Abstract
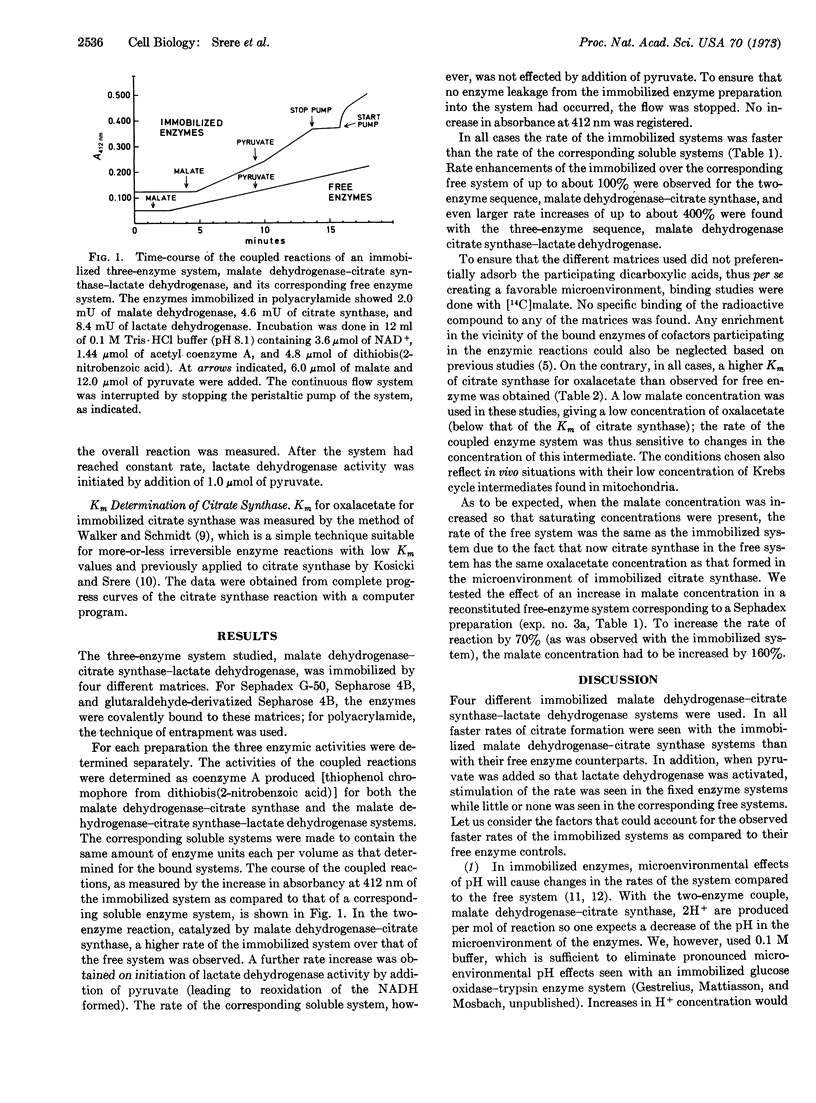
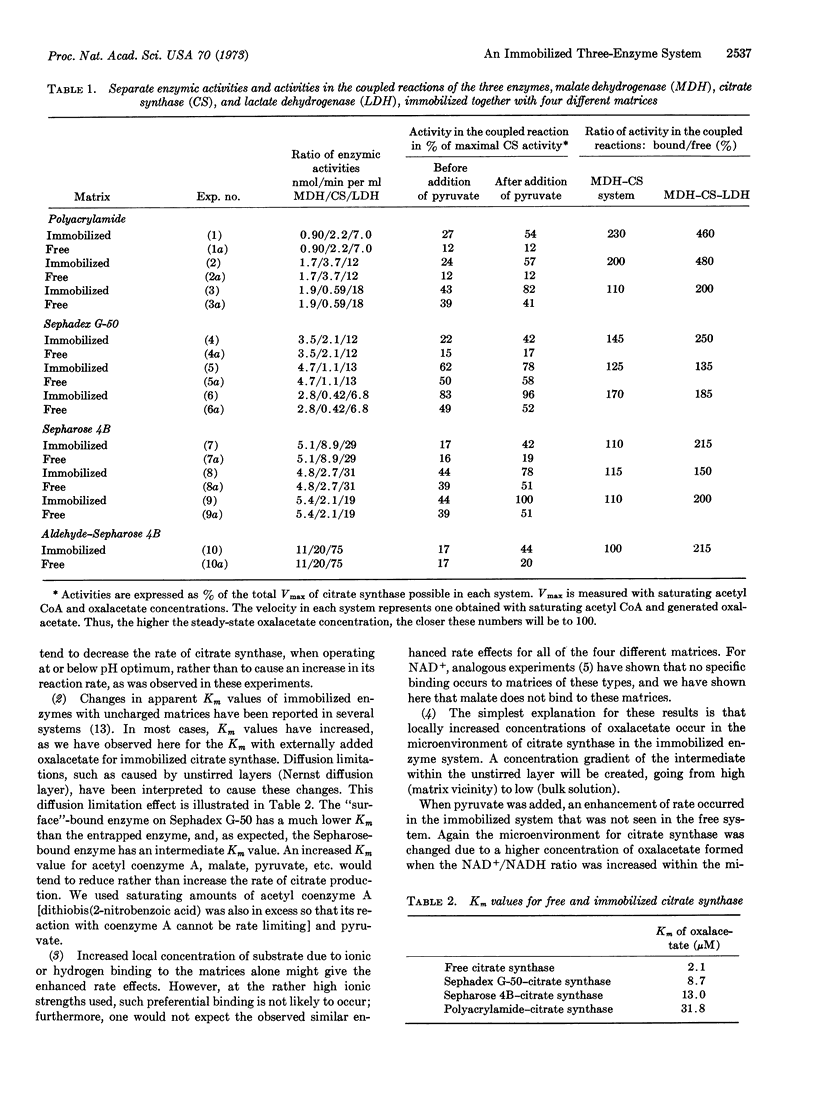
An immobilized three-enzyme system, malate dehydrogenase (EC 1.1.1.37)-citrate synthase (EC 4.1.3.7)-lactate dehydrogenase (EC 1.1.1.27), was investigated as a model for the rate of oxalacetate production and utilization in mitochondria. Lactate dehydrogenase is included to mimic the NADH-utilizing system of mitochondria. This three-enzyme system was immobilized in three different ways (1) on Sephadex G-50 (surface coupling), (2) on Sepharose 4B (internal-external coupling), and (3) entrapped in polycrylamide gel. The rate of citrate production from malate, NAD+, and acetyl CoA was determined continuously in a flow system. Up to about 100% rate enhancements were observed when the immobilized system was compared to identical systems of free enzyme. An even more pronounced increase of rate of up to about 400% compared to the soluble system was measured after addition of pyruvate (to reoxidize formed NADH). These results are interpreted in relation to microenvironmental changes of oxalacetate production and the possible organization of enzymes of the Krebs cycle.
Keywords: malate dehydrogenase, citrate synthase, lactate dehydrogenase, Krebs cycle
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- Bowman R. H. Effects of diabetes, fatty acids, and ketone bodies on tricarboxylic acid cycle metabolism in the perfused rat heart. J Biol Chem. 1966 Jul 10;241(13):3041–3048. [PubMed] [Google Scholar]
- GOLDSTEIN L., LEVIN Y., KATCHALSKI E. A WATER-INSOLUBLE POLYANIONIC DERIVATIVE OF TRYPSIN. II. EFFECT OF THE POLYELECTROLYTE CARRIER ON THE KINETIC BEHAVIOR OF THE BOUND TRYPSIN. Biochemistry. 1964 Dec;3:1913–1919. doi: 10.1021/bi00900a022. [DOI] [PubMed] [Google Scholar]
- Gestrelius S., Mattiasson B., Mosbach K. Studies on pH-activity profiles of an immobilized two-enzyme system. Biochim Biophys Acta. 1972 Aug 28;276(2):339–343. doi: 10.1016/0005-2744(72)90993-x. [DOI] [PubMed] [Google Scholar]
- KOSICKI G. W., SRERE P. A. Kinetic studies on the citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2560–2565. [PubMed] [Google Scholar]
- Katchalski E., Silman I., Goldman R. Effect of the microenvironment on the mode of action of immobilized enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;34:445–536. doi: 10.1002/9780470122792.ch7. [DOI] [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat liver mitochondria. I. Relationships with the citric acid cycle and with the mitochondrial energy state. Biochim Biophys Acta. 1972;283(1):1–15. doi: 10.1016/0005-2728(72)90092-8. [DOI] [PubMed] [Google Scholar]
- Mattiasson B., Mosbach K. Studies on a matrix-bound three-enzyme system. Biochim Biophys Acta. 1971 Apr 14;235(1):253–257. doi: 10.1016/0005-2744(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Srere P. A. Purification of rat heart and rat liver citrate synthases. Physical, kinetic, and immunological studies. J Biol Chem. 1971 May 25;246(10):3217–3223. [PubMed] [Google Scholar]
- Mosbach K., Mattiasson B. Matrix-bound enzymes. II. Studies on a matrix-bound two-enzyme-system. Acta Chem Scand. 1970;24(6):2093–2100. doi: 10.3891/acta.chem.scand.24-2093. [DOI] [PubMed] [Google Scholar]
- Müllhofer G., Kuntzen O. Intracellular compartmentalization in the perfused rat liver. Examination of the existence of a uniform mitochondrial oxaloacetate pool as an intermediate of both gluconeogenesis and the citric acid cycle. Hoppe Seylers Z Physiol Chem. 1972 Sep;353(9):1461–1476. doi: 10.1515/bchm2.1972.353.2.1461. [DOI] [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- Srere P. A. An eclectic view of metabolic regulation: control of citrate synthase activity. Adv Enzyme Regul. 1970;9:221–233. doi: 10.1016/s0065-2571(71)80046-8. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T. Control mechanisms of gluconeogenesis and ketogenesis. II. Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4617–4627. [PubMed] [Google Scholar]


