Abstract
Acinetobacter calcoaceticus–baumannii complex is a common cause of hospital-acquired infections (HAIs) globally, remarkable for its high rate of antibiotic resistance, including to carbapenems. There are few data on the resistance of A. baumannii in Vietnam, which are essential for developing evidence-based treatment guidelines for HAIs. Antibiotic susceptibility testing was conducted by VITEK®2, and pulsed-field gel electrophoresis (PFGE) was performed on 66 clinical A. baumannii complex isolates recovered during 2009 at the National Hospital of Tropical Diseases (NHTD), a referral hospital in Hanoi, Vietnam. Basic demographic and clinical data were collected and analysed using descriptive statistics. Most isolates came from lower respiratory tract specimens (59; 89.4%) from intensive care unit (ICU) patients [64/65 (98.5%) with available data] who had been admitted to NHTD for ≥2 days [42/46 (91.3%) with available data]. More than 90% of the isolates were resistant to the tested β-lactamase/β-lactamase inhibitors, cephalosporins, carbapenems, fluoroquinolones and trimethoprim/sulfamethoxazole. Moreover, 25.4% (16/63) were resistant to all tested β-lactams, quinolones and aminoglycosides. All isolates remained sensitive to colistin and 58.7% were susceptible to tigecycline. Of the 66 isolates, 49 could be classified into eight PFGE types (A–H). Every PFGE type, except D, had cluster(s) of three or more isolates with a temporal relationship. In conclusion, these data suggest a significant rise in A. baumannii antibiotic resistance in Vietnam. Clustering within PFGE types supports cross-transmission of A. baumannii within the ICU at NHTD. Increased research and resources in optimising treatment, infection control and antibiotic stewardship are needed.
Keywords: Acinetobacter baumannii, Ventilator-associated pneumonia, Hospital-acquired infection, Antibiotic resistance, Genotype
1. Introduction
Acinetobacter calcoaceticus–baumannii complex is emerging as one of the most common causes of hospital-acquired infections (HAIs) in intensive care units (ICUs) worldwide and is often resistant to multiple antibiotic classes, complicating treatment [1]. Acinetobacter baumannii is a strictly aerobic, non-motile, Gram-negative bacillus belonging to the A. calcoaceticus–baumannii complex within the family Moraxellaceae of the order Gammaproteobacteria. Identification by phenotypic methods or DNA–DNA hybridisation does not reliably distinguish A. baumannii from other members of the A. calcoaceticus–baumannii complex (henceforth referred to as A. baumannii) [1]. This poses challenges clinically because A. baumannii can be pathogenic whereas A. calcoaceticus is environmental. The exact reservoir of A. baumannii remains undefined [2]. In contrast to other Acinetobacter spp., A. baumannii is uncommon in nature compared with the hospital environment [1]. A. baumannii is able to survive on dry surfaces in hospital environments for up to 4 months [3].
A. baumannii infection occurs when the immunological barriers of the host are breached (e.g. mechanical ventilation) and is hence considered an opportunistic pathogen [4]. A. baumannii causes various types of HAI, including ventilator-associated pneumonia (VAP), bacteraemia, urinary tract infection, meningitis, and infections complicating burn wounds [5]. Treatment is difficult due to the high rates of antibiotic resistance worldwide, including resistance to carbapenems [2]. However, few data are available on the molecular epidemiology and antibiotic resistance of A. baumannii infections in Asia, including Vietnam. Rapid development in Vietnam has been accompanied by the increasing availability of complex healthcare, including ICUs, and accompanied by HAIs. As the isolation of A. baumannii is reported to be particularly common in VAP, it is crucial to know more regarding the resistance and epidemiology of these bacteria in a resource-constrained setting such as Vietnam. This study describes the antibiotic susceptibility and molecular epidemiology of A. baumannii isolates from a referral hospital in Hanoi, Vietnam.
2. Materials and methods
2.1. Design
This was a retrospective study examining the molecular epidemiology and antibiotic susceptibility of clinical A. baumannii isolates collected from hospitalised patients from January to December 2009 at the National Hospital for Tropical Diseases (NHTD) in Hanoi. The NHTD is a tertiary referral hospital for adult infectious diseases with a catchment area that covers Hanoi and the surrounding northern provinces. At the time of the study, NHTD had 150 inpatient beds including 15 ICU beds, and ca. 4000 inpatient admissions annually. The study was approved by the Scientific and Ethical Committee of NHTD.
2.2. Isolates
In this study, 66 stored A. baumannii isolates cultured from unique patients admitted to NHTD in 2009 were viable and available for study, from a total of 99 A. baumannii isolates. For each patient, the first isolate was selected and isolates from sterile sites were chosen over respiratory samples, whenever possible. A. baumannii isolates were identified by standard microbiological methods, including API 20 NE (bioMérieux, Marcy-l’Étoile, France). PCR targeting the blaOXA-51-like gene was conducted later and was positive in 48 of the 48 available isolates [6]. Isolates were stored in glycerol medium at −70 °C. In total, 63 isolates were subjected to antibiotic susceptibility testing according to Clinical and Laboratory Standards Institute (CLSI) guidelines using the VITEK®2 system with card AST-N089 22237 (bioMérieux) for the following antibiotics: ampicillin; amoxicillin/clavulanic acid; piperacillin/tazobactam; ceftazidime; cefotaxime; cefepime; imipenem; meropenem; amikacin; gentamicin; tobramycin; ciprofloxacin; levofloxacin; trimethoprim/sulfamethoxazole (SXT); colistin; and tigecycline. This antibiotic card interprets tigecycline susceptibility according to US Food and Drug Administration (FDA) breakpoints for Enterobacteriaceae. In total, 58 isolates were typed by pulsed-field gel electrophoresis (PFGE) as previously described using the restriction enzyme ApaI [7], [8]. In addition, data were collected on specimen source and date of collection. From the patient chart, data were collected on patient demographics, location, and brief clinical data such as type of infection.
2.3. Analysis
Susceptibility data were analysed using WHOnet 5.5 software (World Health Organization, Geneva, Switzerland). Amikacin data are not shown as these are considered to be unreliable when tested by the VITEK system [9]. Analysis of PFGE results was done visually using the criteria of Tenover et al. [10] by two readers (VDT and HW) and discrepancies were resolved by a third reader (QD). Isolates with PFGE patterns that were indistinguishable (zero genetic differences) or closely related (one genetic difference) were grouped together. Isolate and patient data were analysed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) using descriptive statistics as appropriate.
3. Results
3.1. Source of the A. baumannii isolates
The 66 A. baumannii isolates came from the lower respiratory tract (59; 89.4%), blood (6; 9.1%) and pus (1; 1.5%). The median patient age was 51 years (range 17–93 years) and the majority of patients were male (48; 72.7%). The 59 A. baumannii isolates from sputum comprised 13.1% of a total of 451 sputum specimens. Almost all patients were admitted to the ICU (64/65 with available data). The reason(s) for ICU admission was available for 43 patients (some patients had more than one reason for ICU admission listed) and included pneumonia in 14 (32.6%), sepsis or septic shock in 10 (23.3%), tetanus in 9 (20.9%), central nervous system infection in 8 (18.6%), and substance abuse, respiratory failure not otherwise specified, dengue fever, and fever not otherwise specified each in 2 patients (4.7%). Most specimens were collected for clinical suspicion of VAP. Most specimens were collected ≥2 days after the patient's admission to NHTD [42/46 (91.3%) where dates of admission and specimen collection were both available] and were therefore compatible with HAI. Patient information for three of the remaining specimens indicated that the patients had been transferred from another hospital; however, the length of stay at the referring hospital was not available.
3.2. Antibiotic susceptibility
In total, 63 isolates underwent antibiotic susceptibility testing using the VITEK®2 system (Table 1). Resistance rates were high, with >90% of isolates being resistant to the tested β-lactamase/β-lactamase inhibitors, cephalosporins, carbapenems, fluoroquinolones and SXT. Moreover, 34.9% of isolates were resistant to both gentamicin and tobramycin, and 25.4% of isolates (n = 16) were resistant to all tested β-lactams, quinolones and aminoglycosides. All isolates remained sensitive to colistin and 58.7% were susceptible to tigecycline.
Table 1.
Antibiotic susceptibility testing of 63 Acinetobacter baumannii isolates at the National Hospital of Tropical Diseases (Hanoi, Vietnam), 2009.
| Antibiotic | Susceptible |
Intermediate |
Resistant |
|||
|---|---|---|---|---|---|---|
| % | n | % | n | % | n | |
| AMC | 0.0 | 0 | 0.0 | 0 | 100.0 | 63 |
| TZP | 1.6 | 1 | 3.2 | 2 | 95.2 | 60 |
| Cefotaxime | 0.0 | 0 | 1.6 | 1 | 98.4 | 62 |
| Ceftazidime | 0.0 | 0 | 1.6 | 1 | 98.4 | 62 |
| Cefepime | 0.0 | 0 | 1.6 | 1 | 98.4 | 62 |
| Imipenem | 7.9 | 5 | 0.0 | 0 | 92.1 | 58 |
| Meropenem | 7.9 | 5 | 0.0 | 0 | 92.1 | 58 |
| Gentamicin | 17.5 | 11 | 27.0 | 17 | 55.6 | 35 |
| Tobramycin | 31.7 | 20 | 25.4 | 16 | 42.9 | 27 |
| Ciprofloxacin | 1.6 | 1 | 0.0 | 0 | 98.4 | 62 |
| Levofloxacin | 1.6 | 1 | 7.9 | 5 | 90.5 | 57 |
| Tigecycline | 58.7 | 37 | 38.1 | 24 | 3.2 | 2 |
| Colistin | 100.0 | 63 | 0.0 | 0 | 0.0 | 0 |
| SXT | 0.0 | 0 | 0.0 | 0 | 100.0 | 63 |
AMC, amoxicillin/clavulanic acid; TZP, piperacillin/tazobactam; SXT, trimethoprim/sulfamethoxazole.
3.3. Pulsed-field gel electrophoresis
Of the 58 isolates that underwent PFGE, 49 were classified into eight PFGE types and were assigned the letters A–H (Supplementary Fig. S1). The letter assignment is not intended to ascribe relatedness among the PFGE types. PFGE type A isolates (n = 6) originated from sputum, type B (n = 7) from sputum (4) and blood (3), type C (n = 15) from sputum (14) and blood (1), and types D (n = 3), E (n = 4), F (n = 4), G (n = 5) and H (n = 5) were all from sputum. The remaining nine isolates, including two blood isolates, did not appear related to other isolates by PFGE.
Supplementary Fig. I related to this article can be found, in the online version, at doi:10.1016/j.jgar.2014.05.003.
Supplementary Fig. I.
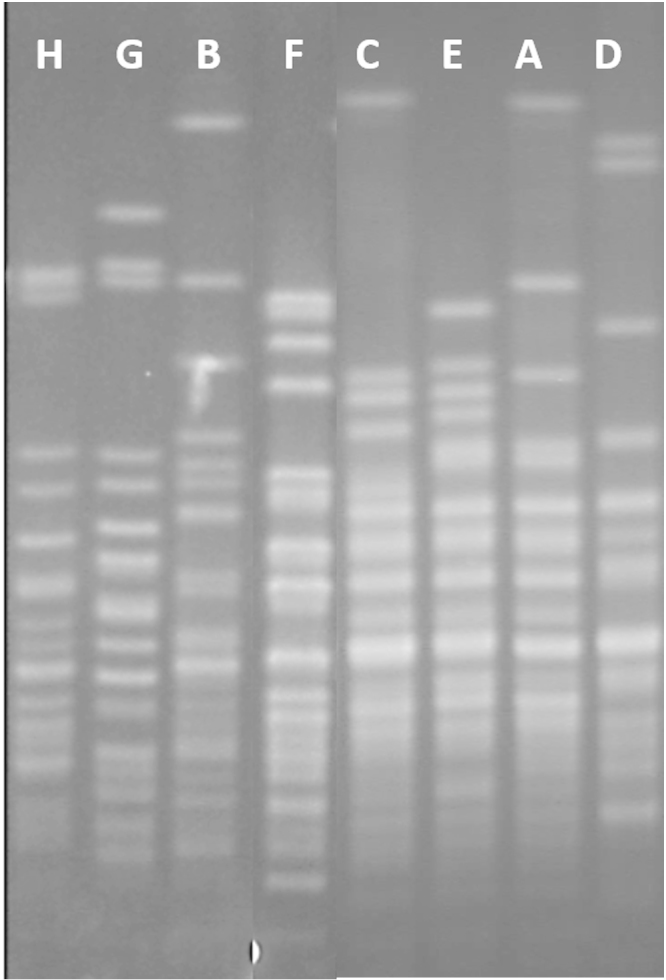
Representative pulsed-field gel electrophoresis (PFGE) patterns of each Acinetobacter baumannii type (A–H), digested with ApaI. Isolates with PFGE patterns that were indistinguishable (zero genetic differences) or closely related (one genetic difference) were grouped into a type. Letter ordering is not intended to ascribe relatedness of types.
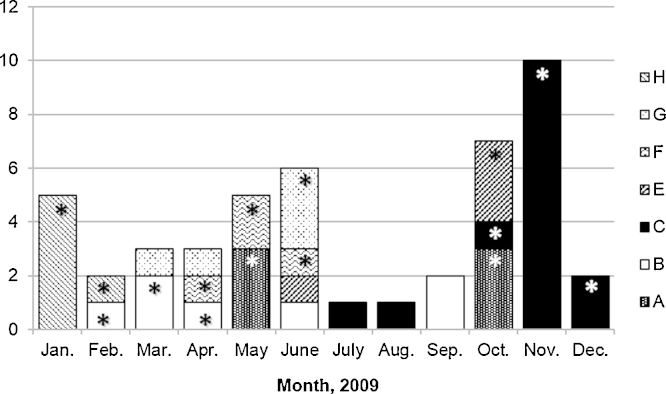
Every PFGE type, except D type, had cluster(s) of three or more isolates that were temporally related, with time spans ranging from 6 days to 67 days (Fig. 1). On average, isolates within each cluster were detected every 2–16.8 days. Type A isolates, which clustered in May and October, had identical antibiotic testing results, with susceptibility only to tigecycline and colistin (Table 2). Isolates within the other PFGE types differed in antibiotic resistance by at least one antibiotic class, most often the aminoglycosides, but also the carbapenems, quinolones and tigecycline. The type B and C clusters included all the blood isolates from those types.
Fig. 1.
Clustering of Acinetobacter calcoaceticus–baumannii complex isolates in 2009 at the National Hospital of Tropical Diseases (Hanoi, Vietnam) within pulsed-field gel electrophoresis (PFGE) types A–C and E–H. * Indicates cluster isolates.
Table 2.
Antibiotic susceptibility patternsa according to pulsed-field gel electrophoresis (PFGE) type.
| Antibiotic/antibiotic class | PFGE type |
|||||||
|---|---|---|---|---|---|---|---|---|
| A (6) | Bb (7) | C (15) | D (3) | E (4) | F (4) | Gb (5) | H (5) | |
| β-Lactamase/β-lactamase inhibitors | R | R | R | R | R | R | R | R |
| Cephalosporins | R | R | R | R | R | R | R | R |
| Carbapenems | R | r/s | R | R | R | R | R | R |
| Gentamicin | R | r/i/s | r/i | R | R | R | r/i/s | r/i |
| Tobramycin | R | r/s | r/i/s | i/s | R | r/i | r/s | i/s |
| Fluoroquinolones | R | R | R | R | r/i | R | R | R |
| Tigecycline | S | S | I | i/s | i/s | S | S | r/i/s |
| Colistin | S | S | S | S | S | S | S | S |
| SXT | R | R | R | R | R | R | R | R |
R, resistant; I, intermediate; S, susceptible; SXT, trimethoprim/sulfamethoxazole.
Susceptibility phenotypes reported in non-capitalised letters and separated by a slash indicate varying susceptibility patterns within that cluster of strains.
Antibiotic testing data were missing for one isolate.
4. Discussion and conclusion
This study presents antibiotic susceptibility data for 63 A. baumannii isolates at a referral hospital in Hanoi, Vietnam, in 2009. The majority of isolates came from sputum samples collected from ICU patients ≥2 days into their admission at NHTD. There were high rates of resistance, including nearly complete resistance to all tested β-lactam antibiotics. Susceptibility to colistin and tigecycline remained high. The VITEK 2 system used in this study is considered reliable for most antibiotics, including colistin [11]. Amikacin was excluded from this analysis as testing for amikacin susceptibility by VITEK 2 was previously shown to produce very major errors more than one-third of the time compared with broth microdilution [9].
Data from northern Vietnam on A. baumannii isolates from 1997 to 1998 showed considerably less resistance, with resistance rates of ca. 50% for cephalosporins, 50% for aminoglycosides, 25% for quinolones and 50% for SXT (Dr. Đoàn Mai Phương, Bach Mai Hospital, Vietnam, unpublished data). These data suggest a sharp increase in A. baumannii resistance over the last 10 years in Vietnam. This is supported by the COMPACT II study, which reported 89.5% carbapenem-non-susceptibility by Etest among 19 A. baumannii isolates from Vietnam, comparable with the current results [12]. In a recent study at a hospital in western China, 31 A. baumannii isolates from the ICU were also highly resistant, including 96.3% to SXT, 92.6% to cefotaxime and 55.6% to carbapenems [13].
These results raise questions regarding what constitutes appropriate empirical and definitive treatment of highly resistant A. baumannii infections, including the use of combination therapy and colistin. In this study, rifampicin was not tested, which is active alone and in combination in in vitro and animal studies [14], [15]. However, clinical data on the use of rifampicin in combination has generally been non-comparative. At NHTD, testing amikacin susceptibility using a more reliable method may broaden the available therapeutic options. Meanwhile, there is increasing reliance on colistin, even though it is not always readily available in Vietnam and is relatively costly. A global colistin usage survey indicated that in Vietnam, no loading dose of colistin is given, which may lead to treatment failure [16]. In general, underdosing of colistin risks the development of bacterial resistance against this last-line drug (unpublished data).
Numerous PFGE types were identified, suggesting a diverse population of A. baumannii rather than the spread of a specific clone. Nevertheless, PFGE types C and H appeared dominant, clustering from October to December and January to February, respectively. Confidence in the smaller clusters was limited by the sample size. The clonality of these PFGE types is supported by similarities in antibiotic susceptibility profiles. The clusters suggest transmission within the ICU with varying A. baumannii types in 2009 and emphasise the importance of rigorous infection control.
This study has limitations that need to be acknowledged. Limitations in microbiological and clinical data could have led to the inclusion of isolates from specimens that represented colonisation and contamination rather than infection. We also did not have details on where patients were located within the ICU to prove environmental, healthcare worker, or direct patient-to-patient spread of infection. Furthermore, we did not distinguish between pathogenic A. baumannii species from the environmental species A. calcoaceticus. A significant number of isolates were either not viable or were not groupable by PFGE, limiting the detection of PFGE types and clusters. However, we do not expect any selection bias in the isolates tested, which do provide us important information on the resistance patterns of A. calcoaceticus–baumannii complex strains in Vietnam.
These data show that A. calcoaceticus–baumannii complex is an important cause of HAIs in ICUs in northern Vietnam. The high rates of antibiotic resistance present a major opportunity for conducting treatment trials to determine the optimal treatment of A. baumannii HAIs, including the best timing and dosing of colistin as well as the role of combination therapy. Given the temporal clustering of related A. baumannii types, adequate resources need to be dedicated towards antibiotic stewardship and infection control activities. We are undertaking further studies on VAP and a nationwide HAI surveillance programme, which will inform future steps.
Funding
This research project was made possible with funds from the Global Antibiotic Resistance Partnership (USA) and the Wellcome Trust (UK).
Competing interests
None declared.
Ethical approval
This study was approved by the Scientific and Ethical Committee of the National Hospital for Tropical Diseases (Hanoi, Vietnam).
References
- 1.Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Perez F., Hujer A.M., Hujer K.M., Decker B.K., Rather P.N., Bonomo R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendt C., Dietze B., Dietz E., Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard A., O’Donoghue M., Feeney A., Sleator R.D. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turton J.F., Woodford N., Glover J., Yarde S., Kaufmann M.E., Pitt T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifert H., Dolzani L., Bressan R., van der Reijden T., van Strijen B., Stefanik D. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J Clin Microbiol. 2005;43:4328–4335. doi: 10.1128/JCM.43.9.4328-4335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seifert H., Schulze A., Baginski R., Pulverer G. Comparison of four different methods for epidemiologic typing of Acinetobacter baumannii. J Clin Microbiol. 1994;32:1816–1819. doi: 10.1128/jcm.32.7.1816-1819.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akers K.S., Chaney C., Barsoumian A., Beckius M., Zera W., Yu X. Aminoglycoside resistance and susceptibility testing errors in Acinetobacter baumannii–calcoaceticus complex. J Clin Microbiol. 2010;48:1132–1138. doi: 10.1128/JCM.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenover F.C., Arbeit R.D., Goering R.V., Mickelsen P.A., Murray B.E., Persing D.H. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo-Ten-Foe J.R., de Smet A.M., Diederen B.M., Kluytmans J.A., van Keulen P.H. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2007;51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiratisin P., Chongthaleong A., Tan T.Y., Lagamayo E., Roberts S., Garcia J. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: results of Asia-Pacific surveillance from the COMPACT II study. Int J Antimicrob Agents. 2012;39:311–316. doi: 10.1016/j.ijantimicag.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Jin H., Xu X.M., Mi Z.H., Mou Y., Liu P. Drug-resistant gene based genotyping for Acinetobacter baumannii in tracing epidemiological events and for clinical treatment within nosocomial settings. Chin Med J (Engl) 2009;122:301–306. [PubMed] [Google Scholar]
- 14.Lim T.-P., Tan T.-Y., Lee W., Sasikala S., Tan T.-T., Hsu L.-Y. In-vitro activity of polymyxin B, rifampicin, tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii in Singapore. PLoS ONE. 2011;6:e18485. doi: 10.1371/journal.pone.0018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pachón-Ibáñez M.E., Docobo-Pérez F., López-Rojas R., Domínguez-Herrera J., Jiménez-Mejias M.E., García-Curiel A. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wertheim H.F. Worldwide colistin and polymyxin usage, the ‘last’ Gram-negative antibiotics: results from an online global survey. 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 31 March–3 April 2012; London, UK: European Society of Clinical Microbiology and Infectious Diseases; 2012. [poster] [Google Scholar]