 116121635–1641 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D.; Johnson P. I.; Camann D.; Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009, 407103425–3429 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Eagle S.; Anthopolos R.; Wolkin A.; Miranda M. L. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect. 2011, 119101454–1459 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K. J.; Greenland S.; Lash T. L.. Modern Epidemiology, 3rd ed.; Lippincott Williams and Wilkins: Philadelphia, 2008; pp 139–142. [Google Scholar]
- Boston Fire Department. Regulation of Upholstered Furniture: BFD IX-10; City of Boston, MA, 1995. [Google Scholar]
- Sjödin A.; Jones R. S.; Lapenza C. R.; Focant J. F.; McGahee E. E.; Patterson D. G. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem. 2004, 7671921–1927. [DOI] [PubMed] [Google Scholar]
- Viera A. J.; Garrett J. M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 375360–363. [PubMed] [Google Scholar]
- Schisterman E. F.; Whitcomb B. W.; Louis G. M.; Louis T. A. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect. 2005, 1137853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice G. M.; Laird N. M. Regression models for mixed discrete and continuous responses with potentially missing values. Biometrics 1997, 531110–122. [PubMed] [Google Scholar]
- Gaskins A. J.; Schisterman E. F. The effect of lipid adjustment on the analysis of environmental contaminants and the outcome of human health risks. Methods Mol. Biol. 2009, 580, 371–381 10.1007/978-1-60761-325-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms L. M.; Sjödin A.; Harden F.; Hobson P.; Jones R.; Edenfield E.; Mueller J. F. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ. Health Perspect. 2009, 11791461–1465 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gari M.; Grimault J. O. Inverse age-dependent accumulation of decabromodiphenyl ether and other PBDEs in serum from a general adult population. Environ. Int. 2013, 54C119–127 10.1016/j.envint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Fleiss J.The Design and Analysis of Clinical Experiments; John Wiley and Sons: New York, 1996. [Google Scholar]
- Watkins D. J.; McClean M. D.; Fraser A. J.; Weinberg J.; Stapleton H. M.; Sjödin A.; Webster T. F. Exposure to PBDEs in the office environment: Evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect. 2011, 11991247–1252 10.1289/ehp.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe T.; Strid A.; Grandjean P.; Weihe P.; Bergman A. Seasonal variation in serum lipids, and incidence and mortality of ischaemic heart disease. J. Atheroscler. Res. 1968, 83591–596. [DOI] [PubMed] [Google Scholar]
- Rastam L.; Hannan P. J.; Luepker R. V.; Mittlemark M. B.; Murray D. M.; Slater J. S. Seasonal variation in plasma cholesterol distributions: Implications for screening and referral. Am. J. Prev. Med. 1992, 86360–366. [PubMed] [Google Scholar]
- Dirtu A. C.; Dirinck E.; Malarvannan G.; Neels H.; Van Gaal L.; Jorens P. G.; Covaci A. Dynamics of organohalogenated contaminants in human serum from obese individuals during one year of weight loss treatment. Environ. Sci. Technol. 2013, 472112441–12449 10.1021/es400657t. [DOI] [PubMed] [Google Scholar]
- Sjödin A.; Jones R. S.; Caudill S. P.; Wong L. Y.; Turner W. E.; Calafat A. M. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003–2008. Environ. Sci. Technol. 2014, 481753–760 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M. E.; Anderson H. A.; Steenport D.; Buelow C.; Imm P.; Knobeloch L. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere 2010, 814517–522 10.1016/j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imm P.; Knobeloch L.; Buelow C.; Anderson H. A. Household exposures to polybrominated diphenyl ethers (PBDEs) in a Wisconsin Cohort. Environ. Health Perspect. 2009, 117121890–1895 10.1289/ehp.0900839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R.; Linderholm L.; Park J. S.; Petreas M.; Guo T.; Privalsky M. L.; Zoeller R. T.; Woodruff T. J. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 2013, 472011776–11784 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangstrom B.; Strid A.; Grandjean P.; Weihe P.; Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ. Health 2005, 4, 12. 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell S. M. Bias in half-life estimates using log concentration regression in the presence of background exposures, and potential solutions. J. Exposure Sci. Environ. Epidemiol. 2012, 223299–303 10.1038/jes.2012.2. [DOI] [PubMed] [Google Scholar]
- Johnson P. I.; Stapleton H. M.; Sjödin A.; Meeker J. D. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ. Sci. Technol. 2010, 44145627–5632 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Eagle S.; Sjödin A.; Webster T. F. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 12071049–1054 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


