Abstract
The aim of the present study was to examine the effect of β2-adrenergic stimulation on skeletal muscle contractile properties, sarcoplasmic reticulum (SR) rates of Ca2+ release and uptake, and Na+–K+-ATPase activity before and after fatiguing exercise in trained men. The study consisted of two experiments (EXP1, n = 10 males, EXP2, n = 20 males), where β2-adrenoceptor agonist (terbutaline) or placebo was randomly administered in double-blinded crossover designs. In EXP1, maximal voluntary isometric contraction (MVC) of m. quadriceps was measured, followed by exercise to fatigue at 120% of maximal oxygen uptake (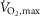 ). A muscle biopsy was taken after MVC (non-fatigue) and at time of fatigue. In EXP2, contractile properties of m. quadriceps were measured with electrical stimulations before (non-fatigue) and after two fatiguing 45 s sprints. Non-fatigued MVCs were 6 ± 3 and 6 ± 2% higher (P < 0.05) with terbutaline than placebo in EXP1 and EXP2, respectively. Furthermore, peak twitch force was 11 ± 7% higher (P < 0.01) with terbutaline than placebo at non-fatigue. After sprints, MVC declined (P < 0.05) to the same levels with terbutaline as placebo, whereas peak twitch force was lower (P < 0.05) and half-relaxation time was prolonged (P < 0.05) with terbutaline. Rates of SR Ca2+ release and uptake at 400 nm [Ca2+] were 15 ± 5 and 14 ± 5% (P < 0.05) higher, respectively, with terbutaline than placebo at non-fatigue, but declined (P < 0.05) to similar levels at time of fatigue. Na+–K+-ATPase activity was unaffected by terbutaline compared with placebo at non-fatigue, but terbutaline counteracted exercise-induced reductions in maximum rate of activity (Vmax) at time of fatigue. In conclusion, increased contractile force induced by β2-adrenergic stimulation is associated with enhanced rate of Ca2+ release in humans. While β2-adrenergic stimulation elicits positive inotropic and lusitropic effects on non-fatigued m. quadriceps, these effects are blunted when muscles fatigue.
). A muscle biopsy was taken after MVC (non-fatigue) and at time of fatigue. In EXP2, contractile properties of m. quadriceps were measured with electrical stimulations before (non-fatigue) and after two fatiguing 45 s sprints. Non-fatigued MVCs were 6 ± 3 and 6 ± 2% higher (P < 0.05) with terbutaline than placebo in EXP1 and EXP2, respectively. Furthermore, peak twitch force was 11 ± 7% higher (P < 0.01) with terbutaline than placebo at non-fatigue. After sprints, MVC declined (P < 0.05) to the same levels with terbutaline as placebo, whereas peak twitch force was lower (P < 0.05) and half-relaxation time was prolonged (P < 0.05) with terbutaline. Rates of SR Ca2+ release and uptake at 400 nm [Ca2+] were 15 ± 5 and 14 ± 5% (P < 0.05) higher, respectively, with terbutaline than placebo at non-fatigue, but declined (P < 0.05) to similar levels at time of fatigue. Na+–K+-ATPase activity was unaffected by terbutaline compared with placebo at non-fatigue, but terbutaline counteracted exercise-induced reductions in maximum rate of activity (Vmax) at time of fatigue. In conclusion, increased contractile force induced by β2-adrenergic stimulation is associated with enhanced rate of Ca2+ release in humans. While β2-adrenergic stimulation elicits positive inotropic and lusitropic effects on non-fatigued m. quadriceps, these effects are blunted when muscles fatigue.
Key Points
From animal models, it is well established that β2-adrenergic stimulation increases contractile force, rates of Ca2+ release and uptake from the sarcoplasmic reticulum, and Na+–K+-ATPase activity of skeletal muscles. However, these effects are unexplored in humans.
Here we report that β2-adrenergic stimulation with the high dose selective β2-adrenoceptor agonist terbutaline elicits positive inotropic and lusitropic effects on non-fatigued m. quadriceps that are associated with enhanced rates of Ca2+ release and uptake from the sarcoplasmic reticulum in trained men.
However, we also observed that the positive inotropic and lusitropic effects of β2-adrenergic stimulation on m. quadriceps were blunted when muscle fatigue developed.
Furthermore, we show that β2-adrenergic stimulation counteracts exercise-induced reductions in Na+–K+-ATPase Vmax (maximum rate of activity) and elevates glycolytic activity during high intensity exercise.
These findings are important for our understanding of the role of β2-adreceptor activation in regulation of ion handling and contractile properties of non-fatigued and fatigued skeletal muscles in humans.
Introduction
The effect of β2-adrenergic stimulation on skeletal muscle contractile properties and ion handling has been intensively investigated in the last couple of decades in animal studies involving pharmacological manipulation with β2-adrenoceptor agonists (Juel, 1988; Cairns & Dulhunty, 1993b). While it is well established that β2-adrenergic stimulation increases contractile force (Bowman & Zaimis, 1958; Cairns & Dulhunty, 1993b; Andersson et al. 2012), rates of Ca2+ release and uptake from the sarcoplasmic reticulum (SR) (Prakash et al. 1999; Rudolf et al. 2006) and Na+–K+-ATPase activity in animal models (Clausen & Flatman, 1980; Juel, 1988; Cairns & Dulhunty, 1994), these effects are inadequately explored in humans.
Upon activation of β2-adrenoceptors, increases in levels of cAMP lead to a marked activation of protein kinase A (PKA; (Roberts & Summers, 1998; Rockman et al. 2002). PKA acts downstream as a covalent modifier on several intramuscular proteins involved in contractile function, including proteins associated with the SR (Slack et al. 1997; Andersson et al. 2012), along with proteins involved in membrane excitability (Clausen & Flatman, 1980; Cairns & Dulhunty, 1994). A major effect of β2-adrenergic stimulation is thus PKA-dependent regulation of SR Ca2+ handling mediated by phosphorylation of ryanodine receptor isoform 1 (RyR1) and SR Ca2+-ATPase (Suko et al. 1993; Slack et al. 1997; Andersson et al. 2012), leading to increased isometric muscle force through higher amplitudes of Ca2+ transients and an accelerated rate of relaxation via an increased rate of Ca2+ uptake (Rudolf et al. 2006; Andersson et al. 2012). Furthermore, stimulation with the selective β2-adrenoceptor agonist terbutaline has been shown by Juel (1988) to delay fatigue in mouse soleus muscles by stimulation of Na+–K+-ATPase. β2-Adrenergic stimulation may as such attenuate contraction-induced accumulation of extracellular K+ and delay development of fatigue caused by membrane inexcitability during repeated tetani in isolated muscles (Clausen & Flatman, 1980; Juel, 1988; Cairns & Dulhunty, 1994). Evidently, β2-adrenergic stimulation affects several aspects of excitation–contraction coupling, modulating force and contractility of skeletal muscles, at least as evidenced in animal models.
In humans, on the other hand, the intramuscular effects of β2-adrenergic stimulation are inadequately explored. Although studies have shown enhancing effects of β2-adrenoceptor agonist on muscle strength, sprinting peak power, and exercise endurance (Collomp et al. 2000, 2005; van Baak et al. 2000; Kalsen et al. 2014a), no studies have investigated the effects of β2-adrenergic stimulation on SR Ca2+ handling and Na+–K+-ATPase activity of non-fatigued and fatigued skeletal muscles in humans. Studies have, however, used percutaneous electrical muscle stimulations to measure the effects of β2-adrenergic stimulation on contractile properties at rest and during fatigue-inducing tasks (Decorte et al. 2008; Crivelli et al. 2011; Crivelli & Maffiuletti, 2014). In contrast to observations in animal studies, most of these studies show no effects of β2-adrenergic stimulation on peak twitch force and M-wave amplitude, suggesting unchanged excitation–contraction coupling in non-fatigued and fatigued muscle (Crivelli et al. 2011; Crivelli & Maffiuletti, 2014). This discrepancy could be explained by the lower doses of β2-adrenoceptor agonists administered in human studies compared with animal studies, since the effect of β2-adrenergic stimulation on amplitudes of Ca2+ transients and tetanic force on skeletal muscles has been shown to be dose dependent (Cairns & Dulhunty, 1993b; Prakash et al. 1999). Administration of higher doses of β2-adrenoceptor agonists, eliciting a larger peripheral response, might affect contractile properties and ion handling of human skeletal muscles.
Besides effects on skeletal muscle contractile properties and ion handling, β2-adrenergic stimulation may also affect muscle metabolism. Infusion of epinephrine (adrenaline) has thus been shown to activate various glycolytic enzymes and to elevate carbohydrate oxidation and glycogen utilization in skeletal muscles (Febbraio et al. 1998; Watt et al. 2001). However, epinephrine is a non-selective agonist with affinity for both α- and β-adrenoceptors, which may be why the effects of selective β2-adrenoceptor stimulation on muscle glycolytic metabolism are less well understood in humans during exercise. Studies have, however, shown that β2-adrenoceptor agonists elevate systemic concentrations of plasma lactate in exercising humans (Collomp et al. 2002; Hostrup et al. 2014b), which suggests a stimulatory action on glycolysis of working skeletal muscles. The elevated rate of glycolysis induced by β2-adrenergic stimulation may on the one hand benefit exercise performance by enlarging the anaerobic energy production, but on the other be detrimental to performance and contractile function through formation of lactate and lowered pH (Lindinger & Heigenhauser, 1991; Fitts, 1994; Hogan et al. 1995; Bangsbo et al. 1996; Kristensen et al. 2005). Moreover, a potential increase in breakdown of muscle glycogen by β2-adrenergic stimulation could also affect SR Ca2+ handling and contractile function, since the SR Ca2+ handling function partly relies on the level of glycogen in close proximity with the SR (Chin & Allen, 1997; Duhamel et al. 2006; Ørtenblad et al. 2011; Nielsen et al. 2014). We recently observed that while β2-adrenergic stimulation enhanced peak power during a 30 s maximal sprint, the effect was blunted when sprints were repeated and fatigue developed (Hostrup et al. 2014a). Still, the interplay between β2-adrenergic actions on contractile function, ion handling and glycolytic activity of non-fatigued and fatigued skeletal muscles of humans has yet to be established.
Thus, the aim of the present study was to investigate the effect of β2-adrenergic stimulation with the high dose selective β2-adrenoceptor agonist terbutaline on the contractile properties, SR Ca2+ handling, and Na+–K+-ATPase activity of non-fatigued and fatigued m. quadriceps in trained males. In addition, the effect of β2-adrenergic stimulation on muscle glycolytic activity during intense exercise was examined.
Methods
Ethical approval
The study was approved by the local ethics committee of Copenhagen, Denmark (H-1-2011-080 and H-1-2012-090). Before inclusion, subjects received oral and written information about aims and contents of the study and possible risks involved. Each subject gave his oral and written informed consent. The study was performed in accordance with the Helsinki II declaration.
Experimental design and subjects
The study consisted of two separate experiments. In experiment 1 (EXP1), the effects of β2-adrenergic stimulation on SR Ca2+ handling, Na+–K+-ATPase activity, and glycolytic metabolism of skeletal muscles were investigated before and after exercise to fatigue. Since SR Ca2+ handling function and Na+–K+-ATPase function are mostly affected by prolonged exercise (McKenna et al. 2008), we chose an exercise protocol that consisted of a 10 min warm-up followed by three 4 min bouts of intermittent exercise at 75% of subjects maximal oxygen uptake (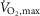 ) with a subsequent intense bout to fatigue at 120% of
) with a subsequent intense bout to fatigue at 120% of 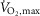 . In experiment 2 (EXP2), the effect of β2-adrenergic stimulation on contractile properties of m. quadriceps was measured with superimposed percutaneous electrical muscle stimulations before and after two fatiguing 45 s sprints. This approach was based on recent observations in which the β2-adrenoceptor agonist salbutamol only enhanced peak power during the first sprint in a repeated Wingate test with three repetitions, indicating that the enhancing effect of β2-adrenergic stimulation was blunted when fatigue developed (Hostrup et al. 2014a).
. In experiment 2 (EXP2), the effect of β2-adrenergic stimulation on contractile properties of m. quadriceps was measured with superimposed percutaneous electrical muscle stimulations before and after two fatiguing 45 s sprints. This approach was based on recent observations in which the β2-adrenoceptor agonist salbutamol only enhanced peak power during the first sprint in a repeated Wingate test with three repetitions, indicating that the enhancing effect of β2-adrenergic stimulation was blunted when fatigue developed (Hostrup et al. 2014a).
In both experiments, using a placebo-controlled randomized crossover design, β2-adrenoceptors were stimulated with high dose selective β2-adrenoceptor agonist (terbutaline). Ten highly trained males (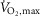 : 67.4 ± 1.6 ml min−1 kg−1) took part in EXP1, and 20 trained males (
: 67.4 ± 1.6 ml min−1 kg−1) took part in EXP1, and 20 trained males (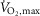 : 56.1 ± 1.1 ml min−1 kg−1) in EXP2. One subject participated in both experiments. Subjects were active in endurance and team-sports. Subjects had no history of respiratory or cardiovascular diseases and had not used β2-adrenoceptor agonists before. Subject characteristics are summarized in Table 1.
: 56.1 ± 1.1 ml min−1 kg−1) in EXP2. One subject participated in both experiments. Subjects were active in endurance and team-sports. Subjects had no history of respiratory or cardiovascular diseases and had not used β2-adrenoceptor agonists before. Subject characteristics are summarized in Table 1.
Table 1.
Subject characteristics
| EXP1 (n = 10) | EXP2 (n = 20) | |
|---|---|---|
| Age (years) | 24.4 ± 1.0 | 23.9 ± 0.9 |
| Height (cm) | 184 ± 3 | 180 ± 1 |
| Weight (kg) | 76.0 ± 2.3 | 75.5 ± 1.9 |
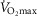 (ml min−1 kg−1) (ml min−1 kg−1) |
67.4 ± 1.6 | 56.1 ± 1.1 |
| Weekly training volume (h week−1) | 9.4 ± 0.9 | 5.2 ± 0.3 |
| Wingate peak power (W) | — | 861 ± 24 |
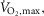 maximal oxygen uptake. Data are presented as means = ± SEM.
maximal oxygen uptake. Data are presented as means = ± SEM.
Experimental protocol
Before the start of the intervention of each experiment, subjects’ 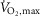 and performance were determined in an incremental test to fatigue on a bike ergometer (Monark 839E, Stockholm, Sweden). Prior to the test, subjects warmed up for 10 min at 150 W. Two minutes after warm-up, subjects performed the incremental test starting at 150 W and increasing by 30 W every 1 min until time of fatigue. Pulmonary gas exchange was measured during the test breath-by-breath with a gas analysing system (Oxycon Pro, CareFusion, CA, USA). During the test, subjects were told to keep a cadence between 80–100 rpm. Time of fatigue was defined as the point where pedaling frequency fell below 70 rpm for more than 5 s despite strong verbal encouragement.
and performance were determined in an incremental test to fatigue on a bike ergometer (Monark 839E, Stockholm, Sweden). Prior to the test, subjects warmed up for 10 min at 150 W. Two minutes after warm-up, subjects performed the incremental test starting at 150 W and increasing by 30 W every 1 min until time of fatigue. Pulmonary gas exchange was measured during the test breath-by-breath with a gas analysing system (Oxycon Pro, CareFusion, CA, USA). During the test, subjects were told to keep a cadence between 80–100 rpm. Time of fatigue was defined as the point where pedaling frequency fell below 70 rpm for more than 5 s despite strong verbal encouragement. 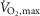 was defined as the highest value recorded in any 30 s period before cessation of exercise. A plateau in the oxygen uptake despite an increased power output and a respiratory exchange ratio above 1.15 were used as criteria for
was defined as the highest value recorded in any 30 s period before cessation of exercise. A plateau in the oxygen uptake despite an increased power output and a respiratory exchange ratio above 1.15 were used as criteria for 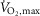 achievement.
achievement.
After the incremental test, subjects were familiarized with the experimental procedures used in each experiment. Furthermore, the subjects in EXP2 completed 30 s of maximal sprinting (Wingate test).
Experiment I
EXP1 consisted of two trials (terbutaline vs. placebo) separated by approximately 1 week. Subjects reported to the laboratory and had a catheter inserted in the antecubital vein for venous blood sampling. In a supine position, two incisions were made in m. vastus lateralis approximately 20–25 cm proximal from patella under local anaesthesia (Xylocain 20 mg ml−1, AstraZeneca, London, UK) for later muscle biopsy sampling. High dose terbutaline (40 × 0.5 mg, Bricanyl Turbohaler, AstraZeneca) or placebo (Placebo Turbohaler, AstraZeneca) was then administered with 100 ml of tap water during supervision. Systemic concentrations of terbutaline have been shown to rise quickly after this dosing regimen and reach their peak within 30 min (Elers et al. 2012). Five minutes after administration of study drugs, subjects warmed up on a bike ergometer (Monark 839E, Stockholm, Sweden) at 150 W for 10 min and 200 W for 5 min. Four minutes after warm-up, subjects’ MVC of m. quadriceps was measured. Before the start of the MVC, subjects performed three submaximal contractions of a duration of 3 s. Subjects then completed three MVCs of 3–4 s duration, each separated by 90 s. Four minutes after the last MVC, subjects were put on a bed and a biopsy was collected from m. vastus lateralis (non-fatigue).
After 10 min of rest, subjects warmed up for 10 min at 150 W followed by three 4 min submaximal exercise bouts at an intensity corresponding to 75% of 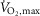 (246 ± 11 W) each interspersed by 4 min of recovery. Immediately following the third submaximal bout, the bike ergometer load was increased to elicit an intensity corresponding to 120% of
(246 ± 11 W) each interspersed by 4 min of recovery. Immediately following the third submaximal bout, the bike ergometer load was increased to elicit an intensity corresponding to 120% of 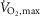 (406 ± 20 W) that subjects performed until fatigue. At time of fatigue, subjects lifted their leg onto the handle bar of the bike and had a biopsy taken from m. vastus lateralis as fast as possible (time of fatigue). Time of fatigue was defined as a drop in cadence (<70 rpm) for more than 5 s despite strong verbal encouragement.
(406 ± 20 W) that subjects performed until fatigue. At time of fatigue, subjects lifted their leg onto the handle bar of the bike and had a biopsy taken from m. vastus lateralis as fast as possible (time of fatigue). Time of fatigue was defined as a drop in cadence (<70 rpm) for more than 5 s despite strong verbal encouragement.
Experiment II
EXP2 consisted of two trials (terbutaline vs. placebo) separated by approximately 1 week. Subjects reported to the laboratory, and terbutaline (5 mg (15 kg BW)−1, Bricanyl Retard, AstraZeneca) or placebo (lactose monohydrate/starch tablets, pharmacy in Copenhagen) was randomly administered during supervision with 100 ml of tap water. The dosing regimen in EXP2 was different from EXP1 in that AstraZeneca was not able to deliver matching placebo at the start of EXP2. Therefore, oral terbutaline was chosen for EXP2 with matching placebo tablets to optimize blinding. The dose of 5 mg (15 kg BW)−1 oral terbutaline was chosen as this would give systemic concentrations of terbutaline of approximately 20 ng ml−1 (Elers et al. 2012), thus being comparable with the dose used in EXP1. After administration of study drugs, a catheter was inserted in the antecubital vein for venous blood sampling. Two hours after administration of the study drug, in which systemic concentrations of oral terbutaline reach their peak (Elers et al. 2012), subjects warmed up on a bike ergometer (Monark 839E, Stockholm, Sweden) at 150 W for 10 min and 200 W for 5 min. After 4 min of recovery, subjects’ contractile properties of non-fatigued m. quadriceps were measured. Before measurements, subjects performed three submaximal contractions of a duration of 3 s. Subjects then performed three MVCs of 3–4 s, each interspersed by 90 s of recovery. During each MVC, superimposed percutaneous electrical muscle stimulations were delivered to m. vastus lateralis and m. rectus femoris by two self-adhesive electrodes (PALS Platinum 5 × 9 cm, Axelgaard Manufacturing CO, Lystrup, Denmark). Electrodes were placed on the skin 25% distal from spina iliaca anterior superior and 25% proximal from patella covering m. vastus lateralis and m. rectus femoris.
To determine the degree of voluntary activation, a single stimulation was delivered during the plateau of each MVC (Merton, 1954). To determine peak twitch force, a single stimulation was delivered 1 s after relaxation of each MVC which also served as the control twitch (Place et al. 2007). Potentiated twitches were used since these have been shown to be more sensitive to fatigue than unpotentiated twitches (Kufel et al. 2002; Place et al. 2007). Muscle stimulations were produced by a constant current stimulator (Digitimer Ltd, Stimulator model DS7AH, Hertfordshire, England) in rectangular pulses of 1 ms. After placement of electrodes, stimulation intensity was progressively increased either until a plateau in peak twitch force was observed or maximal stimulator output (999 mA) was achieved, or if subjects felt pain. Thus, a stimulation intensity of 999 mA, despite no plateau in peak twitch force, was considered the arbitrary maximum. The same stimulation intensity was used for the same subject throughout the intervention. During MVC measurements, subjects received verbal encouragement with no visual feedback.
After measurements of contractile properties of non-fatigued m. quadriceps, subjects performed two bouts of 45 s sprinting with a constant workload of 560 ± 11 W (corresponding to 85–90% of subjects mean power during the Wingate test) on a bike ergometer with 4 min of recovery in between. Immediately following both the first and second 45 s sprint, subjects’ MVC was measured with superimposed muscle stimulations as described above. The time delay between cessation of each 45 s maximal sprint and measurement of MVC was 15 ± 2 s.
Maximal voluntary isometric contraction
For MVC measurements, subjects were positioned on a chair with an adjustable back, with their thighs parallel to the floor and their right leg positioned with a knee joint angle of 90 deg of flexion. To ensure that subjects remained in the same position during each MVC, three Velcro strips were tied around the chest, hip and thighs. The body positions of the subjects were recorded and used for each measurement. Isometric contraction force was recorded using a strain gauge (Tedea-Huntleigh, UK) strapped around the right ankle just above the malleoli. The strain gauge signal was fed to an amplifier that was connected to a computer. Data were recorded at 1 kHz in LabChart 7 (ADInstruments, CA, USA).
In EXP1, MVC was recorded as the highest force (N) during the contraction. In EXP2, the following parameters were determined:
MVC (recorded as in EXP1); peak twitch force (highest force (N) during a stimulated single twitch 1 s after relaxation from a MVC); half-relaxation time (time (ms) from peak twitch force until force reached half of peak twitch force); time to peak tension (time (ms) from single twitch stimulation until peak twitch force was reached); voluntary activation (calculated as: [1 – (size of superimposed twitch/size of control twitch)] × 100; Merton, 1954).
Treatment and biochemical analysis of biopsies
Following collection of muscle biopsies in EXP1, one piece was immediately frozen in liquid nitrogen and stored at –80°C for in vitro Na+–K+-ATPase activity assay, Western blot analysis, and determination of glycogen content and lactate concentrations. A second piece (∼30 mg) was quickly homogenized (Omni 2000) in ice-cold buffer (300 mm sucrose, 1 mm EDTA, 10 mm NaN3, 40 mm Tris-base, and 40 mm l-histidine; pH 7.8), and frozen in liquid nitrogen and stored at –80°C for analysis of SR vesicle Ca2+ handling.
Muscle glycogen and lactate
Samples were freeze-dried at –20°C for at least 48 h and at room temperature for at least 2 h. Muscle samples were analysed for total water by weighing the samples before and after freeze-drying. Freeze-dried muscle tissue was dissected free from blood and connective tissue under a microscope. For determination of muscle lactate, a piece of the muscle sample (∼1 mg dry wt) was homogenized in a buffer containing 0.6 m perchloric acid and 1 mm EDTA, neutralized to pH 7.0 with 2.2 m KHCO3, and stored at –80°C until analysed for muscle lactate fluorometrically (Lowry & Passonneau, 1972). For determination of muscle glycogen, ∼2 mg dry weight muscle tissue was placed in 1 m HCl and hydrolysed at 100°C for 3 h. Glycogen content was then measured (Lowry et Passoneau; 1972). For both assays, samples were measured in duplicates. Due to inadequate muscle tissue yield from one subject, muscle glycogen and lactate were not determined for the subject. One subject's glycogen data were removed from the analysis due to analytical problems showing unphysiologically low muscle glycogen content after MVC (<25 mmol (kg dry wt)−1). Hence, data of muscle glycogen and lactate were determined for eight and nine subjects, respectively.
SR vesicle Ca2+ handling
SR Ca2+ uptake and release were measured in muscle homogenate as previously described (Ørtenblad et al. 2011). Briefly, muscle homogenate (70 μl) was mixed with 2 ml assay buffer (165 mm KCl, 22 mm Hepes, 7.5 mm oxalate, 11 mm NaN3, 5.5 μm N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), 20 μm CaCl2, and 2 mm MgCl2, (pH 7.0 at 37 ºC)), and the reaction was initiated by adding 5 mm ATP. [Ca2+] was determined fluorometrically (20 Hz, Ratiomaster RCM, Photon Technology International, Brunswick, NJ, USA) using the fluorescent Ca2+ indicator indo-1 (1 μm). When [Ca2+] reached a plateau, SR Ca2+ uptake was blocked by adding cyclopiazonic acid (40 μm) and Ca2+ release was initiated by adding 4-chloro-m-cresol (5 mm), and the fluorescence followed for at least 30 s. The SR Ca2+ uptake function was estimated as the ATP-supported SR vesicle uptake and is given as the tau (τ) value, calculated after curve fitting in the whole range of [Ca2+] during the uptake, where τ is defined as the time for the free [Ca2+] to decrease by 63% of the initial free [Ca2+] as previously described (Ørtenblad et al. 2011). In order to estimate the uptake at specific [Ca2+], the Ca2+ uptake was also measured as the derivative of the curve fit at 400 nm and 200 nm [Ca2+]. Ca2+ uptake at these concentrations were chosen as a functional measure, with 400 nm being near Km. for the Ca2+-ATPase and 200 nm being nearer resting [Ca2+] of approximately 30 nm (Westerblad & Allen, 1993a, b1993b). Assays of uptake and release rates of Ca2+ were performed in triplicate. Before Ca2+ assay, protein content of muscle homogenates was measured in triplicate using a standard kit (Pierce BCA protein reagent no. 23225). The homogenate protein content averaged 13.0 ± 0.6 mg ml−1, with no significant difference between trials or time points, or in samples from the same subject.
The values obtained for Ca2+ release and uptake rates are relative and expressed as arbitrary units of Ca2+ min−1 (g protein−1). Due to the inter-individual variation in SR Ca2+ release and uptake, figures and statistics were determined by relative Ca2+ release and uptake rates (% of placebo at non-fatigue).
Na+-dependent Na+–K+-ATPase activity
Na+-stimulated Na+–K+-ATPase activity was determined by measuring ATP hydrolysis as previously described (Juel, 2009; Juel et al. 2013). Released Pi was detected using a malachite based Biomol Green reagent (Biomol no. AK-111, Enzo Life Sciences). Membrane fractionation was needed to reduce background activity of other ATPases (Ca2+-ATPase). After mincing, muscles were homogenized for 30 s (Polytron PT 2100). Crude homogenate was then centrifuged at 3000 g for 30 min, and the resulting supernatant was centrifuged at 190,000 g for 90 min. The final pellet was re-suspended and used for the ATPase assay. The relative amount of protein in the 190,000 g fraction of muscle homogenate did not differ between treatments at non-fatigue and at time of fatigue, being 1.86 ± 0.19 with placebo and 2.13 ± 0.36 with terbutaline at non-fatigue, and 2.10 ± 0.18 and 1.97 ± 0.24 at time of fatigue, respectively. Na+–K+-ATPase Vmax and Km were determined from a Hill plot (Juel et al. 2013).
Western blotting
Western blotting was performed as previously described (Juel, 2009). Western blotting with an anti-FXYD1 antibody (CP68) was used to determine the degree of phosphorylation of phospholemmen at serine68 (PLMSer68). An α-subunit antibody was used to quantify the total pool of Na+–K+-ATPase at non-fatigue and at time of fatigue.
Analysis of serum terbutaline
A blood sample (4 ml) was drawn immediately prior to the MVC measurements in each experiment to determine the systemic concentrations of serum terbutaline. Concentrations of terbutaline were analysed by the WADA accredited doping control laboratory in Norway (Norwegian Doping Control Laboratory) at Oslo University Hospital. Samples were shipped (World Courier) on dry ice from Copenhagen. Concentrations of serum terbutaline were analysed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) as described by Elers et al. (2012). Serum terbutaline was determined for 10 subjects in EXP1 and for nine in EXP2.
Statistics
Data were analysed in SPSS statistical software, version 22 (IBM Software, Chicago, IL, USA). Data were tested for normality with the Shapiro-Wilk test and Q-Q plots. Data were normally distributed and are expressed as means = ± SEM (standard error of the mean). In EXP1, differences between treatments in rates of Ca2+ release and uptake, Na+–K+-ATPase Km and Vmax, phosphorylation of PLMSer68, and muscle lactate were tested with a repeated measures analysis of variance (ANOVA) with treatment and time as the two factors. Differences in MVC, time to fatigue during exercise at 120% of 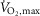 , and net glycogen breakdown between treatments were tested in a Student's paired t test. In EXP2, differences in voluntary activation, MVC, peak twitch force, half-relaxation time, and time to peak twitch force were tested with an ANOVA with treatment and time as the two factors. In the case of a significant ANOVA, a Bonferroni correction was used as a post hoc test. The level of significance was set to α ≤ 0.05.
, and net glycogen breakdown between treatments were tested in a Student's paired t test. In EXP2, differences in voluntary activation, MVC, peak twitch force, half-relaxation time, and time to peak twitch force were tested with an ANOVA with treatment and time as the two factors. In the case of a significant ANOVA, a Bonferroni correction was used as a post hoc test. The level of significance was set to α ≤ 0.05.
Results
Serum terbutaline
Systemic concentrations of serum terbutaline at the time of MVC measurements were 22.0 ± 0.6 ng ml−1 (0.10 μm) and 17.1 ± 2.4 ng ml−1 (0.08 μm) in EXP1 and EXP2, respectively.
Contractile properties of non-fatigued and fatigued m. quadriceps
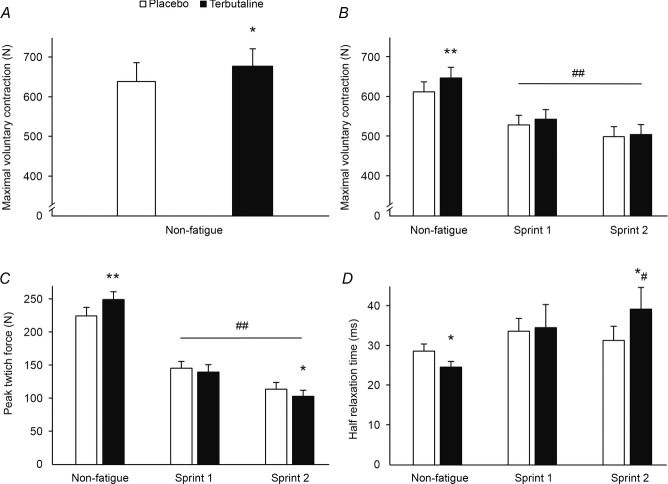
Non-fatigued MVCs were 6 ± 3 and 6 ± 2% higher (P < 0.05 and P < 0.01) with terbutaline compared with placebo in EXP1 (Fig. 1A) and EXP2 (Fig. 1B), respectively.
Figure 1. Contractile properties of m. quadriceps with terbutaline and placebo.
A, maximal voluntary contraction of non-fatigued m. quadriceps in EXP1 (n = 10). B–D, contractile properties of m. quadriceps measured with a single muscle stimulation during the plateau of a maximal voluntary contraction and 1 s following relaxation conducted before (non-fatigue) and after two 45 s fatiguing sprints (sprint 1 and sprint 2) in EXP2 (n = 20). B, maximal voluntary contraction. C, peak twitch force. D, half-relaxation time. Data are presented as means = ± SEM. *Different (P < 0.05) from placebo. **Different (P < 0.01) from placebo. #Different (P < 0.05) from non-fatigue. ##Different (P < 0.01) from non-fatigue.
In EXP2, voluntary activation of non-fatigued m. quadriceps was not different between treatments, being 97 ± 1% for both treatments. Peak twitch force was 11 ± 7% higher (P < 0.05) with terbutaline than placebo at non-fatigue (Fig. 1C). No differences were observed in time to peak twitch force between treatments at non-fatigue (terbutaline: 75.0 ± 1.2 ms, placebo: 73.9 ± 1.3 ms), whereas half-relaxation time was 2.6 ± 1.3 ms shorter (P < 0.05) with terbutaline than placebo (Fig. 1D).
No differences were observed in MVC between treatments after the first and second sprint (Fig. 1B). After the second sprint, MVC was decreased (P < 0.01) by 22 ± 2% with terbutaline and by 19 ± 2% with placebo compared with non-fatigue. MVC declined (P < 0.05) more with terbutaline (–143 ± 13 N) than placebo (–113 ± 12 N) after the second sprint compared with non-fatigue. Voluntary activation of m. quadriceps was not different after the first and second sprint compared with non-fatigue or between treatments, being 93 ± 2% for both treatments after the first sprint and 94 ± 3% for both treatments after the second sprint. Peak twitch force was not different between treatments after the first sprint whereas peak twitch force was lower (P < 0.05) with terbutaline than placebo after the second sprint (Fig. 1C). Peak twitch force declined by 59 ± 3% (P < 0.01) with terbutaline and by 49 ± 3% (P < 0.01) with placebo after the second sprint compared with non-fatigue. The decrease in peak twitch force was higher with terbutaline than placebo after the first (–110 ± 11 vs. –79 ± 8 N, P < 0.05) and second sprint (–147 ± 11 vs. –111 ± 9 N, P < 0.01). No differences were observed in time to peak twitch force between treatments after the first and second sprint, which were 78.1 ± 3.7 and 78.5 ± 2.8 ms with terbutaline and 76.8 ± 2.0 and 81.1 ± 2.3 ms with placebo, respectively. Half-relaxation time was not different between treatments after the first sprint, but after the second sprint it was 5.2 ± 2.9 ms slower (P < 0.05) with terbutaline than placebo (Fig. 1D). Moreover, half-relaxation time was 9.7 ± 4.2 ms slower (P < 0.05) after the second sprint with terbutaline compared with non-fatigue, whereas no differences were observed in half-relaxation time with placebo after the first and second sprint compared with non-fatigue.
Exercise performance at 120% of 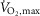
No differences were observed in time to fatigue during exercise at 120% of 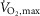 between treatments in EXP1, which were 127 ± 10 s with terbutaline and 144 ± 9 s with placebo.
between treatments in EXP1, which were 127 ± 10 s with terbutaline and 144 ± 9 s with placebo.
Rate of SR vesicle Ca2+ release and uptake
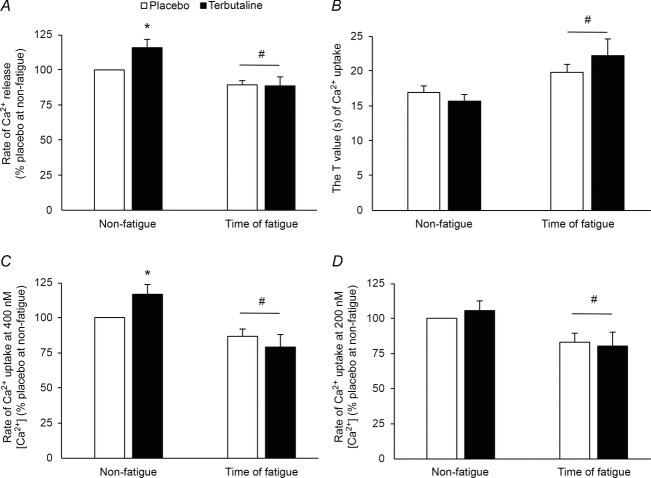
Rate of Ca2+ release was 15 ± 5% higher (P < 0.05) with terbutaline than placebo after MVC (non-fatigue; 2.5 ± 0.1 vs. 2.1 ± 0.1 μmol Ca2+ (g protein min)−1; Fig. 2A). At time of fatigue, rate of Ca2+ release was decreased (P < 0.05) compared with non-fatigue with no difference between terbutaline and placebo (1.9 ± 0.1 vs. 1.9 ± 0.1 μmol Ca2+ (g protein min)−1; Fig. 2A).
Figure 2. SR Ca2+ handling of m. vastus lateralis with terbutaline and placebo.
SR Ca2+ release and uptake were measured fluorometrically in crude homogenate from m. vastus lateralis at non-fatigue and at time of fatigue after three bouts of 4 min exercise at 75% of  and a bout to fatigue at 120% of
and a bout to fatigue at 120% of  with terbutaline and placebo in EXP1 (n = 10). A, rate of Ca2+ release. B, the tau value of Ca2+ uptake. C, rate of Ca2+ uptake at 400 nm [Ca2+]. D, rate of Ca2+ uptake at 200 nm [Ca2+]. Data are presented as means = ± SEM. *Different (P < 0.05) from placebo. #Different (P < 0.05) from non-fatigue.
with terbutaline and placebo in EXP1 (n = 10). A, rate of Ca2+ release. B, the tau value of Ca2+ uptake. C, rate of Ca2+ uptake at 400 nm [Ca2+]. D, rate of Ca2+ uptake at 200 nm [Ca2+]. Data are presented as means = ± SEM. *Different (P < 0.05) from placebo. #Different (P < 0.05) from non-fatigue.
No differences were observed in the τ value of Ca2+ uptake between terbutaline and placebo at non-fatigue (Fig. 2B). At time of fatigue, the tau (τ) value was lower (P < 0.05) compared with non-fatigue, with no difference between the treatments (Fig. 2B). Rate of Ca2+ uptake at 400 nm [Ca2+] was 14 ± 5% higher (P < 0.05) with terbutaline than placebo at non-fatigue (4.1 ± 0.2 vs. 3.6 ± 0.2 μmol Ca2+ (g protein min)−1; Fig. 2C), whereas no differences were observed in rate of Ca2+ uptake at 200 nm [Ca2+] (1.9 ± 0.1 vs. 1.8 ± 0.1 μmol Ca2+ (g protein min)−1; Fig. 2D). At time of fatigue, the rate of Ca2+ uptake at 400 nm [Ca2+] was reduced (P < 0.05) compared with non-fatigue, with no difference between terbutaline (2.7 ± 0.2 μmol Ca2+ (g protein min)−1) and placebo (3.1 ± 0.2 μmol Ca2+ (g protein min)−1) (Fig. 2C). The rate of Ca2+ uptake at 200 nm [Ca2+] decreased (P < 0.05) at time of fatigue compared with non-fatigue, with no difference between terbutaline (1.4 ± 0.1 μmol Ca2+ (g protein min)−1) and placebo (1.5 ± 0.1 μmol Ca2+ (g protein min)−1) (Fig. 2D).
Na+-dependent Na+–K+-ATPase activity
The effects of treatment and fatigue on Na+–K+-ATPase activity at different concentrations of Na+ are presented Fig. 3. No differences were observed in Na+–K+-ATPase Vmax between treatments at non-fatigue, Vmax being 348 ± 43 and 388 ± 33 nmol ATP h−1 (mg protein)−1 with terbutaline and placebo, respectively. At time of fatigue, Na+–K+-ATPase Vmax was unchanged with terbutaline (354 ± 20 nmol ATP h−1 (mg protein)−1), whereas Vmax was lowered (P < 0.05) by 25% with placebo (293 ± 49 nmol ATP h−1 (mg protein)−1). Hill coefficients at non-fatigue and at time of fatigue were 1.7 and 1.7, respectively, with terbutaline, and 1.3 and 1.3, respectively, with placebo. With terbutaline, Km was 6.6 ± 1.5 mm at non-fatigue and 3.7 ± 0.4 mm at time of fatigue, whereas it was 4.7 ± 0.9 mm at non-fatigue and 9.1 ± 3.6 mm at time of fatigue with placebo, with no differences between treatment and time.
Figure 3. Na+-dependent Na+–K+-ATPase activity with terbutaline and placebo.
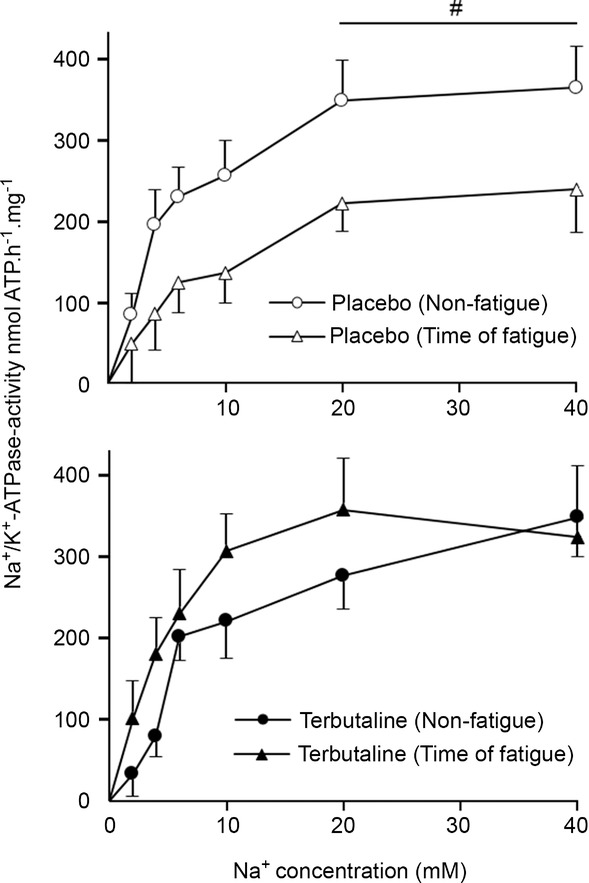
Na+-stimulated Na+–K+-ATPase activity was determined by measuring ATP hydrolysis in muscle homogenate from m. vastus lateralis at non-fatigue and at time of fatigue after three bouts of 4 min exercise at 75% of 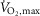 and a bout to fatigue at 120% of
and a bout to fatigue at 120% of 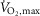 with terbutaline and placebo in EXP1 (n = 10). Data are presented as means = ± SEM. #Different (P < 0.05) from time of fatigue.
with terbutaline and placebo in EXP1 (n = 10). Data are presented as means = ± SEM. #Different (P < 0.05) from time of fatigue.
Phosphorylation of PLMSer68
No differences were observed in phosphorylation of PLMSer68 between treatments at non-fatigue and at time of fatigue, although a slight tendency (P = 0.11) towards an increase in phosphorylation (40 ± 26%) was observed with terbutaline compared with placebo at non-fatigue. At time of fatigue, phosphorylation of PLMSer68 was increased (P < 0.05) by 44 ± 19% with placebo compared with non-fatigue, whereas no difference was observed with terbutaline.
Muscle glycogen and lactate
Muscle glycogen was not different between treatments at non-fatigue (terbutaline vs. placebo: 476 ± 47 vs. 462 ± 52 mmol (kg dry wt)−1). Net muscle glycogen breakdown was 39 ± 13% higher (P < 0.05) during exercise with terbutaline than placebo (Fig. 4A). Muscle lactate was not different between treatments at non-fatigue, whereas net muscle lactate accumulation was 14 ± 6% higher (P < 0.05) with terbutaline than placebo at time of fatigue (Fig. 4B).
Figure 4. Net glycogen breakdown and lactate accumulation with terbutaline and placebo.
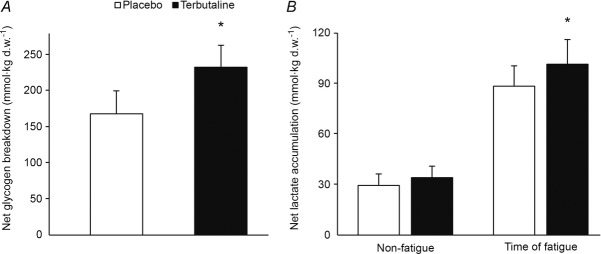
Measured fluorometrically in muscle homogenate from m. vastus lateralis. A, net glycogen breakdown (mmol (kg dry wt)−1) after three bouts of 4 min exercise at 75% of 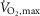 and a bout to fatigue at 120% of
and a bout to fatigue at 120% of 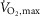 with terbutaline and placebo in EXP1 (n = 8). B, muscle lactate concentrations (mmol (kg dry wt)−1) before (non-fatigue) and after (time of fatigue) three bouts of 4 min exercise at 75% of
with terbutaline and placebo in EXP1 (n = 8). B, muscle lactate concentrations (mmol (kg dry wt)−1) before (non-fatigue) and after (time of fatigue) three bouts of 4 min exercise at 75% of 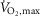 and a bout to fatigue at 120% of
and a bout to fatigue at 120% of 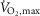 with terbutaline and placebo in EXP1 (n = 9). Data are presented as means = ± SEM. *Different (P < 0.05) from placebo.
with terbutaline and placebo in EXP1 (n = 9). Data are presented as means = ± SEM. *Different (P < 0.05) from placebo.
Discussion
The major findings of the present study were that β2-adrenergic stimulation with high dose selective β2-adrenoceptor agonist terbutaline enhanced SR Ca2+ handling and contractile properties of non-fatigued m. quadriceps, and counteracted exercise-induced reductions in Na+–K+-ATPase Vmax. Furthermore, that the enhancements in SR Ca2+ handling and contractile properties induced by stimulation with terbutaline where blunted as fatigue developed. In addition, β2-adrenergic stimulation elevated net muscle glycogen breakdown and net muscle lactate accumulation during exercise.
Contractile properties of m. quadriceps and SR Ca2+ handling at non-fatigue
In the present study, acute stimulation of β2-adrenoceptors with terbutaline modulated contractile properties of non-fatigued m. quadriceps, inducing a positive inotropic effect (i.e. increased MVC and peak twitch force) that was associated with an enhanced rate of Ca2+ release. While the association between increased contractile force and higher Ca2+ release are well described in animal models following β2-adrenergic stimulation (Cairns & Dulhunty, 1993a,b1993b; Andersson et al. 2012), the present study is the first to describe it in humans. Our observations of a 15 and 11% increase in Ca2+ release and peak twitch force, respectively, with terbutaline are comparable with those observed in animal models (Andersson et al. 2012). These effects are likely to be mediated by PKA-dependent phosphorylation of the Ca2+ release channel, RyR1, increasing its opening probability for subsequent release of Ca2+ and thus elevating the binding of Ca2+ on troponin C (Suko et al. 1993; Andersson et al. 2012). Accordingly, Andersson et al. (2012) observed that a phosphorylation site of PKA on RyR1Ser2844A was required for the increases in Ca2+ release and contractile force associated with β2-adrenergic stimulation. Moreover, since we measured the rate of Ca2+ release in an in vitro assay controlled for pH and other ionic and metabolic factors, the increased rate of Ca2+ release observed with terbutaline could only occur if phosphorylation of SR proteins remained in the muscle homogenate. Furthermore, increased Ca2+ availability in the SR induced by stimulatory actions of terbutaline on Ca2+ uptake could not account for the enhanced rate of Ca2+ release since release of Ca2+ was triggered when the [Ca2+] in the muscle homogenate had reached a plateau (nadir) which was similar between treatments. Given that skeletal muscle Ca2+ sensitivity has been shown to be unaffected by cAMP-dependent PKA activation (Fabiato & Fabiato, 1978; Cairns et al. 1993; Ha et al. 1999) and that β2-adrenergic stimulation is without an effect on the degree of voluntary activation, as evidenced in this and other studies (Decorte et al. 2008; Crivelli et al. 2011), our observations of increased contractile force of m. quadriceps with terbutaline may be attributed to peripheral mechanisms related to the SR system, presumably to an enhanced rate of Ca2+ release mediated by PKA-dependent phosphorylation of RyR1.
Although our observations of augmented contractile force of non-fatigued m. quadriceps are consistent with observations from isolated muscles in rodents (Van Der Heijden et al. 1998; Andersson et al. 2012), most studies in humans show no positive inotropic effect of β2-adrenergic stimulation on contractile force of m. quadriceps and m. soleus (Decorte et al. 2008; Crivelli et al. 2011, 2013). Consequently, it has been debated whether the observed inotropic effects of β2-adrenergic stimulation on isolated skeletal muscles of rodents also apply to humans. However, the discrepancy could be attributed to the dose of β2-adrenoceptor agonist used, since inotropic effects of β2-adrenergic stimulation on skeletal muscles have been shown to be dose dependent (Cairns & Dulhunty, 1993b; Prakash et al. 1999). The doses administered in the present study were well-above the therapeutic range administered in previous human studies (Crivelli et al. 2013; Crivelli & Maffiuletti, 2014), and though the systemic concentrations of terbutaline observed (0.08-0.10 μm) were below those used in most animal studies, they corresponded with the lowest concentration of terbutaline (0.10 μm) shown by Cairns & Dulhunty (1993b) to augment force. Our observations of increased contractile force in two different populations of trained subjects (EXP1 and EXP2) and with two different dosing regimens are also consistent with Kalsen et al. (2014a) who found that supratherapeutic inhalation of three β2-adrenoceptor agonists increased isometric muscle force in elite swimmers. Likewise, van Baak et al. (2000) observed that oral salbutamol increased the isokinetic muscle strength of active men at a dose that was equivalent to that shown by van der Heijden et al. (1998) to augment contractility of isolated rat diaphragm muscle. Although the discrepancies in human studies also may be related to differences in training status and fibre type composition among subjects (Bowman & Zaimis, 1958; Al-Jeboory & Marshall, 1978; Cairns & Dulhunty, 1993b), the present study suggests that β2-adrenergic stimulation causes positive inotropy in humans on a par with that observed in other species (Bowman & Zaimis, 1958; Cairns & Dulhunty, 1993b; Andersson et al. 2012).
Consistent with previous studies (Crivelli et al. 2011; Crivelli & Maffiuletti, 2014), we observed a positive lusitropic effect (shortened half-relaxation time) of β2-adrenergic stimulation on non-fatigued m. quadriceps. The lusitropic effect of β2-adrenergic stimulation on rate of force relaxation has been shown to be attributed to β2-adrenergic effects on the rate of SR Ca2+ uptake in animal models, which is why our observations of increased rate of Ca2+ uptake at 400 nm [Ca2+] with terbutaline may explain the shorter half-relaxation time of non-fatigued m. quadriceps in humans. Notably, we observed no effect of β2-adrenergic stimulation on the rate of Ca2+ uptake at 200 nm [Ca2+]. However, since the rate of Ca2+ uptake measured at 400 nm [Ca2+] was near SR Ca2+-ATPase Km for Ca2+ where changes in the affinity induced by phosphorylation of phospholamban will have a more pronounced effect on the rate of Ca2+ uptake, this may be why no effect was observed at 200 nm [Ca2+], which was near resting [Ca2+] of 30 nm (Westerblad & Allen, 1993a,b1993b). Although the lusitropic effect observed was lower than that reported in previous studies in animals, the 14 ± 2% shorter half-relaxation time with terbutaline in the present study corresponded with the ≈11% shorter half-relaxation time of m. quadriceps observed by Crivelli et al. (2011) following β2-adrenergic stimulation with salbutamol in men. The discrepancies in the β2-adrenergic response on the rate of relaxation that exist in the literature may be related to the muscle investigated, since β2-adrenergic effects on the rate of relaxation are most pronounced in slow-twitch fibres (Cairns & Dulhunty, 1993b). This also seems to be the case in humans where Crivelli et al. observed that the β2-adrenergic effect on the rate of relaxation was higher in m. soleus than m. quadriceps (Crivelli et al. 2011, 2013). The fibre-specific differences are likely to be linked to the expression of phospholamban in slow-twitch fibres, as PKA-dependent phosphorylation of phospholamban augments the SR Ca2+-ATPase (Lindemann et al. 1983; Slack et al. 1997).
Contractile properties of m. quadriceps and SR Ca2+ handling during fatigue
Despite enhanced contractile properties of m. quadriceps and SR Ca2+ handling at non-fatigue, we observed no effect of terbutaline on time to fatigue during exercise at 120% of 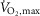 . This observation is inconsistent with previous studies showing increased time to fatigue during submaximal exercise in humans (Collomp et al. 2000; van Baak et al. 2000) and fatigue-resilient effects in animal models in vitro (Juel, 1988; Cairns & Dulhunty, 1994). It has been suggested that side-effects related to high dose β2-adrenoceptor agonist may blunt potential ergogenic effects on exercise performance (van Baak et al. 2000; Sanchez et al. 2013). However, the high dose administered in the present study only gave minor side-effects (tremor and tachycardia) and we found no association between side-effects and time to fatigue. Furthermore, since the duration of action of terbutaline is up to 6 h and its half-life is 11–16 h, we do not believe the lack of an effect of terbutaline on time to fatigue at 120% of
. This observation is inconsistent with previous studies showing increased time to fatigue during submaximal exercise in humans (Collomp et al. 2000; van Baak et al. 2000) and fatigue-resilient effects in animal models in vitro (Juel, 1988; Cairns & Dulhunty, 1994). It has been suggested that side-effects related to high dose β2-adrenoceptor agonist may blunt potential ergogenic effects on exercise performance (van Baak et al. 2000; Sanchez et al. 2013). However, the high dose administered in the present study only gave minor side-effects (tremor and tachycardia) and we found no association between side-effects and time to fatigue. Furthermore, since the duration of action of terbutaline is up to 6 h and its half-life is 11–16 h, we do not believe the lack of an effect of terbutaline on time to fatigue at 120% of 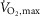 was due to the 70 min period between administration of the study drug and time to fatigue measurements. A more likely possibility relates to our observations that terbutaline increased muscle glycogen breakdown and lactate accumulation, and that the positive inotropic and lusitropic effects of terbutaline were blunted when fatigue developed. As such, MVC and rates of SR Ca2+ uptake and release declined to similar levels as placebo with fatigue, and in addition, peak twitch force and half-relaxation time were respectively lower and longer with terbutaline in fatigued m. quadriceps compared with placebo. These observations partly corresponds with the findings by Hostrup et al. (2014a) showing that salbutamol only enhanced peak power during the first sprint in a repeated Wingate test with three repetitions (Hostrup et al. 2014a). Furthermore, Crivelli & Maffiuletti (2014) also observed that the positive lusitropic effect of β2-adrenergic stimulation was blunted with fatigue. So while β2-adrenergic stimulation elicits positive inotropic and lusitropic effects in the early stages of exercise, these effects are blunted when exercise progresses and fatigue develops.
was due to the 70 min period between administration of the study drug and time to fatigue measurements. A more likely possibility relates to our observations that terbutaline increased muscle glycogen breakdown and lactate accumulation, and that the positive inotropic and lusitropic effects of terbutaline were blunted when fatigue developed. As such, MVC and rates of SR Ca2+ uptake and release declined to similar levels as placebo with fatigue, and in addition, peak twitch force and half-relaxation time were respectively lower and longer with terbutaline in fatigued m. quadriceps compared with placebo. These observations partly corresponds with the findings by Hostrup et al. (2014a) showing that salbutamol only enhanced peak power during the first sprint in a repeated Wingate test with three repetitions (Hostrup et al. 2014a). Furthermore, Crivelli & Maffiuletti (2014) also observed that the positive lusitropic effect of β2-adrenergic stimulation was blunted with fatigue. So while β2-adrenergic stimulation elicits positive inotropic and lusitropic effects in the early stages of exercise, these effects are blunted when exercise progresses and fatigue develops.
The lack of an effect of β2-adrenergic stimulation on contractile properties and SR Ca2+ handling with fatigue in the present study may be attributed to several factors related to muscle fatigue. Firstly, it may reflect a protective mechanism to prevent metabolic catastrophe and ATP depletion by which SR Ca2+ handling function and force production are attenuated (MacIntosh et al. 1983, 2012; Allen et al. 2008; MacIntosh & Shahi, 2011), and though β2-adrenergic stimulation benefits muscle force production and relaxation in the early stages of exercise, it also elevates ATP utilization by myosin-ATPase and Ca2+-ATPase. Secondly, SR [Ca2+] decreases substantially during tetanic contractions (Rudolf et al. 2006) and higher amplitudes of Ca2+ transients in the initial phase of exercise induced by β2-adrenergic stimulation might disrupt Ca2+ handling later on. This is also supported by the opposite phenomenon that the RyR blocker dantrolene increases fatigue resistance despite the fact that dantrolene reduces Ca2+ release and peak tetanic force in non-fatigued muscles (Seebacher et al. 2012). Thirdly, our observations of reduced SR Ca2+ handling function may also reflect critically low muscle glycogen content, since studies in rodent single fibres and humans have pointed to a modulating role of glycogen availability and glycolytic flux on SR Ca2+ handling (Ørtenblad et al. 2011; Nielsen et al. 2014). The increase in glycogen breakdown observed with terbutaline might therefore partly explain the large decrease in rate of Ca2+ uptake and slowing of relaxation with fatigue (Westerblad & Allen, 1993a). Moreover, although we did not measure muscle pH, the higher muscle lactate accumulation with fatigue with terbutaline was probably associated with a lowered pH (Sahlin et al. 1976), which may affect relaxation time (Westerblad & Allen, 1993b; Westerblad et al. 1997). While muscle lactate itself does not seem to play a significant role in muscle fatigue (Allen et al. 2008), elevated lactate production induced by β2-adrenergic stimulation may evoke a larger sensation of pain associated with muscle fatigue (Pollak et al. 2014), hence affecting performance. Nevertheless, the numerous metabolic and ionic factors involved in muscle fatigue makes it difficult to determine the exact cause by which the effects of β2-adrenergic stimulation were blunted with fatigue in the present study.
Na+–K+-ATPase activity at non-fatigue and at time of fatigue
To our knowledge, the present study is the first to investigate the effect of β2-adrenergic stimulation on skeletal muscle Na+–K+-ATPase activity in humans before and after exercise to fatigue. In isolated muscles of rodents, β2-adrenergic stimulation increases uptake of K+ via stimulation of the Na+–K+-ATPase mediated by a higher affinity for Na+ (reduced Km; Kockskamper et al. 2000; Clausen, 2003, 2008). Although we observed a non-significant (P = 0.11) increase (40%) in phosphorylation of PLMSer68 by β2-adrenergic stimulation at non-fatigue, Km for Na+ was unchanged compared with placebo. In contrast to our observations, Juel et al. (2014) observed increased Na+–K+-ATPase activity at 5 mm Na+ in rat and human muscle membranes upon addition of cAMP (1 mm). However, in that study the Na+–K+-ATPase was stimulated by addition of cAMP into the assay in vitro (Juel et al. 2014) and not by administration of a β2-adrenoceptor agonist as in the present study. The inconsistency may also be related to differences in the time when biopsies were sampled, as the non-fatigue biopsies were taken following a warm-up and MVC measurements in the present study and not at rest. It may be that the warm-up and MVC induced a high basal phosphorylation of PLMSer68 at non-fatigue. Nevertheless, other effects of β2-adrenergic stimulation can affect Na+–K+-ATPase and studies in isolated cardiac myocytes suggest that cAMP signalling also may inhibit Na+–K+-ATPase through glutathionylation of the β1 subunit (White et al. 2010; Galougahi et al. 2013). Moreover, higher amplitudes of Ca2+ transients induced by β2-adrenergic stimulation might inhibit Na+–K+-ATPase, since pulsatile increases in intracellular [Ca2+] have been linked to a reduction in Na+–K+-ATPase activity (Sulova et al. 1998). Thus, β2-adrenergic stimulation affects Na+–K+-ATPase in several ways, which may explain why we observed no effects at non-fatigue.
A common feature of fatiguing exercise is exercise-induced reductions in Na+–K+-ATPase activity (Leppik et al. 2004; McKenna et al. 2008). Given that the content of Na+–K+-ATPase is unchanged with fatiguing exercise, exercise-induced reductions in Na+–K+-ATPase activity have been referred to as Na+–K+-ATPase inactivation which seems to be an outcome of reactive oxidative species (oxidative stress), known to reduce Na+–K+-ATPase activity in myocardial cells and in skeletal muscles (McKenna et al. 2006, 2008; Figtree et al. 2009). Interestingly, we observed that β2-adrenergic stimulation counteracted exercise-induced reductions in Na+–K+-ATPase Vmax, whereas Vmax declined by 25% with placebo. While it seems apparent that a reduction in Na+–K+-ATPase function with placebo would be detrimental to performance by decreasing re-uptake of contraction-induced accumulation of extracellular K+, time to fatigue at 120% of 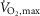 was not different between treatments. Therefore, from the present observations it would not seem that Na+–K+-ATPase Vmax is a good marker of performance, at least during intense exercise at 120% of
was not different between treatments. Therefore, from the present observations it would not seem that Na+–K+-ATPase Vmax is a good marker of performance, at least during intense exercise at 120% of 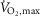 . Still our observations only describe this phenomenon in vitro and functional aspects of Na+–K+-ATPase inactivation in vivo are less clear.
. Still our observations only describe this phenomenon in vitro and functional aspects of Na+–K+-ATPase inactivation in vivo are less clear.
While the large reduction in Vmax at time of fatigue with placebo has been reported in several previous studies (McKenna et al. 2006, 2008), it is more difficult to explain the lack of change in Vmax with terbutaline at time of fatigue. This cannot be explained by Na+–K+-ATPase content, since we observed no changes in the relative protein content or in Na+–K+-ATPase subunit α1 expression between treatments and time points. Furthermore, Na+–K+-ATPase α-isoform mRNA and protein expression were recently shown to be unaffected by β2-adrenergic stimulation (Yin et al. 2014). On the other hand, recent observations by Yin et al. (2014) suggest that short-term stimulation of β2-adrenoceptors modulate the translocation of available Na+–K+-ATPase α-isoforms to the plasma membrane in cardiac myocytes, thus altering the content of functional plasma membrane-localised Na+–K+-ATPases (Yin et al. 2014). Therefore, it may be that β2-adrenergic stimulation counteracts exercise-induced reductions in Vmax by modulation of the content of functional membrane-localised Na+–K+-ATPases, thus counteracting Na+–K+-ATPase inactivation mediated by oxidative stress. Nonetheless, the role of β2-adrenergic stimulation in the regulation of Na+–K+-ATPase activity during exercise needs to be investigated further before such conclusions can be drawn. Indeed, other mechanisms may involve differences in the phosphorylation status of the various subunits of the Na+–K+-ATPase and other metabolic pathways affected by β2-adrenergic stimulation.
Perspectives
While β2-adrenergic stimulation apparently elicits positive inotropy and lusitropy in skeletal muscles, the functional implications for exercise performance are not entirely clear. β 2-Adrenergic stimulation has been shown to enhance sprint performance (Collomp et al. 2005; Hostrup et al. 2014a), which is not surprising as the positive inotropic and lusitropic effects would benefit force production and rate of relaxation, both of which are linked with sprint ability. On the other hand, most studies including the present, show no effects of β2-adrenergic stimulation on time to fatigue during exercise lasting more than 2 min (Sanchez et al. 2013; Hostrup et al. 2014a; Kalsen et al. 2014b), which contrasts with the fatigue-resilient effects observed in animal models during repeated muscle stimulations (Juel, 1988; Cairns & Dulhunty, 1994). This discrepancy may be explained by the state of muscle fatigue in animal models compared with humans. Fatigue-resilient effects of β2-adrenergic stimulation are most pronounced when fatigue is severe (>50% drop in tetanic force; Juel, 1988; Cairns & Dulhunty, 1994), whereas the state of muscle fatigue in humans rarely reach this level (19% drop in MVC with placebo in the present study). Furthermore, pharmacological manipulation targets all tissues in the human body and effects of β2-adrenergic stimulation on other tissues may affect whole-body exercise performance, whereas animal models usually investigate isolated muscles removed from their normal biological system. The exact role of β2-adrenergic stimulation on ion handling and fatigue in humans should therefore be elaborated in future studies.
Glossary
- cAMP
cyclic adenosine monophosphate
- EXP1
experiment 1
- EXP2
experiment 2
- MVC
maximal voluntary contraction
- PKA
protein kinase A
- RyR1
ryanodine receptor isoform 1
- SR
sarcoplasmic reticulum
Additional information
Competing interests
None declared.
Author contributions
The experiments were performed at the Department of Nutrition, Exercise and Sports, University of Copenhagen. All authors contributed to the conception and design of the experiments, collection, analysis and interpretation of data, and drafting the manuscript or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
Funding
The study was supported by grants from the Danish Ministry of Culture and the World Anti-doping Agency.
References
- Al-Jeboory AA, Marshall RJ. Correlation between the effects of salbutamol on contractions and cyclic AMP content of isolated fast-and slow-contracting muscles of the guinea pig. Naunyn-Schmiedeberg's Arch Pharmacol. 1978;305:201–206. doi: 10.1007/BF00498811. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Andersson DC, Betzenhauser MJ, Reiken S, Umanskaya A, Shiomi T, Marks AR. Stress-induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor. J Physiol. 2012;590:6381–6387. doi: 10.1113/jphysiol.2012.237925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WC, Zaimis E. The effects of adrenaline, noradrenaline and isoprenaline on skeletal muscle contractions in the cat. J Physiol. 1958;144:92–107. doi: 10.1113/jphysiol.1958.sp006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Dulhunty AF. Beta-adrenergic potentiation of E–C coupling increases force in rat skeletal muscle. Muscle Nerve. 1993a;16:1317–1325. doi: 10.1002/mus.880161208. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Dulhunty AF. The effects of beta-adrenoceptor activation on contraction in isolated fast- and slow-twitch skeletal muscle fibres of the rat. Br J Pharmacol. 1993b;110:1133–1141. doi: 10.1111/j.1476-5381.1993.tb13932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Dulhunty AF. Beta-adrenoceptor activation shows high-frequency fatigue in skeletal muscle fibers of the rat. Am J Physiol Cell Physiol. 1994;266:C1204–C1209. doi: 10.1152/ajpcell.1994.266.5.C1204. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Westerblad H, Allen DG. Changes of tension and [Ca2+]i during beta-adrenoceptor activation of single, intact fibres from mouse skeletal muscle. Pflugers Arch. 1993;425:150–155. doi: 10.1007/BF00374515. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol. 1997;498:17–29. doi: 10.1113/jphysiol.1997.sp021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. Na+–K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T. Clearance of extracellular K+ during muscle contraction–roles of membrane transport and diffusion. J Gen Physiol. 2008;131:473–481. doi: 10.1085/jgp.200809971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na–K-transport in rat soleus muscle. Br J Pharmacol. 1980;68:749–755. doi: 10.1111/j.1476-5381.1980.tb10868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collomp K, Candau R, Collomp R, Carra J, Lasne F, Prefaut C, De Ceaurriz J. Effects of acute ingestion of salbutamol during submaximal exercise. Int J Sports Med. 2000;21:480–484. doi: 10.1055/s-2000-7422. [DOI] [PubMed] [Google Scholar]
- Collomp K, Candau R, Millet G, Mucci P, Borrani F, Prefaut C, De Ceaurriz J. Effects of salbutamol and caffeine ingestion on exercise metabolism and performance. Int J Sports Med. 2002;23:549–554. doi: 10.1055/s-2002-35530. [DOI] [PubMed] [Google Scholar]
- Collomp K, Le Panse B, Portier H, Lecoq AM, Jaffre C, Beaupied H, Richard O, Benhamou L, Courteix D, De Ceaurriz J. Effects of acute salbutamol intake during a Wingate test. Int J Sports Med. 2005;26:513–517. doi: 10.1055/s-2004-821223. [DOI] [PubMed] [Google Scholar]
- Crivelli G, Borrani F, Capt R, Gremion G, Maffiuletti NA. Actions of β2-adrenoceptor agonist drug on human soleus muscle contraction. Med Sci Sports Exerc. 2013;45:1252–1260. doi: 10.1249/MSS.0b013e318284706a. [DOI] [PubMed] [Google Scholar]
- Crivelli G, Maffiuletti NA. Actions of β2-adrenoceptor agonist drug on neuromuscular function after fatigue. Med Sci Sports Exerc. 2014;46:247–256. doi: 10.1249/MSS.0b013e3182a54ee3. [DOI] [PubMed] [Google Scholar]
- Crivelli G, Millet GP, Gremion G, Borrani F. Effects of salbutamol on the contractile properties of human skeletal muscle before and after fatigue. Acta Physiol. 2011;203:311–320. doi: 10.1111/j.1748-1716.2011.02302.x. [DOI] [PubMed] [Google Scholar]
- Decorte N, Verges S, Flore P, Guinot M, Wuyam B. Effects of acute salbutamol inhalation on quadriceps force and fatigability. Med Sci Sports Exerc. 2008;40:1220–1227. doi: 10.1249/MSS.0b013e31816b87aa. [DOI] [PubMed] [Google Scholar]
- Duhamel TA, Perco JG, Green HJ. Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1100–R1110. doi: 10.1152/ajpregu.00858.2005. [DOI] [PubMed] [Google Scholar]
- Elers J, Hostrup M, Pedersen L, Henninge J, Hemmersbach P, Dalhoff K, Backer V. Urine and serum concentrations of inhaled and oral terbutaline. Int J Sports Med. 2012;33:1026–1033. doi: 10.1055/s-0032-1311590. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Cyclic AMP-induced enhancement of calcium accumulation by the sarcoplasmic reticulum with no modification of the sensitivity of the myofilaments to calcium in skinned fibres from a yeast skeletal muscle. Biochim Biophys Acta. 1978;539:253–260. doi: 10.1016/0304-4165(78)90012-0. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J Appl Physiol. 1998;84:465–470. doi: 10.1152/jappl.1998.84.2.465. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Liu CC, Bibert S, Hamilton EJ, Garcia A, White CN, Chia KK, Cornelius F, Geering K, Rasmussen HH. Reversible oxidative modification: a key mechanism of Na+–K+ pump regulation. Circ Res. 2009;105:185–193. doi: 10.1161/CIRCRESAHA.109.199547. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Galougahi KK, Liu CC, Garcia A, Fry NA, Hamilton EJ, Rasmussen HH, Figtree GA. Protein kinase-dependent oxidative regulation of the cardiac Na+–K+ pump: evidence from in vivo and in vitro modulation of cell signalling. J Physiol. 2013;591:2999–3015. doi: 10.1113/jphysiol.2013.252817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TN, Posterino GS, Fryer MW. Effects of terbutaline on force and intracellular calcium in slow-twitch skeletal muscle fibres of the rat. Br J Pharmacol. 1999;126:1717–1724. doi: 10.1038/sj.bjp.0702482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Gladden LB, Kurdak SS, Poole DC. Increased [lactate] in working dog muscle reduces tension development independent of pH. Med Sci Sports Exerc. 1995;27:371–377. [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Auchenberg M, Bangsbo J, Backer V. Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J Med Sci Sports. 2014a doi: 10.1111/sms.12298. DOI: 10.1111/sms.12298. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Bangsbo J, Hemmersbach P, Karlsson S, Backer V. High-dose inhaled terbutaline increases muscle strength and enhances maximal sprint performance in trained men. Eur J Appl Physiol. 2014b doi: 10.1007/s00421-014-2970-2. DOI: 10.1007/s00421-014-2970-2. [DOI] [PubMed] [Google Scholar]
- Juel C. The effect of beta 2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiol Scand. 1988;134:209–216. doi: 10.1111/j.1748-1716.1988.tb08481.x. [DOI] [PubMed] [Google Scholar]
- Juel C. Na+–K+-ATPase in rat skeletal muscle: muscle fiber-specific differences in exercise-induced changes in ion affinity and maximal activity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R125–132. doi: 10.1152/ajpregu.90760.2008. [DOI] [PubMed] [Google Scholar]
- Juel C, Nordsborg NB, Bangsbo J. Exercise-induced increase in maximal in vitro Na–K-ATPase activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1161–R1165. doi: 10.1152/ajpregu.00591.2012. [DOI] [PubMed] [Google Scholar]
- Juel C, Nordsborg NB, Bangsbo J. Purinergic effects on Na,K-ATPase activity differ in rat and human skeletal muscle. PLoS One. 2014;9:e91175. doi: 10.1371/journal.pone.0091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsen A, Hostrup M, Bangsbo J, Backer V. Combined inhalation of beta2 -agonists improves swim ergometer sprint performance but not high-intensity swim performance. Scand J Med Sci Sports. 2014a;24:814–822. doi: 10.1111/sms.12096. [DOI] [PubMed] [Google Scholar]
- Kalsen A, Hostrup M, Karlsson S, Hemmersbach P, Bangsbo J, Backer V. Effect of inhaled terbutaline on substrate utilization and 300-kcal time trial performance. J Appl Physiol. 2014b doi: 10.1152/japplphysiol.00635.2014. DOI: 10.1152/japplphysiol.00635.2014. [DOI] [PubMed] [Google Scholar]
- Kockskamper J, Erlenkamp S, Glitsch HG. Activation of the cAMP-protein kinase A pathway facilitates Na+ translocation by the Na+–K+ pump in guinea-pig ventricular myocytes. J Physiol. 2000;523:561–574. doi: 10.1111/j.1469-7793.2000.t01-2-00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M, Albertsen J, Rentsch M, Juel C. Lactate and force production in skeletal muscle. J Physiol. 2005;562:521–526. doi: 10.1113/jphysiol.2004.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+–K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. β-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJ. The roles of ion fluxes in skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:246–253. doi: 10.1139/y91-038. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis, New York, USA: Academic Press; 1972. A collection of metabolite assays; pp. 146–218. [Google Scholar]
- MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue – regulation of excitation–contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125:2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Shahi MR. A peripheral governor regulates muscle contraction. Appl Physiol Nutr Metab. 2011;36:1–11. doi: 10.1139/H10-073. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR, Stainsby WN, Gladden LB. Fatigue from incompletely fused tetanic contractions in skeletal muscle in situ. J Appl Physiol. 1983;55:976–982. doi: 10.1152/jappl.1983.55.3.976. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl disturbances and Na+–K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-Acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Cheng AJ, Ortenblad N, Westerblad H. Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol. 2014;592:2003–2012. doi: 10.1113/jphysiol.2014.271528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B, Holmberg HC. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol. 2011;589:711–725. doi: 10.1113/jphysiol.2010.195982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place N, Maffiuletti NA, Martin A, Lepers R. Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve. 2007;35:486–495. doi: 10.1002/mus.20714. [DOI] [PubMed] [Google Scholar]
- Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol. 2014;99:368–380. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, van der Heijden HF, Gallant EM, Sieck GC. Effect of beta-adrenoceptor activation on [Ca2+]i regulation in murine skeletal myotubes. Am J Physiol Cell Physiol. 1999;276:C1038–1045. doi: 10.1152/ajpcell.1999.276.5.C1038. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Summers RJ. Cyclic AMP accumulation in rat soleus muscle: stimulation by β2- but not β3-adrenoceptors. Eur J Pharmacol. 1998;348:53–60. doi: 10.1016/s0014-2999(98)00021-1. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Rudolf R, Magalhaes PJ, Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Borrani F, Le Fur MA, Le Mieux A, Lecoultre V, Py G, Gernigon C, Collomp K, Candau R. Acute supra-therapeutic oral terbutaline administration has no ergogenic effect in non-asthmatic athletes. Eur J Appl Physiol. 2013;113:411–418. doi: 10.1007/s00421-012-2447-0. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Pollard SR, James RS. How well do muscle biomechanics predict whole-animal locomotor performance? The role of Ca2+ handling. J Exp Biol. 2012;215:1847–1853. doi: 10.1242/jeb.067918. [DOI] [PubMed] [Google Scholar]
- Slack JP, Grupp IL, Luo W, Kranias EG. Phospholamban ablation enhances relaxation in the murine soleus. Am J Physiol Cell Physiol. 1997;273:C1–6. doi: 10.1152/ajpcell.1997.273.1.C1. [DOI] [PubMed] [Google Scholar]
- Suko J, Maurer-Fogy I, Plank B, Bertel O, Wyskovsky W, Hohenegger M, Hellmann G. Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim Biophys Acta. 1993;1175:193–206. doi: 10.1016/0167-4889(93)90023-i. [DOI] [PubMed] [Google Scholar]
- Sulova Z, Vyskocil F, Stankovicova T, Breier A. Ca2+-induced inhibition of sodium pump: effects on energetic metabolism of mouse diaphragm tissue. Gen Physiol Biophys. 1998;17:271–283. [PubMed] [Google Scholar]
- van Baak MA, Mayer LH, Kempinski RE, Hartgens F. Effect of salbutamol on muscle strength and endurance performance in nonasthmatic men. Med Sci Sports Exerc. 2000;32:1300–1306. doi: 10.1097/00005768-200007000-00018. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden HF, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Salbutamol enhances isotonic contractile properties of rat diaphragm muscle. J Appl Physiol. 1998;85:525–529. doi: 10.1152/jappl.1998.85.2.525. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Howlett KF, Febbraio MA, Spriet LL, Hargreaves M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol. 2001;534:269–278. doi: 10.1111/j.1469-7793.2001.t01-1-00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993a;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993b;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CN, Liu CC, Garcia A, Hamilton EJ, Chia KK, Figtree GA, Rasmussen HH. Activation of cAMP-dependent signaling induces oxidative modification of the cardiac Na+–K+ pump and inhibits its activity. J Biol Chem. 2010;285:13712–13720. doi: 10.1074/jbc.M109.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Guo HC, Yu D, Wang HC, Li JX, Wang YL. Mechanisms of isoform-specific Na/K pump regulation by short- and long-term adrenergic activation in rat ventricular myocytes. Cell Physiol Biochem. 2014;33:1681–1697. doi: 10.1159/000362951. [DOI] [PubMed] [Google Scholar]