Abstract
The role played by recently discovered novel cytokine IL-33 in controlling T-helper (Th)1 and Th2 cytokines under conditions of diabetic nephropathy (DN) is less well studied. In the present study, we estimated the levels of IL-33 along with both Th1 and Th2 cytokines in the serum of normal glucose tolerant (NGT), diabetic subjects with (DN) or without nephropathy (DM) and correlated it with the clinical risk factors of diabetes and nephropathy. 222 study subjects were recruited from the Chennai Urban Rural Epidemiology Study (CURES): 61 NGT, 79 DM and 82 DN. IL-33 level was estimated by ELISA while other Th1 (IL-12, IFN-gamma and IL-2) and Th2 (IL-4, IL-5 and IL-13) cytokines were measured using a Bio-plex bead assay. DM subjects showed a mixed Th1-Th2 profile (increased IFN-g, IL-12, IL-4 and IL-13 and decreased IL-33) while DN subjects showed enhanced Th1 profile (increased IFN-g, IL-2 and IL-12) with suppression of Th2 cytokine (decreased IL-33 and IL-13). The IL-33 levels showed a serial decline with increasing severity of insulin resistance and microalbuminuria. DN was associated with enhanced Th1 response and suppression of Th2 responses which might be due to inreased levels of IL-12 and decreased levels of IL-33 cytokines respectively.
Keywords: Diabetic nephropathy, IL-33, IL-2, IL-12, IFN-γ, IL-4, IL-5, IL-13
Introduction
Type-2 diabetic nephropathy (DN) is the most common cause of end-stage renal disease among patients undergoing renal dialysis [1]. Apart from the traditional metabolic and hemodynamic risk factors, chronic inflammation is increasingly being recognized as a major risk factor for DN [2,3]. Macrophages have long been recognized as key players in insulin resistance (IR) [4] and vascular (both micro and macro) complications in diabetes [5]. Recently, T cells are increasingly being recognized as key players in macrophage recruitment and activation, there by setting in the stage for chronic inflammation [6]. Th1, Th2, Th17, Treg, and cytotoxic T cells have been implicated in the development and progression of DN [6].
The role played by pro-inflammatory cytokines such as TNF-alpha, IL-6 and IL-1beta in DN has been well studied [7]. However, the role played by Th1 and Th2 cytokines in DN is less well explored. From recent animal studies it appears that, while Th1 cytokines promote IR, Th2 cytokines ameliorate it [8]. Whether the same cytokines are involved in kidney damage as seen in DN is not known. However, increased infiltration of activated T cells and aberrant expression of T cell cytokines in diabetic kidney have been reported [9]. While IL-12 has been identified as a master regulator of Th1 differentiation, the corresponding master regulator of Th2 differentiation had proved to be elusive. Recently IL-33 has emerged as the master regulator of Th2 differentiation [10] and few animal studies have implicated its involvement in obesity [11], insulin resistance [12] and kidney disease [13]. In the present study, our objective was to estimate the levels of the newly discovered IL-33 along with both Th1 and Th2 cytokines in subjects with DN and to correlate it with clinical risk factors associated with DM (IR and glycated haemoglobin (HbA1c)) and DN (Urea, creatinine, GFR and microalbuminuria).
Methods
Study population
All the participants were recruited from the Chennai Urban Rural Epidemiology Study (CURES), an ongoing large epidemiological study conducted on a representative population of Chennai (formerly Madras). The methodology of the study has been published elsewhere [14]. Briefly, in Phase-1 of the urban component, 26,001 individuals were recruited based on a systematic random sampling technique. Fasting plasma glucose (FPG) was determined using the One Touch Basic glucometer (Lifescan, Johnson & Johnson, Milpitas, CA) in all subjects. In Phase-2, detailed studies of diabetic complications, including nephropathy and retinopathy were performed, and in Phase-3, every 10th individual in Phase 1 was invited to participate in more detailed studies. The study subjects of this study were recruited from Phase-2 and -3.
Inclusion and exclusion criteria
The inclusion criteria were patients within the normal range of white blood cell to minimize the confounding effect of infection. The exclusion criteria were patients with type-1 diabetes and patients with a previous diagnosis of urolithiasis, liver cirrhosis, congestive heart failure, chronic lung diseases, chronic infections and viral hepatitis. Institutional ethical committee approval from the Madras Diabetes Research Foundation Ethics Committee was obtained (MDRF-EC/SOC/2009/05) and written informed consent was obtained from all the study participants. The study was conducted as per the declaration of Helsinki. Classification of NGT (99 or below dg/dL) and DM (126 or above dg/dL) were based on FPG.
Control groups
This study is a twin study which was conducted as part of Th1-Th2 cytokine serum cytokine profiling in subjects with diabetic complications. The NGT and DM groups without complications have already been reported as control groups in one of our recent publications on diabetic-Coronary artery disease (DM-CAD) [15]. The IL-33 levels in NGT and DM have not been reported previously.
Anthropometric and biochemical parameters
Anthropometric measurements including height, weight and waist circumference were obtained using standardized techniques. The body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters. FPG (glucose oxidase-peroxidase method), serum cholesterol (cholesterol oxidase-peroxidase-amidopyrine method), serum triglycerides (glycerol phosphate oxidase-peroxidase-amidopyrine method), high density lipoprotein cholesterol (HDL-C) (direct method-polyethylene glycol-pretreated enzymes), urea and creatinine (Jaffe’s method) were measured using a Hitachi-912 Autoanalyser (Hitachi, Mannheim, Germany). Glycated hemoglobin (HbA1c) was estimated by high pressure liquid chromatography using a variant machine (Bio-Rad, Hercules, CA). The intra- and inter assay coefficient of variation for the biochemical assays ranged between 3.1% and 5.6%.
Diagnosis of diabetic nephropathy
Albumin concentration was measured in a fasting urine sample using an immunoturbidometric assay (Hitachi 902 autoanalyser; Roche Diagnostics) as described earlier [16]. Subjects were classified as normoalbuminuric (≤29 μg), microalbuminuric (30-229 μg) or albuminuric/ overt nephropathy (≥300 μg) based on albumin excretion per mg of creatinine. As per these criteria, 68 of the 82 DN subjects were microalbuminuric while the remaining 14 were albuminuric. The mean inter- and intra-assay coefficients of variation were 3.5% and 4.2%, respectively as determined in our laboratory. Data on serum creatinine, age, sex, and race were then used to calculate the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [17].
Estimation of serum cytokine level
The levels of Th1 and Th2 cytokines (IL-2, IL-12, IFN-γ, IL-4, IL-5, and IL-13) in the serum were measured using a Bio-Plex multiplex cytokine bead assay system (Bio-Rad). The lower detection limits were 16 pg/mL for IL-2, 0.3 pg/mL for IL-4, 2.08 pg/mL for IL-5, 2.78 pg/mL for IL-12, 2.22 pg/mL for IL-13, 2.14 pg/mL for IFN- γ. IL-33 levels were estimated by ELISA as per the instructions of the kit (eBiosciences). The lower detection limit was 2 pg/mL. The intra- and inter-assay coefficients of variation for multiplex assay were less than 5% as determined in our lab.
Sample size calculation
It is a cross-sectional observational study. Initially, 20 NGT, 20 DM and 20 DN subjects were used for analysis. On the basis of the preliminary results, with a confidence interval of 95%, an estimated P value <0.05, and a power of 80%, the present sample size of 50 per group was calculated. However, we included few more samples in each group to account for the wide variation generally seen among serum biomarkers (based on our previous experience) and derived the present sample size which includes 61 NGT, 79 DM and 82 DN.
Statistical analyses
Student t-test was used to compare groups for continuous variables which followed normal distribution, whereas χ2 test or Fisher exact test (as appropriate) was used to compare proportions. Kruskal-Wallis test was used for multiple parameters that did not show normal distribution. Spearman’s correlation analysis was carried out to determine the association of clinical paramaters with the cytokines. Multivariate logistic regression analysis was used to determine the association of cytokines with the study groups. Multiple comparisons were corrected using the Holm’s correction for each set of analysis. All the analyses were done using SPSS statistical package (Version 20.0; SPSS, Chicago, IL) and p value less than 0.05 was considered significant.
Results
Patient characteristics
Table 1 shows the clinical and biochemical characteristics of the study subjects. Age was significantly higher in DN groups compared to NGT and DM (P = 0.001). BMI showed a linear increase from NGT to DN group (P < 0.001). Subjects with DN had higher blood pressure (both systolic and diastolic) compared to NGT and DM groups (P < 0.001). The FPG and HbA1c values were significantly higher in the DM and DN groups (P < 0.001). DN subjects had significantly lower levels of HDL (P = 0.041), LDL (P = 0.001) and higher levels of VLDL (P < 0.001) and triglycerides (P < 0.001), while the cholesterol levels were not significantly different between the groups. DN subjects also had significantly elevated levels of urea (P = 0.003), creatinine (P = 0.011), microalbuminuria (P < 0.001) and GFR (P = 0.002). Based on GFR values and as per the renal association criteria, 54 (66%) were in stage 1 (GFR ≥ 90), 19 (23%) in stage 2 (GFR = 60-89), 8 (10%) in stage 3 (GFR = 30-59) and 1 (1%) in stage 4 (GFR = 15-29) of CKD. None were in stage 5 (GFR ≤ 15).
Table 1.
Clinical and biochemical characteristics of the study subjects
| Clinical Parameters | NGT (n = 61) | DM (n = 79) | DN (n = 82) | P Value |
|---|---|---|---|---|
| Age (Year) | 46.2±16 | 54.7±13.4 | 56.3±10.9 | 0.001 |
| Gender (F/M) | 30/31 | 30/49 | 36/46 | NS |
| BMI (kg/m2) | 22.9±5.0 | 25.2±4.2 | 27.4±5.6 | <0.001 |
| Systolic BP (mm Hg) | 118.9±20.7 | 126.7±18.2 | 137.4±14.4 | <0.001 |
| Diastolic BP (mm Hg) | 72.6±11.3 | 78.5±10 | 83.38±9.4 | <0.001 |
| FPG (mg/dL) | 84.5±8.8 | 158.7±67.1 | 166±59.4 | <0.001 |
| Hb1Ac levels (%) | 5.3±0.4 | 8.3±2.0 | 9.55±17.6 | <0.001 |
| Cholesterol (mg/dL) | 178.5±53.1 | 177.6±45.0 | 164.4±38 | 0.138 |
| Triglyceride (mg/dL) | 108.1±50.8 | 164.6±92.5 | 174.4±107.1 | <0.001 |
| HDL (mg/dL) | 42.9±7.9 | 40.1±8.5 | 39.32±7.6 | 0.041 |
| LDL (mg/dL) | 114.1±51.5 | 103.2±39.9 | 88.94±31.2 | 0.001 |
| VLDL (mg/dL) | 21.4±10.1 | 31.3±13.3 | 33.90±20.3 | <0.001 |
| Urea (mg/dL) | 20.6±5.1 | 23.5±9.5 | 26.82±11.7 | 0.003 |
| Creatinine (mg/dL) | 0.76±0.10 | 0.88±0.24 | 0.92±0.29 | 0.011 |
| Malb (mg/dL) | 17.2±47.5 | 19.6±20.6 | 107.1±73.5 | <0.001 |
| GFR | 96.99±18.9 | 93.06±23.1 | 78.06±21.5 | 0.002 |
| Treatment | Nil | OHD | Statins, OHD and Insulin |
BMI-Body mass index, BP-blood pressure, FPG-fasting plasma glucose, Hb1Ac-glycated haemoglobin, HDL-high-density lipoprotein, LDL-low-density lipoprotein, VLDL-very low-density lipoprotein, Malb-Micoalbuminaria, GFR-glomerular filtration rate, OHD-Oral hypoglycaemic drugs and NS-Non significant.
Serum cytokine profile
To determine the serum Th1 cytokine profile, the levels of IL-2, IL-12 and IFN-gamma were estimated in the study groups (Figure 1). As shown in Figure 1, the Th1 cytokine IFN-gamma levels were found to be increased in DM and stayed higher in DN (GM (Range): NGT-7.3 (0.56-90) pg/ml vs. DM-297 (0.57-9495) Vs DN-108.9 (1.12-5013) pg/ml; P = 0.0011). The IL-12 levels showed a liner increase from NGT to DM to DN (GM (Range): NGT-2.1 (0.23-10.7) pg/ml vs. DM-2.8 (0.26-224) pg/ml vs. DN-2.9 (0.24-58) pg/ml; P < 0.05). The IL-2 levels were significantly elevated in DN compared to DM and NGT (GM (Range): NGT-2.3 (0.37-7.7) pg/ml Vs DM-2.1 (0.21-28.1) pg/ml vs. DN-3.7 (0.51-150); P < 0.05). Similarly, to determine the serum Th2 cytokine profile the levels of IL-33, IL-4, IL-5 and IL-13 were estimated in the study groups (Figure 2). As can be seen in Figure 2, the IL-33 levels showed a linear decline from NGT to DM to DN (GM (Range): NGT-348.0 (318.2-380.6) pg/ml vs. DM-118.6 (72.6-193.7) pg/ml vs. DN-45.98 (27.98-75.56); P < 0.05). The IL-4 levels were significantly elevated in DM but not in DN groups compared to NGT (GM (Range): NGT-0.12 (0.01-0.52) pg/ml vs. DM-0.24 (0.06-95) pg/ml vs. DN-0.12 (0.01-9.8); P < 0.05). IL-13 levels were found to be significantly increased in DM and decreased in DN representing Th2 suppression (GM) (Range): NGT-2.1 (0.60-6.24) pg/ml Vs DM-17.52(0.40-747.4) pg/ml vs. DN 3.36 (0.28-130.4) pg/ml; P < 0.001). IL-5 levels were not significantly altered among the groups. The Th1-Th2 cytokine profile (except for IL-33) in NGT and DM has recently been reported in our twin publication on DM-CAD [15].
Figure 1.
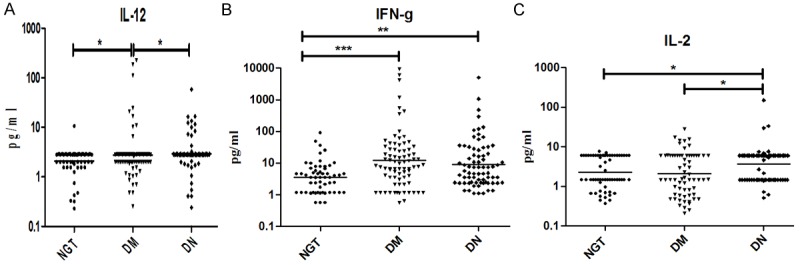
T-helper 1 serum cytokine levels in NGT, DM and DN subjects: (A) Interleukin (IL)-12, (B) Interferon-g (IFN-g) and (C) IL-2. The geometric mean is represented by the horizontal bars. P values were calculated by Kruskal-Wallis one-way analysis of variance. P < 0.05 was considered significant.
Figure 2.
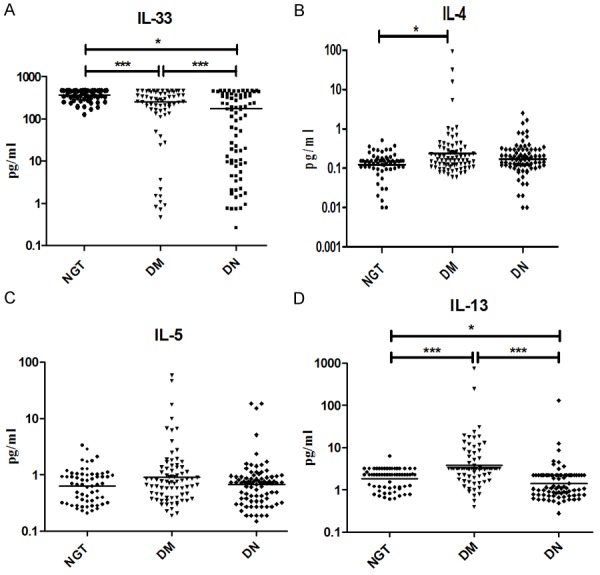
T-helper 2 serum cytokine levels in NGT, DM and DN subjects: (A) interleukin (IL)-33, (B) IL-4, (C) IL-5 and (D) IL-13. The geometric mean is represented by the horizontal bars. P values were calculated by Kruskal-Wallis one-way analysis of variance. P < 0.05 was considered significant.
Correlation analysis
Table S1 shows Spearman’s correlation between the mean cytokine levels and the biochemical parameters of the study subjects. Age did not show significant correlation with any of the cytokines. BMI (r = -.151; P = 0.036), systolic (r = -.161; P = 0.025) and diastolic BP (r = -0.158; P = 0.028) showed significant correlation with IL-33. FPG showed positive correlation with IL-2 (r = 0.15, P = 0.032), IL-12 (r = 0.12, P = 0.011), IFN-gamma (r = 0.17; P = 0.013), IL-4 (r = 0.16, P = 0.021) and IL-5 (r = 0.17; P = 0.010) and negative correlation with IL-33 (r = -0.284; P < 0.001). Cholesterol showed negative correlation with IFN-gamma (r = -0.16, P = 0.019), IL-5 (r = -0.15, P = 0.025) and positive correlation with IL-13 (r = 0.17, P = 0.011). While triglyceride showed negative correlation (r = -0.216; P = 0.003), HDL levels (r = .147; P = 043) showed positive correlation with IL-33. LDL showed negative correlation with IL-2 (r = -0.144, P = 0.041), IFN-gamma (r = -0.160, P = 0.019) and IL-4 (r = -0.164, P = 0.016) and positive correlation with IL-33 (r = 0.153; P = 0.036) and IL-13 (r = 0.170, P = 0.013). VLDL showed a negative correlation only with IL-33 (r = -.189; P = 0.012) and positive correlation with IL-13 (r = 0.187, P = 0.008). Glycated hemoglobin showed a positive correlation with IL-12 (r = 0.15, P = 0.023), IFN-gamma (r = 0.19; P = 0.006), IL-4 (r = 0.18, P = 0.007) and IL-5 (r = 0.14; P = 0.045) and negative correlation with IL-33 (r = -0.32; P < 0.001). While urea showed a negative correlation with IL-13 (r = -0.18, P = 0.008), creatinine showed a positive correlation with IL-2 (r = 0.19, P = 0.007) and negative correlation with IL-13 (r = -0.29, P < 0.001). Microalbuminuria showed positive correlation with IL-13 (r = -0.26; P < 0.001) and negative correlation with IL-33 (r = -.308; P < 0.001). GFR showed a negative correlation with IL-13 (r = -0.15; P = 0.031).
Multivariable logistic regression analyses
Table S2 shows the results of multivariate logistic regression analysis with disease phenotype as dependent variable and log transformed cytokine levels as independent variables. With NGT and DM as dependent variables, IL-33 (OR = 0.994; 95% CI = 0.991-0.998; P = 0.001) and IL-13 (OR = 1.005; 95% CI = 1.002-1.008; P = 0.003) were the only cytokines which showed significant association with DM. The significance of the association with IL-13 was not affected when adjusted for FPG but was lost when adjusted for HbA1c. With respect to IL-33, the significance was lost when adjusted for FGP and HbA1c. With DM and DN as dependent variables, IL-12 (OR = 1.250; 95% CI = 1.1-1.3; P = 0.001) and IFN-gamma (OR = 1.020; 95% CI = 1.011-1.030; P = 0.001) showed a positive association while IL-33 (OR = 0.997; 95% CI = 0.994-0.998; P = 024), IL-4 (OR = 0.199; 95% CI = 0.045-0.88; P = 0.034) and IL-13 (OR = 0.427; 95% CI = 0.300-0.609; P = 0.001) showed a negative association with DN. The associations remained significant even after adjusting for FPG and HbA1c.
Discussion
DN is characterized by chronic, low grade non-specific inflammation, which has long been associated with sterile innate immune responses mediated by pro-inflammatory cytokines (TNF-alpha, IL-6 and IL-1beta) [18]. However, recent identification of T cells in the diabetic kidney had induced renewed interest in studying T cell cytokines in this disease condition [19]. While IL-12 serves as the master controller of Th1 differentiation, IL-33 has recently been identified as a master controller of Th2 differentiation. However compared to IL-12 the role played by IL-33 in DN is not known. As a first step towards this goal we evaluated the serum Th1-Th2 cytokine levels along with IL-33 levels in NGT, DM and DN subjects. The salient findings of the study are: 1) DM subjects showed a mixed Th1-Th2 phenotype with significantly elevated levels of IFN-gamma, IL-12, IL-4 and IL-13 and decreased levels of IL-33, 2) DN subjects showed a clear enhanced Th1 phenotype with significantly elevated levels of IFN-gamma, IL-12 and IL-2 and suppressed levels of Th2 cytokine (IL-33 and IL-13) and 3) While most Th1 cytokines (IFN-gamma and IL-2) served as significant risk factors against DN, most Th2 cytokines (IL-4, IL-13 and IL-33) showed significant protection again DN (as determined by regression analysis).
The DM group showed a mixed Th1-Th2 profile with increased levels of IFN-gamma, IL-12, IL-4 and IL-13 and reduced levels of IL-33. Previously, we have reported the same immune phenotype in subjects with metabolic syndrome, a major risk factor for diabetes (if not already present) [20]. However the IL-33 levels were not reported in that study [20]. IFN-gamma had been implicated not only in obesity but also with worsening of insulin resistance [21]. Animal studies have reported early infiltration of CD4+ and CD8+ T cells with a Th1 phenotype into the visceral adipose tissue in diet-induced obese (DIO) mice [22]. Further, immune mediated depletion of Th1 cells in DIO mice showed increased glucose tolerance and insulin sensitivity [23]. IL-12 was found to be elevated in obese subjects with insulin resistance [24] and also in diabetic subjects with cardiovascular complications [25]. Our study is well in agreement with these reports with increased levels of both IL-12 and IFN-gamma in the DM group. Along with Th1 cytokines, a significant up-regulation of Th2 cytokines IL-4 and IL-13 was seen in the DM group. However IL-33 which has recently been identified as a master regulator of Th2 differentiation was downregulated in DM subjects. The role played by Th2 cytokines in IR is still an enigma. Moreover, data available on the Th2 serum cytokines in DM are scant. In a recent study, decreased serum levels of IL-13 in T2DM subjects was reported and was implicated in impaired glucose uptake and metabolism [26]. Chang et al., have demonstrated the role played by IL-4 in improving insulin sensitivity and glucose tolerance in DIO mice [27]. Recently IL-33 was found to be negatively associated with BMI and serum lipid parameters in non diabetic subjects [12]. Thus with the current findings and in the light of available literature, the increased levels of Th2 cytokines in DM implicate a counter measure to inhibit Th1 immunity and there by IR (with IL-33 being an exception).
The DN group showed a clear Th1 profile (with significantly elevated levels of IFN-gamma, IL-12 and IL-2) with concomitant Th2 suppression (with significant lower levels of Th2 cytokine IL-33, IL-4 and IL-13). Apart from IL-13, a small but statistically non-significant decrease in the IL-4 levels was also seen in the DN group compared to DM. Thus, when diabetic subjects develop nephropathy, the systemic inflammation becomes more Th1 like from a mixed Th1-Th2 profile. Diabetic nephropathy is the pathological outcome of end stage renal failure because of changed hemodynamics, hypertrophy of glomerular structures, thickening of the basement membrane, oxidative stress and inflammatory cytokines [28]. Diabetic kidney was found to promote pro-inflammatory actions and was implicated in T cell recruitment and activation [29]. Activated T cells are thought to be part of an immune-mediated attack associated with the development of proteinuria in diabetic nephropathy [30]. Our results are in agreement with the report of Wu et al., [24] which demonstrates significant Th1 profile at the serum cytokine levels in DN subjects. However, while they found no difference in serum Th2 cytokines we found a strong downmodulation of these cytokine in DN subjects. Arababadi et al., has previously reported increased serum levels of IFN-gamma and decreased levels of IL-17 in DN [26]. However the levels of Th2 cytokines were not reported in their study. In the present study, the exact cause for low levels of Th2 cytokines in DN is currently not known. However the significant decline in serum IL-33 levels, as seen in DN subjects could be one of the major factors associated with the decline of Th2 cytokines. It was also seen that, compared to effectors Th2 cytokines (IL-4 and IL-13), a steep decline in the master regulator (IL-33) was seen even in the DM stage. It is important to note that at least 77% of the DN subjects were in early stage (1 or 2) of the disease which could be a reason for the modest decline in IL-4 levels. The negative correlation seen between IL-33 and IL-13 with most of the diabetogenic and renal parameters and a negative association of IL-33 and IL-13 with DN indicates some protective association between Th2 cytokines and DN.
In conclusion, from the present study and other available data, it seems, transition from NGT to DM is associated with mixed Th1 and Th2 immune response, while transition from DM to DN (which occurs during late stages of the disease) is associated with strong enhancement of Th1 and down regulation of Th2 cytokine responses. The major limitation of our study is its cross-sectional nature, which means that no cause and effect relationship can be drawn. Despite the study being cross-sectional in nature, it gains importance in the context of it being conducted in a high-risk ethnic population where no report on this topic is currently available. Upregulation of Th1 master regulator (IL-12) and down regulation of Th2 master regulator (IL-33) much before the development of DN could be one of the major factors responsible for the enhanced Th1 and suppressed Th2 profile as seen in DN subjects.
Acknowledgements
We thank the epidemiology team members of MDRF for conducting the CURES field studies. This is the 134th publication from CURES. The project was funded by DAE-BRNS grant (2012/37B/11/BRNS/1936).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Striker GE, Agodoa LL, Held P, Doi T, Conti F, Striker LJ. Kidney disease of diabetes mellitus (diabetic nephropathy): perspectives in the United States. J Diabet Complications. 1991;5:51–52. doi: 10.1016/0891-6632(91)90014-g. [DOI] [PubMed] [Google Scholar]
- 2.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 3.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep. 2006;6:463–468. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 5.Shikata K, Makino H. Role of macrophages in the pathogenesis of diabetic nephropathy. Contrib Nephrol. 2001:46–54. doi: 10.1159/000060147. [DOI] [PubMed] [Google Scholar]
- 6.Wu CC, Sytwu HK, Lu KC, Lin YF. Role of T cells in type 2 diabetic nephropathy. Exp Diabetes Res. 2011;2011:514738. doi: 10.1155/2011/514738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ, Liu Y, Zhang J, Goswami C, Lin H, Dominguez JH. Comprehensive genomic profiling in diabetic nephropathy reveals the predominance of proinflammatory pathways. Physiol Genomics. 2013;45:710–719. doi: 10.1152/physiolgenomics.00028.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Zeyda M, Wernly B, Demyanets S, Kaun C, Hammerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, Prager G, Wojta J, Stulnig TM. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond) 2013;37:658–665. doi: 10.1038/ijo.2012.118. [DOI] [PubMed] [Google Scholar]
- 12.Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, Al-Arouj M, Behbehani K, Dehbi M, Dermime S. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014;15:19. doi: 10.1186/1471-2172-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caner S, Usluogullari CA, Balkan F, Buyukcam F, Kaya C, Sacikara M, Koca C, Ersoy R, Cakir B. Is IL-33 useful to detect early stage of renal failure? Ren Fail. 2014;36:78–80. doi: 10.3109/0886022X.2013.832313. [DOI] [PubMed] [Google Scholar]
- 14.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)--study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–870. [PubMed] [Google Scholar]
- 15.Madhumitha H, Mohan V, Deepa M, Babu S, Aravindhan V. Increased Th1 and suppressed Th2 serum cytokine levels in subjects with diabetic coronary artery disease. Cardiovasc Diabetol. 2014;13:1. doi: 10.1186/1475-2840-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, Mohan V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30:2019–2024. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro JF, Mora C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. ScientificWorldJournal. 2006;6:908–917. doi: 10.1100/tsw.2006.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon JY, Jeong KH, Lee TW, Ihm CG, Lim SJ, Lee SH. Aberrant recruitment and activation of T cells in diabetic nephropathy. Am J Nephrol. 2012;35:164–174. doi: 10.1159/000334928. [DOI] [PubMed] [Google Scholar]
- 20.Surendar J, Mohan V, Rao MM, Babu S, Aravindhan V. Increased levels of both Th1 and Th2 cytokines in subjects with metabolic syndrome (CURES-103) Diabetes Technol Ther. 2011;13:477–482. doi: 10.1089/dia.2010.0178. [DOI] [PubMed] [Google Scholar]
- 21.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 22.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 23.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra M, Kumar H, Bajpai S, Singh RK, Tripathi K. Level of serum IL-12 and its correlation with endothelial dysfunction, insulin resistance, proinflammatory cytokines and lipid profile in newly diagnosed type 2 diabetes. Diabetes Res Clin Pract. 94:255–261. doi: 10.1016/j.diabres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Alvarez K, Solis-Lozano L, Leon-Cabrera S, Gonzalez-Chavez A, Gomez-Hernandez G, Quinones-Alvarez MS, Serralde-Zuniga AE, Hernandez-Ruiz J, Ramirez-Velasquez J, Galindo- Gonzalez FJ, Zavala-Castillo JC, De Leon-Nava MA, Robles-Diaz G, Escobedo G. Serum IL-12 is increased in Mexican obese subjects and associated with low-grade inflammation and obesity-related parameters. Mediators Inflamm. 2013:967067. doi: 10.1155/2013/967067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang LQ, Franck N, Egan B, Sjogren RJ, Katayama M, Duque-Guimaraes D, Arner P, Zierath JR, Krook A. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in Type 2 diabetic patients involves microRNA let-7. Am J Physiol Endocrinol Metab. 2013;305:E1359–66. doi: 10.1152/ajpendo.00236.2013. [DOI] [PubMed] [Google Scholar]
- 27.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int J Obes (Lond) 2011;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 28.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 29.Wu CC, Chen JS, Lu KC, Chen CC, Lin SH, Chu P, Sytwu HK, Lin YF. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411:700–704. doi: 10.1016/j.cca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Bending JJ, Lobo-Yeo A, Vergani D, Viberti GC. Proteinuria and activated T-lymphocytes in diabetic nephropathy. Diabetes. 1988;37:507–511. doi: 10.2337/diab.37.5.507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


