Abstract
Bupropion is an effective abstinence aid for cessation of smoking and possibly other drug use as well. There is evidence that bupropion improves attention and impulse control in certain patient populations, and improvements in these processes could mediate its efficacy as an abstinence aid. In the present study, we tested the effects of acute bupropion on measures of attention and impulsivity in healthy adults with d-amphetamine included as a positive control. Twenty-two nonsmokers (11 women) and 11 smokers (4 women) completed four 4-h sessions where they received placebo, bupropion (150 or 300 mg) or d-amphetamine (20 mg) in capsules. Ninety minutes after capsule administration, participants were tested on attention with a Simple Reaction Time Task (SRT) and on impulsivity with the Stop Task, a Delay and Probability Discounting Task (DPD), and the Balloon Analogue Risk Task (BART). Participants also completed mood questionnaires during sessions. Bupropion (150 mg) decreased lapses in attention on the SRT, but did not affect performance on the Stop Task, DPD or BART. d-Amphetamine decreased lapses in attention and speeded sensory motor processing time on the SRT but did not significantly affect responding on the Stop Task or DPD. On the BART, d-amphetamine tended to decrease risk taking in men but increased risk taking in women. Bupropion (300 mg) and d-amphetamine increased ratings of arousal. These results suggest that bupropion improves attention without affecting impulsive behavior in healthy adults. Improvements in attention may contribute to the effectiveness of bupropion as a pharmacotherapy for smoking.
Keywords: Bupropion, d-Amphetamine, Attention, Smoking Abstinence, Impulsive Behavior, Human
Introduction
The purpose of this study was to examine the effects of bupropion on attention and impulsivity. Bupropion is effective in promoting abstinence during smoking cessation (Foulds, Steinberg, Williams, & Ziedonis, 2006; Frishman, Mitta, Kupersmith, & Ky, 2006; Richmond & Zwar, 2003; Zwar & Richmond, 2002), and there is also evidence that bupropion may aid in cessation of other drugs such as cocaine and methamphetamine (Elkashef et al., 2007; Margolin et al., 1995; Newton et al., 2005; Poling et al., 2006). However, it is not clear what underlies these beneficial effects. Bupropion improves attention and reduces impulsive behavior in patients with depressive symptoms and those with Attention Deficit Hyperactivity Disorder (ADHD; (Barrickman et al., 1995; Becker & Dufresne, 1982; Wilens et al., 2005), raising the possibility that its therapeutic effects in smoking cessation may be related to improved attention or impulse control (de Wit & Richards, 2004; Warner & Shoaib, 2005). A better understanding of the direct effects of bupropion on attention and impulsivity in healthy adults may yield greater insight into the therapeutic actions of this drug.
Bupropion, first marketed as an antidepressant, is a weak reuptake inhibitor of norephinephrine and dopamine, and consistent with this, it produces mild stimulant-like effects in both humans and laboratory animals (Cousins, Stamat, & de Wit, 2001; Cryan, Bruijnzeel, Skjei, & Markou, 2003; de la Garza & Johanson, 1987; Kamien & Woolverton, 1989; Rush, Kollins, & Pazzaglia, 1998; Terry & Katz, 1997). Bupropion also functions as a nicotinic receptor antagonist and blocks the antinociceptive, motor, hypothermic and convulsive effects of nicotine (Slemmer, Martin, & Damaj, 2000). In particular, it blocks the effects of nicotine on alpha(3)beta(2), alpha(4)beta(2), and alpha(7) neuronal acetylcholine nicotinic receptors (nAChRs).
The mechanisms by which bupropion aids smoking abstinence are not known. Mixed effects of bupropion have been reported on the reinforcing properties of nicotine and cigarette smoking. In rats, some studies have reported that bupropion increases nicotine self-administration (Rauhut, Dwoskin, & Bardo, 2005; Rauhut, Neugebauer, Dwoskin, & Bardo, 2003) whereas others reported decreases (Bruijnzeel & Markou, 2003; Glick, Maisonneuve, & Kitchen, 2002). In humans, although chronic administration of bupropion during quit attempts reduces smoking, acute administration increased smoking in dependent smokers not intending to quit (Cousins et al., 2001). There are also mixed reports on the effects of bupropion on cigarette craving in abstinent smokers, with some studies reporting decreases and others reporting no effects (Shiffman et al., 2000; Teneggi et al., 2005).
There is evidence that bupropion may both improve attention and reduce impulsive behavior (Barrickman et al., 1995; Becker & Dufresne, 1982; Wilens et al., 2005), and these effects may be important for the beneficial effects of this drug on abstinence (de Wit & Richards, 2004; Warner & Shoaib, 2005). Bupropion improves ratings of attention in both depressed and normal individuals (Becker & Dufresne, 1982; Gobbi, Slater, Boucher, Debonnel, & Blier, 2003), and reduces ratings of inattention and impulsive behavior in children and adults with ADHD (Barrickman et al., 1995; Wilens et al., 2005). In the present study, we tested the effects of bupropion on attention and several forms of impulsive behavior. The doses of bupropion tested were limited to 150 and 300 mg due to safety concerns with higher doses (Pesola & Avasarala, 2002; Richmond & Zwar, 2003). Attention was measured with a simple reaction time task and analyzed to distinguish between processes related to sensory motor processing time and lapses in attention (de Wit, In press; Sabol, Richards, Broom, Roach, & Hausknecht, 2003). Three validated and operationalized measures of impulsivity were examined: behavioral inhibition, delay discounting, and risk taking. Behavioral inhibition refers to the ability to withhold a prepotent response, delay discounting measures preference for immediate versus delayed rewards, and risk taking measures the tendency to engage in a rewarded behavior that also involves probabilistic losses (Ainslie, 1975; Bechara, Damasio, Damasio, & Anderson, 1994; Lejuez et al., 2002; Logan, 1981).
The participant sample included both men and women, and smokers and nonsmokers. Although we did not expect that the effects of bupropion would differ in men and women, there was reason to believe that the drug might have differential effects in smokers and nonsmokers because of differences in bupropion metabolism (Lee, Miksys, Palmour, & Tyndale, 2006; Miksys, Lerman, Shields, Mash, & Tyndale, 2003). We hypothesized that bupropion, like amphetamine, would improve attention, decrease impulsive behavior, and increase subjective ratings of stimulant like effects in all participants. d-Amphetamine (20 mg) was included in this study as a positive control to compare to previous studies (de Wit, Crean, & Richards, 2000; de Wit, Enggasser, & Richards, 2002).
Methods
Participants
Healthy nonsmokers (11 men, 11 women) and smokers (7 men, 4 women) aged 18 to 45 years participated. Participants were recruited by means of posters, advertisements in newspapers, and word-of-mouth referrals. Eligibility was ascertained during a telephone interview screening process, followed by an in-person screening. Participants completed a psychiatric symptom checklist (SCL-90; Derogatis, 1983), the Michigan Alcoholism Screening Test (MAST; Selzer, 1971), a detailed health and drug-use questionnaire, underwent a semi-structured psychiatric screening interview based on the Structured Clinical Interview for DSM-IV Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1996), and had an electrocardiogram and a physical examination. Volunteers were excluded if they met criteria for major Axis I DSM-IV diagnoses (APA, 2000), had less than high school education or medical problems, were taking medications, or if their body mass index was outside of the range 19–26 kg/m2. Smokers were required to smoke at least 5 cigarettes per day for at least 3 months to be eligible. Nonsmokers were required to have never smoked on a daily basis. Ex-smokers (individuals who ever smoked on a daily basis) were excluded from the study.
Before participating in the study, participants attended an orientation session where they provided written informed consent and were familiarized with the experimental procedures. The consent form stated that the purpose of the study was to investigate the effects of commonly used drugs on mood and performance. For blinding purposes, participants were advised that they might receive any of several classes of drugs and the drugs’ associated side effects were listed. Participants were instructed to abstain from alcohol and other drugs except their normal amounts of caffeine for 24 hours before and 6 hours after each session. Their compliance was verified by testing breath alcohol levels (BAL), and urine samples for amphetamines, cocaine, phencyclidine, opiates, and cannabis. Participants were instructed not to eat for two hours before the session. Smokers were allowed to smoke as normal the night before, but asked not to smoke the morning of the session. Female participants were tested for pregnancy before each session. The study was approved by the University of Chicago Institutional Review Board.
Design
This study utilized a 4-session, double-blind, placebo-controlled, within-subject design. Capsules containing placebo, 150 mg bupropion, 300 mg bupropion or 20 mg d-amphetamine were administered in random order on the 4 test sessions. Sessions were conducted from 9 am to 1 pm, and scheduled approximately one week apart.
Procedure
Volunteers were tested individually in comfortably furnished rooms with a television/VCR, magazines, and a computer for administering questionnaires and tasks. When no dependent measures were being obtained participants were allowed to watch television, movies, or read but they were not allowed to work or study.
Upon arrival for each session at 9 am, a urine sample was obtained for drug and pregnancy screening, and BAL’s and expired CO levels were checked. Participants completed pre-capsule subjective effects questionnaires (described in detail below) and vital signs were recorded. Smokers smoked a single cigarette after these pre-capsule measures. Participants then ingested a capsule containing placebo, bupropion (150 or 300 mg) or d-amphetamine (20 mg) under double-blind conditions. Forty and eighty minutes later they repeated the subjective effects questionnaires and vital signs were again recorded. Behavioral measures of attention and impulsivity were obtained between 90 and 150 min after capsule ingestion, coinciding with the peak plasma concentrations of bupropion and peak behavioral effects of d-amphetamine on the impulsivity tasks (de Wit et al., 2002; Lai & Schroeder, 1983). The tests, which are described in detail below, included a Simple Reaction Time Task (Bleiberg et al., 2004; Bleiberg, Kane, Reeves, Garmoe, & Halpern, 2000), the Stop Task (Logan, Cowan, & Davis, 1984), a Delay and Probability Discounting Task developed by Richards et al. (1999), and the Balloon Analogue Risk Task (Lejuez et al., 2002). All tasks were completed via computer, in randomized order. After finishing these tasks (about 150 min after capsule ingestion), participants again completed the subjective effects questionnaires and vital signs were recorded. Finally they completed an end-of-session questionnaire, were paid money earned in the impulsivity tasks, and were transported home. After completing all 4 sessions, participants attended a debriefing session and were given a final payment for their participation.
Drugs
Bupropion hydrochloride (75 & 100 mg tablets, immediate release; Wellbutrin, GlaxoSmithKline, USA) and d-amphetamine sulfate (5 mg tablet; Dexedrine, GlaxoSmithKline, USA) were administered in opaque gelatin capsules (size 00) with dextrose filler. Placebo capsules contained only dextrose.
Dependent Measures
Attention
Simple Reaction Time Task (SRT)
The SRT is an attention task taken from the Automated Neuropsychological Assessment Metrics computerized test battery (Bleiberg et al., 2004; Bleiberg et al., 2000). In this task, a large asterisk-like symbol appeared on the monitor screen at variable time intervals averaging every 5 s and participants were required to press a mouse button as quickly as possible when it was presented. The task took approximately 10 minutes to complete.
The SRT was analyzed by individual trials (100) to determine the mean, estimated mode, and deviation from the mode for reaction times to distinguish drug effects on sensory motor processing time from lapses in attention (de Wit, In press; Sabol et al., 2003). The deviation from the mode is equivalent to the modal minus the mean value of the distribution of RT’s and represents lapses in attention, whereas the mode best represents the typical fast reaction times. Thus, a drug that affects sensory motor processing time would alter the reaction time mode, whereas a drug that affects lapses in attention would alter the deviation from the mode. Reaction times on measures such as the SRT are typically positively skewed, where the majority of reaction times are relatively fast. Lapses in attention result in occasional long reaction times, which cause the mean RT to be slower (greater) than the RT mode.
Trials in which participants failed to respond to the stimulus were assigned a value of 1500 ms, the maximum duration of the go stimulus. The estimated mode was determined by grouping the reaction times in 10 ms bins and computing a running frequency for each bins. The mid point of the 10 ms bin with the highest frequency of response times was considered the mode. The deviation from the mode was determined by subtracting the estimated mode from the mean of the reaction times.
Impulsivity
Stop Task (Logan et al., 1984)
The Stop Task is designed to assess the ability to inhibit a prepotent response. Participants are instructed to respond as quickly as possible when a certain letter (go signal) appears on a computer screen, and to inhibit their responses when a tone is heard (stop signal). On each trial, one of two discrete go signals are presented (letters ‘X’ or ‘O’) to which participants make corresponding responses (pressing ‘Z’ or ‘?/’ keys, respectively). Successful discrimination of the two go signals was used to indicate that participants were fully attending to the task on every trial. The tone is presented randomly on 25% of trials and at different delays following the letter presentation. The delays to the stop signal are adjusted until the participant inhibits his or her responses on approximately 50% of trials. At this 50% criterion the Stop Reaction Time can be calculated by subtracting the final mean delay at which the tone is presented from the mean Go Reaction Time or latency to respond to the letter presentation. Both Go Reaction Time and Stop Reaction Time are measured in milliseconds. The task took approximately twelve minutes to complete.
Delay and Probability Discounting Task (DPD; Richards et al., 1999)
This task measures the discounting or devaluation of rewards by delay and probability (uncertainty). Participants have the opportunity to choose between different amounts of money available after different delays or with different probabilities. The procedure consists of approximately 100 questions, such as: (1) Would you prefer $10.00 in 30 days or $2.00 at the end of the session, or (2) Would you prefer $5.00 for sure or $10.00 with a 25% chance? The task uses an adjusting amount procedure (see Richards et al., 1999), to derive an indifference point at which the delayed and immediate options (for delay discounting) or probabilistic and certain options (for probability discounting) are judged to be of equivalent subjective value for a respondent. The obtained delay and probability indifference points are then plotted to form two separate discount functions. An area under the curve (AUC; Myerson, Green, & Warusawitharana, 2001) was calculated for each discount function for each session for each participant. AUC values can range from 1 (no discounting) to 0 (maximum discounting). The task took approximately 20 minutes to complete and one trial was randomly selected at the end of the session and the participant was paid for the response from that trial.
Balloon Analogue Risk Task (BART; Lejuez et al., 2002)
This task is designed to measure risk taking, and has been validated in samples of known risk takers (Aklin, Lejuez, Zvolensky, Kahler, & Gwadz, 2005; Lejuez, Aklin, Jones et al., 2003; Lejuez, Aklin, Zvolensky, & Pedulla, 2003). In this task version, participants are required to “pump up” a series of 30 balloons on a computer screen. Each pump was worth 1, 5, or 25 cents, which accumulated during a trial. This worth was clearly displayed to the participant on each trial. Participants could stop pumping at any time and bank their accumulated money. However, if they continued to pump the balloon would occasionally “explode” resulting in the loss of the money accumulated on that trial. Thus, more pumps on a trial were taken to be an indicator of greater risk-taking. The dependent measure was the average number of pumps on trials when the balloon did not explode (adjusted average number of pumps). The task took approximately 10 minutes to complete. At the end of each session participants were paid the average amount of money earned across all trials.
Measures of Subjective Effects
Addiction Research Center Inventory (ARCI; Martin et al., 1971)
The ARCI is a standardized questionnaire consisting of 49 true/false statements, designed to measure subjective effects of specific classes of abused drugs. This version of the ARCI consists of five empirically derived scales, which measure drug-induced euphoria (Morphine-Benzedrine Group; MBG), stimulant-like effects (Amphetamine; A, and Benzedrine Group; BG), sedation (Pentobarbital-Chlorpromazine; PCAG), and dysphoria and somatic effects (Lysergic Acid; LSD).
Profile of Mood States (Johanson & Uhlenhuth, 1980; McNair, Lorr, & Droppleman, 1971)
The POMS consists of 72 adjectives commonly used to describe mood states. Participants indicate how they feel at that moment in relation to each of the adjectives using a five-point scale ranging from “not at all” [0] to “extremely” [4]. The POMS consists of 8 scales: Friendliness, Anxiety, Depression, Fatigue, Anger, Elation, Confusion, Vigor and two derived scales Arousal, and Positive mood.
Drug Effects Questionnaire (DEQ; Fraser, Van Horn, Martin, Wolbach, & Isbell, 1961)
The DEQ consists of four questions concerning drug effects. On 100 mm lines labeled “not at all” to “extremely” participants indicate the extent to which they feel the drug, how high they feel, if they like the drug, and if they want more of the drug.
Vital Signs
Blood pressure and heart rate measures were recorded using a Digital Blood Pressure Monitor Dinamap 1846SX (Critikon, Tampa, FL).
Primary Data Analysis
Data analyses were conducted using SPSS ® version 10. First we examined the effects of gender and smoking on task performance and responses to the drugs to determine the use of these variables as covariates in further analyses. Then, using sex and smoking as covariates when appropriate, we examined the effects of bupropion and amphetamine on task performance and mood using one factor analyses of variance (ANOVA) with the four drug conditions. The primary comparisons were between each active drug and placebo. On the BART, price was included as a within subject factor. For the DPD the k and h values were normalized using a log-10 transformation because the data were skewed. For subjective effects and vital signs, peak changes from pre capsule baseline values were compared. Post-hoc t-tests with Bonferroni correction were used when significant main effects or interactions were obtained. The significance level was p < 0.05.
Results
Participants
The demographic characteristics and current and lifetime recreational drug use histories reported by participants are summarized in Table 1. Most participants were in their early 20’s, most had at least some college and were currently fulltime students. The 11 smokers were light to moderate smokers, none of whom smoked more than 20 cigarettes per day on average. A minority of the participants had tried illicit drugs, and their average weekly alcohol consumption was about 6 drinks per week.
Table 1.
Participant Demographic and Drug Use Summary (n=33).
| Age (years) Mean ± SD | 23.45 ± 4.6 |
| Weight (lbs: mean ± SD) | 150.6 ± 20.9 |
| Sex (n; male/ female) | 18 (55%) / 15 (45%) |
| Race/Ethnicity | |
| Caucasian | 23 (70%) |
| African-American | 4 (12%) |
| Asian/ Native American | 5 (15%) /1 (3%) |
| Education (n) | |
| High school | 1 (3%) |
| Some college | 19 (58%) |
| College degree | 13 (39%) |
| Full time student | 23 (70%) |
| Current drug use | |
| Alcohol (mean ± SD; drinks/week) | 6.2 ± 5.0 |
| Caffeine (mean ± SD; drinks/week) | 8.3 ± 7.4 |
| Cigarettes (mean ± SD; cigarettes/day) | |
| Nonsmokers (n=22) | 0 |
| Smokers (n=11) | 10.7 ± 3.4 |
| Marijuana (n: >0.5 cigarettes/week) | 5 (15%) |
| Lifetime recreational drug use | |
| Stimulants (n: ever used) | 13 (40%) |
| Tranquilizers (n: ever used) | 1 (3%) |
| Hallucinogens (n: ever used) | 11 (33%) |
| Opiates (n: ever used) | 4 (12%) |
| Marijuana | |
| Never used (n) | 5 (15%) |
| Used < 10 times (n) | 11 (33%) |
| Used 10–50 times (n) | 9 (27%) |
| Used > 50 times (n) | 8 (24%) |
| Inhalants (n: ever used) | 5 (15%) |
Attention
SRT
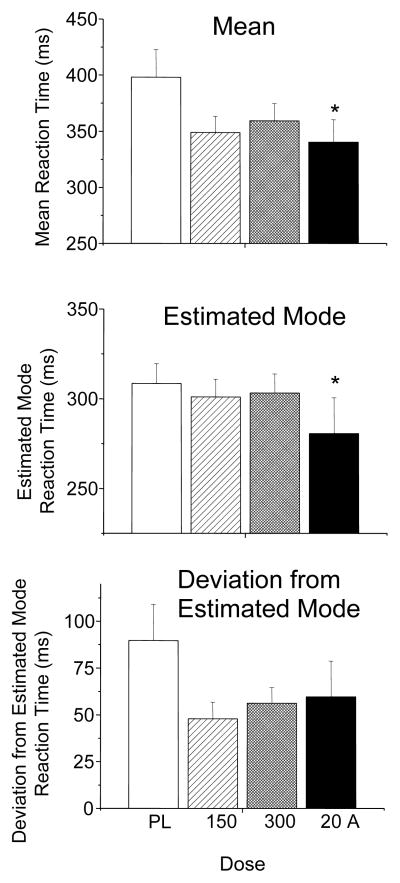
Data was lost from one nonsmoker male due to experimenter error. There were moderate performance improvements with both doses of bupropion and d-amphetamine on the SRT across all subjects. d-Amphetamine and to a lesser extent bupropion decreased mean reaction times [main effect of drug; F (3, 93) = 4.248, p = 0.007]. Post hoc tests revealed d-amphetamine significantly decreased mean reaction times, and there was a trend for decreases with both doses of bupropion (Figure 1 upper panel). There was significant main effect of drug on estimated modal reaction times [F (3, 90) = 5.431, p = 0.002], and post hoc tests revealed d-amphetamine significantly decreased estimated modal reaction times (Figure 1 middle panel). Thus, d-amphetamine, but not bupropion, speeded sensory motor processing time. All drugs decreased deviations from estimated modal reaction times [main effect of drug; F (3, 93) = 4.246, p = 0.007], and post hoc tests revealed trends towards decreased deviations from the estimated mode with both doses of bupropion and d-amphetamine (Figure 1 lower panel). Thus, both bupropion and d-amphetamine tended to decrease lapses in attention.
Figure 1.
Upper panel: Mean (± SEM) Simple Reaction Time Task (SRT) reaction times. Middle panel: Estimated mode reaction times(± SEM). Lower panel: deviations from estimated mode reactions times (± SEM). * indicates significant difference from placebo.
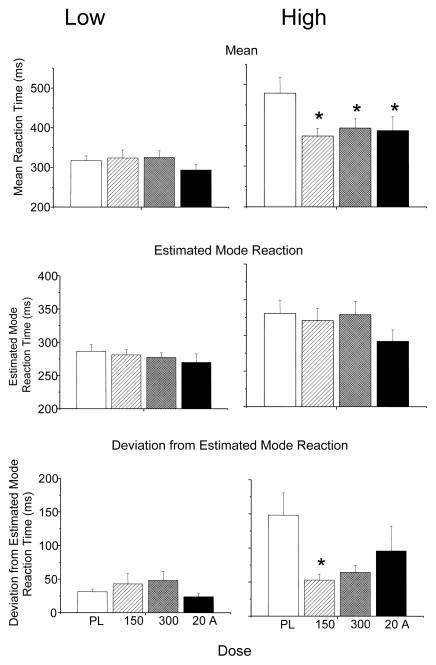
There was a wide interindividual variability in deviations from estimated mode reaction times across participants under placebo conditions. To further explore the effects of bupropion and d-amphetamine on deviation from estimated modal reaction time, two groups were formed by median split of the deviation from the estimated mode data from placebo sessions (Low Deviation & High Deviation groups). The Low Deviation group consisted of 6 male nonsmokers, 5 female nonsmokers, 4 male smokers and no female nonsmokers. The High Deviation group consisted of 3 male nonsmokers, 6 female nonsmokers, 3 male smokers, and all 4 female smokers. Due to potential ceiling effects in the Low Deviation group because of near optimal baseline performance, the effects of bupropion and amphetamine on SRT reaction time mean, estimated mode, and deviation from estimated mode were analyzed separately in these two groups.
In the Low Deviation group, there were no effects of bupropion or d-amphetamine on reaction time mean, deviation from the mode, or deviation from the estimated mode (Figure 2, left panels). In contrast, the High Deviation group showed significant improvements with bupropion or d-amphetamine on each measure (Figure 2 right panels). Bupropion and d-amphetamine decreased mean reaction time [main effect of drug; F (3, 45) = 5.455, p = 0.003]. Post hoc tests revealed bupropion (150 & 300 mg) and d-amphetamine significantly decreased mean reaction time (Figure 2 upper right panel). There was a trend towards a main effect of drug on estimated modal reaction time [F (3, 45) = 2.600, p = 0.061] that appeared driven by a decrease in modal reaction time follow d-amphetamine administration (Figure 2 right middle panel). There was also a significant main effect of drug on deviation from estimated modal reaction time [F (3, 45) = 3.906, p = 0.015], and post hoc tests revealed significantly decreased deviation from estimated mode with bupropion (150 mg) and trends towards decreases with bupropion (300 mg) and d-amphetamine (Figure 1 lower panel).
Figure 2.
Median split simple reaction time task data for individuals with lower deviations from estimated mode reaction times (Low Deviation; left column) and higher deviations from the estimated mode reaction times (High Deviations; right column). Upper row: Mean (± SEM) Simple Reaction Time Task (SRT) reaction times. Middle row: Estimated mode reaction times(± SEM). Lower row: deviations from estimated mode reactions times (± SEM). * indicates significant difference from placebo.
Impulsivity
Stop Task
Neither bupropion nor amphetamine affected performance on the Stop Task (Go or Stop Reaction Time; Table 2). Stop Task session data were lost from 1 smoker male and 2 smoker females due to experimenter error, and 1 nonsmoker male and 1 smoker male were not included in the analysis because they discriminated the two go signals less than 80% of the time on one or more sessions. Stop Task data were analyzed in the remaining 28 participants. Although Stop Reaction Time appeared lower after both 150 mg bupropion and 20 mg amphetamine, these decreases were not statistically significant. Participants were also divided by median split into individuals with slow and fast Stop Reaction Times based on their placebo session, because previous studies indicated that the effects of amphetamine were most pronounced in individuals with initially slow Stop Reaction Times (de Wit et al., 2000). However, neither drug significantly decreased Stop Reaction Times even in participants with slow Stop Reaction Times.
Table 2.
Mean (± SEM) scores on Stop Task & DPD following placebo, 150 mg bupropion, 300 mg bupropion, and 20 mg d-amphetamine. There were no significant effects of drug treatment. On the Stop Task, 5 participants were excluded for lost or invalid data on one or more sessions. For the Delay and Probability Discounting Task, 1 participant was excluded for lost data on one session. On the BART, data from 3 participants were lost. Adjusted average number of pumps on the BART are shown averaged across all three price conditions (1, 5 & 25 cents).
| Placebo | 150 mg bupropion | 300 mg bupropion | 20 mg d-amphetamine | |
|---|---|---|---|---|
| Stop Task (n = 28) | ||||
| Go Reaction Time | 548.1 ± 28.2 | 534.5 ± 27.0 | 543.3 ± 27.0 | 541.9 ± 29.0 |
| Stop Reaction Time | 196.1 ± 11.3 | 183.6 ± 8.5 | 190.8 ± 13.9 | 182.4 ± 11.0 |
| Delay Discounting (n = 32) | ||||
| AUC values | 0.53 ± 0.05 | 0.54 ± 0.05 | 0.53 ± 0.05 | 0.52 ± 0.05 |
| Probability Discounting (n = 32) | ||||
| AUC values | 0.45 ± 0.02 | 0.45 ± 0.02 | 0.45 ± 0.02 | 0.45 ± 0.10 |
| BART (n=32) | ||||
| Mean Adjusted average number of pumps | 42.7 ± 8.1 | 44.1 ± 7.9 | 41.7 ± 7.5 | 43.2 ± 7.8 |
DPD
DPD session data was lost from one nonsmoker male due to experimenter error and this individual was removed from the analysis. Neither bupropion nor amphetamine affected discounting of hypothetical delayed or probabilistic rewards on the DPD. Table 2 shows AUC values for all conditions. Neither bupropion (150 or 300 mg) nor d-amphetamine affected AUC values.
A preliminary analysis found discounting data from 14 participants were not well-described by the hyperbolic discount function of Mazur (1987) in one or more sessions, Previous studies have also reported discounting data is not uniformly well-described by this function (de Wit, Flory, Acheson, McCloskey, & Manuck, 2007; Myerson et al., 2001; Odum & Rainaud, 2003). There were no apparent relationships with data not conforming to the hyperbolic discounting function and smoking status, gender, drug treatment or session order.
BART
BART session data were lost from 1 nonsmoker male, 1 nonsmoker female and 1 smoker male due to computer errors, and these participants were removed from the analysis. Participants made fewer pumps as the price increased [1, 5 or 25 cents; main effect of price; F (2, 56) = 4.777, p = 0.012; data not shown]. However, there was no main effect of drug treatment on the number of pumps at any of the three prices.
Subjective Effects, and Cardiovascular Responses
The three drugs produced expected changes in subjective effects and cardiovascular function (Table 3 & 4). d-Amphetamine produced its prototypic stimulant effects, whereas bupropion (300 mg) produced similar but fewer and less pronounced changes in mood states, and bupropion (150 mg) produced no significant effects on mood or subjective state. d-Amphetamine produced expected increases in heart rate and blood pressure. Bupropion (300 mg) increased heart rate but not blood pressure, and bupropion (150 mg) had no effects on these measures.
Table 3.
Subjective effects (mean ± SEM) measured as peak change from pre-capsule baseline on the Profile of Mood States (POMS) following placebo, 150 mg bupropion, 300 mg bupropion, and 20 mg d- amphetamine. Data are shown for 30 participants (data from 3 participants were lost). Significant main effects of drug treatment were found for all values listed.
| Placebo | 150 mg bupropion | 300 mg bupropion | 20 mg d-amphetamine | |
|---|---|---|---|---|
| Profile of Mood | ||||
| States | ||||
| Friendliness | −1.58 ± 0.88 | −0.87 ± 1.07 | −0.67 ± 0.83 | 2.55 ± 0.95* |
| Anxiety | 0.03 ± 0.52 | 1.03 ± 0.60 | 1.88 ± 0.68 | 3.15 ± 1.07* |
| Fatigue | 0.58 ± 0.88 | −0.39 ± 0.80 | −2.45 ± 0.68* | −2.36 ± 0.77 |
| Elation | −0.94 ± 0.69 | −0.84 ± 0.66 | 0.03 ± 0.70 | 4.42 ± 0.92* |
| Vigor | −0.27 ± 0.94 | 0.07 ± 0.92 | 2.52 ± 0.95 | 8.18 ± 1.44* |
| Arousal | −1.03 ± 1.78 | 0.36 ± 1.82 | 5.70 ± 1.68* | 13.67 ± 2.26* |
| ARCI | ||||
| A | 0.49 ± 0.27 | 0.58 ± 0.30 | 1.50 ± 0.38 | 4.25 ± 0.46* |
| BG | −0.67 ± 0.37 | −0.23 ± 0.40 | 0.09 ± 0.47 | 2.69 ± 0.65* |
| MBG | −0.09 ± 0.42 | 0.41 ± 0.48 | 1.46 ± 0.61 | 5.81 ± 0.88* |
| PCAG | 0.97 ± 0.70 | −0.21 ± 0.59 | −0.31 ± 0.72 | −1.03 ± 0.78* |
| Drug Effects | ||||
| Questionnaire | ||||
| Feel | 0.19 ± 0.04 | 0.16 ± 0.03 | 0.32 ± 0.04* | 0.52 ± 0.04* |
| Like | −0.04 ± 0.03 | −0.03 ± 0.03 | 0.03 ± 0.04* | 0.13 ± 0.05* |
| High | 0.09 ± 0.02 | 0.08 ± 0.03 | 0.18 ± 0.04 | 0.33 ± 0.05* |
| Want More | 0.06 ± 0.05 | 0.16 ± 0.06 | 0.17 ± 0.06* | 0.42 ± 0.07* |
indicates significant difference from placebo ( p ≤ 0.01).
Table 4.
Cardiac measures (mean ± SEM) summarized as peak change from pre-capsule baseline following placebo, 150 mg bupropion, 300 mg bupropion, and 20 mg d-amphetamine. Significant main effects of drug treatment were found for all values listed. Data shown for all 33 participants.
| Placebo | 150 mg bupropion | 300 mg bupropion | 20 mg d-amphetamine | |
|---|---|---|---|---|
| Cardiac Measures | ||||
| Heart Rate | −8.42 ± 1.85 | −3.76 ± 1.85 | 3.18 ± 1.76* | 1.76 ± 2.70* |
| Systolic BP | −7.38 ± 2.72 | −2.61 ± 2.22 | −0.06 ± 2.72 | 24.27 ± 2.12* |
| Diastolic BP | −1.30 ± 1.91 | −1.33 ± 1.33 | 2.91 ± 1.73 | 11.30 ± 2.24* |
indicates significant difference from placebo ( p ≤ 0.01).
Gender and smoking differences
Men and women did not differ on any of the measures in the absence of drug. However, differential effects of gender on d-amphetamine effects were found on the SRT and the BART (Table 5). On the SRT, d-amphetamine decreased modal SRT in women more than men [d-amphetamine by gender interaction; F (1, 30) = 7.311, p = 0.011]. On the BART, d-amphetamine decreased the number of pumps in men but increased the number of pumps in women [d-amphetamine by gender interaction; F (1,28) = 6.299, p = 0.018]. Neither sex nor smoking status affected responses on any other measures.
Table 5.
Gender differences (mean ± SEM) on the SRT and BART following placebo, 150 mg bupropion, 300 mg bupropion, and 20 mg d-amphetamine. Data are shown for 32 participants on the SRT (data from 1 participant lost) and 30 participants on the BART (data from 3 participants were lost). Significant amphetamine by gender interactions were found on both measures.
| Placebo | 150 mg bupropion | 300 mg bupropion | 20 mg d-amphetamine | |
|---|---|---|---|---|
| SRT – Estimated modal reaction time | ||||
| Men (n = 17) | 318.1± 14.4 | 293.1± 10.0 | 308± 15.5 | 297.5± 13.7 |
| Women (n = 15) | 297.5 ± 17.2 | 309.8 ± 18.1 | 297.6± 15.1 | 261.3 ± 14.7 |
| BART – Mean adjusted average number of pumps | ||||
| Men (n = 16) | 46.2 ± 5.3 | 47.8 ± 5.5 | 43.4 ± 4.2 | 42.6 ± 4.6 |
| Women (n = 14) | 39.1 ± 3.2 | 39.4 ± 2.3 | 39.2 ± 2.8 | 43.9 ± 4.0 |
Discussion
This study investigated the effects of bupropion on attention and impulsive behavior. Contrary to our hypothesis, bupropion did not affect performance on a measure of sensory motor processing time or any of the three standardized measures of impulsivity: behavioral inhibition, delay discounting, and risk taking. Bupropion (150 mg) significantly improved performance on a measure of lapses in attention in individuals who performed poorly at baseline (High Deviation group). It is possible that the therapeutic efficacy of bupropion as an abstinence aid may be related at least in part to beneficial effects on lapses in attention.
Neither bupropion nor d-amphetamine improved performance on the Stop Task or on delay and probability discounting. On the Stop Task, the mean Stop Reaction Times under placebo and 20 mg d-amphetamine conditions are comparable to what has been observed previously with this task (de Wit et al., 2000; de Wit et al., 2002), and the lack of significance may be related to the small, relatively diverse participant sample (smokers and nonsmokers, women in both phases of the menstrual cycle). The possibility remains that bupropion would significantly improve Stop Task performance in larger, more homogeneous sample, and this should be explored in future studies. Delay and probability discounting have demonstrated validity as an index of individual differences in impulsivity, but the sensitivity of these measures to acute pharmacological challenges is unclear (e.g. Crean, de Wit, & Richards, 2000; Giordano et al., 2002; Richards et al., 1999). Thus, the lack of effect of either bupropion or d-amphetamine on this measure may be related to the relative insensitivity of the task. Alternatively, either drug may reduce measures of impulsivity in individuals exhibiting initially higher levels of impulsive behavior than the subjects tested here. Field et al (2006) recently reported that acute nicotine deprivation increased delay discounting in dependent smokers. Thus, it might be interesting to determine whether bupropion or d-amphetamine would improve delay discounting in acutely nicotine deprived smokers.
The present findings suggest bupropion and d-amphetamine may have differential effects on lapses in attention and sensory motor processing time, as indicated by effects on SRT performance, and a follow-up investigation using lower doses of amphetamine may resolve this question. In the present study, doses of bupropion higher than 300 mg were not included because of safety concerns. Subjective and cardiovascular measures indicated that both doses of bupropion produced much weaker stimulant effects than 20 mg d-amphetamine. Interestingly, the 150 mg dose of bupropion produced the most robust effects on the SRT task, yet this dose produced no significant subjective or cardiovascular effects. It is possible that lower doses of d-amphetamine would also reveal this apparent dissociation between subjective and behavioral effects.
d-Amphetamine appeared to decrease risk-taking (i.e., decreased pumps) on the BART in men, but increase risk taking in women. Interestingly, these effects are opposite to the sexually dimorphic effects of sleep deprivation on this task (Acheson, Richards, & de Wit, 2007). In that study sleep deprivation decreased risk taking in women and tended to increase it in men, suggesting that fatigue and arousal may have differential effects on risk taking in men and women. However, this conclusion is tentative as the effects of amphetamine on risk taking were small, and apparent increases in pump presses on the BART may also be attributable to other factors, such as willingness to expend effort.
Limitations of this study included aspects of the participant sample and drug doses used. The sample was relatively small and heterogeneous (male, female, smoker, nonsmoker), which may have contributed variability to the results. The cigarette smokers were light to moderate smokers, and it is possible that greater effects of bupropion may be observed in heavier smokers. Although the effects of bupropion on attention and mood were generally modest, it is plausible that even modest improvements in attention and mood may benefit abstinent smokers during quit attempts. Although two previous studies reported minimal or no effects of chronic bupropion dosing on simple cognitive measures (Paul, Gray, Kenny, & Lange, 2002; Shiffman et al., 2000), it is possible that chronic dosing with bupropion would have decreased impulsive behaviors on the selected tasks or produced greater effects on the attention task. Paul et al (2002) reported no effects of chronic bupropion (150 or 300 mg) on a cognitive test battery in healthy adults, although this does not rule out effects on more direct measures of attention or on measures of impulsivity. Shiffman et al. (2000) observed no effects of chronic bupropion (150 or 300 mg) on a simple reaction time task in abstinent smokers, however this procedure only consisted of 10 trials which may not have been sufficient to induce lapses in attention. In the present study, bupropion (150 mg) decreased lapses in attention on the SRT only in individuals with high lapses at baseline (High Deviation group). It may be that individuals with few lapses (Low Deviation group) were performing at near maximal levels, thus providing little opportunities for the drug to improve performance. It is possible that reaction time procedures which induce more lapses in attention across all participants, such as a significantly longer task with many more trials, would show more robust effects of bupropion.
In summary, in this study bupropion (150 mg) significantly improved attention in individuals who performed poorly under baseline conditions but did not affect impulsive behavior on several standardized tasks. Impairments in attention are common among newly abstinent smokers and other individuals who abstain from habitual use of drugs (Barr et al., 2006; Cargiulo, 2007; Kalechstein, De La Garza, Mahoney, Fantegrossi, & Newton, 2007; Lundqvist, 2005; Rogers & Robbins, 2001; Shiffman, West, & Gilbert, 2004). It is possible that this impaired attention contributes to the drug user’s difficulty in abstaining: Abstinence requires sustained and prolonged vigilance to suppress repeated and continuing tendencies to use a drug, and return to former habitual behaviors. Alternatively, it is possible that attention impairments may be unpleasant to abstinent drug users, which may encourage a return to drug use. Thus, it seems reasonable that the attention-enhancing effects of bupropion may be important for the beneficial effects of this drug as a pharmacotherapy for smoking and abuse of other drugs. It will be of interest in future studies to further examine the effects of bupropion and d-amphetamine on attention in both healthy controls and smokers and/or other drug using populations.
Acknowledgments
This work was supported by DA09133 and T32DA07255. Lisa Vicini and Heather Philips provided excellent technical assistance.
References
- Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82(4):463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther. 2005;43(2):215–228. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31(5):301–313. [PMC free article] [PubMed] [Google Scholar]
- Barrickman LL, Perry PJ, Allen AJ, Kuperman S, Arndt SV, Herrmann KJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649–657. doi: 10.1097/00004583-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Becker RE, Dufresne RL. Perceptual changes with bupropion, a novel antidepressant. Am J Psychiatry. 1982;139(9):1200–1201. doi: 10.1176/ajp.139.9.1200. [DOI] [PubMed] [Google Scholar]
- Bleiberg J, Cernich AN, Cameron K, Sun W, Peck K, Ecklund PJ, et al. Duration of cognitive impairment after sports concussion. Neurosurgery. 2004;54(5):1073–1078. doi: 10.1227/01.neu.0000118820.33396.6a. discussion 1078–1080. [DOI] [PubMed] [Google Scholar]
- Bleiberg J, Kane RL, Reeves DL, Garmoe WS, Halpern E. Factor analysis of computerized and traditional tests used in mild brain injury research. Clin Neuropsychol. 2000;14(3):287–294. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50(1):20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Cargiulo T. Understanding the health impact of alcohol dependence. Am J Health Syst Pharm. 2007;64(5 Suppl 3):S5–11. doi: 10.2146/ajhp060647. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 2001;157(3):243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharmacol. 2000;8(2):155–162. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168(3):347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther. 1987;243(3):955–962. [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology. doi: 10.1111/j.1369-1600.2008.00129.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114(4):830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Flory J, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42:111–121. [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Derogatis L. SCL-90-R Manual-II. Townson, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology (Berl) 2006;186(2):255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I/P (version 2.0) New York: Biometrics Research Department; 1996. [Google Scholar]
- Foulds J, Steinberg MB, Williams JM, Ziedonis DM. Developments in pharmacotherapy for tobacco dependence: past, present and future. Drug Alcohol Rev. 2006;25(1):59–71. doi: 10.1080/09595230500459529. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- Frishman WH, Mitta W, Kupersmith A, Ky T. Nicotine and non-nicotine smoking cessation pharmacotherapies. Cardiol Rev. 2006;14(2):57–73. doi: 10.1097/01.crd.0000172309.06270.25. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Berl) 2002;163(2):174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002;448(2–3):185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Slater S, Boucher N, Debonnel G, Blier P. Neurochemical and psychotropic effects of bupropion in healthy male subjects. J Clin Psychopharmacol. 2003;23(3):233–239. doi: 10.1097/01.jcp.0000084023.22282.03. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71(3):269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189(4):531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. A pharmacological analysis of the discriminative stimulus properties of d-amphetamine in rhesus monkeys. J Pharmacol Exp Ther. 1989;248(3):938–946. [PubMed] [Google Scholar]
- Lai AA, Schroeder DH. Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry. 1983;44(5 Pt 2):82–84. [PubMed] [Google Scholar]
- Lee AM, Miksys S, Palmour R, Tyndale RF. CYP2B6 is expressed in African Green monkey brain and is induced by chronic nicotine treatment. Neuropharmacology. 2006;50(4):441–450. doi: 10.1016/j.neuropharm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, et al. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003;11(1):26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Logan GD. Attention, automaticity, and the ability to stop a speeded choice response. In: Long J, Baddeley AD, editors. Attention and Performance IX. Hillsdale, NJ: Lawerence Erlbaum; 1981. [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81(2):319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting amount procedure for studying delayed reinforcement. In: Commons JEMML, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior: The Effects of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Hillsdale: Lawrence Erlbaum Associates; 1987. pp. 55–73. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states: Educational and Industrial Testing Service 1971 [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45(1):122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, et al. Bupropion Reduces Methamphetamine-Induced Subjective Effects and Cue-Induced Craving. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Odum AL, Rainaud CP. Discounting of delayed hypothetical money, alcohol, and food. Behavioural Processes. 2003;64(3):305–313. doi: 10.1016/s0376-6357(03)00145-1. [DOI] [PubMed] [Google Scholar]
- Paul MA, Gray G, Kenny G, Lange M. The impact of bupropion on psychomotor performance. Aviat Space Environ Med. 2002;73(11):1094–1099. [PubMed] [Google Scholar]
- Pesola GR, Avasarala J. Bupropion seizure proportion among new-onset generalized seizures and drug related seizures presenting to an emergency department. J Emerg Med. 2002;22(3):235–239. doi: 10.1016/s0736-4679(01)00474-7. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res. 2005;7(6):901–907. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169(1):1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of Alcohol. J of Exp Anal of Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22(2):203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11(2):250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6(1):32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Richards JB, Broom SL, Roach JT, Hausknecht K. Effects of stimulus salience and methamphetamine on choice reaction time in the rat: central tendency versus distribution skew. Behav Pharmacol. 2003;14(7):489–500. doi: 10.1097/00008877-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127(12):1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148(1):33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295(1):321–327. [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Effects of sustained-release (SR) bupropion on craving and withdrawal in smokers deprived of cigarettes for 72 h. Psychopharmacology (Berlin) 2005;183(1):1–12. doi: 10.1007/s00213-005-0145-x. [DOI] [PubMed] [Google Scholar]
- Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion in rats. Psychopharmacology (Berl) 1997;134(2):201–212. doi: 10.1007/s002130050443. [DOI] [PubMed] [Google Scholar]
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10(3):219–231. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Haight BR, Horrigan JP, Hudziak JJ, Rosenthal NE, Connor DF, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry. 2005;57(7):793–801. doi: 10.1016/j.biopsych.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Zwar N, Richmond R. Bupropion sustained release. A therapeutic review of Zyban. Aust Fam Physician. 2002;31(5):443–447. [PubMed] [Google Scholar]