Abstract
Introduction
Somatostatin receptor scintigraphy (SRS; Octreoscan) is used in neuroendocrine tumors to locate the primary tumor site and delineate extent of disease. SRS has decreased sensitivity for small bowel neuroendocrine tumors (SBNETs). The reasons for SRS nonlocalization are not clear. We sought to determine factors that correlate with successful primary tumor localization by SRS in patients with resected SBNETs, and also identify factors that confound interpretation of SRS reports.
Methods
Records of patients with resected SBNETs were reviewed for SRS results, tumor size, multifocality, N, and M status. Somatostatin receptor 2 (SSTR2) expression was analyzed in resected tumors by quantitative PCR. SRS reports were reviewed and categorized as localizing the primary tumor or not. A nuclear medicine physician independently reviewed available images.
Results
Of 37 patients with preoperative SRS, the primary tumor was localized in 37%. Of all the factors tested, only small tumor size correlated significantly with SRS non-localization. Overexpression of SSTR2 was not significantly different between tumors that were or were not localized by SRS, regardless of tumor size. There were three instances where the SRS report did not agree with the nuclear medicine physician's interpretation as to whether SRS localized the primary tumor. In each case, uptake in mesenteric adenopathy was a confounding factor.
Conclusions
SBNETs less than 2 cm are most likely to be missed by SRS. SSTR2 expression did not correlate with SRS non-localization of the primary tumor. Uptake in mesenteric nodes may help indicate an SBNET primary, but can also interfere with its visualization within the small bowel.
Keywords: Small bowel neuroendocrine tumor, somatostatin scintigraphy, Octreoscan, SSTR2 expression
Introduction
Small bowel neuroendocrine tumors (SBNETs) are characterized by their small size, indolent growth, and propensity to metastasize to the liver. Approximately 15% of patients present with liver metastases where the primary tumor site is unknown. The vast majority of these are ultimately found in the small bowel.1 Despite advanced presentation, surgery remains the first line of treatment for this disease, as resection of the primary tumor and aggressive debulking of liver metastases are associated with greater overall and progression free survival.2-7 Additionally, patients with unknown primary tumors have worse overall survival compared to patients with known primary sites.8 Therefore, a thorough search for the primary tumor should be undertaken to afford the patient the best outcome.
Identifying SBNETs is difficult to do endoscopically, and therefore the patient's workup usually includes radiographic imaging. CT scans are useful for detecting liver lesions, metastatic adenopathy associated with SBNETs, and occasionally, the primary tumors themselves.9 Somatostatin receptor scintigraphy (SRS) is another useful method to look for neuroendocrine tumors (NETs), as it surveys the whole body for somatostatin receptor (SSTR) positive lesions.10 Somatostatin receptors are expressed on a variety of normal and neoplastic tissues and are upregulated in more than 80% of well-differentiated gastrointestinal (GI) NETs.11 In humans, there are five known receptor subtypes, designated SSTR types 1 through 5. SSTR1 and 2 are the most common subtypes expressed on GI NETs.11-13
The most widely used SRS radioligand is 111In-DPTA-octreotide (Octreoscan), which detects primary and metastatic NETs by binding primarily to SSTR2, but also weakly to SSTR5.12-14 Octreoscan has evolved over the years with improvement in its anatomic localization. The most basic SRS provides only a whole body planar image. SRS combined with single-photon emission computed tomography (SPECT) provides axial images like a standard CT, but does not provide the same anatomic detail as a CT. Recently, SRS combined with SPECT and CT has become the standard of care. This SRS modality combines the nuclear medicine image with the CT images and provides the best SRS-based localization of SSTR2-expressing tumors.15 An even more sensitive somatostatin analogue imaging modality combines positron emission tomography (PET) with a 68Ga-labeled somatostatin analogue, but this has very limited availability in the United States, restricting its use as part of the standard work up for NETs.16,17
Although the introduction of SRS has led to significant improvement in NET diagnostics,18 it fails to identify the primary NET in many patients. The detection rate for all NETs by SRS is greater than 75%, but it has decreased sensitivity for SBNETs.10 Patients with SBNETs often have bulky mesenteric adenopathy, which takes up the radioligand and can obscure detection of the primary SBNET. Most analyses of SRS sensitivity do not attempt to separate the primary tumor from mesenteric adenopathy, which may inflate the specificity ascribed to SRS in detecting primary SBNETs. Given the importance of the identification of the primary tumor in SBNETs, we set out to determine whether specific clinicopathologic factors correlate with successful localization of the primary tumor by SRS.
Methods
Patient selection
Patients undergoing surgery for SBNETs were consented for enrollment in an IRB-approved Neuroendocrine Tumor Registry. Patients with SBNETs who were operated upon at the University of Iowa Hospital between 2005 and 2013 and who also had SRS performed preoperatively were included in this study. The clinicopathologic data gathered from each patient's record was: primary tumor size, Tumor Node Metastasis (TNM) stage, single versus multifocal tumors, the number of total lymph nodes excised at the time of surgery, the size of the largest lymph node, and the number of positive lymph nodes found in the surgical specimen. If the disease was multifocal, the largest primary tumor measured in the surgical pathology report was used as the primary tumor size. The clinicopathologic factors of patients with successful preoperative localization by SRS of their primary SBNET were compared to those with nonlocalizing SRS imaging using Fisher's exact test and Welch's t-test.
SRS Report Review
Imaging reports from preoperative SRS scans were obtained from each patient, reviewed independently by three reviewers, and scored as either localizing the primary tumor or nonlocalizing. An SRS was considered localizing if (1) the nuclear medicine report unequivocally stated that the primary tumor was located in the small bowel, (2) if the report indicated that there was uptake in the abdomen that could be correlated with a mass within the small bowel found on preoperative CT, or (3) the abdominal uptake seen on SRS correlated with the location of the small bowel mass discovered at the time of surgery as described in the operative note. Surgical pathology and operative reports were also used to aid in the determination of the success of SRS. We attempted to differentiate mesenteric lymphadenopathy from the primary tumor, if possible, by examining the accompanying preoperative CT scans and operative reports. If the abdominal uptake appeared to be from a mesenteric mass rather than the primary tumor, the scan was considered non-localizing.
Nuclear Medicine Physician SRS Review
Where available, preoperative images were obtained and reviewed in a blinded fashion by a nuclear medicine radiologist and categorized as either localizing or non-localizing, depending upon whether the radiologist could unequivocally identify the primary tumor. These results were compared to the reviewers’ interpretations of the SRS reports to evaluate for concordance. In cases of discordance, the reviewers and nuclear medicine physician evaluated the images and report together to determine the cause of disagreement. These cases were also reviewed by a second nuclear medicine physician to reach a final interpretation.
Quantification of SSTR2 Expression
Total RNA was isolated from RNAlater-preserved tumor tissue using the Trizol method (Life Technologies, Carlsbad, CA) and reverse transcribed to cDNA. SSTR2 expression was measured in triplicate by quantitative PCR (qPCR) using Taqman reagents (Life Technologies) on StepOne-Plus and 7900 HT-Fast RT-PCR platforms (Life Technologies). SSTR2 expression was determined by normalization of the SSTR2 mean threshold cycle (Ct) to the average Ct of the internal controls, GAPDH and POLR2A, and the normalized value was calculated (dCT). Relative expression of SSTR2 in SBNETs compared to SSTR2 expression in normal small bowel tissue collected from each patient (ddCT) was computed by subtracting the dCT of tumor tissue from the dCT of the corresponding normal tissue. The relative SSTR2 expression of tumor tissue was calculated as a fold-change compared to normal tissue using 2(−ddCT). Expression levels were compared between the two groups using Welch's t-test.
Results
Clinicopathologic characteristics
Of 80 patients with pathologically-confirmed SBNETs operated upon at the University of Iowa Hospital and Clinics in the Neuroendocrine Tumor Registry Database, 37 (46.3%) had preoperative SRS. Of these, 24 patients were male and 13 were female. All but 1 patient had N1 disease at the time of diagnosis, and all but 3 patients had M1 disease. Twenty-two patients (59.5%) had multifocal primary tumors, as noted in the surgical pathology report and the operative note, and the average number of primary tumor foci for all 37 patients was 5.5 (range 1 – 47). The average primary tumor size was 2.0 cm (range 0.6 cm – 5.5 cm). The mean number of lymph nodes resected during surgery was 14.5 (range 2 – 51), and the mean number of positive lymph nodes in these patients was 4.8 (range 0 – 31).
SRS report analysis and subgroup characteristics
Of the 37 SRS cases, results in 24 patients (65%) were classified as non-localizing, and 13 (35%) were classified as localizing (Table 1). The average tumor size in the group where SRS was non-localizing was 1.69 cm compared to 2.65 cm in the localizing group (p = 0.01). Of the patients with non-localizing SRS, 17 of 24 (71%) had tumors less than 2 cm, whereas only 4 of 13 (31%) in the SRS localizing group had tumors less than 2 cm (p = 0.02). The SRS localizing group had a greater number of patients with multifocal disease, a greater number of lymph nodes excised at surgery, a higher lymph node ratio (number of positive lymph nodes divided by the total number of lymph nodes excised), and higher SSTR2 expression compared to the nonlocalizing group, but these differences were not significant. There was little difference in the number of patients with N1 or M1 disease in each group, as nearly all had nodal and liver metastases (p = 0.37, p = 1.0, respectively).
Table 1.
Factors associated with SRS localizing the primary SBNET.
| SRS Non-localizing | SRS Success | p-value | |
|---|---|---|---|
| Number of cases | 24 (65%) | 13 (35%) | |
| Mean tumor size (cm) | 1.69, SEM 0.15 | 2.65, SEM 0.32 | 0.01 |
| Number of cases with tumors < 2 cm | 17 (70.8%) | 4 (30.8%) | 0.02 |
| Presence of multifocal disease | 11 (45.8%) | 11 (84.6%) | 0.09 |
| Presence of N1 disease | 24 (100%) | 12 (92.3%) | 0.37 |
| Presence of Ml disease | 22 (91.7%) | 12 (92.3%) | 1 |
| Mean # positive lymph nodes | 4.13 | 5.78 | 0.51 |
| Mean # positive/total nodes | 0.32 | 0.36 | 0.62 |
| SSTR2 expression versus normal tissue | +2.0 fold, SEM 1.4 | +2.9 fold, SEM 1.3 | 0.41 |
| SSTR2 expression versus normal tissue in tumors < 2 cm | +1.9 fold, SEM 1.5 | +3.0 fold, SEM 1.8 | 0.52 |
P-value < 0.05 is considered significant. SEM = standard error of measurement.
The SRS reports were also examined for trends in the types of abdominal uptake they identified (Table 2). In the SRS localizing group, 3 of 13 (23%) cases noted mesenteric uptake, whereas in the non-localizing group, 10 of 24 (42%) cases demonstrated this (p = 0.44). There were three cases in the localizing group where the SRS detected the primary tumor and mesenteric adenopathy (23%). There were three cases (13%) in the non-localizing group that failed to show increased uptake of radioligand anywhere in the body. The proportion of cases with hepatic uptake was nearly identical between the two groups, with 69% of those in the localizing and 71% of the non-localizing group demonstrating uptake in this region (p = 1.0).
Table 2.
Somatostatin analogue uptake detected on the SRS reports for various abdominal locations.
| Primary tumor | Mesenteric adenopathy | Liver metastases | No uptake | |
|---|---|---|---|---|
| Localizing (n = 13) | 13 | 3 (23%) | 9 (69%) | NA |
| Non-localizing (n = 24) | NA | 10 (42%) | 17 (71%) | 3 (13%) |
| p-value | ----- | 0.44 | 1 | ----- |
P-value < 0.05 is considered significant.
SSTR2 expression of primary SBNETs
SSTR2 expression was found in all tumors. In the SRS non-localizing group, SSTR2 expression was 2.0 fold greater than the SSTR2 expression in adjacent normal small bowel. In the localizing group, the relative SSTR2 expression was slightly greater, at 2.9 fold greater than normal small bowel tissue. The expression difference between the groups was not significant (p = 0.41). When restricted to tumors less than 2 cm in size, the non-localizing group had 1.9 fold greater SSTR2 expression compared to normal small bowel, and the localizing group had 3.0 fold greater SSTR2 expression (p = 0.52).
Nuclear medicine physician SRS image analysis
Of the 31 available images for independent interpretation by our nuclear medicine physician, there were 7 planar SRS, 10 SPECT and 14 SPECT-CT (Fig. 1). One study was interpreted per patient. Six patients did not have images available for review. The determination by the nuclear medicine physician as to whether the primary tumor was localized agreed with the analysis of the SRS reports in all but 3 cases (90%). In all of these cases, the patient had SPECTCT imaging and the report was interpreted as non-localizing, with the abdominal uptake described as within a mesenteric mass rather than a primary tumor.
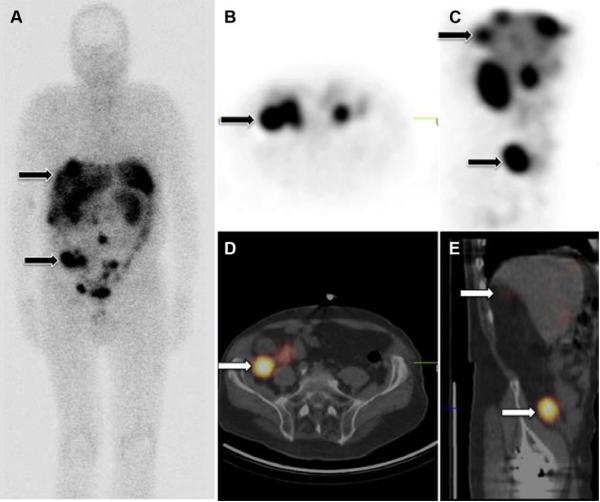
Fig 1. Comparison of 3 types of SRS images from same patient.
(a) Whole body planar image, AP view. Top arrow indicates liver metastases. Bottom arrow indicates primary tumor. (b) SPECT image, axial. Arrow indicates primary SBNET. (c) SPECT image, sagittal. Top arrow indicates liver metastases. Bottom arrow indicates primary tumor. (d) SPECT-CT, axial. Arrow indicates primary SBNET. (e) SPECT-CT, sagittal. Top arrow indicates liver metastases. Bottom arrow indicates primary tumor.
Discussion
The purpose of this study was two-fold. First, given the widespread use of SRS and its decreased sensitivity in SBNET patients, we wanted to determine which factors contribute to the modality's success in locating primary SBNETs. Second, to identify factors on SRS reports that may contribute to provider difficulty in interpreting SRS imaging, we compared the SRS reports to an independent nuclear medicine physician's interpretation of the actual SRS images to identify potential sources of disagreement.
To the first aim, we attempted to expand on the few findings previously published regarding SRS non-localization in NETs. In 1997, Lebtahi et al. reported that 61% (28/46) of patients with suspected gastrointestinal NETs had neuroendocrine lesions that were detected by SRS-SPECT but not otherwise identified by other imaging techniques. In their cohort of patients with known GI NETs, 15% had negative SRS-SPECT results despite the primary tumor site being identified by a different imaging modality. They postulated that tumor size was likely important in the ability of SRS-SPECT to detect the primary tumor, as the SRS detection rate was 38% for those patients with primary tumors less than 1 cm versus 92% for those with primary tumors greater than 1 cm.19
Intuitively, one might speculate that the underlying reason for the inability of SRS to detect a neuroendocrine tumor relates to SSTR2 expression of that tumor, as it serves as the basis for the imaging technique. In 2006, O'Toole et al. examined the SSTR2 expression of a variety of gastrointestinal neuroendocrine tumors and found that in a subset of patients with corresponding SRS imaging, there was a trend towards higher SSTR2 expression in patients with positive SRS scans compared to those with negative SRS scans.20 They postulated that this difference could be due to variable SSTR2 expression on different sized NETs, and did find that in a group of patients with primary tumors less than 3 cm and positive SRS scans (n = 10), the mean SSTR2 density on the tumors was significantly higher compared to small tumors (< 3 cm) with negative scans.
We found that tumor size was the only factor significantly correlated with localization of primary SBNETs by SRS. Interestingly, SSTR2 expression was not significantly different between the two groups. If SSTR2 is expressed similarly at the mRNA level in both groups, it is possible that there are differences in the translation of SSTR2 transcripts or in their degradation, which has been suggested from in vitro studies.12 The results of the current study suggest that it is tumor volume, rather than SSTR2 gene expression levels, that is the most important factor for localization of primary SBNETs by SRS.
As there were 3 different SRS modalities included in this study, we thought that this could confound the interpretation as to whether the SRS localized the primary tumor. If the SRS non-localization group had a greater proportion of planar SRS studies compared to the localization group, the outcome could have been influenced by scan quality. There were more SPECT-CT images in the SRS localization group, but it was not significantly different from the proportion of SPECT-CT in the SRS non-localizing group (58% vs. 39%, p = 0.19). The difference in the proportion of planar SRS included in each group was not significant either (8% in localizing vs. 33% in non-localizing, p = 0.19). Therefore, the type of SRS imaging was not a significant factor in SRS success or failure in this series.
The second aim of this study was to determine what might complicate a provider's ability to interpret whether the SRS can identify the primary tumor site. One known confounder to interpreting imaging in patients with SBNETs is the presence of mesenteric adenopathy. This bulky disease takes up the SRS radioligand and can make it difficult to identify the primary tumor as a separate entity from the mesenteric mass. Often, there is no attempt made to separate the mesenteric mass from the primary tumor in SRS reports. We feel that the presence of a mesenteric mass on SRS or CT is a strong indicator that a NET is of small bowel origin, and commonly explore patients based upon this finding, even when the primary tumor site has not been determined. In a previous paper, we defined a positive scan as showing uptake in mesenteric nodes and/or primary tumors, because we did not believe that these two can be consistently and reliably distinguished by this type of imaging.9 In this study, we specifically tried to look at whether the primary tumor could be differentiated from mesenteric masses, to better define the detection rate for primary tumors by SRS. In the 3 cases where the nuclear medicine physician differed in his interpretation of the scans versus the printed reports, uptake in mesenteric nodes was clearly the complicating factor. In each case, the operating surgeon felt in retrospect that the uptake was in mesenteric nodes rather than a discrete small bowel primary tumor, while the nuclear medicine physician felt that the primary tumor could be identified separately from the mesenteric mass. In all of these cases, there were both a large mesenteric mass and smaller SBNET primaries found within the bowel intraoperatively.
Overall, approximately one third of the patients in this study had mesenteric lymphadenopathy detected on their SRS—23% in the localizing group and 42% in the nonlocalizing group. This is a substantial number of patients in each group, but the difference in the proportions of patients with mesenteric disease was not significant. This suggests that factors such as small tumor size are the true confounders with regard to SRS localization of the primary SBNET. Furthermore, one should not assume that the mention of abdominal uptake on SRS reports means that the primary SBNET is indistinguishable from the mesenteric mass. In up to 25% of patients, the primary tumor could be identified separately from the mesenteric disease. This reinforces the notion that even if the presence of mesenteric adenopathy is used as an indicator of an SBNET and triggers exploration in cases of unknown primary tumors, these nodes do not necessarily also identify the location of primary tumor(s) within the small bowel. In this series, 85% of those with localization by SRS had multifocal primary tumors, and therefore meticulous palpation of the small bowel during abdominal exploration is crucial to ensure discovery and resection of all primary tumors.
SRS is a useful tool in the workup of neuroendocrine disease, but is limited in its ability to localize the primary tumor in patients with small (< 2 cm) SBNETs. SSTR2 expression differences at the mRNA level do not explain the differences in SRS localization of primary SBNETs, although there could be differences in SSTR2 protein expression that were not addressed in this study. The presence of lymphadenopathy complicates the issue of SRS interpretation, although the primary tumor can be identified as a separate entity in some cases if the images are carefully reviewed. Differentiating primary SBNETs from mesenteric masses may become easier with wider adoption of SRS SPECT-CT, or in the future with the use of 68Ga-DOTA PET imaging.
Acknowledgments
Supported by NIH 5T32#CA148062-03 (JEM, SKS)
Footnotes
None of the authors have any conflicts of interest
Author contributions:
Jessica Maxwell: Conception and design, analysis and interpretation, data collection, writing the article
Scott Sherman: Conception and design, analysis and interpretation, critical revisions
Yusuf Menda: Analysis and interpretation, critical revisions
Donghong Wang: Data collection
Thomas O'Dorisio: Data collection, critical revisions
James Howe: Conception and design, analysis and interpretation, critical revisions
An earlier version of this work as presented as an abstract at the Oncology Session of the Academic Surgical Congress in San Diego, CA on February 4, 2014.
REFERENCES
- 1.Wang SC, Parekh JR, Zuraek MB, et al. Identification of Unknown Primary Tumors in Patients with Neuroendocrine Liver Metastases. Arch Surg. 2010;145(3):276–80. doi: 10.1001/archsurg.2010.10. [DOI] [PubMed] [Google Scholar]
- 2.Givi B, Pommier SJ, Thompson AK, et al. Operative Resection of Primary Carcinoid Neoplasms in Patients with Liver Metastases Yields Significantly Better Survival. Surgery. 2006;140(6):891–7. doi: 10.1016/j.surg.2006.07.033. discussion 97-8. [DOI] [PubMed] [Google Scholar]
- 3.Huang LC, Poultsides GA, Norton JA. Surgical Management of Neuroendocrine Tumors of the Gastrointestinal Tract. Oncology. 2011:794–803. [PubMed] [Google Scholar]
- 4.Knigge U, Hansen CP. Surgery for Gep-Nets. Best Pract Res Clin Gastroenterol. 2012;26(6):819–31. doi: 10.1016/j.bpg.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Zappa M, Abdel-Rehim M, Hentic O, et al. Liver-Directed Therapies in Liver Metastases from Neuroendocrine Tumors of the Gastrointestinal Tract. Target Oncol. 2012;7(2):107–16. doi: 10.1007/s11523-012-0219-8. [DOI] [PubMed] [Google Scholar]
- 6.Landry CS, Scoggins CR, McMasters KM, Martin RC., 2nd Management of Hepatic Metastasis of Gastrointestinal Carcinoid Tumors. J Surg Oncol. 2008;97(3):253–8. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 7.Norton JA, Warren RS, Kelly MG, et al. Aggressive Surgery for Metastatic Liver Neuroendocrine Tumors. Surgery. 2003;134(6):1057–63. doi: 10.1016/j.surg.2003.07.025. discussion 63-5. [DOI] [PubMed] [Google Scholar]
- 8.Kirshbom PM, Kherani AR, Onaitis MW, et al. Carcinoids of Unknown Origin: Comparative Analysis with Foregut, Midgut, and Hindgut Carcinoids. Surgery. 1998;124(6):1063–70. doi: 10.1067/msy.1998.93105. [DOI] [PubMed] [Google Scholar]
- 9.Dahdaleh FS, Lorenzen A, Rajput M, et al. The Value of Preoperative Imaging in Small Bowel Neuroendocrine Tumors. Ann Surg Oncol. 2013;20(6):1912–7. doi: 10.1245/s10434-012-2836-y. [DOI] [PubMed] [Google Scholar]
- 10.Kwekkeboom DJ, Krenning EP, Scheidhauer K, et al. Enets Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Somatostatin Receptor Imaging with (111)in-Pentetreotide. Neuroendocrinology. 2009;90(2):184–9. doi: 10.1159/000225946. [DOI] [PubMed] [Google Scholar]
- 11.Reubi JC. Somatostatin and Other Peptide Receptors as Tools for Tumor Diagnosis and Treatment. Neuroendocrinology. 2004;80(Suppl 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 12.Reubi J, Waser B, Schaer J-C, Laissue J. Somatostatin Receptor Sst1-Sst5 Expression in Normal and Neoplastic Human Tissues Using Receptor Autoradiography with Subtype-Selective Ligands. Eur J Nucl Med Mol Imaging. 2001;28(7):836–46. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 13.Reubi JC, Krenning E, Lamberts SWJ. Distribution of Somatostatin Receptors in Normal and Tumor Tissue. Metabolism. 1990;39(9):78–81. doi: 10.1016/0026-0495(90)90217-z. [DOI] [PubMed] [Google Scholar]
- 14.Theodoropoulou M, Stalla GK. Somatostatin Receptors: From Signaling to Clinical Practice. Front Neuroendocrinol. 2013;34(3):228–52. doi: 10.1016/j.yfrne.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Histed SN, Lindenberg ML, Mena E, et al. Review of Functional/Anatomical Imaging in Oncology. Nucl Med Commun. 2012;33(4):349–61. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68ga-Dotatoc Pet and 111in-Dtpaoc (Octreoscan) Spect in Patients with Neuroendocrine Tumours. Eur J Nucl Med Mol Imaging. 2007;34(10):1617–26. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel M, Decristoforo C, Kendler D, et al. 68ga-Dota-Tyr3-Octreotide Pet in Neuroendocrine Tumors: Comparison with Somatostatin Receptor Scintigraphy and Ct. J Nucl Med. 2007;48(4):508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 18.Krenning EP, Breeman WAP, Kooij PP, et al. Localisation of Endocrine-Related Tumours with Radioiodinated Analogue of Somatostatin. The Lancet. 1989 Feb;:242–44. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 19.Lebtahi R, Cadiot G, Sarda L, et al. Clinical Impact of Somatostatin Receptor Scintigraphy in the Management of Patients with Neuroendocrine Gastroenteropancreatic Tumors. J Nucl Med. 1997;38:853–58. [PubMed] [Google Scholar]
- 20.O'Toole D, Saveanu A, Couvelard A, et al. The Analysis of Quantitative Expression of Somatostatin and Dopamine Receptors in Gastro-Entero-Pancreatic Tumours Opens New Therapeutic Strategies. Eur J Endocrinol. 2006;155(6):849–57. doi: 10.1530/eje.1.02307. [DOI] [PubMed] [Google Scholar]