Abstract
The predominant isoform of β-adrenoceptor (β-AR) in skeletal muscle is β2-AR and that in the cardiac muscle is β1-AR. We have reported that Epac1 (exchange protein directly activated by cAMP 1), a new protein kinase A-independent cAMP sensor, does not affect cardiac hypertrophy in response to pressure overload or chronic isoproterenol (isoprenaline) infusion. However, the role of Epac1 in skeletal muscle hypertrophy remains poorly understood. We thus examined the effect of disruption of Epac1, the major Epac isoform in skeletal muscle, on masseter muscle hypertrophy induced by chronic β2-AR stimulation with clenbuterol (CB) in Epac1-null mice (Epac1KO). The masseter muscle weight/tibial length ratio was similar in wild-type (WT) and Epac1KO at baseline and was significantly increased in WT after CB infusion, but this increase was suppressed in Epac1KO. CB treatment significantly increased the proportion of myosin heavy chain (MHC) IIb at the expense of that of MHC IId/x in both WT and Epac1KO, indicating that Epac1 did not mediate the CB-induced MHC isoform transition towards the faster isoform. The mechanism of suppression of CB-mediated hypertrophy in Epac1KO is considered to involve decreased activation of Akt signalling. In addition, CB-induced histone deacetylase 4 (HDAC4) phosphorylation on serine 246 mediated by calmodulin kinase II (CaMKII), which plays a role in skeletal muscle hypertrophy, was suppressed in Epac1KO. Our findings suggest that Epac1 plays a role in β2-AR-mediated masseter muscle hypertrophy, probably through activation of both Akt signalling and CaMKII/HDAC4 signalling.
Key Points
Epac (exchange protein directly activated by cyclic AMP (cAMP)), a PKA-independent cAMP sensor, plays important roles in multiple cellular processes, but its role in the pathogenesis of skeletal muscle hypertrophy and myosin heavy chain (MHC) transition is poorly understood.
Chronic stimulation of β2-adrenoceptor (β2-AR) with clenbuterol (CB), a selective β2-AR agonist, induced masseter muscle hypertrophy in wild-type (WT) mice, but not in Epac1-null mice (Epac1KO), even if slow-to-fast MHC isoform transition was similarly induced by CB treatment in both WT and Epac1KO.
Disruption of Epac1 inhibited development of masseter muscle hypertrophy concomitantly with decreased phosphorylation of Akt and its downstream molecules 70 kDa ribosomal S6 kinase 1 and eukaryotic initiation factor 4E-binding protein 1, and also, in parallel, glycogen synthase kinase-3β.
Disruption of Epac1 decreased histone deacetylase 4 (HDAC4) phosphorylation on serine 246 mediated by calmodulin kinase II (CaMKII), which plays a role in skeletal muscle hypertrophy.
We conclude that Epac1 induces β2-AR-mediated masseter muscle hypertrophy without influencing slow-to-fast MHC isoform transition, probably via activation of Akt and its downstream molecules and increase of CaMKII-mediated HDAC4 phosphorylation.
Introduction
For many decades, it was believed that the major target of cyclic AMP (cAMP) signaling is protein kinase A (PKA). Recently, exchange protein directly activated by cAMP (Epac) was identified as a new PKA-independent sensor. Epac has two isoforms (Epac1 and Epac2). Epac1 is ubiquitously expressed, including in skeletal muscle, and has a single cAMP-binding site, whereas Epac2 contains a second cAMP-binding site and is localized to brain and endocrine tissue (de Rooij et al. 1998; Kawasaki et al. 1998). We have recently demonstrated that Epac1 does not affect the development of cardiac hypertrophy in response to pressure-overload or chronic isoproterenol infusion, using Epac1-deficient mice (Epac1KO) (Okumura et al. 2014). However, the role of Epac1 in skeletal muscle hypertrophy remains poorly understood.
Although all three β-adrenergic receptor (β1-, β2-, and β3-AR) are expressed in the cytoplasmic membrane of skeletal muscle, β2-subtype is predominant, while β1-AR accounts for less than 10%, together with small populations of α-AR and β3-AR (Kim et al. 1991). In contrast to skeletal muscle, the predominant receptor subtype expressed in heart is β1-AR, together with approximately 20% β2-AR (Woo and Xiao, 2012). All adrenoceptors belong to the guanine nucleotide-binding G protein-coupled receptor family. The G protein-adenylyl cyclase-cAMP is the best characterized of the β2-AR signaling pathways and is generally thought to be responsible for β2-AR-mediated hypertrophy and increase of muscle strength with slow-to-fast myosin heavy chain (MHC) isoform transition in skeletal muscle (Ohnuki et al. 2013a). The hypertrophic response of skeletal muscle following treatment with a chronic β2-agonist such as clenbuterol (CB) is associated with an increase of protein synthesis, a decrease of protein degradation, or a combination of both mechanisms (Lynch and Ryall, 2008).
Unlike β1-AR, which couples only to Gsα, β2-AR also couples to pertussis toxin-sensitive Giα protein in skeletal muscle (Gosmanov et al. 2002). β2-AR-Giα signaling not only inhibits PKA activity, but also stimulates the Gβγ-mediated phosphoinositol 3-kinase (PI3K)-Akt and extracellular signaling regulated kinase 1/2 (ERK1/2) signaling pathway (Zhu et al. 2001; Shi et al. 2007). It was recently reported by us that Akt phosphorylation and subsequent activation of mammalian target of rapamycin (mTOR) are involved in masseter muscle hypertrophy and ERK1/2 phosphorylation exerts an opposing effects on mechanical-overload-induced masseter muscle hypertrophy (Umeki et al. 2013). It was recently reported that β-AR signaling activated Epac1 signaling in rat skeletal muscle and β-AR-induced Epac1 activation potentiated insulin-stimulated Akt phosphorylation on serine 473, as well as phosphorylation of its downstream factors 70-kDa ribosomal S6 kinase 1 (S6K1) on threonine 389 (Brennesvik et al. 2005). However, the role of Epac1 in skeletal muscle hypertrophy has not been examined yet. We thus hypothesized that Epac1, a major skeletal muscle isoform, might play a role in masseter muscle hypertrophy and MHC isoform transition by linking cAMP signaling and Akt signaling or ERK signaling, and we tested this hypothesis using Epac1KO (Okumura et al. 2014).
Methods
Mice and experimental protocols
We have previously reported the generation of Epac1KO (ACC. No. CDB0542K: http://www.cdb.riken.jp/arg/mutant%20mice%20list.htm) (Suzuki et al. 2010). All experiments were performed on C57BL/6 and CBA mixed-background homozygous Epac1KO (6.8 ± 0.3-month-old, n = 12) and their wild-type (WT) littermates (6.4 ± 0.4-month-old, n = 12). This study was approved by the Animal Care and Use Committees of Yokohama City University School of Medicine and Tsurumi University.
CB (Sigma, St. Louis, MO, USA) was dissolved in saline to prepare a 0.6 mg ml−1 stock solution and the appropriate volume of this solution to provide the desired dose (2 mg kg−1) was added to 0.2 ml of saline to prepare the solution for intraperitoneal (i.p.) injection (Pearen et al. 2009; Goodman et al. 2011). CB was administered i.p. once daily for 3 weeks, and control mice received an identical volume of saline only (Wong et al. 1998). (Fig. 1A). In order to minimize the adverse effects associated with repeated i.p. administrations such as infection, inflammation, pain, and adhesions within the abdominal cavity, we followed the recommended protocols for the intraperitoneal injection of mice (Turner et al. 2011a, 2011b; Machholz et al. 2012). Briefly, we used the recommended gauge and length of needle (22 gauge, 1 inch), scrubbed the injection site gently with 2% chlorhexidine-moistened cotton wool, prepared CB with the recommended volume of saline (maximum 10 ml kg−1), and changed the site of injection every time. In addition, body weight, food and water intake were monitored for all animals throughout the 3 weeks of the experimental period.
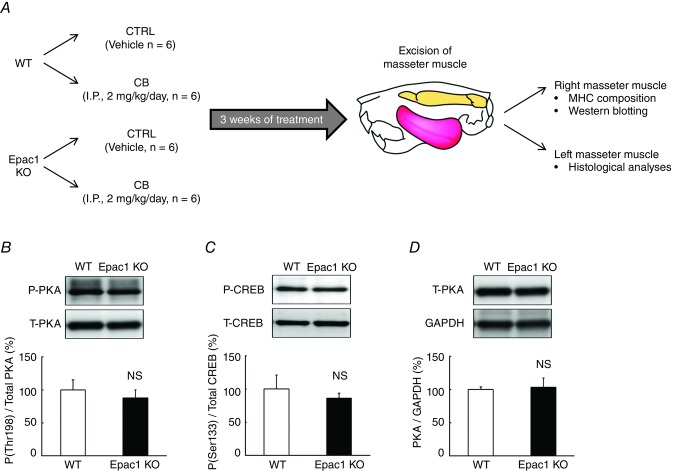
Figure 1. Experimental procedure and cAMP/PKA signaling in Epac1KO.
A, clenbuterol (CB) was administered once daily for 3 weeks via intraperitoneal injection (i.p.) at a dose of 2 mg/kg, dissolved in saline. Age-matched control mice (CTRL) received an identical volume of saline only. B-D, cAMP/PKA signaling in masseter muscle of Epac1KO was investigated by examining the expression of PKA-catalytic units phosphorylated at threonine (Thr) 198 (B), cAMP response element binding protein (CREB) phosphorylated on serine (Ser) 133 (C), and total PKA-catalytic units (D), using total homogenate prepared from the masseter muscle of Epac1KO and WT. No significant difference was observed between WT and Epac1KO at baseline (n = 6 each, P = NS (not significant) vs. WT by unpaired t test). The amount of expression in WT treated with saline was taken as 100% in each determination and representative immunoblotting results are shown for phosphorylated and total PKA-catalytic unit, phosphorylated and total CREB, and PKA-catalytic units and GAPDH.
The dose of CB used in this study has been reported to increase skeletal mass efficiently without affecting body weight (Ryall et al. 2002). After the completion of each treatment, mice were anaesthetized with isoflurane and the left and right masseter muscles were each excised and weighed, frozen in liquid nitrogen, and stored at −80ºC for later analysis. The muscle mass (mg) and the ratio of muscle mass to tibial length (mm) were used as indexes of muscle growth. After tissue extraction, the mice were killed by cervical dislocation (Goodman et al. 2011).
Western blotting
The right masseter muscle excised from the mice (Fig. 1A) was homogenized in a Polytron (Kinematica AG, Lucerne, Switzerland) in ice-cold RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA: 25 mm Tris-HCl (pH 7.6), 150 mm NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) without addition of inhibitors (Yu et al. 2011), and the homogenate was centrifuged at 13000 x g for 10 min at 4°C. The supernatant was collected and the protein concentration was measured using a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein (5 μg) were subjected to 12.5% SDS-polyacrylamide gel electrophoresis and blotted onto 0.2 mm PVDF membrane (Millipore, Billerica, MA, USA).
Western blotting was conducted with commercially available antibodies (Okumura et al. 2003a; Okumura et al. 2003b; Okumura et al. 2008; Okumura et al. 2009; Bai et al. 2012). The primary antibodies against CREB (#9197), phospho-CREB (Ser-133, #9198), Akt (#9272), phospho-Akt (Ser-473, #9271), S6K1 (#9202), phospho-S6K1 (Thr-389, #9205), 4E-BP1 (#9644), phospho-4E-BP1 (Thr-37/46, #2855), GSK-3β (#12456) phospho-GSK-3β (Ser-19, #5558), CaMKII (#3362), phospho-CaMKII (Thr-286, #3361), HDAC4 (#7628), phospho-HDAC4 (Ser-246, #3443), ERK1/2 (#4695), phospho-ERK1/2 (Thr-202/Tyr-204, #4370) were purchased from Cell Signaling Technology (Boston, MA, USA) and the primary antibodies against PKA-catalytic subunit (sc-903), phospho-PKA-catalytic subunit (Thr-198, sc-32968), GAPDH (sc-25778), NFATc1 (sc-13033), phospho-NFATc1 (Ser-259, sc-32979), NFATc3 (sc-8321), phospho-NFATc3 (Ser-265, sc-32982) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) (Lunde et al. 2011). Horseradish peroxidase-conjugated anti-rabbit IgG (GB Healthcare, NA934) was used as a secondary antibody. The primary and secondary antibodies were diluted in Tris-buffered saline (pH 7.6) with 0.1% Tween 20 and 5% bovine serum albumin. The blots were visualized with enhanced chemiluminescence solution (ECL Prime Western Blotting Detection Reagent, GE Healthcare, Piscataway, NJ, USA) and scanned with a densitometer (LAS-1000, Fuji Photo Film, Tokyo, Japan).
Diameter and cross-sectional area of muscle fibres
The left masseter muscle, excised as a whole (Fig. 1A), was embedded in Tissue-Tek OCT compound (Sakura Finetec, Torrance, CA, USA) in a slightly stretched state so as to maintain a length close to the resting length (L0), and stored it at -80°C until sectioning, as reported (Bruusgaard et al. 2012). Cross sections (10 μm thick) were cut from the middle portion of the left masseter muscle with a cryostat (CM1900, Leica Microsystems, Nussloch, Germany) at −20ºC. The section were air-dried and fixed with 4% paraformaldehyde in 0.1 m phosphate-buffered saline (pH 7.5). The sections were then stained with hematoxylin and eosin (HE) and observed under a light microscope (BX61, Olympus Co., Tokyo, Japan). Micrographs were taken with a digital camera (DP-72, Olympus Co.) connected to a personal computer. The cross-sectional size of muscle fibres was evaluated by measuring the minimal diameter of muscle fibres (in order to correct for obliquely cut muscle fibres) and the cross-sectional area (CSA) (Kiliaridis et al. 1988; Okumura et al. 2003b). The minimal diameter and CSA of 100 muscle fibres in the superficial portion were measured with image analysis software (Image J 1.45) and averaged to obtain the mean values in each mouse.
MHC composition
MHC isoform composition in masseter muscle was analysed by means of SDS-PAGE, followed by silver staining of the bands of each MHC isoform (Silver Staining Kit, GE Healthcare, Uppsala, Sweden). The stained bands were scanned with a densitometer (LAS-1000, Fuji Photo Film, Tokyo, Japan). To determine the MHC composition, the relative proportion of each MHC isoform was calculated as a percentage of total MHC content using the integrated dye density of the bands (Ohnuki et al. 1999; Ohnuki and Saeki, 2008; Ohnuki et al. 2009; Ohnuki et al. 2013b).
Histochemistry
Myofibrillar actomyosin ATPase (mATPase) staining with pre-incubation at pH 4.6 or pH 10.6, as well as NADH-tetrazodium reductase (NADH-TR) staining, was performed as described previously (Hamalainen and Pette, 1993; Sartorius et al. 1998). mATPase staining with pre-incubation at pH 4.6 enables distinction of type IIA fibre (light) and type IID/X (dark) or type IIB fibres (dark) and mATPase staining with pre-incubation at pH 10.6 enables distinction of type IIB fibres (light) and type IIA (dark) or type IID/X fibres (dark). NADH-TR staining visualizes the oxidative capacity of muscle fibres.
Statistical analysis
Data are expressed as means ± SEM. The statistical significance of difference was determined using student's unpaired t test (Fig. 1B–D) or a two-way ANOVA (genotype and treatment main effects, and interaction effect) where appropriate (Figs 2A–C, E–F, 3B, 4B, 5, and 6). Tukey's post hoc test was used to examine simple treatment main effects and to identify significant differences between Control and CB-treated groups in WT or Epac1KO (Figs 2A–C, E–F, 3B, 4B, 5, and 6). The criterion of significance was taken as P < 0.05.
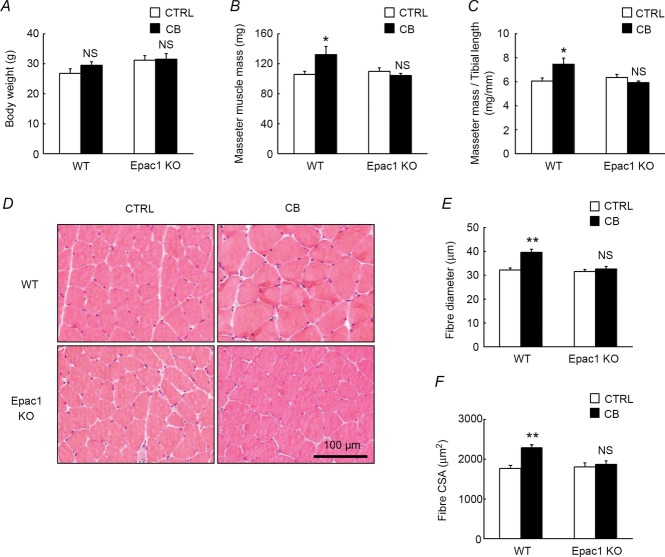
Figure 2. Effects of CB on body weight, masseter mass and histological analysis.
Body weight (A), masseter muscle mass (B), masseter muscle mass to tibial length ratio (C), cross sections of masseter muscle (D), fibre diameter(E) and fibre cross-sectional area (CSA) (F) of masseter muscle prepared from CB-treated (CB) and age-matched control (CTRL) WT and Epac1KO. A, no significant difference in body weight was observed between the control (CTRL) and CB-treated group in either WT (CTRL vs. CB: 27 ± 1.6 vs. 29 ± 1.2 g, P = NS by Tukey's post hoc test, n = 6) or Epac1KO (CTRL vs. CB: 31 ± 1.5 vs. 32 ± 1.9 g, P = NS by Tukey's post hoc test, n = 6). B and C, both the masseter muscle mass and the masseter muscle mass to tibial length ratio were significantly increased by the CB treatment in WT (masseter muscle mass: CTRL vs. CB: 106 ± 4.4 vs. 133 ± 10.5 mg; masseter muscle mass/tibial length (mg/mm): CTRL vs. CB: 6.1 ± 0.2 vs. 7.5 ± 0.5, *P <0.05 by Tukey's post hoc test, n = 6), but these increases were suppressed in Epac1KO (masseter muscle: CTRL vs. CB: 110 ± 4.9 vs. 104 ± 2.4 mg; masseter muscle mass/tibial length (mg/mm): CTRL vs. CB: 6.4 ± 0.3 vs. 5.9 ± 0.1, P = NS by Tukey's post hoc test, n = 6). D–F, typical cross-sections of HE staining (D), fibre diameter (E), and fibre cross sectional area (CSA) (F) of masseter muscle in control and CB-treated WT and Epac1KO. Fibre diameter was significantly increased by CB treatment in WT (from 32 ± 0.8 to 40 ± 1.3 mm, **P < 0.01 by Tukey's post hoc test, n = 6), but no increase was observed in Epac1KO (from 32 ± 0.8 to 33 ± 1.0 mm, P = NS by Tukey's post hoc test, n = 6) (E). Fibre CSA was also similar in WT and Epac1KO at baseline (WT vs. Epac1KO: 1762 ± 80 vs. 1800 ± 107 μm2, P = NS by Tukey's post hoc test, n = 6) (F). It was significantly increased by CB treatment in WT (from 1762 ± 80 to 2284 ± 80 μm2, **P < 0.01 by Tukey's post hoc test, n = 6), while this increase was suppressed in Epac1KO (from 1800 ± 107 to 1866 ± 92 μm2, P = NS by Tukey's post hoc test, n = 6).
Figure 3. Effects of CB on MHC composition in masseter muscle.
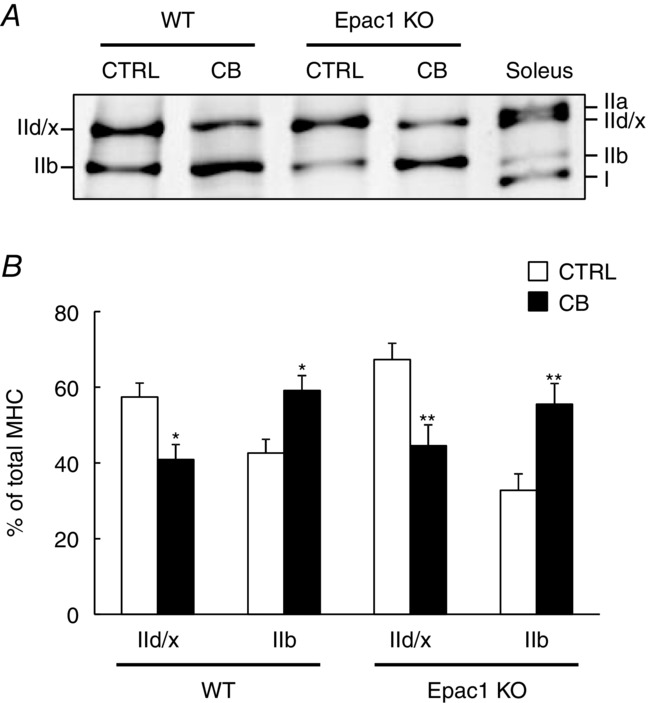
Typical SDS-PAGE profiles of MHC isoforms of masseter muscle and soleus muscle (A) and the average MHC compositions of masseter muscle (B) prepared from CB-treated (CB) and age-matched control (CTRL) in WT and Epac1KO. The relative proportion of each MHC isoform was expressed as a percentage of total MHC content (% of total MHC). In both WT and Epac1KO, the proportion of MHC-IId/x was significantly decreased (WT: from 57 ± 3.7 to 41 ± 4.0%, *P < 0.05 by Tukey's post hoc test, n = 6, Epac1KO: from 67 ± 4.4 to 45 ± 5.5%, **P < 0.01 by Tukey's post hoc test, n = 6), while that of MHC IIb was significantly increased (WT: from 43 ± 3.7 to 59 ± 4.0%, *P < 0.05 by Tukey's post hoc test, n = 6, Epac1KO: from 33 ± 4.4 to 55 ± 5.5%, **P < 0.01 by Tukey's post hoc test, n = 6) after CB treatment (B).
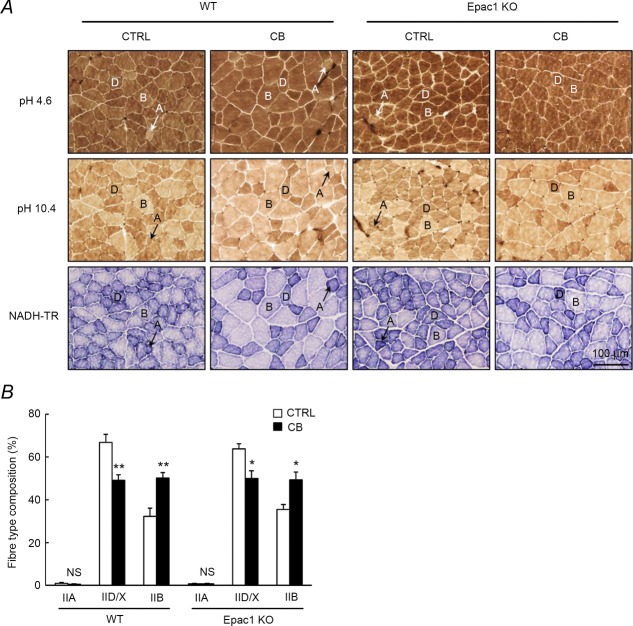
Figure 4. Effects of CB on fibre type composition in masseter muscle.
A, typical cross sections of histochemical staining for mATPase with pre-incubation at pH 4.6 (upper), or with pre-incubation at pH 10.4 (middle), and NADH-TR staining (lower) of masseter muscle in control and CB-treated WT (left) and Epac1KO (right). The oxidative capacity of muscle fibres, as presented by NADH-TR staining, showed the following tendency: IIA (dark) > IID/X > IIB (light). Key: A, type IIA; B, type IIB; D, type IID/X. B, CB treatment did not alter the proportion of type IIA fibre (IIA) in both WT (from 1.0 ± 0.4 to 0.5 ± 0.2%, P = NS by Tukey's post hoc test, n = 6) and Epac1KO (from 0.6 ± 0.3 to 0.7 ± 0.3%, P = NS by Tukey's post hoc test, n = 6). The proportion of type IID/X fibre (IID/X) was significantly decreased in both WT (from 67 ± 3.8 to 49 ± 2.4%, **P < 0.01 by Tukey's post hoc test, n = 6) and Epac1KO (from 64 ± 2.4 to 50 ± 3.6%, *P < 0.05 by Tukey's post hoc test, n = 6) after the CB treatment. The proportion of type IIB fibre (IIB) was significantly increased in both WT (from 32 ± 3.9 to 50 ± 2.4%, **P < 0.01 by Tukey's post hoc test, n = 6) and Epac1KO (from 36 ± 2.2 to 49 ± 3.7%, *P < 0.05 by Tukey's post hoc test, n = 6) after the CB treatment.
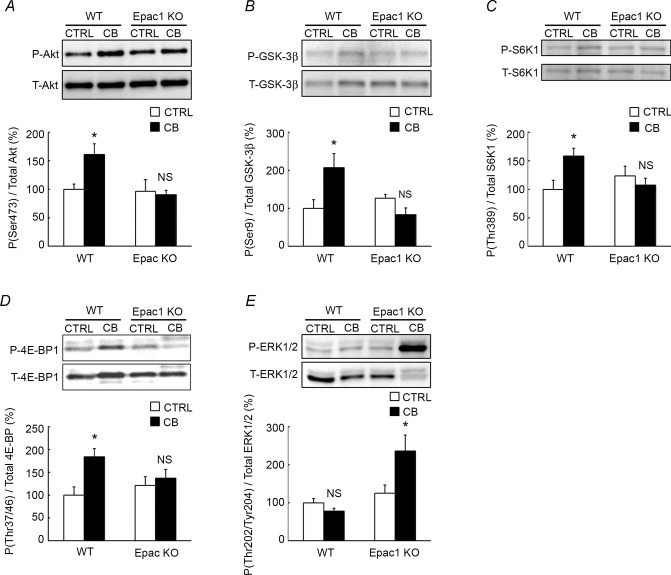
Figure 5. Activities of Akt or ERK1/2 signalling in WT and Epac1KO in response to chronic CB treatment.
A–D, the activities of molecules involved in Akt signalling were examined by measuring phosphorylated and total Akt (A), GSK-3β (B), S6K1 (C), and 4E-BP1 (D) in masseter muscle of WT and Epac1KO after chronic CB treatment (2 mg kg−1 day−1i.p.) for 3 weeks. Significant activation of these molecules was observed in WT (*P < 0.05 by Tukey's post hoc test, n = 5–6), but not in Epac1KO (P = NS by Tukey's post hoc test, n = 5–6). E, phosphorylation of ERK1/2 was not different between control (CTRL) and CB-treated group in WT (from 100 ± 12 to 79 ± 8%, P = NS by Tukey's post hoc test, n = 5), but it was significantly increased by approximately two-fold by CB treatment in Epac1KO (from 126 ± 21 to 236 ± 42%, *P < 0.05 by Tukey's post hoc test, n = 5–6). The amount of expression in WT treated with saline was taken as 100% in each determination and representative immunoblotting results are shown for phosphorylated and total Akt, GSK-3β, S6K1, 4E-BP1, ΕΡΚ1/2.
Figure 6. Activities of CaMKII/HDAC4 or calcineurin/NFAT signaling in WT and Epac1KO in response to chronic CB treatment.
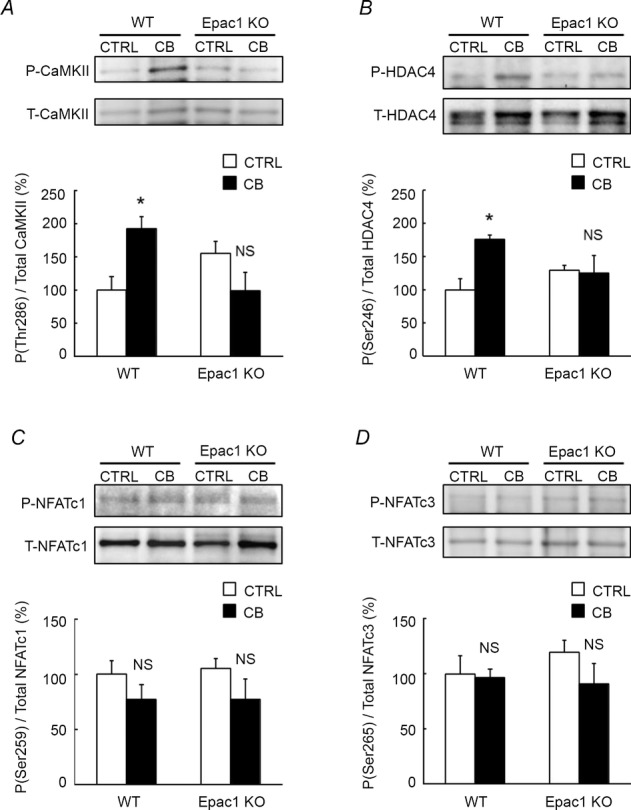
A-B, phosphorylated and total CaMKII (A) and HDAC4 (B) in masseter muscle of WT and Epac1KO were examined after chronic CB treatment (2 mg kg−1 day−1i.p.) for 3 weeks. Phosphorylation of both CaMKII and HDAC4 was significantly increased in WT (*P < 0.05 by Tukey's post hoc test, n = 5–6), but the increases were suppressed in Epac1KO (P = NS, n = 5–6 by Tukey's post hoc test). C-D, phosphorylated and total NFATc1 (C) and NFATc3 (D) in masseter muscle of WT and Epac1KO were examined after CB treatment for 3 weeks. Phosphorylation of NFATc1 and NFATc3 tended to be decreased, though not significantly, in both WT and Epac1KO (P = NS by Tukey's post hoc test, n = 6)
Results
PKA signalling was not altered in Epac1KO
We first examined whether or not cAMP/PKA signalling in masseter muscle of Epac1KO was altered by examining the expression of PKA signalling proteins in total homogenates prepared from masseter muscles of Epac1KO and WT controls (Altarejos and Montminy, 2011). PKA-catalytic units phosphorylated at threonine 198 (WT vs. Epac1KO: 100 ± 15 vs. 88 ± 12%, P = NS (not significant) by unpaired t test, n = 6 each) (Fig. 1B), cAMP response element binding protein (CREB) phosphorylated on serine 133 (WT vs. Epac1KO: 100 ± 21 vs. 86 ± 7%, P = NS by unpaired t test, n = 6 each) (Fig. 1C), and total PKA-catalytic units (WT vs. Epac1KO: 100 ± 4 vs. 103 ± 15%, P = NS by unpaired t test, n = 6 each) (Fig. 1D). Thus, we found no significant difference between Epac1KO and WT control. These data indicated that cAMP/PKA signalling was not altered in the masseter muscle of Epac1KO.
CB-induced masseter muscle hypertrophy was inhibited in Epac1KO
Body weight, masseter muscle mass, and masseter muscle mass to tibial length ratio were examined in 3-week CB-treated and age-matched control WT and Epac1KO mice (Fig. 2). Body weight (Fig. 2A, genotype and treatment main effects, and interaction effect, P = NS by two-way ANOVA) was similar in the control and CB-treated groups of WT (Control vs. CB: 27 ± 1.6 vs. 29 ± 1.2 g, P = NS by Tukey's test, n = 6) as well as Epac1KO (Control vs. CB: 31 ± 1.5 vs. 32 ± 1.9 g, P = NS by Tukey's test, n = 6). However, the masseter muscle mass (Fig. 2B, significant interaction effect, P < 0.05 by two-way ANOVA) and the masseter muscle mass to tibial length ratio (Fig. 2C, significant interaction effect, P < 0.05 by two-way ANOVA) were significantly increased by CB treatment in WT (masseter muscle mass: Control vs. CB: 106 ± 4.4 vs. 133 ± 10.5 mg; masseter muscle mass/tibial length ratio (mg/mm): Control vs. CB: 6.1 ± 0.2 vs. 7.5 ± 0.5, P <0.05 by Tukey's test, n = 6). These increases were suppressed in Epac1KO (masseter muscle mass: Control vs. CB: 110 ± 4.9 vs. 104 ± 2.4 mg; masseter muscle/tibial length ratio (mg/mm): Control vs. CB: 6.4 ± 0.3 vs. 5.9 ± 0.1, P = NS by Tukey's test, n = 6).
Histological analysis showed no abnormal organization of masseter muscle (such as fibrosis) in either WT or Epac1KO (Fig. 2D) and the fibre diameter (Fig. 2E, significant interaction effect, P < 0.01 by two-way ANOVA) was similar in WT and Epac1KO at baseline (WT vs. Epac1KO: 32 ± 0.8 vs. 32 ± 0.8 μm, P = NS by Tukey's test, n = 6). However, it was significantly increased by CB treatment in WT (from 32 ± 0.8 to 40 ± 1.3 μm, P < 0.01 by Tukey's test, n = 6), while this increase was suppressed in Epac1KO (from 32 ± 0.8 to 33 ± 1.0 μm, P = NS by Tukey's test, n = 6), as was the case for masseter muscle mass.
Fibre CSA (Fig. 2F, significant interaction effect, P < 0.01 by two-way ANOVA) was also similar in WT and Epac1KO at baseline (WT vs. Epac1KO: 1762 ± 80 vs. 1800 ± 107 μm2, P = NS by Tukey's test, n = 6). It was significantly increased by CB treatment in WT (from 1762 ± 80 to 2284 ± 80 μm2, P < 0.01 by Tukey's test, n = 6), while this increase was suppressed in Epac1KO (from 1800 ± 107 to 1866 ± 92 μm2, P = NS by Tukey's test, n = 6), as was the case for masseter muscle mass as well as fibre diameter.
These data indicate that Epac1 plays an important role in the development of masseter muscle hypertrophy in response to β2-AR stimulation with CB.
CB-induced MHC isoform transition was not altered in Epac1KO
The average MHC isoform compositions in masseter muscle obtained from CB-treated and age-matched control WT and Epac1KO mice were examined by SDS-PAGE analysis (Fig. 3). The masseter muscle is composed primarily of MHC-IId/x and MHC-IIb, which harness anaerobic metabolism to generate ATP, whereas soleus muscle is composed primarily of MHC-I and MHC-IIa, which utilize oxidative phosphorylation as their energy source, with only a little MHC-IIb (Fig. 3A). CB treatment promoted MHC isoform transition towards faster isoforms in both WT and Epac1KO (Fig. 3B, significant treatment main effect in MHC-IId/x and MHC-IIb, P < 0.01 by two-way ANOVA), i.e. the proportion of MHC-IId/x was significantly decreased (WT: from 57 ± 3.7 to 41 ± 4.0%, P < 0.05 by Tukey's test, n = 6; Epac1KO: from 67 ± 4.4 to 45 ± 5.5%, P < 0.01 by Tukey's test, n = 6), while that of MHC-IIb was significantly increased (WT: from 43 ± 3.7 to 59 ± 4.0%, P < 0.05 by Tukey's test, n = 6; Epac1KO: from 33 ± 4.4 to 55 ± 5.5%, P < 0.01 by Tukey's test, n = 6). These data indicated that Epac1 did not influence the slow-to-fast MHC isoform transition in the masseter muscle in response to CB treatment.
In order to confirm the results of SDS-PAGE analysis, we also performed the histochemical staining for mATPase with acid pre-incubation at pH 4.6 (Fig. 4A, upper), which enables the distinction of type IIA fibre (containing MHC-IIa) (light) and type IID/X fibre (containing MHC-IId/x) (dark) or type IIB fibre (containing MHC-IIb) (dark), as well as staining for mATPase with alkaline pre-incubation at pH 10.4 (Fig. 4A, middle), which enables distinction of type IIB fibre (light) and type IID/X fibre (dark) or type IIA fibre (dark), in addition to NADH-TR staining (Farber et al. 1954) (Fig. 4A, lower). The proportion of type IIA fibre (Fig. 4B, genotype and treatment main effects, and interaction effect in IIA, P = NS by two-way ANOVA) was small (∼ 1%) and similar between control and CB-treated mice in both WT (1.0 ± 0.4 vs. 0.5 ± 0.2%, P = NS by Tukey's test, n = 6) and Epac1KO (0.6 ± 0.3 vs. 0.7 ± 0.3%, P = NS by Tukey's test, n = 6). These results indicate that the masseter muscle was primarily composed of type IID/X fibre and type IIB fibre, in agreements with the SDS-PAGE analysis (Fig. 3). CB treatment significantly decreased the proportion of type IID/X fibre (Fig. 4B, significant treatment main effect in IID/X, P < 0.01 by two-way ANOVA) in both WT (from 67 ± 3.8 to 49 ± 2.4%, P < 0.01 by Tukey's test, n = 6) and Epac1KO (from 64 ± 2.4 to 50 ± 3.6%, P < 0.05 by Tukey's test, n = 6), but it significantly increased the proportion of type IIB fibre (Fig. 4B, significant treatment main effect in IIB, P < 0.01 by two-way ANOVA) in both WT (from 32 ± 3.9 to 50 ± 2.4%, P < 0.01 by Tukey's test, n = 6) and Epac1KO (from 36 ± 2.2 to 49 ± 3.7%, P < 0.05 by Tukey's test, n = 6). The results of NADH-TR staining showed that the oxidative capacity of muscle fibres followed the pattern IIA (dark) > IID/X > IIB (light), as shown previously (Fig. 4A, lower) (Hamalainen and Pette, 1993; Sartorius et al. 1998). These data indicated that Epac1 did not influence the slow-to-fast MHC isoform transition in the masseter muscle in response to CB treatment, in accordance with the SDS-PAGE findings.
CB-mediated Akt pathway activation was attenuated in Epac1KO
Activation of β2-AR was shown to activate Akt via the Giα-Gβγ-PI3K pathway in cardiac myocytes (Zhu et al. 2001). Recently, we reported that Akt/mTOR is involved in both development of hypertrophy and fast-to-slow MHC isoform transition in masseter muscle in response to mechanical overload stress (Umeki et al. 2013). However, the role of Epac1 in β2-AR-mediated masseter muscle hypertrophy and activation of the Akt/mTOR pathway remains poorly understood.
Thus, we first examined the Akt phosphorylation on serine 473 (Fig. 5A, significant interaction effect, P < 0.05 by two-way ANOVA) and confirmed that it was significantly increased in WT, but not in Epac1KO (WT vs. Epac1KO: from 100 ± 9.4 to 161 ± 19%, P < 0.05 by Tukey's test, n = 5 vs. from 96 ± 21 to 90 ± 7.7%, P = NS by Tukey's test, n = 5–6).
We also examined the phosphorylation of Akt downstream target, glycogen synthase kinase-3β (GSK-3β) (Fig. 5B, significant interaction effect, P < 0.01 by two-way ANOVA), because GSK-3β activity is negatively regulated by Akt activity (Hardt and Sadoshima, 2002; Okumura et al. 2007). The phosphorylation level of GSK-3β at serine 9 was significantly increased in masseter muscle of WT (from 100 ± 23 to 206 ± 38%, P < 0.05 by Tukey's test, n = 5–6), but this increase was suppressed in Epac1KO (from 127 ± 10 to 83 ± 18%, P = NS by Tukey's test, n = 5–6).
We next examined activation of the Akt/mTOR pathway in terms of phosphorylation of S6K1 on threonine 389 (Fig. 5C, significant interaction effect, P < 0.05 by two-way ANOVA) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) on threonine 37/46 (Fig. 5D, significant interaction effect, P < 0.05 by two-way ANOVA). We found that these phosphorylations were significantly increased by CB treatment in masseter muscle of WT (S6K1: from 100 ± 16 to 159 ± 14%; 4E-BP1: from 100 ± 18 to 184 ± 18, P < 0.05 by Tukey's test, n = 5–6), but these increases were suppressed in Epac1KO (S6K1: from 124 ± 16 to 107 ± 12%; 4E-BP1: from 121 ± 19 to 138 ± 19%, P = NS by Tukey's test, n = 5). These data indicated that CB-mediated activation of Epac1/Akt/mTOR signalling might play an important role in the development of masseter muscle hypertrophy.
ERK pathway was attenuated in Epac1KO
We also examined the phosphorylation of p44/42 mitogen-activated protein kinase (also known as ERK1/2) on threonine 202/tyrosine 204, because β2-AR activation has been shown to phosphorylate ERK1/2 via the Giα-Gβγ pathway in skeletal muscle (Zhu et al. 2001) (Fig. 5E, significant interaction effect, P < 0.05 by two-way ANOVA). Also, ERK1/2 phosphorylation was reported to be necessary for regulating the mass of skeletal muscle by us and another group (Penna et al. 2010a; Umeki et al. 2013).
Phosphorylation of ERK1/2 in masseter muscle was similar between the control and CB-treated groups in WT (Control vs. CB: 100 ± 12 vs. 79 ± 8%, P = NS by Tukey's test, n = 5), but CB induced a significant increase of phosphorylation by approximately 2-fold in Epac1KO (Control vs. CB: 126 ± 21 vs. 236 ± 42%, P < 0.05 by Tukey's test, n = 5) (Fig. 5E). These data indicate that Epac1 decreased the CB-mediated activation of ERK1/2 signalling in masseter muscle.
CaMKII/HDAC4 pathway was attenuated in Epac1KO
Phosphorylation of histone deacetylase 4 (HDAC4) on serine 265/266 mediated by PKA leads to induction of skeletal muscle atrophy, whereas phosphorylation on serine 246 mediated by Epac-activated calmodulin kinase II (CaMKII) leads to induction of skeletal muscle hypertrophy (Liu and Schneider, 2013).
We thus examined the phosphorylation of CaMKII on threonine 286 (Fig. 6A, significant interaction effect, P < 0.01 by two-way ANOVA) and HDAC4 on serine 246 (Fig. 6B, significant interaction effect, P < 0.05 by two-way ANOVA) in CB-treated masseter muscle of WT and Epac1KO. These phosphorylations were significantly increased in WT (CaMKII: from 100 ± 20 to 192 ± 18%, n = 6, P < 0.05 by Tukey's test; HDAC4: from 100 ± 16 to 176 ± 6.6%, n = 5–6, P < 0.05 by Tukey's test), but the increases were suppressed in Epac1KO (CaMKII: from 156 ± 18 to 99 ± 28%, P = NS by Tukey's test, n = 6; HDAC4: from 130 ± 7.2 to 125 ± 27%, P = NS by Tukey's test, n = 5). These data suggest that Epac1 plays an important role in development of masseter muscle hypertrophy through the regulation of CaMKII/HDAC4 activity, in addition to activation of Akt/mTOR signalling.
Calcineurin-NFAT signalling was not altered in Epac1KO
Calcineurin is a calcium/calmodulin-regulated protein phosphatase that acts on the transcription factors of the nuclear factor of activated T cells (NFAT) family, causing them to be translocated to the nucleus, where they induce transcriptional activation. We have previously demonstrated that calcineurin-NFAT signalling has a role in preservation of masseter muscle mass (Arai et al. 2005). Therefore, we examined the role of Epac1 in calcineurin-NFAT signalling activation in response to chronic CB treatment. We found that phosphorylation of NFATc1 on serine 259 (Fig. 6C, genotype and treatment main effects, and interaction effects, P = NS by two-way ANOVA) and NFATc3 on serine 265 (Fig. 6D, genotype and treatment main effects, and interaction effects, P = NS by two-way ANOVA) tended to be decreased, though not significantly, in both WT and Epac1KO (NFATc1: WT: from 100 ± 12 to 77 ± 13%, Epac1KO: from 105 ± 9.1 to 77 ± 18%. NFATc3: WT: from 100 ± 16 to 97 ± 7.9%, Epac1KO: from 119 ± 11 to 91 ± 19%, P = NS by Tukey's test, n = 6) (Lunde et al. 2011). These data are consistent with the idea that Epac1 did not influence the activation of calcineurin-NFAT signalling in masseter muscle before or after CB treatment.
Discussion
Most skeletal muscle growth-promoting agonists, such as CB and salbutamol, are highly selective for β2-AR, and their action is thought to occur through Gsα-AC-cAMP-PKA signalling via β2-AR with slow-to-fast MHC isoform transition (Li et al. 2012; Ohnuki et al. 2013a). Recently, it was reported that β2-AR couples not only to Gsα, but also to Giα in skeletal muscle, leading to stimulation of Gβγ-mediated Akt signalling as well as ERK1/2 signalling (Fig. 7) (Zhu et al. 2001; Gosmanov et al. 2002; Shi et al. 2007). We have recently demonstrated that both CB, a lipophilic β2-AR agonist, and salbutamol, a hydrophilic β2-AR agonist, similarly induce masseter muscle hypertrophy with slow-to-fast MHC isoform transition, indicating that hypertrophy might be mediated through direct muscle β2-AR stimulation, not through CNS β2-AR stimulation (Ohnuki et al. 2013a). However, the relationship between cAMP signalling and Akt or ERK1/2 signalling in skeletal muscle hypertrophy as well as MHC isoform transition remains poorly understood.
Figure 7. Schematic summary of the proposed role of Epac1 in masseter muscle hypertrophy.
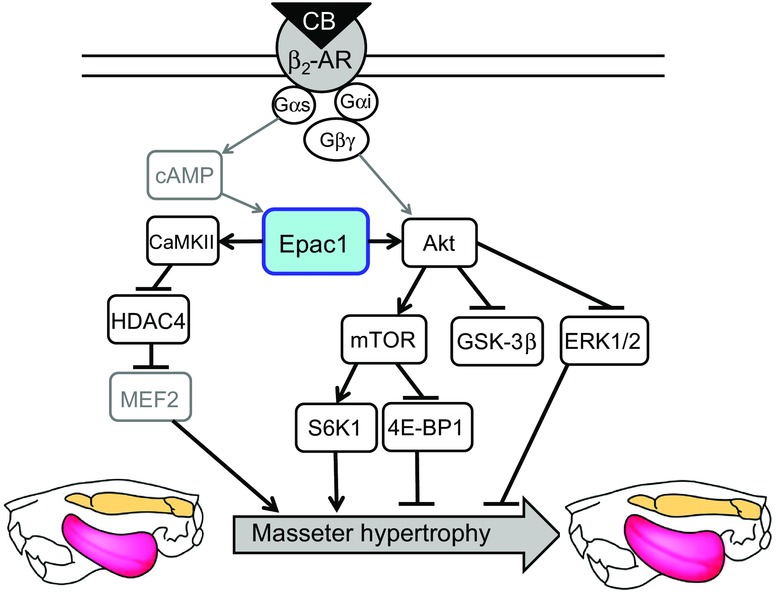
This scheme illustrates the proposed relationship between Epac1 and CB-mediated masseter muscle hypertrophy, mediated by activation of both Akt and CaMKII/HDAC4 signalings. Solid black lines represent findings in this study and solid grey lines represent findings reported previously (Kawasaki et al., 1998, de Rooij et al., 1998, Zhu et al., 2001, Liu et al., 2013).
We hypothesized that Epac1, which was recently identified as a PKA-independent cAMP sensor and a major skeletal muscle isoform, might play an important role in masseter muscle hypertrophy and MHC isoform transition by linking cAMP signalling and Akt signalling or ERK signalling, and we aimed to test this hypothesis using Epac1-null mice (Okumura et al. 2014).
We first found that development of CB-mediated masseter muscle hypertrophy was suppressed in Epac1KO without any change of the slow-to-fast MHC isoform transition. Importantly, phosphorylation of Akt on serine 473 and its downstream molecules S6K1 on serine 389 and 4E-BP1 on threonine 37/46, and, in parallel, GSK-3β on serine 9 by CB treatment were all inhibited in Epac1KO without affecting the MHC isoform transition towards faster isoforms. These data indicated that CB-mediated masseter muscle hypertrophy might develop as a result of activation of cAMP/Epac1/Akt signalling, rather than cAMP/PKA signalling, because the cAMP/PKA signalling in masseter muscle was intact in both WT and Epac1KO (Fig. 1B), as demonstrated previously in heart (Okumura et al. 2014). Conversely, Epac1 did not affect the MHC isoform transition toward faster isoforms induced by CB treatment in masseter muscle.
We recently demonstrated that phosphorylation of ERK1/2 on threonine 202/tyrosine 204 was reduced more in hypertrophied masseter muscle exposed to mechanical overload and the effect was attenuated by rapamycin, a selective mTOR inhibitor (Umeki et al. 2013). Our current data showed that ERK1/2 phosphorylation in masseter muscle was not different between WT and Epac1KO at baseline. However, it was significantly increased by approximately two-fold in masseter muscle of Epac1KO in response to CB treatment, though it remained unchanged in the hypertrophied masseter muscle of WT. Importantly, ERK activation was recently reported to be a critical contributor to muscle atrophy (Penna et al. 2010a). We thus anticipated that CB-mediated ERK1/2 phosphorylation in Epac1KO might be induced through the opposing effect of PI3-Akt signalling on ERK1/2 signalling, and up-regulation of ERK activity might be associated with the less effective CB-mediated hypertrophy in Epac1KO (Rommel et al. 1999; Penna et al. 2010b).
HDAC4 moves between cytoplasm and nuclei in cells prepared from flexor digitorum brevis muscle of CD-1 mice (Liu and Schneider, 2013). HDAC4 phosphorylation at serine 265/266 mediated by PKA induces nuclear influx, leading to inhibition of myocyte enhancer factor 2 (MEF2) activity, while phosphorylation at serine 246 mediated by CaMKII induces nuclear efflux, leading to activation of MEF2, which has a role in muscle hypertrophy (Fig. 7) (Potthoff et al. 2007; Cardinale et al. 2010). Importantly, Epac mediates the CaMKII-mediated HDAC4 phosphorylation on serine 246 (Liu and Schneider, 2013). We thus examined the phosphorylation of CaMKII on threonine 286 and HDAC4 on serine 246 in masseter muscle and found that these phosphorylations were significantly increased by CB treatment in masseter muscle of WT, but not in Epac1KO.
Taken together, the present finding indicate a causal relationship between Epac1 and CB-mediated masseter muscle hypertrophy and further suggest that this relationship might be mediated by the activation of both Akt signalling and CaMKII/HDAC4 signalling. As markers of the activation of Akt signalling, we examined the phosphorylation status of Akt itself and its downstream targets such as S6K1, 4E-BP1, and GSK-3β. Also, as markers of the activation of CaMKII/HDAC signalling, we examined the phosphorylation status of CaMKII and HDAC4. Phosphorylation of these markers was significantly increased in the masseter muscle of WT after CB infusion, but these increases were suppressed in Epac1KO.
Further studies will be required to determine whether activations of these signalling pathways were induced via stimulation of β2-AR expressed in myofibres or vessel because direct myofibre β2-AR stimulation was reported to increase protein synthesis and to decrease protein degradation, resulting in a net increase in myofibrillar protein content through activation of the Akt pathway or CaMKII/HDAC4 pathway (Choo et al. 1992; Joassard et al. 2013a; Joassard et al. 2013b; Liu and Schneider, 2013). Also, vascular β2-AR stimulation in the masseter muscle was reported to evoke vasodilatation in the skeletal muscle and to induce endothelial nitric oxide synthase expression through the activation of the Akt and/or HDAC pathway (Rossig et al. 2002; Lee, 2002 Osuka et al. 2009; Ishii et al. 2010; Banquet et al. 2011; Bharti et al. 2012). Importantly, myocardial blood flow is pivotal for the development and maintenance of hypertrophied myocardium (Sano et al. 2007). In order to clarify the mechanisms involved at the molecular level, in vitro experiments using cultured skeletal muscle fibres and/or endothelial cells isolated from WT and Epac1KO might be a fruitful approach Liu and (Schneider, 2013; Liu et al. 2014), and we are planning studies along this line.
In view of the current finding that disruption of Epac1 inhibited the development of CB-mediated masseter muscle hypertrophy, we consider that pharmacological activation of Epac1 might be an alternative approach for the treatment of masticatory dysfunction due to masseter muscle wasting and weakness.
Acknowledgments
We are grateful to Ms Yoko Shinoda (Tsurumi University, Yokohama, Japan) for assistance with graphics for publication.
Glossary
- AR
adrenergic receptor
- cAMP
cyclic adenosine 3′,5′-cyclic monophosphate
- CaMKII
calmodulin kinase II
- CB
clenbuterol
- CREB
cAMP response element binding protein
- CSA
cross-sectional area
- 4E-BP1
eukaryotic initiation factor 4E-binding protein 1
- Epac
exchange protein activated by cAMP
- Epac1KO
Epac1-deficient mice
- ERK1/2
extracellular signalling regulated kinase 1/2
- GSK-3β
glycogen synthase kinase-3β
- HDAC4
histone deacetylase 4
- HE
haematoxylin and eosin
- i.p.
intraperitoneal injection
- mATPase
myofibrillar actomyosin ATPase
- MEF2
myocyte enhancer factor 2
- MHC
myosin heavy chain
- mTOR
mammalian target of rapamycin
- NADH-TR
NADH-tetrazodium reductase
- NFAT
nuclear factor of activated T cells
- NS
not significant
- PI3K
phosphoinositol 3-kinase
- PKA
protein kinase A
- S6K1
70 kDa ribosomal S6 kinase 1
- WT
wild-type.
Additional information
Competing interests
None declared.
Author contributions
Y.O. and S.O. conceived and designed the research; Y.O., D.U., H.J. and W.C. performed the experiments; Y.O., Y.M., K. Shiozawa, K. Suita, Y.S., T.F., Y.I. and S.O. analysed the data; Y.O. and S.O. wrote the manuscript. All authors have read and approved the final version of the manuscript. All experiments were carried out at Tsurumi University and Yokohama City University.
Funding
This study was supported in part by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (S.O., D.U., Y.M., T.F., Y.I.), a Grant-in-Aid for Scientific Research on Innovative Areas (22136009) (Y.I., S.O.), Takeda Science Foundation (S.O, Y.I.), Yokohama Foundation for Advancement of Medical Science (S.O., T.F.), Mitsubishi Pharma Research Foundation (S.O.), and Research for Promoting Technological Seeds A (discovery type) (S.O.), Yokohama Academic Foundation (Y.O., S.O.), 2010 Commercialization Promotion Program for Biotechnology-related Studies (S.O.), Grant for Research and Development Project II of Yokohama City University (S.O.), and Suzuken Memorial Foundation (S.O.).
References
- Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai C, Ohnuki Y, Umeki D, Saeki Y. Effects of bite-opening and cyclosporin A on the mRNA levels of myosin heavy chain and the muscle mass in rat masseter. Jpn J Physiol. 2005;55:173–179. doi: 10.2170/jjphysiol.R2123. [DOI] [PubMed] [Google Scholar]
- Bai Y, Tsunematsu T, Jiao Q, Ohnuki Y, Mototani Y, Shiozawa K, Jin M, Cai W, Jin HL, Fujita T, Ichikawa Y, Suita K, Kurotani R, Yokoyama U, Sato M, Iwatsubo K, Ishikawa Y, Okumura S. Pharmacological stimulation of type 5 adenylyl cyclase stabilizes heart rate under both microgravity and hypergravity induced by parabolic flight. J Pharmacol Sci. 2012;119:381–389. doi: 10.1254/jphs.12102fp. [DOI] [PubMed] [Google Scholar]
- Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V. Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal. 2011;23:1136–1143. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Bharti S, Singh R, Chauhan SS, Hussain T, Al-Attas OS, Arya DS. Phosphorylation of Akt/GSK-3β/eNOS amplifies 5-HT2B receptor blockade mediated anti-hypertrophic effect in rats. FEBS Lett. 2012;586:180–185. doi: 10.1016/j.febslet.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal. 2005;17:1551–1559. doi: 10.1016/j.cellsig.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol (1985) 2012;113:290–296. doi: 10.1152/japplphysiol.00436.2012. [DOI] [PubMed] [Google Scholar]
- Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol on skeletal muscle are mediated by β2-adrenoceptor activation. Am J Physiol Endocrinol Metab. 1992;263:E50–E56. doi: 10.1152/ajpendo.1992.263.1.E50. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Farber E, Sternberg WH, Dunlap CE. Tetrazolium stains for diphosphopyridine nucleotide (DPN) diaphorase and triphosphopyridine nucleotide (TPN) diaphorase in animal tissue. Proc Soc Exp Biol Med. 1954;86:534–537. doi: 10.3181/00379727-86-21156. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589:5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for β-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1025–C1032. doi: 10.1152/ajpcell.00096.2002. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993;41:733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Sadoshima J. Glycogen synthase kinase-3β: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- Ishii H, Niioka T, Izumi H. Vagal visceral inputs to the nucleus of the solitary tract: involvement in a parasympathetic reflex vasodilator pathway in the rat masseter muscle. Brain Res. 2010;1312:41–53. doi: 10.1016/j.brainres.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Joassard OR, Amirouche A, Gallot YS, Desgeorges MM, Castells J, Durieux AC, Berthon P, Freyssenet DG. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. Int J Biochem Cell Biol. 2013a;45:2444–2455. doi: 10.1016/j.biocel.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Joassard OR, Durieux AC, Freyssenet DG. β2-Adrenergic agonists and the treatment of skeletal muscle wasting disorders. Int J Biochem Cell Biol. 2013b;45:2309–2321. doi: 10.1016/j.biocel.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kiliaridis S, Engström C, Thilander B. Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Arch Oral Biol. 1988;33:187–193. doi: 10.1016/0003-9969(88)90044-1. [DOI] [PubMed] [Google Scholar]
- Kim YS, Sainz RD, Molenaar P, Summers RJ. Characterization of β1- and β2-adrenoceptors in rat skeletal muscles. Biochem Pharmacol. 1991;42:1783–1789. doi: 10.1016/0006-2952(91)90516-8. [DOI] [PubMed] [Google Scholar]
- Lee TJ. Sympathetic modulation of nitrergic neurogenic vasodilation in cerebral arteries. Jpn J Pharmacol. 2002;88:26–31. doi: 10.1254/jjp.88.26. [DOI] [PubMed] [Google Scholar]
- Li Y, He J, Sui S, Hu X, Zhao Y, Li N. Clenbuterol upregulates histone demethylase JHDM2a via the β2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Cell Signal. 2012;24:2297–2306. doi: 10.1016/j.cellsig.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Liu QS, Wang HF, Sun AK, Huo XP, Liu JL, Ma SH, Peng N, Hu J. A comparative study on inhibition of total astragalus saponins and astragaloside IV on TNFR1-mediated signaling pathways in arterial endothelial cells. PloS One. 2014;9:e101504. doi: 10.1371/journal.pone.0101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schneider MF. Opposing HDAC4 nuclear fluxes due to phosphorylation by β-adrenergic activated protein kinase A or by activity or Epac activated CaMKII in skeletal muscle fibres. J Physiol. 2013;591:3605–3623. doi: 10.1113/jphysiol.2013.256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde IG, Kvaloy H, Austbo B, Christensen G, Carlson CR. Angiotensin II and norepinephrine activate specific calcineurin-dependent NFAT transcription factor isoforms in cardiomyocytes. J Appl Physiol (1985) 2011;111:1278–1289. doi: 10.1152/japplphysiol.01383.2010. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Ryall JG. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- Machholz E, Mulder G, Ruiz C, Corning BF, Pritchett-Corning KR. Manual restraint and common compound administration routes in mice and rats. J Vis Exp. 2012;67:e2771. doi: 10.3791/2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki Y, Kawai N, Tanaka E, Langenbach GE, Tanne K, Saeki Y. Effects of increased occlusal vertical dimension on daily activity and myosin heavy chain composition in rat jaw muscle. Arch Oral Biol. 2009;54:783–789. doi: 10.1016/j.archoralbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Saeki Y. Jaw-opening muscle contracts more economically than jaw-closing muscle in rat. Arch Oral Biol. 2008;53:193–198. doi: 10.1016/j.archoralbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Saeki Y, Yamane A, Kawasaki K, Yanagisawa K. Adaptation of guinea-pig superficial masseter muscle to an increase in occlusal vertical dimension. Arch Oral Biol. 1999;44:329–335. doi: 10.1016/s0003-9969(98)00128-9. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Umeki D, Cai W, Kawai N, Mototani Y, Shiozawa K, Jin HL, Fujita T, Tanaka E, Saeki Y, Okumura S. Role of masseter muscle β2-adrenergic signaling in regulation of muscle activity, myosin heavy chain transition, and hypertrophy. J Pharmacol Sci. 2013a;123:36–46. doi: 10.1254/jphs.12271fp. [DOI] [PubMed] [Google Scholar]
- Ohnuki Y, Yamada T, Mototani Y, Umeki D, Shiozawa K, Fujita T, Saeki Y, Okumura S. Effects of protein kinase A on the phosphorylation status and transverse stiffness of cardiac myofibrils. J Pharmacol Sci. 2013b;123:279–283. doi: 10.1254/jphs.13110sc. [DOI] [PubMed] [Google Scholar]
- Okumura S, Fujita T, Cai W, Jin M, Namekata I, Mototani Y, Jin H, Ohnuki Y, Tsuneoka Y, Kurotani R, Suita K, Kawakami Y, Hamaguchi S, Abe T, Kiyonari H, Tsunematsu T, Bai Y, Suzuki S, Hidaka Y, Umemura M, Ichikawa Y, Yokoyama U, Sato M, Ishikawa F, Izumi-Nakaseko H, Adachi-Akahane S, Tanaka H, Ishikawa Y. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J Clin Invest. 2014;124:2785–2801. doi: 10.1172/JCI64784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, Vatner SF, Ishikawa Y. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res. 2003a;93:364–371. doi: 10.1161/01.RES.0000086986.35568.63. [DOI] [PubMed] [Google Scholar]
- Okumura S, Suzuki S, Ishikawa Y. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: effects of targeted disruption of the type 5 adenylyl cyclase gene. J Pharmacol Sci. 2009;109:354–359. doi: 10.1254/jphs.08r26fm. [DOI] [PubMed] [Google Scholar]
- Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci USA. 2003b;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Tsunematsu T, Bai Y, Jiao Q, Ono S, Suzuki S, Kurotani R, Sato M, Minamisawa S, Umemura S, Ishikawa Y. Type 5 adenylyl cyclase plays a major role in stabilizing heart rate in response to microgravity induced by parabolic flight. J Appl Physiol (1985) 2008;105:173–179. doi: 10.1152/japplphysiol.01166.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases Akt signal with chronic catecholamine stress. Circulation. 2007;116:1776–1783. doi: 10.1161/CIRCULATIONAHA.107.698662. [DOI] [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Yoshida J, Takayasu M. Modification of endothelial nitric oxide synthase through AMPK after experimental subarachnoid hemorrhage. J Neurotrauma. 2009;26:1157–1165. doi: 10.1089/neu.2008.0836. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Ryall JG, Lynch GS, Muscat GE. Expression profiling of skeletal muscle following acute and chronic β2-adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics. 2009;10:e448. doi: 10.1186/1471-2164-10-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G, Rossi Fanelli F, Baccino FM, Costelli P. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved. Int J Cancer. 2010a;127:1706–1717. doi: 10.1002/ijc.25146. [DOI] [PubMed] [Google Scholar]
- Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PloS One. 2010b;5:e13604. doi: 10.1371/journal.pone.0013604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest. 2007;117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nuñez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S. Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res. 2002;91:837–844. doi: 10.1161/01.res.0000037983.07158.b1. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Gregorevic P, Plant DR, Sillence MN, Lynch GS. β2-Agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1386–R1394. doi: 10.1152/ajpregu.00324.2002. [DOI] [PubMed] [Google Scholar]
- Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Lu BD, Acakpo-Satchivi L, Jacobsen RP, Byrnes WC, Leinwand LA. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J Cell Biol. 1998;141:943–953. doi: 10.1083/jcb.141.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Zeng C, Ricome A, Hannon KM, Grant AL, Gerrard DE. Extracellular signal-regulated kinase pathway is differentially involved in β-agonist-induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol. 2007;292:C1681–C1689. doi: 10.1152/ajpcell.00466.2006. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–24259. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011a;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- Turner PV, Pekow C, Vasbinder MA, Brabb T. Administration of substances to laboratory animals: equipment considerations, vehicle selection, and solute preparation. J Am Assoc Lab Anim Sci. 2011b;50:614–627. [PMC free article] [PubMed] [Google Scholar]
- Umeki D, Ohnuki Y, Mototani Y, Shiozawa K, Fujita T, Nakamura Y, Saeki Y, Okumura S. Effects of chronic Akt/mTOR inhibition by rapamycin on mechanical overload-induced hypertrophy and myosin heavy chain transition in masseter muscle. J Pharmacol Sci. 2013;122:278–288. doi: 10.1254/jphs.12195fp. [DOI] [PubMed] [Google Scholar]
- Wong K, Boheler KR, Bishop J, Petrou M, Yacoub MH. Clenbuterol induces cardiac hypertrophy with normal functional, morphological and molecular features. Cardiovasc Res. 1998;37:115–122. doi: 10.1016/s0008-6363(97)00190-9. [DOI] [PubMed] [Google Scholar]
- Woo AY, Xiao RP. β-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin. 2012;33:335–341. doi: 10.1038/aps.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, He Y, Zhang X, Peng Z, Yang Y, Zhu R, Bai J, Tian Y, Li X, Chen W, Fang D, Wang R. The rat IgGFcβBP and Muc2 C-terminal domains and TFF3 in two intestinal mucus layers bind together by covalent interaction. PloS One. 2011;6:e20334. doi: 10.1371/journal.pone.0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]