Abstract
Although active listening is an influential behavior, which can affect the social responses of others, the neural correlates underlying its perception have remained unclear. Sensing active listening in social interactions is accompanied by an improvement in the recollected impressions of relevant experiences and is thought to arouse positive feelings. We therefore hypothesized that the recognition of active listening activates the reward system, and that the emotional appraisal of experiences that had been subject to active listening would be improved. To test these hypotheses, we conducted functional magnetic resonance imaging (fMRI) on participants viewing assessments of their own personal experiences made by evaluators with or without active listening attitude. Subjects rated evaluators who showed active listening more positively. Furthermore, they rated episodes more positively when they were evaluated by individuals showing active listening. Neural activation in the ventral striatum was enhanced by perceiving active listening, suggesting that this was processed as rewarding. It also activated the right anterior insula, representing positive emotional reappraisal processes. Furthermore, the mentalizing network was activated when participants were being evaluated, irrespective of active listening behavior. Therefore, perceiving active listening appeared to result in positive emotional appraisal and to invoke mental state attribution to the active listener.
Keywords: Active listening, Emotional appraisal, Functional magnetic resonance imaging, Reward system
Active listening, which is defined as empathic understanding, unconditional positive regard, and congruence behavior (Rogers, 1959), can improve the social behavior of others. In this sense, it is an influential component of social behavior. Perceiving active listening behavior in a partner can facilitate a positive interaction in terms of future behaviors associated with relevant experiences (Rogers, 1959). In particular, active listening behavior as a part of psychotherapy positively affects therapeutic personality changes (Duan & Hill, 1996; Gladstein, 1977), which are accompanied by an improvement in the impression of relevant experiences (Rogers, 1957). Thus, perceiving active listening might improve one’s impression of an experience. However, the neural mechanisms underlying the perception of active listening are not well understood, particularly with respect to improving the impression of experiences.
Active listening behavior can facilitate positive interpersonal relationships (Duan & Hill, 1996; Gladstein, 1977; Rogers, 1957, 1959). As an active listening attitude is perceived as unconditional positive regard (Rogers, 1959), the target identifies this conduct as social acceptance through engaging in mentalizing about active listener. Positive social interactions are fundamentally rewarding for humans (Baumeister & Leary, 1995). Similarly, being in receipt of active listening behavior might be perceived as a positive, rewarding event. As a therapeutic skill, active listening often involves mirroring the behavior of others (Rogers, 1959), which arouses an empathic mind-set in the target (Stel, Van Baaren, & Vonk, 2008; Stel & Vonk, 2010). In this sense, mirroring is a key element of active listening. Mirroring has a positive influence on the target, can lead to prosocial behaviors (Chartrand & Bargh, 1999), and influences activation of the reward system via the medial prefrontal cortex (mPFC), a component of the mentalizing system (Kuhn et al., 2010). Various types of reward, including monetary (Elliott, Newman, Longe, & Deakin, 2003; Fliessbach et al., 2007; Knutson, Adams, Fong, & Hommer, 2001) and social (Izuma, Saito, & Sadato, 2008) rewards, activate the ventral striatum and are accompanied by positive feelings. In addition, the mPFC is a key node for social cognition (Frith & Frith, 2010), which requires mentalizing ability (Amodio & Frith, 2006; Frith & Frith, 1999; Gallagher et al., 2000) for the perception of social reward (Izuma et al., 2008). Here, we hypothesized that perceiving active listening activates positive feelings through mentalizing, represented in the ventral striatum and the mPFC.
In addition to generating social reward, being in receipt of an attitude of active listening improves the evaluation of topics associated with the ongoing social interaction (Rogers, 1957). This impression improvement in response to perceiving active listening is the result of emotional reappraisal. Emotional appraisal can be evaluated via accompanying somatic representation (Gray, Harrison, Wiens, & Critchley, 2007; Lamm & Singer, 2010; Preuschoff, Quartz, & Bossaerts, 2008), which comprises interoceptive information that is represented in the insula (Craig, 2002, 2003). Because emotionally relevant experiences are evaluated by retrieving appropriate cues (Gray et al., 2007; Schachter & Singer, 1962), the positive feelings aroused by receiving active listening might act as cues for emotional reappraisal. In this sense, receiving active listening improves the impression of topics associated with ongoing social interaction through emotional reappraisal by using the aroused positive feelings as evaluation cues. Thus, we hypothesized that active listening behavior would result in increased insula activation as well as ventral striatal activation in a way that might be related to improvement in the impression of relevant experiences.
To test these hypotheses, we conducted a functional magnetic resonance imaging (fMRI) experiment in which participants viewed assessments of their personal episodes made by evaluators who were, or were not, engaging in active listening (with and without active listening conditions). As perceiving reward is a result of mentalizing behavior (perceivers do not know the intent of an evaluator at the time that mentalization begins), mentalizing should be invoked irrespective of the listening attitude. Thus, we expected that both the with active listening and the without active listening conditions would invoke mPFC activation, implying that mentalizing is conducted similarly for both. Furthermore, we predicted that active listening would activate the striatum and the anterior insula in comparison to assessment without active listening.
EXPERIMENTAL METHODS
Subjects
In total, 22 subjects (13 males) with a mean ± standard error of the mean (SEM) age of 21.72 ± 0.59 years (21.92 ± 0.67 years for males and 21.44 ± 1.04 years for females) participated in the experiment. All subjects had normal or corrected-to-normal visual acuity and were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). All subjects provided written informed consent and were monetarily compensated. The protocol was approved by the ethical committee of the National Institute for Physiological Sciences, Okazaki, Japan. The experiments were undertaken in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
The following four subjects were excluded from the analysis: one male who reported that he did not believe that the evaluators assessed his experiences; one male who showed excessive head movements (>4 mm) during a run; and two subjects who did not complete all of the runs due to physical problems. Data from 18 subjects (10 males) were therefore analyzed.
Apparatus for visual presentation
Visual stimuli were presented using Presentation Software 14.4 (Neurobehavioral Systems, Inc., Albany, CA, USA) implemented on a personal computer (dc7900; Hewlett-Packard Japan Ltd., Tokyo, Japan). A liquid-crystal display (LCD) projector (CP-SX12000; Hitachi Ltd., Tokyo, Japan) located outside and behind the scanner projected the stimuli through a waveguide to a translucent screen, which the subjects viewed via a mirror attached to the bed of the MRI scanner. The projector had a spatial resolution of 1024 × 768 pixels and a refresh rate of 60 Hz. The distance between the screen and each subject’s eyes was approximately 175 cm, and the visual angle was 13.8° (horizontal) × 10.4° (vertical). Responses were collected via an optical button box (HHSC-2x2; Current Designs Inc., Philadelphia, PA, USA).
Task design
Subjects attended two experimental sessions, conducted on separate days. On the first day, subjects were instructed to select themes for, and to write, eight short essays based on emotional episodes from their own lives. They then briefly described the eight episodes in front of a video camera, for which subjects were required to speak for a minimum of 20 s. The participants were told that evaluators would assess each video clip to determine whether they agreed with the subjects’ emotional state during each episode.
On the second day, fMRI was used to measure the neural activation of the 22 subjects while viewing scenes of evaluators assessing their video-recorded life episode descriptions. The title of the subject’s life episode was initially presented for 2.5 s. Then, a 20-s video clip of an evaluator assessing the life episode description was shown, with the result displayed on the lower part of the screen. Subjects were told that they were observing assessment scenes of their speeches, which were conducted between the first and the second experimental days. However, in fact the video clips of evaluators were recorded prior to the first experimental day. The ostensible evaluators (in fact actors) were required to perform the task with or without active listening behavior six times, respectively. There were 10 ostensible evaluators. The experiment included three conditions: “with active listening” (four evaluators × six episodes = 24 video clips); “without active listening” (four evaluators × six episodes = 24 video clips); and control (two evaluators × six episodes = 12 times). In the “with active listening” and the “without active listening” conditions, six performances of each ostensible evaluator corresponded to one of six episodes. Each episode described by a participant was paired with a different video clip of each ostensible evaluator. In the control condition, photographs of the ostensible evaluators were presented rather than video clips. Subjects viewed the photographs, which were presented along with prerecorded audio clips and the assessment results, for 20 s (similar to the other two conditions). The ostensible evaluators for the three conditions (assessment with active listening, assessment without active listening, and control) were counterbalanced among subjects. The assessment result was displayed using a visual analog scale. The subjects were told that the score indicated the extent to which the evaluator agreed with emotions that participants depicted in each story, and ranged from 0 (not at all) to 100 (very much). However, in actuality the score was a random positive value, ranging from 50 to 80; this range was chosen in order to reduce the possibility of the subjects noticing the experimental manipulation. As part of the assessment, subjects listened to their own speech presented simultaneously with the video clip of the ostensible evaluator’s assessment. The subjects’ audio clip, which was recorded on the first day, was used to remind them of their emotions at the time when the episode occurred; for this purpose, subjects were not required to listen to the whole speeches, and so only the last 20 s of the audio clip was presented using a block study design. Subjects were required to focus on the evaluation scene for a 20-s period. They were then allowed 2.5 s to evaluate the emotional response of the just-described life episode, at the time it had occurred in their past, using a visual analog scale ranging from 100 (positive) to 0 (negative) with their right index and middle fingers. They were instructed to score the emotional response to the life episode irrespective of the assessment provided by the ostensible evaluator. Finally, a fixation cross was presented for 15 s (Figure 1).
Figure 1.
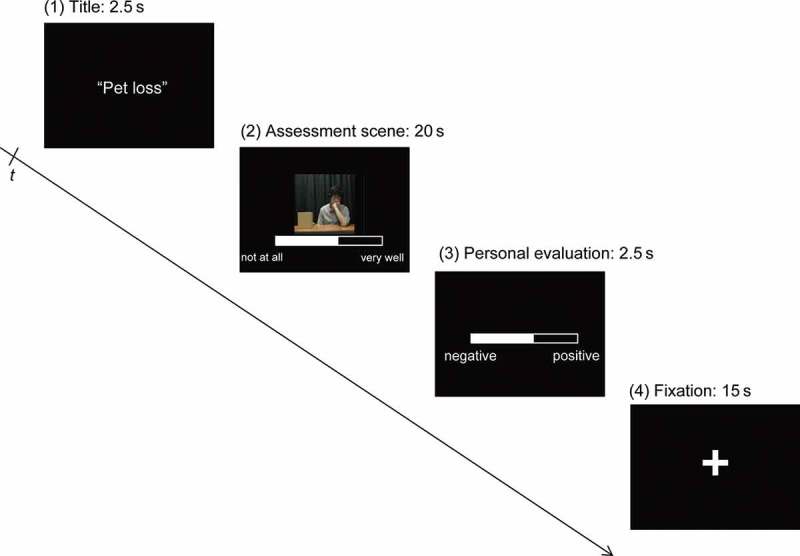
Time chart for stimulus presentation. (1) The title of the subject’s story was presented at the start of the stimulus sequence for 2.5 s. (2) The assessment episode by ostensible evaluators was shown for 20 s. The assessment result was shown on the lower part of the screen throughout this period. During the assessment episode, subjects were also presented with the final 20 s of an audio file of their own speech, which was recorded on the first experimental day, and where they described an emotional life event. (3) In the third phase, subjects were given 2.5 s to rate the emotional response of the life episode at the time it occurred. (d) A fixation cross was then presented for 15 s.
The subjects were required to record audio clips for a total of eight stories, to increase the possibility of producing at least six episodes that were longer than 20 s. Audio clips that were shorter than 20 s could not be used in the present fMRI experiment. Six stories were then chosen from the eight recorded for each subject based on duration (longer than 20 s) and order of recording. Events reported with more primacy were assumed to be more vivid or emotionally salient for the subjects, and therefore better suited for reminding them of the emotional states at the time when the event occurred.
Each story was presented 10 times along with a different evaluator’s assessment (four in the assessment with active listening condition and four in the assessment without active listening condition) or photograph (two in the control condition). The order of the three conditions (assessment with active listening, assessment without active listening, and control) was counterbalanced within the subjects. In addition, the presentation order was predetermined and counterbalanced among the subjects. In total, each subject participated in six runs, which comprise 10 blocks (for each of the 10 evaluators’ assessments).
Before and after the fMRI experiment, subjects were required to evaluate their impression of each of the ostensible evaluators on a scale ranging from 100 (positive) to 0 (negative) after viewing their photographs for 5 s.
Prior to the fMRI experiments, subjects were told that they were required to participate with one of the ostensible evaluators in other cooperation tasks. Subjects were told that the experimenters would select partners for them based on scores that they provide indicating their willingness to cooperate with each evaluator. These ratings were collected after the fMRI experiment, using a visual analog scale ranging from 100 (very willing) to 0 (not at all willing).
Visual stimulus validation
To confirm the validity of each evaluator’s performance, five independent raters (two males) also scored each assessment video clip in the active listening and without active listening conditions (10 ostensible evaluators × 6 performances = 60 video clips for each). These raters were asked to indicate their overall impression of the evaluator’s performance, scored on a scale ranging from 5 (with active listening) to 1 (without active listening) based on behavioral (for example, “Is the facial expression appropriate for listening?”) and semantic (for example, “Is the manner serious?”) considerations (Anme et al., 2013). The evaluators rated assessments with active listening behavior significantly more positive than assessments without active listening behavior (average ± SEM scores = 4.62 ± 0.12 and 1.55 ± 0.11, respectively; paired t test; t(9) = 16.47, p < .001).
fMRI data acquisition
A 3-T scanner (Verio; Siemens Ltd., Erlangen, Germany) was used in the fMRI examination. A subject’s head was immobilized within a 32-element phased-array head coil. fMRI was performed using an echo planar imaging (EPI) gradient-echo sequence (echo time [TE] = 30 ms; repetition time [TR] = 2500 ms; field of view [FOV] = 192 × 192 mm2; flip angle = 80°; matrix size = 64 × 64 pixels; 39 slices; slice thickness = 3 mm; and total number of volumes = 166). A whole-brain high-resolution, T1-weighted anatomical MR image using magnetization-prepared rapid acquisition gradient echo (MP-RAGE) was also acquired for each subject (TE = 2.97 ms; TR = 1800 ms; FOV = 256 × 256 mm2; flip angle = 9°; matrix size = 256 × 256 pixels; and slice thickness = 1 mm).
fMRI data analysis
We used SPM8 revision 3684 (The Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm) in MATLAB 2010a (MathWorks Inc., Natick, MA, USA) to analyze the functional images. The first four volumes of each fMRI run were discarded because the signal was unsteady. We initially performed motion correction, normalization to the Montréal Neurological Institute (MNI) T1 template, and spatial smoothing (8 mm). After the realignment processes, we checked head-movement parameters. Task-related activation was statistically evaluated on a voxel-by-voxel basis using a general linear model with five regressors (title: 2.5 s; assessment scene [with active listening behavior] = 20 s; assessment scene [without active listening behavior] = 20 s; assessment scene [control] = 20 s; and personal evaluation = 2.5 s), including the six motion parameters at the individual level, to generate contrast images. These were incorporated into the random-effects analysis at the group level (Friston et al., 1994).
Four subjects were excluded from the fMRI data analysis (see above), and so 18 subjects were included. To investigate the neural activation in response to viewing the evaluation scene and the modulation due to evaluators’ active listening behavior, we analyzed three contrast images: with active listening behavior > control; without active listening behavior > control; and with active listening behavior > without active listening behavior. The three contrast images were submitted to one-sample t tests for second-level analysis. In the analyses, we conducted whole-brain analyses. The overlapping activations of the first two contrasts were used to investigate the common neural correlates underlying the viewing of assessments of participant’s experiences regardless of the ostensible evaluator’s behavior, and in comparison to only seeing evaluators’ photographs during assessment. The third contrast was used to examine the neural correlates that were specifically modulated by the active listening behavior of the ostensible evaluators. Thresholds of significant activation were set at uncorrected p < .01 at the voxel level and false discovery rate (FDR) corrected (Chumbley, Worsley, Flandin, & Friston, 2010) p < .05 at the cluster level (Huettel, Song, & McCarthy, 2009).
Behavioral data analysis
To investigate the effect of perceiving active listening behavior, we analyzed the subjects’ impressions of the ostensible evaluators, their willingness to cooperate with the ostensible evaluators, and changes in their impressions of their personal experiences. We initially conducted a two-way (time [before/after the experiments] × target [evaluators with active listening behavior/evaluators without active listening behavior/control]) repeated measures analysis of variance (rmANOVA) for the impressions of ostensible evaluators. Because all combinations of the three conditions before the experiments showed similar results (all cases: Bonferroni corrected p = 1.000), and the impression of the control condition did not change significantly before and after the experimental session (Bonferroni corrected p = .122), the effect of active listening behavior was analyzed relative to the control condition in the subsequent analysis. We conducted paired t tests to examine the changes in the willingness to cooperate and the emotional responses of personal experiences due to the behavior (with or without active listening) of the evaluators.
RESULTS
Ratings
Prior to the fMRI experiment, the average impression rating (±SEM) for evaluators with active listening behavior, evaluators without active listening behavior, and controls were 58.21 ± 1.90, 57.51 ± 2.10, and 57.61 ± 2.67, respectively. After the fMRI experiment, the average impression rating (±SEM) for evaluators with active listening behavior, evaluators without active listening behavior, and controls were 70.08 ± 2.00, 42.93 ± 2.76, and 54.92 ± 1.68, respectively. rmANOVA indicated interaction effects (F(2,16) = 35.03, p < .001). Post hoc analysis showed that, following the fMRI experiment, the impression of evaluators with active listening behavior and those without were given the highest and the lowest ratings, respectively (Bonferroni corrected p < .01 for control versus evaluators without active listening behavior and Bonferroni corrected p < .001 for control versus evaluators with active listening behavior and for evaluators with active listening behavior versus those without active listening behavior). The subjects’ impression of an evaluator demonstrating active listening behavior after the fMRI experiments was significantly increased (Bonferroni corrected p < .001). By contrast, the impression of an evaluator without active listening behavior after the fMRI experiments was significantly decreased (Bonferroni corrected p < .001). Because every combination of the three conditions before the experiments and two impression results for the control condition did not show significant differences, the behavior effect was analyzed using the value relative to the control condition in the subsequent analysis.
The average (±SEM) willingness to cooperate ratings for evaluators demonstrating active listening behavior relative to the control (that is, the active listening behavior effect for the willingness to cooperate) was 12.00 ± 3.17, whereas that for the non-active listening behavior effect was −14.86 ± 2.99. Subjects rated greater willingness to cooperate with evaluators who had demonstrated active, compared to non-active, listening behavior (paired t test; t(17) = 6.10, p < .001).
Due to a technical problem while measuring the rating data during the fMRI experiment, two subjects’ data were excluded from the analysis of the emotional response ratings. The average (±SEM) rating provided by subjects of the recollected emotional response of life episodes while viewing evaluations with active listening behavior relative to the control was 1.08 ± 0.84, whereas that for the non-active listening behavior relative to control was −0.45 ± 0.98. Episodes which had been assessed by actively listening evaluators were rated as more positive than episodes which had been assessed by non-actively listening evaluators (paired t test; t(15) = 2.17, p < .05).
fMRI
The fMRI results showed that viewing an assessment provided by an actively listening or a non-actively listening evaluator, as compared to the control condition, commonly activated the mPFC, the right inferior frontal gyrus (IFG), the anterior insula, the superior temporal sulcus (STS), and the lateral visual cortex (uncorrected p < .01 at voxel level and FDR corrected p < .05 at cluster level, Figure 2 and Table 1).
Figure 2.
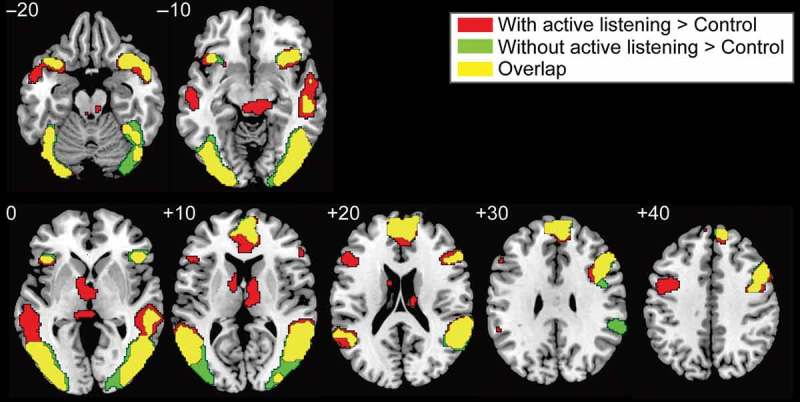
Significant activation while viewing an ostensible evaluator’s assessment relative to the control condition. Thresholds were set at uncorrected p < .01 at the voxel level and FDR corrected p < .05 at the cluster level. Red, green, and yellow indicate the activation during an ostensible evaluator’s assessment with active listening behavior, without active listening behavior, and the overlapping activation, respectively. Overlapping activation during the ostensible evaluators’ assessments with and without active listening behaviors occurred in the mPFC, IFG, STS, and anterior insula.
TABLE 1 .
Significant activation while viewing an ostensible evaluator’s assessment scene relative to the control condition
| Side | Label | Cluster p (FDR) | Cluster size | x | y | z | t Value |
|---|---|---|---|---|---|---|---|
| With active listening behavior > control | |||||||
| Right | Lateral visual area/STS | <0.001 | 6554 | 48 | −74 | 0 | 6.70 |
| Both | mPFC | <0.001 | 2258 | 6 | 50 | 16 | 6.59 |
| Left | Lateral visual area/STS | <0.001 | 4226 | −38 | −78 | −10 | 6.52 |
| Both | Midbrain | 0.026 | 452 | −4 | −28 | −12 | 6.41 |
| Left | Anterior insula/IFG | 0.001 | 1093 | −52 | 24 | 22 | 5.71 |
| Both | Ventral striatum/thalamus | 0.002 | 921 | −4 | −6 | 0 | 5.39 |
| Right | IFG | <0.001 | 1498 | 42 | 12 | 36 | 4.98 |
| Left | Cerebellum | 0.017 | 533 | −8 | −74 | −44 | 4.86 |
| Left | IFG | 0.017 | 556 | −34 | 2 | 42 | 4.46 |
| Both | SMA | 0.042 | 371 | 6 | 14 | 64 | 3.27 |
| Without active listening behavior > control | |||||||
| Left | Lateral visual area/STS | <0.001 | 3985 | −40 | −82 | −4 | 9.24 |
| Right | Lateral visual area/STS | <0.001 | 6103 | 44 | −76 | −4 | 8.22 |
| Right | Anterior insula | 0.002 | 1019 | 36 | 18 | −20 | 6.98 |
| Both | mPFC | <0.001 | 1810 | −2 | 54 | 22 | 6.28 |
| Left | Anterior insula | 0.049 | 430 | −38 | 18 | −16 | 4.42 |
| Right | IFG | 0.001 | 1236 | 48 | 24 | 32 | 4.29 |
Notes: Thresholds were set at uncorrected p < .01 at the voxel level and false discovery rate (FDR) corrected p < .05 at the cluster level. STS = superior temporal sulcus. mPFC = medial prefrontal cortex. IFG = inferior frontal gyrus. SMA = supplementary motor area.
The active listening behavior condition significantly activated the ventral striatum/thalamus and the right insula/IFG compared with the non-active listening behavior condition (uncorrected p < .01 at voxel level and FDR corrected p < .05 at cluster level) (Figure 3 and Table 2).
Figure 3.
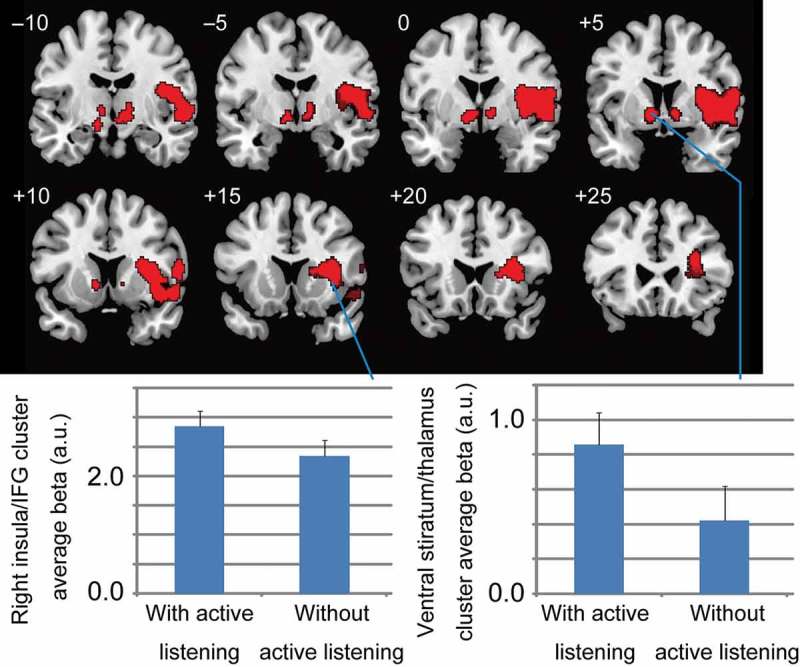
Significant activation during an ostensible evaluator’s assessment with active listening behavior compared to without active listening behavior. Thresholds were set at uncorrected p < .01 at the voxel level and false discovery rate (FDR) corrected p < .05 at the cluster level. Two significant activation clusters occurred: one in the insula and the right inferior frontal gyrus (IFG), and the other in the ventral striatum and thalamus. The lower half shows the average estimated beta values in the two significant clusters related to the two conditions (with and without active listening attitudes). The cluster average beta value was calculated using MarsBaR (http://marsbar.sourceforge.net). These bar-graphs are based on raw data and detail the significant activations.
TABLE 2 .
Significant activation while viewing an ostensible evaluator’s assessment with or without active listening behavior
| Side | Label | Cluster p (FDR) | Cluster size | x | y | z | t Value |
|---|---|---|---|---|---|---|---|
| With active listening behavior > without active listening behavior | |||||||
| Right | insula/IFG | <0.001 | 4035 | 28 | 16 | 10 | 5.28 |
| Both | ventral striatum/thalamus | 0.028 | 576 | −10 | 2 | −4 | 4.84 |
Notes: Thresholds were set at uncorrected p < .01 at the voxel level and false discovery rate (FDR) corrected p < .05 at the cluster level. Reported significant activation was restricted; significant activation required a larger average beta value in a significant cluster while viewing an ostensible evaluator’s assessment scene with or without active listening behavior than the control condition. The cluster average beta value was calculated using MarsBaR (http://marsbar.sourceforge.net). IFG = inferior frontal gyrus.
DISCUSSION
Behavioral data
The present results suggest that active listening behavior helped to establish a good rapport with an evaluator via positive impression formation. Participants also indicated that they were more willing to cooperate with an evaluator who had actively listened to them, as compared to one who had not. In addition, and consistent with a previous study, which demonstrated that recognizing active listening behavior by a therapist during psychotherapy led to an improvement in the impression towards relevant experiences (Rogers, 1957), our current results suggest that the emotional appraisal of an experience is positively changed in response to perceiving active listening by another. To the best of our knowledge, this is the first evidence that emotional appraisal (in this case, emotional appraisal of a personal life experience) is positively changed by perceiving active listening of the recounting of that experience. Our results also suggest that this positive reappraisal might occur after relatively short (20 s) experiences of active listening.
Ventral striatal activation caused by active listening recognition
As expected, the ventral striatum was activated when active listening was perceived. The striatum plays a key role in reward processing (O’Doherty, 2004). The ventral striatum can represent various types of reward including abstract rewards, such as a warm glow (that is, a pleasant feeling that accompanies a helping behavior) (Harbaugh, Mayr, & Burghart, 2007; Telzer, Masten, Berkman, Lieberman, & Fuligni, 2010), praise from others (Izuma et al., 2008), and monetary gain (Elliott et al., 2003; Izuma et al., 2008; Knutson et al., 2001). In this sense, the ventral striatum may represent a common currency of reward (Izuma et al., 2008). As positive social interactions are fundamentally rewarding for humans (Baumeister & Leary, 1995), the present ventral striatum activation may represent the abstract reward of being actively listened to by another.
The striatum is divided into two major parts: ventral and dorsal (O’Doherty et al., 2004). The ventral striatum plays the “critic” role in reward prediction, whereas the dorsal striatum plays the “actor” role and maintains information about the rewarding outcomes of actions to enable them to be chosen more frequently (O’Doherty et al., 2004). The ventral striatum is thought to more directly represent actual reward by evaluating the difference between expected and received reward (Van Der Meer, Johnson, Schmitzer-Torbert, & Redish, 2010). In line with the proposed functional segregation of the striatum, the ventral striatum is activated during the experience of receiving a reward, whereas the dorsal striatum is activated during reward anticipation (Salimpoor, Benovoy, Larcher, Dagher, & Zatorre, 2011). According to this account, the ventral striatum activation observed in the present study is more likely to represent the acquisition of abstract reward aroused by the perception of active listening behavior.
The ventral striatum contributes to reward-related learning processes (O’Doherty et al., 2004). Positive social interaction (explicit feedback) is learned by similar reward-related learning processes through ventral striatal activation (Jones et al., 2011); the present ventral striatal activation could therefore be utilized for social learning processes. During emotional appraisal, humans get to know the present emotional states through learning mechanisms (Lamm & Singer, 2010). In this sense, reward representation in the ventral striatum could be subject to emotional reappraisal processes, which was expected for subjects’ experiences after viewing the ostensible evaluators’ assessments.
We found that the emotional response of recollected experiences was improved by active listening. This suggests that recognition of active listening promotes a positive emotional appraisal of communicated material. Because an emotional appraisal requires emotional generation through physical arousal (James, 1894) and the retrieval of emotionally relevant cues from the environment (Schachter & Singer, 1962), reward-related information might act as a relevant cue for emotional reappraisal.
Right anterior insular activation caused by active listening recognition
Observing assessments by an evaluator engaging in active listening behavior, compared to an evaluator not engaging in active listening, enhanced right insula activation. The insula plays a role in feelings by representing current and predictive internal states (Singer, Critchley, & Preuschoff, 2009) as well as past internal states (Craig, 2009). By comparing current and/or past and predictive internal states, the anterior insula is thought to represent information about emotional states (Lamm & Singer, 2010). Through this kind of internal states comparison process, we can learn for risk prediction (Preuschoff et al., 2008), pain anticipation (Ploghaus et al., 1999), or anxiety proneness (Paulus & Stein, 2006). By utilizing the learning mechanisms in the anterior insula (Seymour et al., 2004), humans might alter emotional appraisals based on present contextual information, such as a current positive feeling aroused by receiving an active listening attitude. Along this line, emotional appraisal modulation due to false physiological feedback activates the right anterior insula (Gray et al., 2007). As active listening behavior in psychotherapy positively affects therapeutic personality changes (Duan & Hill, 1996; Gladstein, 1977), which are accompanied by an improvement in the impression of relevant experiences (Rogers, 1957), changes of the emotional appraisal of subjects’ life episodes were aroused by active listening behavior. As the absolute value of the difference in the pleasantness rating from the control condition during assessments with the active listening condition was larger than that during assessments without the active listening condition, the load of the emotional reappraisal process during assessments with the active listening condition was thought to be high. The present right anterior insula activation (with active listening > without active listening) might have reflected the high load of emotional reappraisal during assessments with the active listening condition. Thus, the enhanced right insula activation observed in response to perceiving active listening might represent an emotional reappraisal process based on interoceptive information related to reward-related ventral striatal activation.
In addition, we found a common significant activation in the anterior insula, close to the active listening-specific anterior insula cluster during assessments with and without active listening behaviors. In the present task, participants were required to recall their emotional status; thus, to some degree, they conducted emotional appraisal even for assessments in the without active listening condition. As physical state arousal is accompanied by emotion, somatic representation in the insula (Craig, 2002, 2003) may act as a gateway to generate emotion (Damasio, 1994; Damasio et al., 2000). As listening attitude modulated the impression of target experiences (Rogers, 1957), it appears to influence emotional appraisal processes. In this sense, the ostensible evaluators’ behavior (whether with or without active listening) might promote emotional reappraisal processes through an emotional regeneration processes. Thus, the subjects might reexperience emotions, which are related to a personal experience during viewing an assessment.
Mentalizing network activation in response to perceiving an evaluator’s listening behavior
To change an emotional appraisal in response to an evaluator’s active listening behavior, relevant contextual information must be retrieved. Thus, mentalization might play a key role through inferring the mental state of the evaluator (Frith & Frith, 1999). The mentalizing system is an orchestration of two functions (Frith & Frith, 2003): mental state inference in the mPFC (Hynes, Baird, & Grafton, 2006; Tamir & Mitchell, 2010) and the understanding of others’ behaviors in the STS (Brass, Schmitt, Spengler, & Gergely, 2007). In this sense, the present common mPFC and STS activations when observing video clips of assessments, compared to static photographs of evaluators, might reflect mentalizing processes which are used to infer the intent of ostensible evaluators. As a result of mentalizing, participants might judge evaluators’ intent as social acceptance for assessments with active listening.
CONCLUSION
We found that recognizing active listening behavior directed towards one’s own emotional episodes changes the emotional appraisal of those episodes and is accompanied by a positive impression of the evaluator. Moreover, positive impression arousal together with ventral striatal activation suggested that the recognition of active listening is represented as a reward. Reward perception while receiving active listening is mediated by inferring the intent of listeners, which invokes mPFC and STS activation. The inference of listeners’ intent is implicitly required when viewing evaluators irrespective of their attitude. Based on the impression improvement of relevant experiences after perceiving active listening, the right anterior insula may support emotional reappraisal processes, which might interact with reward representation processes.
Acknowledgments
This study was partly supported by Scientific Research on Innovative Areas grants [#23101507] (to H.K.) and [#22101007] (to H.C.T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), by a Grant-in-Aid for Scientific Research (S) [#21220005] (to N.S.) and by Grants-in-Aid for Young Scientists (B) [#23700505] and [#25750407] (to H.K.) from the Japan Society for the Promotion of Science. Part of this study was supported by “Development of biomarker candidates for social behavior,” carried out under the Strategic Research Program for Brain Sciences of MEXT.
REFERENCES
- Amodio D. M., Frith C. D. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anme T., Tokutake K., Tanaka E., Mochizuki Y., Wu B., Watanabe T., Sadato N. Validity and reliability of the index of active listening (IAL) Journal of Applied Medical Sciences. 2013;2(2):21–29. [Google Scholar]
- Baumeister R. F., Leary M. R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Brass M., Schmitt R. M., Spengler S., Gergely G. Investigating action understanding: Inferential processes versus action simulation. Current Biology. 2007;17(24):2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Chartrand T. L., Bargh J. A. The chameleon effect: The perception-behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76(6):893–910. doi: 10.1037/0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Chumbley J., Worsley K., Flandin G., Friston K. Topological FDR for neuroimaging. Neuroimage. 2010;49(4):3057–3064. doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. D. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A. D. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig A. D. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio A. R. Descartes’ error and the future of human life. Scientific American. 1994;271(4):144. doi: 10.1038/scientificamerican1094-144. [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Grabowski T. J., Bechara A., Damasio H., Ponto L. L., Parvizi J., Hichwa R. D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Duan C., Hill C. E. The current state of empathy research. Journal of Counseling Psychology. 1996;43:261–274. doi: 10.1037/0022-0167.43.3.261. [DOI] [Google Scholar]
- Elliott R., Newman J. L., Longe O. A., Deakin J. F. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. The Journal of Neuroscience. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K., Weber B., Trautner P., Dohmen T., Sunde U., Elger C. E., Falk A. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318(5854):1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P., Worsley K. J., Poline J.-P., Frith C. D., Frackowiak R. S. J. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1994;2(4):189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- Frith C. D., Frith U. Interacting minds – A biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C. D. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U., Frith C. D. The social brain: Allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1537):165–176. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H. L., Happe F., Brunswick N., Fletcher P. C., Frith U., Frith C. D. Reading the mind in cartoons and stories: An fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gladstein G. A. Empathy and counseling outcome: An empirical and conceptual review. The Counseling Psychologist. 1977;6:70–79. doi: 10.1177/001100007700600427. [DOI] [Google Scholar]
- Gray M. A., Harrison N. A., Wiens S., Critchley H. D. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS ONE. 2007;2(6):e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh W. T., Mayr U., Burghart D. R. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Huettel S. A., Song A. W., McCarthy G. Functional magnetic resonance imaging. Sunderland, MA: Sinauer Associates; 2009. [Google Scholar]
- Hynes C. A., Baird A. A., Grafton S. T. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–383. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D. N., Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- James W. Discussion: The physical basis of emotion. Psychological Review. 1894;1(5):516–529. doi: 10.1037/h0065078. [DOI] [PubMed] [Google Scholar]
- Jones R. M., Somerville L. H., Li J., Ruberry E. J., Libby V., Glover G., Casey B. J. Behavioral and neural properties of social reinforcement learning. The Journal of Neuroscience. 2011;31(37):13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C. M., Fong G. W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Muller B. C., Van Baaren R. B., Wietzker A., Dijksterhuis A., Brass M. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Social Neuroscience. 2010;5(4):384–392. doi: 10.1080/17470911003633750. [DOI] [PubMed] [Google Scholar]
- Lamm C., Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214(5–6):579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- O’Doherty J. P. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty J. P., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R. J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus M. P., Stein M. B. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Tracey I., Gati J. S., Clare S., Menon R. S., Matthews P. M., Rawlins J. N. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Preuschoff K., Quartz S. R., Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. The Journal of Neuroscience. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. The necessary and sufficient conditions of therapeutic personality change. Journal of Consulting Psychology. 1957;21(2):95–103. doi: 10.1037/h0045357. [DOI] [PubMed] [Google Scholar]
- Rogers C. A theory of therapy, personality and interpersonal relationships as developed in the client-centered framework. New York, NY: McGraw Hill; 1959. [Google Scholar]
- Salimpoor V. N., Benovoy M., Larcher K., Dagher A., Zatorre R. J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nature Neuroscience. 2011;14(2):257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Schachter S., Singer J. E. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Seymour B., O’Doherty J. P., Dayan P., Koltzenburg M., Jones A. K., Dolan R. J., Frackowiak R. S. Temporal difference models describe higher-order learning in humans. Nature. 2004;429(6992):664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H. D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stel M., Van Baaren R. B., Vonk R. Effects of mimicking: Acting prosocially by being emotionally moved. European Journal of Social Psychology. 2008;38:965–976. doi: 10.1002/ejsp.472. [DOI] [Google Scholar]
- Stel M., Vonk R. Mimicry in social interaction: Benefits for mimickers, mimickees, and their interaction. British Journal of Psychology. 2010;101(2):311–323. doi: 10.1348/000712609X465424. [DOI] [PubMed] [Google Scholar]
- Tamir D. I., Mitchell J. P. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):10827–10832. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E. H., Masten C. L., Berkman E. T., Lieberman M. D., Fuligni A. J. Gaining while giving: An fMRI study of the rewards of family assistance among white and Latino youth. Social Neuroscience. 2010;5(5–6):508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Meer M. A., Johnson A., Schmitzer-Torbert N. C., Redish A. D. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67(1):25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


