Abstract
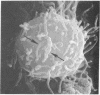
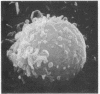
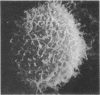
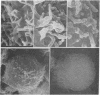
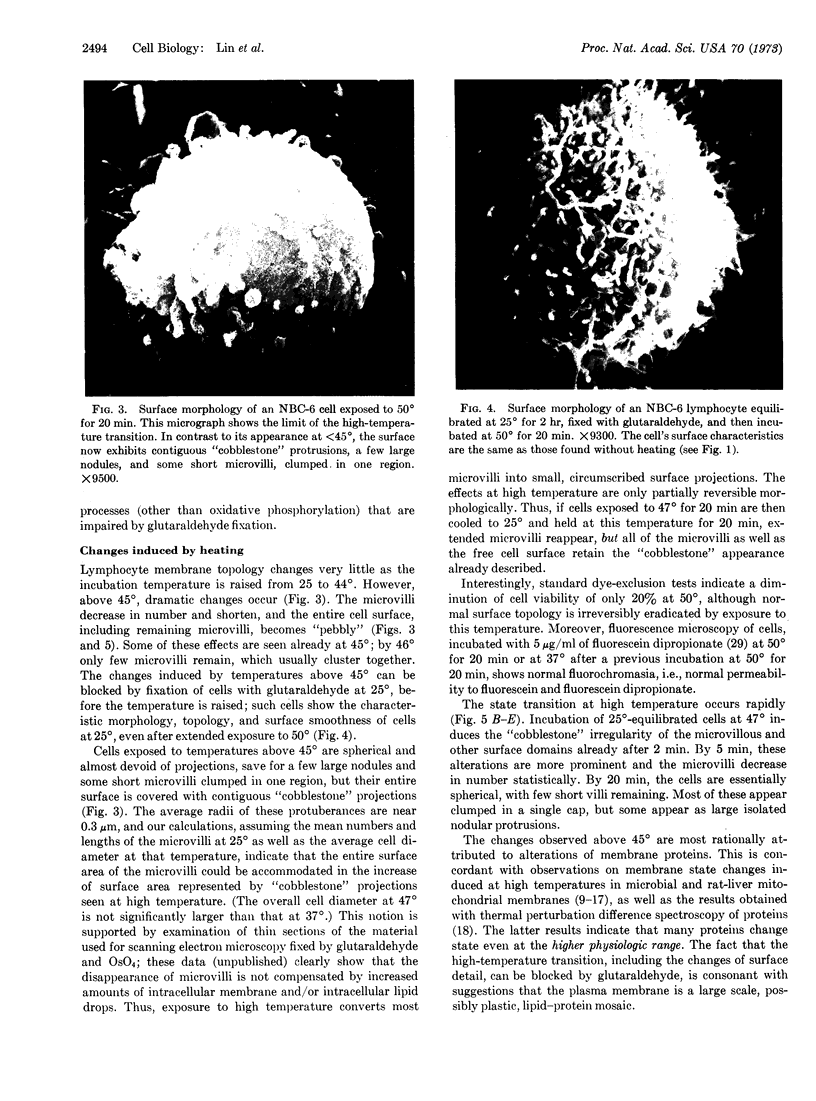
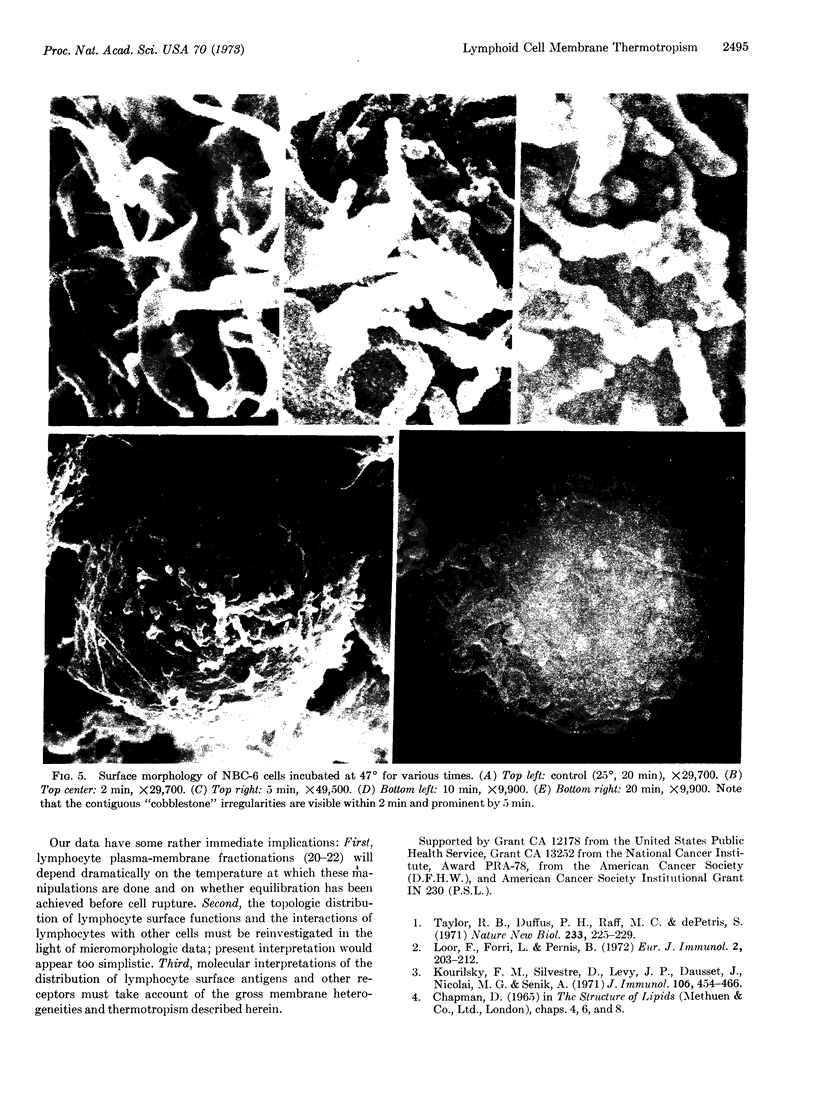
Temperature-induced variations in the surface morphology of cultured lymphocytes were evaluated by scanning electron microscopy. At 25-37° the cells' surfaces are largely obscured by numerous undulating microvilli of various lengths but uniform diameter. Temperature changes alter the number of microvilli, their lengths, diameters, distribution, branching, and fusing. Typically, chilling to 0-4° markedly reduces the number of microvilli and increases the diameter of the survivors in a reversible process. In contrast, heating the cells to about 45° rapidly and irreversibly transforms the ordinarily smooth membrane surface into one with a “cobblestone” morphology. At the same time most microvilli disappear and the few that remain clump into a cap. The data suggest that the low-temperature effects reflect a change in the physical state of membrane lipids, while the high-temperature alterations represent thermotropic protein transitions.
Keywords: plasma membrane, microvilli, lipid transition, protein transition
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Isolation and composition of human thymocyte plasma membrane. Biochim Biophys Acta. 1972 Jul 3;274(1):22–27. doi: 10.1016/0005-2736(72)90276-3. [DOI] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazyk J. F., Steim J. M. Phase transitions in mammalian membranes. Biochim Biophys Acta. 1972 Jun 20;266(3):737–741. doi: 10.1016/0006-3002(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Chapman D., Urbina J. Phase transitions and bilayer structure of Mycoplasma laidlawii B. FEBS Lett. 1971 Jan 12;12(3):169–172. doi: 10.1016/0014-5793(71)80060-1. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber E., Resch K., Wallach D. F., Imm W. Isolation and characterization of lymphocyte plasma membranes. Biochim Biophys Acta. 1972 May 9;266(2):494–504. doi: 10.1016/0005-2736(72)90105-8. [DOI] [PubMed] [Google Scholar]
- Kourilsky F. M., Silvestre D., Levy J. P., Dausset J., Nicolai M. G., Senik A. Immunoferritin study of the distribution of HL-A antigens on human blood cells. J Immunol. 1971 Feb;106(2):454–466. [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Leach S. J., Smith J. A. Thermal perturbation difference spectroscopy of proteins. Int J Protein Res. 1972;4(1):11–19. doi: 10.1111/j.1399-3011.1972.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J., Sturtevant J. M., Tsong T. Y. Characterization of the plasma membrane of Mycoplasma laidlawii. VII. Phase transitions of membrane lipids. Biochim Biophys Acta. 1970;219(1):114–122. doi: 10.1016/0005-2736(70)90066-0. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter E., Gulik-Krzywicki T., Kaback H. R. Correlations between fluorescence, x-ray diffraction, and physiological properties in cytoplasmic membrane vesicles isolated from Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):466–477. doi: 10.1016/0005-2736(72)90192-7. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]