Abstract
objectives: To evaluate the expression of PBK/TOPK (PDZ-binding kinase/T-LAK cell-originated protein kinase) and its clinical significance in cervical cancer and cervical intraepithelial neoplasia. Methods: PBK/TOPK expression was detected in 28 cases of low-grade cervical intraepithelial neoplasia (CINI), 62 cases of high-grade intraepithelial neoplasia and 80 cases of cervical cancer by immunohistochemistry (IHC). Then, the correlation between PBK/TOPK expression and clinicopathological features was quantitatively analyzed by measuring the positive unit (PU). Results: PBK/TOPK expression was significantly greater in cervical cancer than that in high-grade intraepithelial neoplasia and CINI (P < 0.05). Meanwhile, PBK/TOPK expression in high-grade intraepithelial neoplasia was significantly higher compared with that in CINI (P < 0.05). In addition, PBK/TOPK expression in cervical cancer significantly correlated with histological type, differentiation, lymph node metastasis, vaginal and cervical invasion, TNM stage and tumor size (P < 0.05). Conclusion: PBK/TOPK expression is closely associated with cervical cancer and cervical intraepithelial neoplasia, which may be served as a useful target for tumor diagnosis and immunotherapy.
Keywords: Cervical carcinoma, CINI, T-LAK, PDZ-binding kinase, quantitative analysis
Introduction
Cervical cancer is one of most common malignancy, whose incidence rate is gradually increasing in Chinese women [1,2]. Although patients with cervical cancer have received surgery resection, radiotherapy and chemotherapy, the treatment effect remains unsatisfied. Recently, immunotherapeutic target has received greater attention, which may be effective in eradicating malignancy.
PBK/TOPK is a MAPKK-like serine/threonine kinase, which is hard to be detected in normal tissues except normal testis and fetal tissue [3,4]. Studies have confirmed that PBK/TOPK prevailed in a variety of malignancies, such as lymphoma, leukemia, breast cancer and malignant peripheral nerve sheath tumors [5-8]. PBK/TOPK over-expression is contributed to cell growth and proliferation in breast cancer, colorectal cancer and Ewing sarcoma [9-11]. In lung cancer and colorectal cancer, patients with high-expression of PBK/TOPK have poorer prognosis [12-15]. Meanwhile, studies have confirmed that PBK/TOPK is correlated with apoptosis [16], inflammation [17] and mitotic regulation [18-20], and may be regulated by c-Myc signaling pathway [21].
However, whether PBK/TOPK expression is associated with cervical cancer and cervical intraepithelial neoplasia remains unknown. In this study, we aimed to evaluate the expression of PBK/TOPK and its clinical significance in cervical cancer and cervical intraepithelial neoplasia.
Materials and methods
Patients
All specimens were collected from the Department of Pathology of Nanfang Hospital, Leiyang People’s Hospital and affiliated hospital of Nanhua university during the year of 2006-2010. It contained 28 cases of CINI, 62 cases of high-grade intraepithelial neoplasia and 80 cases of cervical cancer. All tissues were obtained from cervical biopsy and surgery resection. None of patients received radiotherapy or chemotherapy before cervical biopsy and surgical resection. Cervical cancer patients aged from 26 to 70 years, with a mean age of 43.9 years. Clinicopathological features including histological type, differentiation, lymph node metastasis, vaginal and uterine invasion, TNM stage and tumor size were assessed in this study. Histological diagnosis was confirmed by two pathologists. The study was approved by the Ethnics Committee of Southern Medical University, Nanhua University and Leiyang People’s Hospital.
Immunohistochemical staining
The procedures were performed as previously described [14]. Anti-PBK/TOPK antibody (dilution 1:100; Epitomic, California, USA) was incubated for 2 h at room temperature. After incubation for 30 min with the secondary biotinylated antibody (Jingqiao, Beijing, China), the sections were stained with diaminobenzidine (Jingqiao, Beijing, China), and counterstained with haematoxylin. The cervical cancer tissues with known positive and negative expression of PBK/TOPK were used to be as positive control and negative control, respectively.
The results were quantitatively analyzed by measuring the positive unit (PU) of PBK/TOPK in cervical cancer and cervical intraepithelial neoplasia tissues. The PU values were measured as described previously [22-25] by using ImagePro Plus image analysis software (Media Cybernetics, Inc., USA). Staining with orange or brown in nucleus or cytoplasm was regarded as positive expression. A total of 10 fields in representative tissue cores of each specimen were randomly obtained at 40× object lens. The average gray level of positive cells was recorded as Gα, and the background gray level of positive cells was recorded as Gβ. Then, the average PU value of each specimen was calculated by using the equation: PU=|Gα-Gβ|×100/Gmax, and Gmax equals 256.
Statistical analysis
All data were presented as means ± s.d and analyzed by SPSS 13.0 (SPSS, Chicago, IL, USA). PU values of PBK/TOPK in different cervical tissues were compared by ANOVA. The chi-square test was used to assess the correlation between PBK/TOPK expression and clinicopathological features. Statistical significance was defined as a P-value of < 0.05 in two-sided test.
Results
Expression of PBK/TOPK in cervical cancer and cervical intraepithelial neoplasia
PBK/TOPK expression was located in the nucleus or cytoplasm as orange or brown staining, but was hard to be detected in CINI tissues (Figure 1). The PU values of PBK/TOPK in cervical cancer and cervical intraepithelial neoplasia were showed in Figure 2. The results showed that PBK/TOPK expression in cervical cancer was significantly greater than that in high-grade intraepithelial neoplasia and CINI, respectively (P < 0.0001, P < 0.0001, respectively). Meanwhile, the expression of PBK/TOPK in high-grade intraepithelial neoplasia was also significantly higher compared with that in CINI (P=0.001).
Figure 1.
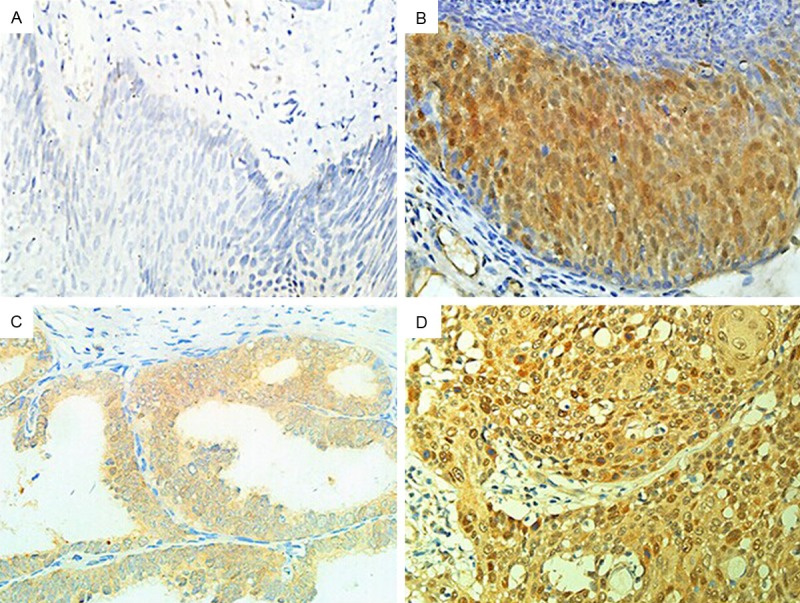
The expression of PBK/TOPK in different types of cervical tissues (40×). A. CINI; B. High-grade cervical intraepithelial neoplasia; C. Adenocarcinoma; D. Squamous carcinoma.
Figure 2.
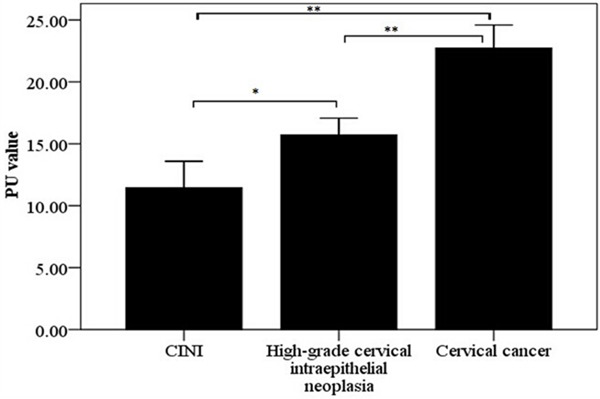
The PU values of PBK/TOPK expression in different types of cervical lesions. *P < 0.05, **P < 0.001.
Correlation between PBK/TOPK expression and clinicopathological features in cervical cancer
Then, we evaluated the correlation between PBK/TOPK expression and clinicopathological features in cervical cancer. The results showed that PBK/TOPK expression was associated with histological type, differentiation, lymph node metastasis, vaginal and cervical invasion, TNM stage and tumor size, but no significant correlation with age (Table 1). The PU values of PBK/TOPK in poorly differentiated tumor were significantly greater than that in well to moderately differentiated tumor (P < 0.0001). Patients with lymph node metastasis presented a higher level of PBK/TOPK PU compared with that those without lymph node metastasis (P=0.001). Meanwhile, The PU values of PBK/TOPK in patients with vaginal and cervical invasion were significantly higher than that those without vaginal and cervical invasion (P=0.008). The PU values of PBK/TOPK in patients with TNM stage II-III were significantly higher in comparison with that those with TNM stage I (P < 0.0001). In addition, PBK/TOPK PU was positively correlated with tumor size (P=0.037).
Table 1.
Correlation between PBK/TOPK expression and clinicopathological features in cervical cancer (X̅ ± s)
| Clinicopathological features | N | PU value | P value |
|---|---|---|---|
| Age (Years) | |||
| < 44 | 42 | 20.98±8.21 | 0.246 |
| ≥ 44 | 38 | 21.26±6.88 | |
| Histological type | |||
| Squamous cell carcinoma | 70 | 21.78±6.79 | 0.012 |
| Adenocarcinoma | 6 | 19.23±11.27 | |
| Adenosquamous carcinoma | 2 | 5.41±0.45 | |
| Carcinoid tumor | 1 | 27.96±0.00 | |
| Small cell carcinoma | 1 | 10.51±0.00 | |
| Differentiation | |||
| Poorly differentiated | 21 | 25.61±4.89 | < 0.0001 |
| Well to moderately differentiated | 55 | 19.82±7.67 | |
| Lymph node metastasis | |||
| Positive | 11 | 28.05±5.31 | 0.001 |
| Negative | 69 | 20.01±7.30 | |
| Vagina and cervix invasion | |||
| Positive | 6 | 24.06±1.65 | 0.008 |
| Negative | 74 | 20.88±7.81 | |
| TNM stage | |||
| I | 65 | 19.82±7.48 | < 0.0001 |
| II-III | 15 | 26.74±5.04 | |
| Tumor size (cm) | |||
| ≤ 1 | 26 | 18.37±8.37 | 0.037 |
| > 1 | 54 | 22.44±6.84 |
Discussion
In this study, we are the first to report PBK/TOPK expression in cervical cancer and cervical intraepithelial neoplasia. In order to avoid subjective bias, PBK/TOPK expression was quantitatively evaluated by measuring the PU value. PU value is the protein expression intensity of positive cells calculated by Image-Pro Plu image analysis software, and the larger value represents the higher level of protein expression [26,27]. Therefore, present results indicated that PBK/TOPK highly expressed in cervical cancer and high-grade intraepithelial neoplasia, but is undetectable in CINI. However, PBK/TOPK level in cervical cancer was significantly higher compared with that in high-grade intraepithelial neoplasia. Moreover, PBK/TOPK has been found to be undetectable in normal cervical tissue [28]. It is well known that low-grade intraepithelial neoplasia (CINI) and high-grade intraepithelial neoplasia have been considered as the precancerous lesion of cervical cancer, which closely associated with the occurrence of cervical cancer [29,30]. Thus, our results confirm that PBK/TOPK expression is associated with the occurrence of cervical cancer, which may be also involved with the progression of cervical intraepithelial neoplasia. In addition, the level of PBK/TOPK expression may be beneficial to distinguish high-grade intraepithelial neoplasia and cervical carcinoma.
Meanwhile, we analyzed the correlation between PBK/TOPK expression and clinicopathological features in cervical cancer. The results showed that PBK/TOPK expression was correlated with histological type, differentiation, lymph node metastasis, vaginal and cervical invasion, TNM stage and tumor size. The expression of PBK/TOPK in different types of cervical cancer suggested that PBK/TOPK might be related to tumor heterogeneity, which was in line with the study reported in non-small cell lung cancer [14]. PBK/TOPK expression in poorly differentiated tumor was dramatically elevated than that in well to moderately differentiated tumor, which suggested that PBK/TOPK expression might be contributed to tumor differentiation. The correlation of PBK/TOPK with tumor size, lymph node metastasis, vaginal and cervical invasion and TNM stage indicated that PBK/TOPK expression might be involved with the progression and invasion of cervical cancer. Furthermore, Shih et al. and Lei et al. also reported that PBK/TOPK correlated with lymph node metastasis and TNM stage and affected patients’ prognosis in lung cancer [12,14]. However, it is not yet clear whether PBK/TOPK expression will affect the prognosis in cervical cancer.
In conclusion, this study showed that PBK/TOPK is closely associated with cervical cancer and cervical intraepithelial neoplasia, which may be served as a useful target for tumor diagnosis and immunotherapy. Of course, our findings also need to be confirmed by further studies.
Acknowledgements
This study was supported by the Science and Technology Projects of Guangdong, China (No. 2010B060300001), the Fund Projects of Key Laboratory in Guangdong Province, China (2013GDDSIPL-01) and the Science and Technology Projects of Guangdong, China (No. 201300000192).
Disclosure of conflict of interest
None.
References
- 1.Li Ni, Zheng RS, Zhang SW, Zhou XN, Zeng HM, Chen WQ. An analysis of incidence and mortality of cervical cancer in China 2003-2007. China Cancer. 2012;21:801–804. [Google Scholar]
- 2.Zhang MQ, Chen MZ. [Analysis of 174 cases with cervical cancer in women under 35 years old] . Zhonghua Fu Chan Ke Za Zi. 2003;38:689–693. [PubMed] [Google Scholar]
- 3.Gaudet S, Branton D, Lue RA. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A. 2000;97:5167–5172. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe Y, Matsumoto S, Kito K, Ueda N. Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J Biol Chem. 2000;275:21525–21531. doi: 10.1074/jbc.M909629199. [DOI] [PubMed] [Google Scholar]
- 5.Simons-Evelyn M, Bailey-Dell K, Toretsky JA, Ross DD, Fenton R, Kalvakolanu D, Rapoport AP. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. Blood Cells Mol Dis. 2001;27:825–829. doi: 10.1006/bcmd.2001.0452. [DOI] [PubMed] [Google Scholar]
- 6.Nandi A, Tidwell M, Karp J, Rapoport AP. Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly down-regulated during terminal differentiation of HL-60 leukemic cells. Blood Cells Mol Dis. 2004;32:240–245. doi: 10.1016/j.bcmd.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Lin ML, Nishidate T, Nakamura Y, Katagiri T. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res. 2006;66:9186–9195. doi: 10.1158/0008-5472.CAN-06-1601. [DOI] [PubMed] [Google Scholar]
- 8.Stricker TP, Henriksen KJ, Tonsgard JH, Montag AG, Krausz TN, Pytel P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol. 2013;26:930–943. doi: 10.1038/modpathol.2012.242. [DOI] [PubMed] [Google Scholar]
- 9.Ayllon V, O’Connor R. PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene. 2007;26:3451–3461. doi: 10.1038/sj.onc.1210142. [DOI] [PubMed] [Google Scholar]
- 10.Zhu F, Zykova TA, Kang BS, Wang Z, Ebeling MC, Abe Y, Ma WY, Bode AM, Dong Z. Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology. 2007;133:219–231. doi: 10.1053/j.gastro.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Herrero-Martín D, Osuna D, Ordóñez JL, Sevillano V, Martins AS, Mackintosh C, Campos M, Madoz-Gúrpide J, Otero-Motta AP, Caballero G, Amaral AT, Wai DH, Braun Y, Eisenacher M, Schaefer KL, Poremba C, de Alava E. Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. Br J Cancer. 2009;101:80–90. doi: 10.1038/sj.bjc.6605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M, Lai JM. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2013;31:2389–2400. doi: 10.1038/onc.2011.419. [DOI] [PubMed] [Google Scholar]
- 13.Wei DC, Yeh YC, Hung JJ, Chou TY, Wu YC, Lu PJ, Cheng HC, Hsu YL, Kuo YL, Chen KY, Lai JM. Overexpression of T-LAK cell-originated protein kinase predicts poor prognosis in patients with stage I lung adenocarcinoma. Cancer Sci. 2012;3:731–738. doi: 10.1111/j.1349-7006.2011.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei B, Liu S, Qi W, Zhao Y, Li Y, Lin N, Xu X, Zhi C, Mei J, Yan Z, Wan L, Shen H. PBK/TOPK expression in non-small-cell lung cancer: its correlation and prognostic significance with Ki67 and p53 expression. Histopathology. 2013;63:696–703. doi: 10.1111/his.12215. [DOI] [PubMed] [Google Scholar]
- 15.Zlobec I, Molinari F, Kovac M, Bihl MP, Altermatt HJ, Diebold J, Frick H, Germer M, Horcic M, Montani M, Singer G, Yurtsever H, Zettl A, Terracciano L, Mazzucchelli L, Saletti P, Frattini M, Heinimann K, Lugli A. Prognostic and predictive value of TOPK stratified by KRAS and BRAF gene alterations in sporadic, hereditary and metastatic colorectal cancer patients. Br J Cancer. 2010;102:151–161. doi: 10.1038/sj.bjc.6605452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zykova TA, Zhu F, Vakorina TI, Zhang J, Higgins LA, Urusova DV, Bode AM, Dong Z. T-LAK cell-originated protein kinase (TOPK) phosphorylation of Prx1 at Ser-32 prevents UVB-induced apoptosis in RPMI7951 melanoma cells through the regulation of Prx1 peroxidase activity. J Biol Chem. 2010;285:29138–29146. doi: 10.1074/jbc.M110.135905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Zhu F, Zykova T, Kim MO, Cho YY, Bode AM, Peng C, Ma W, Carper A, Langfald A, Dong Z. T-LAK cell-originated protein kinase (TOPK) phosphorylation of MKP1 protein prevents solar ultraviolet light-induced inflammation through inhibition of the p38 protein signaling pathway. J Biol Chem. 2011;286:29601–29609. doi: 10.1074/jbc.M111.225813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto S, Abe Y, Fujibuchi T, Takeuchi T, Kito K, Ueda N, Shigemoto K, Gyo K. Characterization of a MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun. 2004;325:997–1004. doi: 10.1016/j.bbrc.2004.10.133. [DOI] [PubMed] [Google Scholar]
- 19.Stricker TP, HenriksenK J, Tonsgard JH, Montag AG, Krausz TN, Pytel P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol. 2013;26:930–943. doi: 10.1038/modpathol.2012.242. [DOI] [PubMed] [Google Scholar]
- 20.Abe Y, Takeuchi T, Kagawa-Miki L, Ueda N, Shigemoto K, Yasukawa M, Kito K. A mitotic kinase TOPK enhances Cdk1/cyclin B1-dependent phosphorylation of PRC1 and promotes cytokinesis. J Mol Biol. 2007;370:231–245. doi: 10.1016/j.jmb.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 21.Hu F, Gartenhaus RB, Zhao XF, Fang HB, Minkove S, Poss DE, Rapoport AP. c-Myc and E2F1 drive PBK/TOPK expression in high-grade malignant lymphomas. Leuk Res. 2013;37:447–454. doi: 10.1016/j.leukres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Shen H. Study on quantitative method of intensity of immunohistochemical staining (II) Journal of Cell and Molecular Immunology. 1994;4:33–35. [Google Scholar]
- 23.Shen H. Study on quantitative method of immunochemical staining (III) Chinese Journal of Histochemistry and Cytochemistry. 1995;4:89–92. [Google Scholar]
- 24.Shen H, Lu YD. Study on quantitative method of immunochemical staining. Journal of Biomedical Engineering. 1993;10:281–284. [Google Scholar]
- 25.Bai XY, Shen H. Mutational analysis of thyroid transcription factor-1 gene (TTF-1) in lung carcinomas. In Vitro Cell Dev Biol Anim. 2008;44:17–25. doi: 10.1007/s11626-007-9062-0. [DOI] [PubMed] [Google Scholar]
- 26.Wan L, Li X, Shen H, Bai X. Quantitative analysis of EZH2 expression and its correlations with lung cancer patients’ clinical pathological characteristics. Clin Transl Oncol. 2013;15:132–138. doi: 10.1007/s12094-012-0897-9. [DOI] [PubMed] [Google Scholar]
- 27.Xu XY, Lin N, Li YM, Zhi C, Shen H. Expression of HAb18G/CD147 and its localization correlate with the progression and poor prognosis of non-small cell lung cancer. Pathol Res Pract. 2013;209:345–352. doi: 10.1016/j.prp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 28.He FR, Huang GS, Wang JH, Wang L, Liu YH, Wang D, Li YF. PBK/TOPK expression in human normal and tumor tissues by tissuem icroarray. J Clin Exp Pathol. 2007;23:450–452. [Google Scholar]
- 29.Boccalon M, Tirelli U, Sopracordevole F, Vaccher E. Intra-epithelial and invasive cervical neoplasia during HIV infection. Eur J Cancer. 1996;32A:2212–2217. doi: 10.1016/s0959-8049(96)00416-9. [DOI] [PubMed] [Google Scholar]
- 30.Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006;118:2048–2055. doi: 10.1002/ijc.21604. [DOI] [PubMed] [Google Scholar]


