Abstract
The aim of this study was to elucidate the effects of zedoary turmeric oil (ZTO) on P450 activities (CYP1A2, CYP2C9, CYP2C19, CYP2B6, CYP2D6 and CYP3A4) in rats with liver cirrhosis induced by thioacetamide (TAA). For the induction of liver cirrhosis, rats were given TAA in their drinking water at a concentration of 0.03% for consecutive 5 weeks and then 0.04% for the next consecutive 5 weeks throughout the establishment of cirrhosis. Then the cirrhotic rats were ip given saline, ZTO 100, 200 and 400 mg/kg, respectively, once daily for 2 weeks. When cirrhosis model was established at week 10, all rats of five groups were administered intragastrically with 15 mg/kg phenacetin, 0.6 mg/kg tolbutamide, 15 mg/kg omeprazole, 15 mg/kg bupropion, 15 mg/kg metoprolol, and 10 mg/kg midazolam. Blood samples were collected at a series of time-points and the concentrations of probe drugs in plasma were determined by HPLC-MS/MS. The degree of liver cirrhosis was assessed by HE staining. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) from the model group increased by approximately 4-fold, and a decreased level of albumin (Alb) was also observed, as compared to the control group (P < 0.05). However, ZTO was found to reverse those changes of serum levels observed in the model group, and the 200 mg/kg ZTO treatment group showed the most obvious reverse tendency with significantly decreased ALT, AST and increased Alb levels (P < 0.05). The results indicated that ZTO with the dose of 100 mg/kg could inhibit the activities of CYP450 isoforms CYP2C9 and CYP2D6 in vivo in cirrhotic rats induced by TAA, while ZTO with the dose of 400 mg/kg could induce the activity of CYP2C19 in vivo in cirrhotic rats induced by TAA. However, ZTO showed no influence on cirrhotic rat hepatic CYP1A2, CYP2B6 and CYP3A4 activity in vivo. This has certain guiding significance to clinical treatment.
Keywords: Zedoary turmeric oil, CYP450, liver cirrhosis, rat, thioacetamide
Introduction
Zedoary turmeric oil (ZTO) is derived from rhizomes of three species of Curcoma including Curcuma phaeocaulis Valeton, Curcuma kwangsiensis S. G. Lee et C. F. Liang, and Curcuma wenyujin Y. H. Chen et C. Ling, and has been linked to protection against liver injury, as well as reported anti-tumor, anti-thrombotic, and antiviral activities [1-4]. As a promising anti-tumor, anti-inflammatory or antivirus agent, ZTO might be in combination with other anti-tumor, anti-inflammatory and antivirus drugs which often have a narrow therapeutic index and a steep dose-toxicity curve [5]. Moreover, ZTO has potent protective effects on chronic liver diseases caused by ongoing hepatic damage [6]. However, it remains uncertain yet whether ZTO influences the effect on cytochrome P450 (CYP450) activities in rats with liver cirrhosis induced by thioacetamide (TAA).
The CYP450s mediate most of phase I drug metabolism. Phase I metabolism can occur during drug absorption, either in the gut wall or in the liver. The presystemic (or first-pass metabolism) clearance determines the fraction of the oral dose that will reach the systemic circulation [7]. When the drug reaches the systemic circulation, it is redistributed to the liver and metabolized by CYP450. Therefore, the presystemic and systemic clearance determines the concentration of drug. The rate of clearance of the drug depends on the activity of CYP450. It is believed that more than 10 CYP450s in the human liver are involved in drug metabolism. Among the various CYP450 isozymes, CYP1A2, CYP2C9, CYP2C19, CYP2B6, CYP2D6 and CYP3A4 are considered to be most important [8,9]. Each enzyme can metabolize a vast array of xenobiotics. CYP3A4 acts on most lipophilic substrates and is known to metabolize > 50% of marketed drugs, while CYP2D6 exhibits a preference for positively charged molecules, usually with a basic nitrogen. CYP2C9 and CYP2C19 metabolizes weakly anionic molecules and CYP1A2 polyaromatic hydrocarbons as well as carcinogen.
Probe drug is one kind of compound specially catalyzed by CYP isoforms, and the activities of CYP isoforms can be reflected by the metabolic rate of probe drug. In this paper, the cocktail probe drugs approach is used to evaluate the induction or inhibition effects of ZTO on the activities of rats CYP450 isoforms such as CYP1A2, CYP2C9, CYP2C19, CYP2B6, CYP2D6 and CYP3A4 in cirrhosis rats, which are reflected by the changes of pharmacokinetic parameters of six specific probe drugs (phenacetin, tolbutamide, omeprazole, bupropion, metroprolol and midazolam).
Materials and methods
Chemicals and reagents
Phenacetin, tolbutamide, omeprazole, bupropion, metoprolol, midazolam (all > 98%) and the internal standard carbamazepine (IS, > 98%) were purchased from Sigma-Aldrich Corporation (St. Louis, USA), thioacetamide (TAA) of 99% purity was supplied by Sinopharm Chemical Reagent Company (Shanghai, China), Zedoary Turmeric oil of 98% purity was supplied by Tianrui Pharmaceutical Company (Zhejiang, China). HPLC grade acetonitrile and methanol were from Merck Company (Darmstadt, Germany). Ultra-pure water (resistance > 18.2 mΩ) prepared by a Millipore Milli-Q purification system (Bedford, MA, USA). All other chemicals were analytical grade and used without further purification.
Apparatus and chromatographic conditions
All analysis was performed with a 1200 Series liquid chromatograph (Agilent Technologies, Waldbronn, Germany) equipped with a quaternary pump, a degasser, an autosampler, a thermostatted column compartment, and a Bruker Esquire HCT ion-trap mass spectrometer (Bruker Technologies, Bremen, Germany) equipped with an electrospray ion source and controlled by ChemStation software (Version B.01.03 [204], Agilent Technologies, Waldbronn, Germany).
Chromatographic separation was achieved on a 150 mm×2.1 mm, 3.5 μm particles, Agilent Zorbax SB-C18 column at 30°C. A gradient elution programme was conducted for chromatographic separation with mobile phase A (0.1% formic acid in water) and mobile phase B (acetonitrile) as follows: 0-1.0 min (10-90% B), 1.0-7.0 min (90-90% B), 7.0-8.0 min (90-10% B), 8.0-11.0 min (10-10% B). The flow rate was 0.4 mL/min. A typical injection volume was 10 μL.
The quantification was performed by the peak-area method. The determination of target ions were performed in SIM mode (m/z 180 for phenacetin, m/z 271 for tolbutamide, m/z 198 for omeprazole, m/z 240 for bupropion, m/z 268 for metoprolol, m/z 326 for midazolam and m/z 237 for IS) and positive ion electrospray ionization interface. Drying gas flow was set to 7 L/min and temperature to 350°C. Nebuliser pressure and capillary voltage of the system were adjusted to 25 psi and 3,500 V, respectively.
Animals
A total of 30 specific pathogen-free male Sprague-Dawley (SD) rats weighing 200-220 g were obtained from Wenzhou Medical University Laboratory Animal Center (Wenzhou, China). All rats were maintained in a light controlled room (light: 07:00-19:00 h, dark: 19:00-07:00 h) kept at a temperature of 22±2°C and a relative humidity of 55±5% and were given free access to diets and water ad libitum. All experimental procedures were conducted according to the Institutional Animal Care guidelines and approved ethically by the Administration Committee of Experimental Animals, Laboratory Animal Center, Wenzhou Medical University.
Induction of liver cirrhosis induced by TAA
The rats were randomly divided into two initial groups: the control (n = 6) and cirrhotic (n = 24) rats. For the induction of liver cirrhosis, rats were given TAA in their drinking water at a concentration of 0.03% for consecutive 5 weeks and then 0.04% for the next consecutive 5 weeks throughout the establishment of cirrhosis. Control rats drinking natural water. Rats were weighed weekly, and blood was collected from the tail vein for biochemical tests of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin (Alb). Serum samples were analyzed to measure the serum activities of ALT, AST and Alb, which was used to evaluate the liver damage. Liver cirrhosis in rats induced by TAA was diagnosed by histology, which was described by Schuppan and Afdhal1 as vascularized fibrotic septa that linked portal tracts with each other and with central veins, leading to hepatocyte islands that were surrounded by fibrotic septa and were devoid of a central vein. At the end of treatment, rats were weighed and sacrificed. It was reported [10-12] that TAA was used as a reproducible animal model of liver cirrhosis.
Treatment experiment
Rats with liver cirrhosis induced by TAA as above were randomly assigned to 4 groups (each had 6 rats): Model group, ZTO 100 mg/kg, 200 mg/kg, 400 mg/kg group. Then the cirrhotic rats were ip given saline, ZTO 100, 200 and 400 mg/kg, respectively, once daily for 2 weeks. Also the control rats were ip given saline.
Histological observation
The whole liver of each rat were excised after euthanasia, rinsed with 0.9% NaCl-injectable solution, blotted dry with tissue paper, and weighed. Two pieces of liver tissues (0.5×0.5×0.5 cm for each) were collected from both left half and right half livers from each rat. Liver tissues were fixed in 10% neutral phosphate-buffered formalin, embedded in paraffin, sectioned at 4-μm/slide, and then processed for routine histological examination with hematoxylin-eosin staining.
Sample preparation
Before analysis, the plasma sample was thawed to room temperature. In a 1.5 mL centrifuge tube, an aliquot of 10 μL of the IS working solution (2 μg/mL) was added to 100 μL of collected plasma sample followed by the addition of 200 μL acetonitrile-methanol (90:10, v/v). The tubes were vortex mixed for 1.0 min. After centrifugation at 16,000 g for 10 min, the supernatant (10 μL) was injected into the LC-MS system for analysis.
Pharmacokinetic study
When cirrhosis model was established at week 10, all rats of five groups were administered intragastrically with 15 mg/kg phenacetin, 0.6 mg/kg tolbutamide, 15 mg/kg omeprazole, 15 mg/kg bupropion, 15 mg/kg metoprolol, and 10 mg/kg midazolam. Then, the blood samples (0.3 mL) were collected from the tail vein into heparinized 1.5 mL polythene tubes at 0, 0.0833, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h, then centrifuge and collect separated plasma, stored at -80°C for used.
Statistical analysis
Plasma concentration versus time data for each rat was analyzed by DAS software (Version 3.0, Drug Clinical Research Center of Shanghai University of Traditional Chinese Medicine and Shanghai BioGuider Medicinal Technology Co., Ltd., Shanghai, China). Pharmacokinetic parameters were expressed as means±SD and were analyzed by ANOVA with a post hoc test (differences between groups) were used. Statistical analyses were performed using SPSS statistical software, version 16.0. A value of P < 0.05 was considered to be statistically significant.
Results
Effects of ZTO on serum concentrations of ALT, AST and Alb in rats with liver cirrhosis
After 10-week administration of TAA, the serum levels of ALT and AST from the model group increased by approximately 4-fold, and a decreased level of Alb was also observed, as compared to the control group (P < 0.05). However, ZTO was found to reverse those changes of serum levels observed in the model group, and the 200 mg/kg ZTO treatment group showed the most obvious reverse tendency with significantly decreased ALT, AST and increased Alb levels (P < 0.05) (shown in Figure 1).
Figure 1.
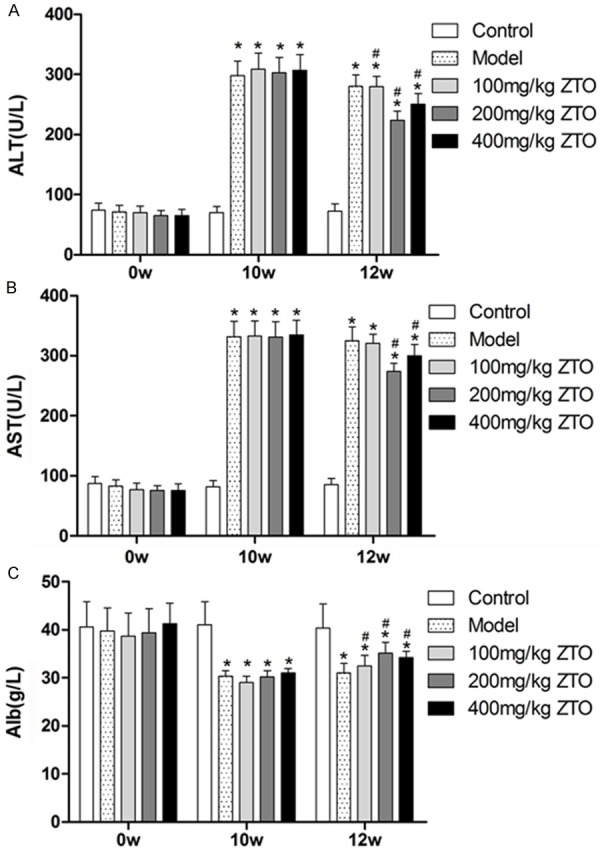
Effects of ZTO on changes in serum concentrations of ALT, AST and Alb by TAA in rats. The activities of alanine aminotransferase (ALT) (A) aspartate aminotransferase (AST) (B) and albumin (Alb) (C) were assayed by using an automated blood chemistry analyzer. (*significant compared to levels at 0 week, P < 0.05; #significant compared to levels at 10 week, P < 0.05).
Effect of ZTO on histopathologic characteristics in liver cirrhosis
To show changes of tissue structure and collagen, liver sections from each group were stained with hematoxylin and eosin (HE) (shown in Figure 2). Normal hepatic cells, central veins and few collagen fibers were shown in the control group. Substantial pseudolobules and necrosis, extensive liver bridging fibrosis and collagen deposition were obviously seen in the model group, but were alleviated in ZTO treatment groups in varying degrees. Treatment group with 200 mg/kg ZTO exhibited few pseudolobules, less liver bridging fibrosis and collagen deposition compared with 100 mg/kg ZTO group and 400 mg/kg ZTO group, indicating the best concentration of ZTO in alleviating liver cirrhosis.
Figure 2.
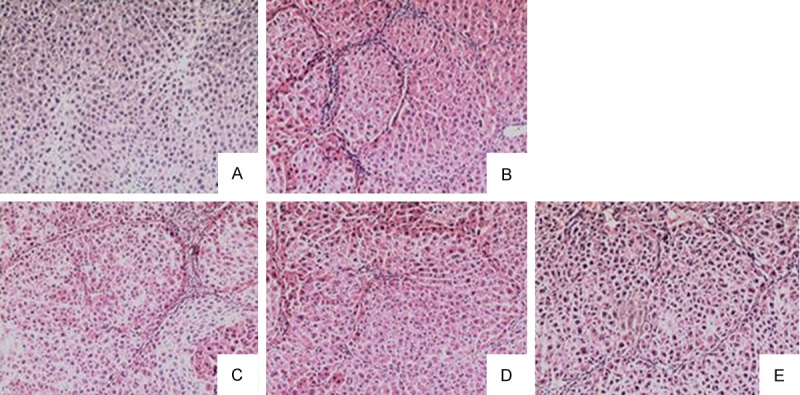
Histological assessment of liver sections by H&E staining (×100). A. Liver sections from the control group; B. Liver sections from the model group; C. Liver sections from the 100 mg/kg ZTO group; D. Liver sections from the 200 mg/kg ZTO group; E. Liver sections from the 400 mg/kg ZTO group.
Pharmacokinetic study
The main pharmacokinetic parameters after oral administration of phenacetin, tolbutamide, omeprazole, bupropion, metroprolol and midazolam from non-compartment model analysis are summarized in Tables 1, 2, 3, 4, 5 and 6. The representative phenacetin, tolbutamide, omeprazole, bupropion, metroprolol and midazolam concentration vs. time profiles of 30 rats are presented in Figure 3.
Table 1.
Mean pharmacokinetic parameters of phenacetin in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 5.96±1.76 | 2.72±1.41* | 4.49±1.35 | 4.45±1.97 | 3.11±0.39* |
| Cmax (ng/ml) | 493.31±155.85 | 284.68±153.95 | 389.47±177.38 | 277.99±185.88 | 400.30±190.36 |
| AUC(0~∞) (ng/h/ml) | 778.04±190.10 | 335.16±122.28** | 449.58±188.13* | 360.66±213.00* | 503.61±222.22 |
| Vz/F (l/kg) | 164.35±24.41 | 183.23±82.52 | 227.30±67.53 | 372.60±272.57 | 145.37±40.65 |
| CLz/F (l/h/kg) | 20.31±5.70 | 49.07±16.16* | 37.59±14.00 | 51.03±24.66 | 33.25±11.79 |
ZTO: Zedoary turmeric oil; t1/2z: half life; Cmax: peak concentration; AUC(0~∞): area under concentration-time curve among 0-infinity time; Vz/F: apparent volume of distribution; CLz/F: clearance;
P < 0.05 vs. control group;
P < 0.01 vs. control group.
Table 2.
Mean pharmacokinetic parameters of tolbutamide in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 3.27±0.58 | 3.68±2.13 | 3.45±0.36 | 2.73±0.57 | 12.57±14.54 |
| Cmax (ng/ml) | 239.37±21.16 | 140.70±33.17** | 224.77±87.85 | 80.55±52.02** | 89.11±8.47** |
| AUC(0~∞) (ng/h/ml) | 1453.20±415.03 | 630.45±287.46* | 1388.32±626.24 | 484.29±221.19* | 289.63±204.17** |
| Vz/F (l/kg) | 2.11±0.79 | 124.35±24.82** | 2.62±1.44## | 5.62±2.42*,## | 1432.02±1693.83 |
| CLz/F (l/h/kg) | 0.45±0.17 | 27.95±13.10** | 0.51±0.25## | 1.49±0.83# | 68.20±35.41 |
P < 0.05 vs. control group;
P < 0.01 vs. control group;
P < 0.05 vs model group;
P < 0.01 vs model group.
Table 3.
Mean pharmacokinetic parameters of omeprazole in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 4.57±2.83 | 4.68±2.56 | 6.49±5.04 | 5.25±1.76 | 10.79±8.17 |
| Cmax (ng/ml) | 348.14±87.57 | 140.31±78.93* | 137.86±43.20* | 87.98±9.62* | 82.54±33.38** |
| AUC(0~∞) (ng/h/ml) | 336.79±123.66 | 117.21±16.70 | 114.28±79.78 | 64.98±3.51# | 67.09±10.21# |
| Vz/F (l/kg) | 324.60±202.89 | 827.96±386.15 | 1747.63±1805.51 | 1771.25±662.96* | 3245.63±2354.78 |
| CLz/F (l/h/kg) | 50.17±23.05 | 129.89±20.16* | 170.86±85.19 | 231.27±12.11**,## | 227.41±37.71**,# |
P < 0.05 vs. control group;
P < 0.01 vs. control group;
P < 0.05 vs model group;
P < 0.01 vs model group.
Table 4.
Mean pharmacokinetic parameters of bupropion in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 3.36±1.24 | 3.34±2.19 | 2.52±1.08 | 6.50±5.29 | 4.97±3.94 |
| Cmax (ng/ml) | 275.57±40.07 | 325.60±47.43 | 232.83±43.21# | 365.52±176.83 | 378.56±71.38 |
| AUC(0~∞) (ng/h/ml) | 434.34±57.83 | 528.15±159.15 | 358.47±67.89 | 563.44±193.38 | 447.89±116.70 |
| Vz/F (l/kg) | 163.77±49.95 | 163.72±156.44 | 160.28±74.74 | 232.71±167.28 | 242.30±184.60 |
| CLz/F (l/h/kg) | 35.04±5.04 | 30.34±9.81 | 43.04±8.46 | 29.37±12.10 | 35.14±9.61 |
P < 0.05 vs model group.
Table 5.
Mean pharmacokinetic parameters of metoprolol in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 2.51±1.40 | 4.29±2.24 | 3.19±0.89 | 5.05±1.53 | 3.91±2.49 |
| Cmax (ng/ml) | 707.07±261.36 | 332.76±43.96* | 709.32±279.85 | 413.85±175.72 | 469.80±283.06 |
| AUC(0~∞) (ng/h/ml) | 1520.40±455.79 | 546.13±132.69** | 1144.57±378.42# | 661.47±185.32* | 803.69±107.10*,# |
| Vz/F (l/kg) | 43.61±41.14 | 189.33±127.82 | 64.72±25.17 | 164.48±4.07** | 101.61±58.14 |
| CLz/F (l/h/kg) | 10.67±3.69 | 28.78±7.23** | 14.15±4.28# | 24.00±7.17* | 18.87±2.35* |
P < 0.05 vs. control group;
P < 0.01 vs. control group;
P < 0.05 vs model group.
Table 6.
Mean pharmacokinetic parameters of midazolam in rat plasma (X̅±S, n = 6-8)
| Parameter | Control | Model | ZTO, 100 mg/kg | ZTO, 200 mg/kg | ZTO, 400 mg/kg |
|---|---|---|---|---|---|
| t1/2z (h) | 2.11±1.04 | 3.89±3.91 | 4.69±3.82 | 5.48±3.80 | 4.01±3.82 |
| Cmax (ng/ml) | 414.03±168.77 | 364.54±87.00 | 502.45±276.17 | 381.80±200.32 | 244.30±9.57 |
| AUC(0~∞) (ng/h/ml) | 458.70±126.48 | 414.45±57.03 | 688.32±314.92 | 359.58±145.42 | 323.02±99.81 |
| Vz/F (l/kg) | 104.32±47.81 | 189.04±172.48 | 113.61±80.28 | 202.57±113.19 | 228.00±274.74 |
| CLz/F (l/h/kg) | 34.90±10.78 | 36.67±5.19 | 17.21±7.88*,# | 32.01±15.79 | 33.16±10.85 |
P < 0.05 vs. control group;
P < 0.05 vs model group.
Figure 3.
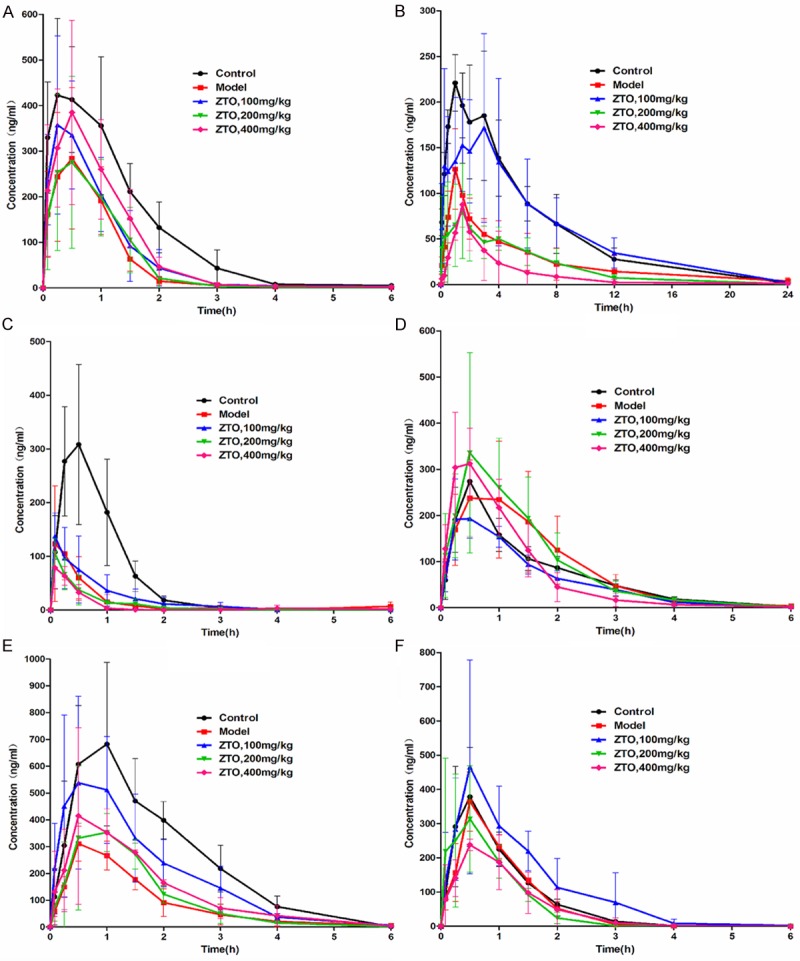
Mean concentration-time curves of six probe drugs in five groups. All rats of five groups were administered intragastrically with 15 mg/kg phenacetin, 0.6 mg/kg tolbutamide, 15 mg/kg omeprazole, 15 mg/kg bupropion, 15 mg/kg metoprolol, and 10 mg/kg midazolam. Then the blood samples (0.3 ml) were collected from the tail veil into heparinized 1.5 ml polythene tubes at 0.083, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12 and 24 h after administration. A. phenacetin; B. tolbutamide; C. omeprazole; D. bupropion; E. metroprolol; and F. midazolam.
Effect of ZTO on CYP1A2 in cirrhotic rats
As could be seen from Table 1, the t1/2 and AUC(0~∞) of phenacetin in model group were decreased significantly by 54.36% and 56.92% (P < 0.05 and P < 0.01) compared to those of control group, CL of phenacetin in model group was increased significantly by 141.61% (P < 0.05) compared to this of control group. After treatment with ZTO, the t1/2 and AUC(0~∞) of phenacetin were increased compared to those of model group, but there were no statistical significance (P > 0.05), CL of phenacetin was comparable among model group and ZTO treated groups. No apparent influences were observed on other pharmacokinetic parameters in these treatment groups. The results indicated that metabolism of phenacetin in model group was evidently speeded up, and ZTO showed no influence on cirrhotic rat hepatic CYP1A2 activity in vivo.
Effect of ZTO on CYP2C9 in cirrhotic rats
As could be seen from Table 2, the Cmax and AUC(0~∞) of tolbutamide in model group were decreased significantly by 41.22% and 56.62% (P < 0.01 and P < 0.05) compared to those of control group, V and CL of tolbutamide in model group were increased significantly by 5793.36% and 6111.11% (P < 0.01) compared to those of control group. After treatment with ZTO 100 mg/kg, V and CL of tolbutamide were decreased significantly by 97.89% and 98.18% (P < 0.01) compared to those of model group. After treatment with ZTO 200 mg/kg, V and CL of tolbutamide were decreased significantly by 95.48% and 94.67% (P < 0.01 and P < 0.05) compared to those of model group. The pharmacokinetic parameters of tolbutamide in rats showed no significant difference between model group and ZTO group at a dose of 400 mg/kg. The results indicated that metabolism of tolbutamide in model group was evidently speeded up, and ZTO with the dose of 100 mg/kg and 200 mg/kg had the potential to inhibit cirrhotic rat hepatic CYP2C9 activity in vivo.
Effect of ZTO on CYP2C19 in cirrhotic rats
As could be seen from Table 3, the Cmax of omeprazole in model group was decreased significantly by 59.70% (P < 0.05) compared to this of control group, and CL of omeprazole in model group was increased significantly by 158.90% (P < 0.05) compared to this of control group. After treatment with ZTO 200 mg/kg and 400 mg/kg, AUC(0~∞) of omeprazole were decreased significantly by 44.56% and 42.76% (P < 0.05) compared to those of model group, and CL of omeprazole were increased significantly by 78.05% and 75.08% (P < 0.01 and P < 0.05). The pharmacokinetic parameters of omeprazole in rats showed no significant difference between model group and ZTO group at a dose of 100 mg/kg. The results indicated that metabolism of omeprazole in model group was evidently speeded up, and ZTO with the dose of 200 mg/kg and 400 mg/kg had the potential to induce cirrhotic rat hepatic CYP2C19 activity in vivo.
Effect of ZTO on CYP2B6 in cirrhotic rats
As could be seen from Table 4, the Cmax of bupropion in ZTO group at a dose of 100 mg/kg was decreased significantly by 28.49% (P < 0.05) compared to this of model group. No apparent influences were observed on other pharmacokinetic parameters in these treatment groups. The results indicated that ZTO showed no influence on rat hepatic CYP2B6 activity in vivo.
Effect of ZTO on CYP2D6 in cirrhotic rats
As could be seen from Table 5, the Cmax and AUC(0~∞) of metoprolol in model group were decreased significantly by 52.94% and 64.08% (P < 0.05 and P < 0.01) compared to those of control group, and CL of metoprolol in model group was increased significantly by 169.73% (P < 0.01) compared to this of control group. After treatment with ZTO 100 mg/kg, AUC(0~∞) of metoprolol was increased significantly by 109.58% (P < 0.05) compared to this of model group, and CL of metoprolol was decreased significantly by 50.83% (P < 0.05) compared to this of model group. After treatment with ZTO 400 mg/kg, AUC(0~∞) of metoprolol was increased significantly by 47.16% (P < 0.05) compared to this of model group. The pharmacokinetic parameters of metoprolol in rats showed no significant difference between model group and ZTO group at a dose of 200 mg/kg. The results indicated that metabolism of metoprolol in model group was evidently speeded up, and ZTO with the dose of 100 mg/kg and 400 mg/kg had the potential to inhibit cirrhotic rat hepatic CYP2D6 activity in vivo.
Effect of ZTO on CYP3A4 in cirrhotic rats
As could be seen from Table 6, the CL of midazolam in ZTO group at a dose of 100 mg/kg were decreased significantly by 50.69% and 53.07% (P < 0.05) compared to those of control and model group. No apparent influences were observed on other pharmacokinetic parameters in these treatment groups. The results indicated that ZTO showed no influence on rat hepatic CYP3A4 activity in vivo.
Discussion
Modern research has shown that CYP enzymes can be induced or inhibited by exogenous materials such as drugs. Changes in CYP levels or activities can affect the concentration of drug in the blood, the pharmacokinetic process and biological medicinal properties [13]. Currently, Europe and the United States require that drug screens and metabolic research based on the CYP system should be included in new drug evaluations. This methodology is also required by the Chinese SFDA for pharmacokinetic research in new chemical drug development.
Curcuma wenyujin Y. H. Chen et C. Ling, which is mainly produced in Wenzhou, Zhejiang Province, is a Chinese herb traditionally used to treat various external or internal inflammatory conditions such as arthritis, colitis and hepatitis [14]. The essential oils are considered as the active part of Curcuma wenyujin, and turmeric is mainly known for its excellent ability to preserve food, and is approved as food additive in most Western countries. Now, ZTO injection is among the most commonly used herbal drugs by chronic disease patients in China. Herbal medicines are often used concomitantly with synthetic drugs in China; ZTO injection is of no exception. It is increasingly important to elucidate/identify potential herb-drug interactions between ZTO and synthetic drugs so that ZTO can be used safely and effectively. Hence, in the present study, we investigated the effect of ZTO on the activity of six major CYP isozymes (CYP1A2, CYP2C9, CYP2C19, CYP2B6, CYP2D6 and CYP3A4) in rats with liver cirrhosis induced by thioacetamide.
In this study, the changes in the enzyme activity of CYP1A2, CYP2C9, CYP2C19 and CYP2D6 increased in cirrhotic rats induced by TAA, the changes in the enzyme activity of CYP2B6 and CYP3A4 seemed to be not considerable in rats with liver cirrhosis induced by TAA. It suggested that ZTO could not influence the activity of CYP1A2 in vivo in cirrhotic rats induced by TAA. In addition, the study also found that ZTO at low dosage may induce the activity of CYP2B6 and inhibit the activity of CYP3A4 in vivo in cirrhotic rats induced by TAA. However it remains uncertain yet whether ZTO at low dosage influences the effect on CYP450 activities in rats with liver cirrhosis induced by thioacetamide. Further more studies are required to fully assess the effects of ZTO at low dosage in terms of CYP.
According to our results, ZTO at low dosage could inhibit the activities of CYP450 isoforms CYP2C9 and CYP2D6 in vivo in cirrhotic rats induced by TAA, while ZTO at high dosage could induce the activity of CYP2C19 in vivo in cirrhotic rats induced by TAA. This has certain guiding significance to clinical treatment. In the case of about 16% of common drugs’ metabolism through CYP2C9, when these drugs are orally given to patients with cirrhosis, we must pay close attention to dosage and appropriately reduce the dose of the drug, otherwise, the plasma drug concentration may be too high and have a poisoning effect [15]. Furthermore, in the case of about 50% of prescription drugs metabolism through CYP2C9, when orally given to patients with cirrhosis, the dose must be increased, so as not to hinder the effect of the drug. However, it is noted that the human case differs from rat; therefore, the result is only provided for clinical reference.
Acknowledgements
This work was supported by fund of science and technology project of Zhejiang province scientific department (2012C37101).
Disclosure of conflict of interest
None.
References
- 1.Xia QHZ, Li SP, Zhang P, Wang J, He LN. The experiment study of the anti-virus effects of zedoary oil on influenzavirus and respiratory syncytial virus. Chinese Pharmacological Bulletin. 2004;20:357–358. [Google Scholar]
- 2.You J, Cui FD, Han X, Wang YS, Yang L, Yu YW, Li QP. Study of the preparation of sustained-release microspheres containing zedoary turmeric oil by the emulsion-solvent-diffusion method and evaluation of the self-emulsification and bioavailability of the oil. Colloids Surf B Biointerfaces. 2006;48:35–41. doi: 10.1016/j.colsurfb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Sun XY, Zheng YP, Lin DH, Zhang H, Zhao F, Yuan CS. Potential anti-cancer activities of Furanodiene, a Sesquiterpene from Curcuma wenyujin. Am J Chin Med. 2009;37:589–596. doi: 10.1142/S0192415X09007077. [DOI] [PubMed] [Google Scholar]
- 4.Yang FQ, Wang YT, Li SP. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J Chromatogr A. 2006;1134:226–231. doi: 10.1016/j.chroma.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 5.Sun DX, Fang ZZ, Zhang YY, Cao YF, Yang L, Yin J. Inhibitory effects of curcumenol on human liver cytochrome P450 enzymes. Phytother Res. 2010;24:1213–1216. doi: 10.1002/ptr.3102. [DOI] [PubMed] [Google Scholar]
- 6.Ali SO, Darwish HA, Ismail NA. Modulatory effects of curcumin, silybin-phytosome and alpha-R-lipoic acid against thioacetamide-induced liver cirrhosis in rats. Chem Biol Interact. 2014;216:26–33. doi: 10.1016/j.cbi.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Yoo HH, Kim NS, Lee J, Sohn DR, Jin C, Kim DH. Characterization of human cytochrome P450 enzymes involved in the biotransformation of eperisone. Xenobiotica. 2009;39:1–10. doi: 10.1080/00498250802509448. [DOI] [PubMed] [Google Scholar]
- 8.Lewis DF. P450 structures and oxidative metabolism of xenobiotics. Pharmacogenomics. 2003;4:387–395. doi: 10.1517/phgs.4.4.387.22752. [DOI] [PubMed] [Google Scholar]
- 9.Daly AK. Pharmacogenetics of the cytochromes P450. Curr Top Med Chem. 2004;4:1733–1744. doi: 10.2174/1568026043387070. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann T, Muller A, Machnik G, Franke H, Schubert H, Dargel R. Biochemical and morphological studies on production and regression of experimental liver cirrhosis induced by thioacetamide in Uje: WIST rats. Z Versuchstierkd. 1987;30:165–180. [PubMed] [Google Scholar]
- 11.Torres MI, Fernandez MI, Gil A, Rios A. Dietary nucleotides have cytoprotective properties in rat liver damaged by thioacetamide. Life Sci. 1998;62:13–22. doi: 10.1016/s0024-3205(97)01033-3. [DOI] [PubMed] [Google Scholar]
- 12.Gu K, Zhao JD, Ren ZG, Ma NY, Lai ST, Wang J, Liu J, Jiang GL. A natural process of cirrhosis resolution and deceleration of liver regeneration after thioacetamide withdrawal in a rat model. Mol Biol Rep. 2011;38:1687–1696. doi: 10.1007/s11033-010-0281-1. [DOI] [PubMed] [Google Scholar]
- 13.Flockhart DA, Oesterheld JR. Cytochrome P450-mediated drug interactions. Child Adolesc Psychiatr Clin N Am. 2000;9:43–76. [PubMed] [Google Scholar]
- 14.Bengmark S, Mesa MD, Gil A. Plant-derived health: the effects of turmeric and curcuminoids. Nutr Hosp. 2009;24:273–281. [PubMed] [Google Scholar]
- 15.Wang X, Han A, Wen C, Chen M, Chen X, Yang X, Ma J, Lin G. The effects of H2S on the activities of CYP2B6, CYP2D6, CYP3A4, CYP2C19 and CYP2C9 in vivo in rat. Int J Mol Sci. 2013;14:24055–24063. doi: 10.3390/ijms141224055. [DOI] [PMC free article] [PubMed] [Google Scholar]


