Abstract
This study is to examine whether the activation of Rho kinase (ROCK) accounts for hemoglobin (Hb)-induced disruption of blood-brain barrier (BBB) after the occurrence of intracerebral hemorrhage. A model of intracerebral injection of Hb was established in rats. Changes in the levels of mRNA of RhoA, ROCK2 and matrix metalloproteinase-9 (MMP-9) were measured using quantitative real-time polymerase chain reaction. Protein expression of RhoA, ROCK2, claudin-5 and MMP-9, as well as ROCK activity, were determined using Western blotting. Immunohistochemical assay was performed to visualize the expression of RhoA, ROCK2, claudin-5 and MMP-9 in endothelial cells. Hb injection produced a significant increase in BBB permeability and water content in the brain. Significant reduction of claudin-5 expression was detected by Western blotting and immunofluorescence in Hb group. The levels of RhoA and ROCK2 were significantly up-regulated from 6 h to 12 h after Hb injection and were concomitant with the increase in ROCK activity. Immunofluorescence double staining showed enhanced p-myosin light chain immunoreactivity but diminished claudin-5 staining in endothelial cells. Significant up-regulation of MMP-9 expression was detected after Hb injection, and statistical analyses further confirmed a positive correlation of MMP-9 expression with ROCK activity. The results showed that ROCK was activated in endothelial cells by Hb. This may account for the early disruption of the BBB via up-regulation of p-myosin light chain expression and aggravation of injuries to TJ proteins. The activation of ROCK may also increase MMP-9 expression, thereby leading to further BBB disruption.
Keywords: Blood-brain barrier, hemoglobin, Rho kinase, tight junction, intracerebral hemorrhage, matrix metalloproteinase
Introduction
The blood-brain barrier (BBB) is an important physiologic barrier that maintains the homeostasis of the central nervous system. Endothelial intercellular junction proteins, especially endothelial tight junction (TJ) proteins, aid the barrier properties of the BBB [1]. Once the integrity of TJs is weakened, the permeability of the BBB is increased. Endothelial TJs consist of cytoplasmic proteins known as zonula occludens as well as transmembrane proteins such as occludin, claudins and junction adhesion molecules [2]. Claudin-5 is considered to be the most important endothelial TJ protein [3]. It constitutes the “backbone” of TJ strands and determines the specificity and selectivity of paracellular barriers [4].
Hemoglobin (Hb) is an essential degradation product of erythrocytes and initiator of the secondary cascade of brain injury [5]. Hb plays a crucial role in the disruption of BBB as well as the formation of cerebral edema after the occurrence of intracerebral hemorrhage (ICH). In a rabbit model of ICH, Hb and heme were observed in the perihematomal region as early as 24 h after the occurrence of ICH [6]. Intracerebral injection of Hb can directly cause disruption of BBB [7] as well as an increase in the water content of the brain [8]. Studies have reported that, upon injection of Hb, TJ proteins of the BBB display different degrees of changes, and that degradation of TJ proteins facilitates capillary leakage and thereby increases the permeability of the BBB [4]. However, the exact mechanism of Hb-induced BBB disruption is not known.
Ras homolog gene family, member A (RhoA) is a small guanosine triphosphate-binding protein known to regulate actin cytoskeleton in the formation of stress fibers. Rho kinase (ROCK) is a downstream effector of RhoA [9], which is shown to mediate cell contraction, inflammation, vascular leakage and BBB maintenance in vivo [10-12]. In recent years, reports indicated that increase in the activity of ROCK decreased the stabilization and increased the permeability of BBB [10,13]. Activation of ROCK and disruption of BBB have been demonstrated in cerebral ischemia [14], subarachnoid hemorrhage [15], autoimmune encephalomyelitis [13], and malignant brain tumors [16]. However, the potential role of ROCK in Hb-induced BBB disruption after the occurrence of ICH is poorly understood. ROCK has two isoforms (ROCK1 and ROCK2) but only ROCK2 is expressed primarily in the brain [11]. ROCK1 is associated with immunological functions, whereas ROCK2 is more important for the function of endothelial muscles and vascular smooth muscles [16]. It has been reported that endothelial ROCK is activated by multiple inflammatory mediators and cytokines such as thrombin, tumor necrosis factor and angiotensin II in the increase in vascular permeability, but the role of Hb in activation of ROCK is little known [10,11]. Two downstream effectors of ROCK, myosin phosphatase target subunit 1 (MYPT1) and myosin light chain (MLC), which can be transformed into phosphorylated myosin phosphatase target subunit 1 (p-MYPT1) and phosphorylated myosin light chain (p-MLC) after ROCK activation, were used to detect ROCK activation by examining the ratio of p-MYPT1 and MYPT1 as well as p-MLC and MLC using Western blotting [16]. Studies have shown that activation of ROCK may up-regulate the expression of matrix metallopeptidase-9 (MMP-9) [17,18], which is an important detrimental factor for BBB injury during the acute phase of stroke [19]. In addition, ROCK inhibitor fasudil has been shown to significantly reduce MMP-9 mRNA expression and alleviate the permeability of BBB [14]. In the present study, we test whether activation of ROCK is involved in changes of TJ proteins and disruption of BBB induced by Hb after the occurrence of ICH, as well as its mechanism of action.
Materials and methods
Animals
Adult male Sprague-Dawley rats (weights 280 ± 20 g) were obtained from the Animal Experimental Center of Guangdong Province (Guangzhou, P.R. China). Surgery was performed as described previously [9]. Rats of the Hb group were anesthetized using pentobarbital (40 mg/kg, intraperitoneally), a 1-mm burr hole was drilled into the cranium, and 20 μL of rat Hb solution (150 mg/mL in sterile saline, Sigma-Aldrich, St Louis, MO, USA) was infused into the right caudate nucleus (3 mm lateral to the midline, 1 mm anterior to the bregma, and 6 mm below the surface of the skull) using a microsyringe after exposure of the skull. Rats in the saline-injected group were injected with only 20 μL sterile saline in the same manner. Rats in the normal control group were not given any treatment. The study protocols were approved by the Animal Ethics Committee of Southern Medical University (Guangzhou, P.R. China).
Measurement of BBB permeability
Under anesthesia, Evans blue dye (EBD, Wako Pure Chemical Industries, Ltd., Osaka, Japan) was injected (4 mL/kg) via femoral vein 2 h before sacrifice. Rats were perfused with saline via the transcardial route. After decapitation, coronal blocks of the ipsilateral hemisphere were dissected and weighed immediately. After homogenization and centrifugation (15,000 rpm for 20 min at room temperature), supernatants was diluted with pure ethanol (1:3). The fluorescence of EBD was measured with an Automatic Microplate Reader (Spectramax M5; Molecular Devices, Silicon Valley, CA, USA). The tissue content of EBD was represented as μg/g brain weight.
Determination of water content in the brain
Animals were decapitated 6 h, 12 h, 24 h and 72 h after surgery. Coronal slices were cut, and ipsilateral cortex was dissected to obtain a sample of basal ganglia. Each sample was weighed immediately after removal (wet weight) and after drying at 100°C for 24 h (dry weight). The percentage of water content in the brain was calculated using the following equation: Water content in the brain = [(wet weight-dry weight)/wet weight] ×100%.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using a GeneJETTM RNA Purification kit (Thermo Fisher, Waltham, MA, USA). First-strand cDNA was synthesized from RNA using Maxima® First Strand cDNA Synthesis kit (Thermo Fisher). The primers were: RhoA, 5’-CCAAAATGAAGCAGGAGCCG-3’ (forward primer) and 5’-ATGAGGCACCCCGACTTTTT-3’ (reverse primer); ROCK2, 5’-CTGAATGAAATGCAGGCTCAA-3’ (forward primer) and 5’-CCCTGGTCCACTGCCTATAC-3’ (reverse primer); MMP-9, 5’-TCCAGCATCTGTATGGTCGTG-3’ (forward primer) and 5’-TGCAGTGGGACACATAGTGG-3’ (reverse primer); glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5’-ATGATTCTACCCACGGCAAG-3’ (forward primer) and 5’-CTGGAAGATGGTGATGGGTT-3’ (reverse primer). PCR was conducted using Power SYBR® Green PCR Master Mix (Life Technologies Corporation, Carlsbad, CA, USA). Standard curves were plotted to calculate the expression of target genes. By comparing with normal levels (set to 1.0), fold-changes in gene expression were evaluated.
Western blotting
Brain tissues around the lesion sites in right caudate nucleus of 4 rats were homogenized and diluted, total protein was measured using a Bicinchoninic Acid Protein Assay kit (Thermo Fisher). Protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies: rabbit anti-RhoA (1:500; Abcam, USA), rabbit anti-ROCK2 (1:100; Abcam, USA), mouse anti-claudin-5 (1:100; Invitrogen, USA), rabbit anti-MYPT1 (1:500; Cell Signaling Technology, USA), rabbit anti-p-MYPT1 (1:500; Cell Signaling Technology, USA), mouse anti-MMP-9 (1:500; Abcam, USA), mouse anti-MLC (1:100; Cell Signaling Technology, USA), and mouse anti-p-MLC (1:100; Cell Signaling Technology, USA). Horseradish peroxidase (HRP)-labeled goat anti-mouse or goat anti-rabbit IgG were used as secondary antibodies (1:1000; Sigma-Aldrich, USA) to detect the primary antibodies. GAPDH was employed as an internal control to determine and normalize the relative intensity of each band. The densities of the bands were semi-quantitatively analyzed using Image-Pro Plus software (Media Cybernetics, Inc., USA).
Immunohistochemistry
The rat brain was perfused, fixed, and then embedded. Serial sections (4 μm) were cut and stored for immunohistochemistry. According to the instructions, the sections were subjected to antigen retrieval, blocked with normal 5% goat serum, and then incubated with primary antibodies (rabbit monoclonal anti-RhoA, 1:50 dilution, Abcam, USA; rabbit monoclonal anti-ROCK2, 1:50, Abcam, USA; mouse monoclonal anti-p-MLC, 1:100, Cell Signaling Technology, USA; mouse monoclonal anti-MMP-9, 1:50, Abcam, USA) and biotinylated goat anti-rabbit IgG secondary antibody, followed by HRP streptavidin reagent and diaminobenzidine solution. Finally, the sections were stained with hematoxylin. Images were obtained using Leica Application Suite (Leica, Wetzlar, Germany).
Immunofluorescence
After being de-waxed and rehydrated using deionized water, antigen retrieval was carried out as described above followed by incubation with 5% donkey serum for 30 min. Incubation with primary antibodies (mouse monoclonal anti-p-MLC, 1:100 dilution; rabbit monoclonal anti-claudin-5, 1:100; Abcam, USA) at 4°C overnight was then performed. After washing with PBS, the samples were incubated with secondary antibodies (Alexa Fluor 594 donkey anti-mouse IgG, 1:100 dilution, Invitrogen, Carlsbad, CA, USA; Alexa Fluor 488 donkey anti-rabbit IgG, 1:100 dilution, Invitrogen) at 37°C for 1 h. Immunostained images were acquired using a fluorescence microscope (Leica). Semi-quantitative analysis was performed using Image-Pro Plus software (Media Cybernetics, Inc., USA).
Statistical analyses
Statistical analyses were performed using SPSS v19.0 (Chicago, IL, USA). Data were presented as means ± SD. Comparison between two groups was assessed using Student’s t-test. Differences among groups were assessed by one-way ANOVA with the least square difference test. Pearson’s correlation analyses were carried out to examine the correlation between ROCK activity and MMP-9 expression. P < 0.05 was considered statistically significant.
Results
BBB permeability is increased by Hb in a time-dependent manner
To determine the permeability of BBB, EBD leakage in brain tissues was measured. EBD content in the saline group remained relatively constant over time. By contrast, BBB permeability in the Hb group was significantly increased at 6, 12, 24 and 72 h compared with those of normal control group or saline group (P < 0.05). Of note, the maximum BBB permeability occurred at 12 h in the Hb group (P < 0.05) (Figure 1A). These data suggested that BBB permeability was increased by Hb in a time-dependent manner.
Figure 1.
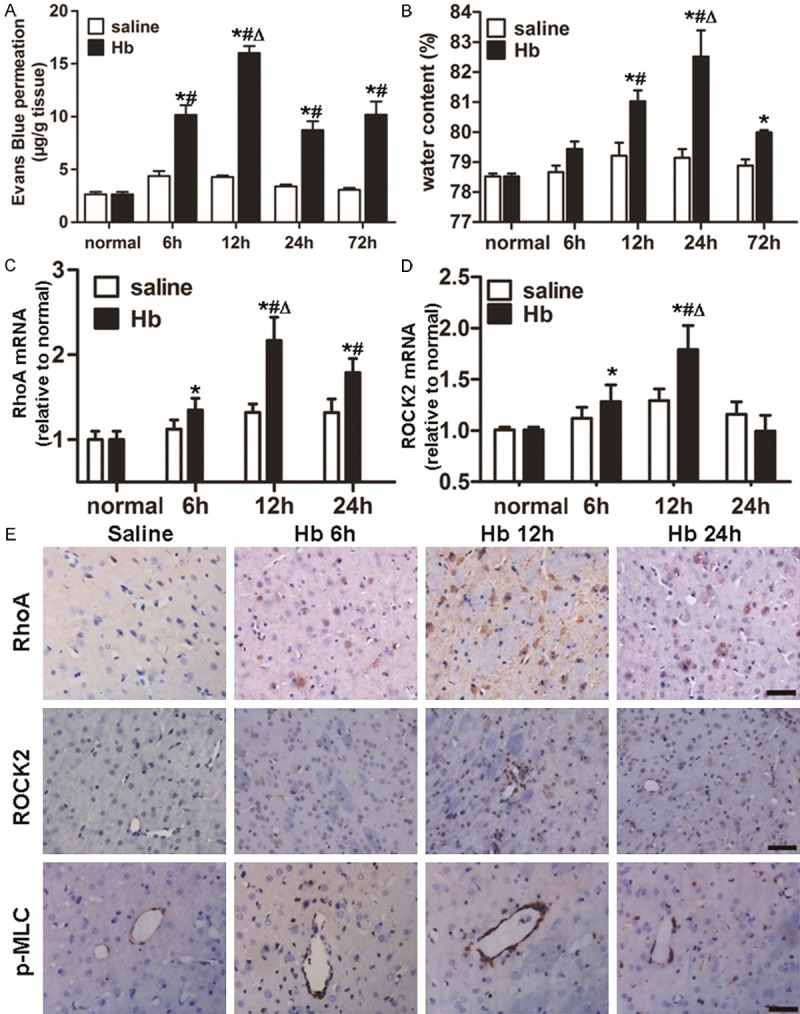
Alterations in BBB permeability, water content in the brain and the expression of RhoA and ROCK2 after Hb injection. (A) Leakage of Evans blue dye and (B) water content in the brain in saline and Hb groups at 6, 12, 24 and 72 h after injection. mRNA expression of (C) RhoA and (D) ROCK2 in saline and Hb groups at 6, 12 and 24 h after injection. Data are means ± SD. *, P < 0.05 compared with normal control group; #, P < 0.05 compared with saline group at respective time points; Δ, P < 0.05 compared with Hb group at other time points. (E) Immunochemical analyses showing RhoA, ROCK2 and p-MLC. Scale bar = 100 μm.
Water content in the brain is enhanced by Hb treatment in a time-dependent manner
To evaluate alterations in water content in the brain, samples of basal ganglia retrieved at 6, 12, 24 and 72 h after surgery were weighed immediately after removal and after drying. The results showed that water content in the brains of Hb group began to increase significantly at 12 h, and reached a value that was higher than those at all other time points at 24 h (P < 0.05) (Figure 1B). These data indicated that water content in the brain was enhanced by Hb treatment in a time-dependent manner.
Expression of RhoA and ROCK2 is enhanced and the level of claudin-5 is reduced by treatment with Hb
To measure the expression of RhoA, ROCK2 and claudin-5, qRT-PCR, Western blotting, and immunohistochemical assays were performed. qRT-PCR data showed that the level of RhoA mRNA in Hb group was increased at 6, 12 and 24 h after Hb treatment, with the highest value observed at 12 h (P < 0.05) (Figure 1C). In addition, the level of ROCK2 mRNA in Hb group was enhanced at 6 and 12 h after Hb treatment, with the strongest effect occurring at 12 h (P < 0.05) (Figure 1D). Western blotting data showed that the trends in changes of RhoA and ROCK2 protein expression were in accordance with their mRNA levels at all time points (Figure 2A-C). Similar changes were also detected by immunohistochemical analysis (Figure 1E). By contrast, claudin-5 expression was gradually decreased to a minimal value at 12 h (the time of the most severe disruption of BBB) and began to recover at 24 h in Hb group (Figure 2A and 2E). Consistent with this observation, the fluorescence intensity of the stain of claudin-5 in Hb group at 12 h was weaker than that in saline group (Figure 2F and 2G). Compared with saline group, there was a stronger expression of p-MLC in the endothelia of microvessels after Hb injection (Figures 1E, 2D, 2F and 2H). These data demonstrated that the expression of RhoA and ROCK2 was enhanced, while the level of claudin-5 was reduced by treatment with Hb.
Figure 2.
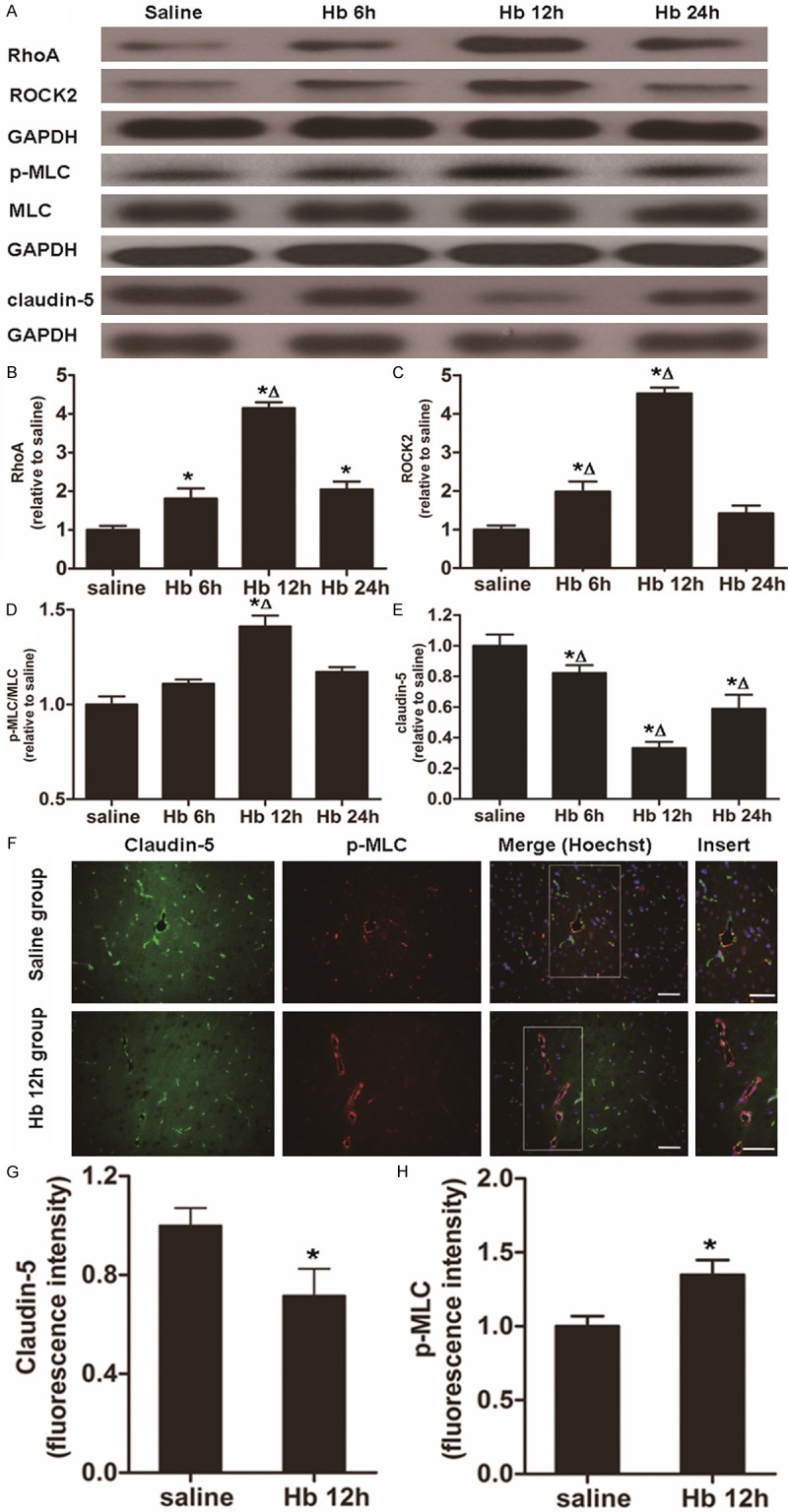
Alterations in protein expression of RhoA, ROCK2 and claudin-5 as well as changes in ROCK activity (p-MLC/MLC). (A) Western blotting analysis of protein expression of RhoA, ROCK2, p-MLC, MLC and claudin-5 at 6, 12 and 24 h after Hb injection. (B-E) Quantification of relative protein expression of (B) RhoA, (C) ROCK2, (D) p-MLC/MLC and (E) claudin-5 at 6, 12 and 24 h after Hb injection. (F) Immunohistochemical analysis of the expression of claudin-5 and p-MLC in Hb group at 12 h. Green, claudin-5; red, p-MLC. Merge means the combination of claudin-5 and p-MLC. Nuclei were stained using Hoechst. Scale bar = 100 μm. (G, H) Quantification of fluorescence intensity of (G) claudin-5 and (H) p-MLC. Data are means ± SD. *, P < 0.05 compared with normal control group; Δ, P < 0.05 compared with Hb group at other time points.
Levels of MMP-9 mRNA and protein are increased by Hb treatment in a time-dependent manner
To determine the expression of MMP-9, we carried out qRT-PCR, Western blotting, and immunohistochemical assays. qRT-PCR data showed that the level of MMP-9 mRNA in Hb group was increased to a peak value at 12 h, and then began to decrease at 24 h after Hb injection, whereas the level of MMP-9 mRNA in saline group was weak at all time points (Figure 3A). Similar trends were observed for MMP-9 protein expression (Figure 3B and 3D). Compared with saline group, stronger intensity of MMP-9 was detected using immunohistochemistry in Hb group (Figure 3E). These data indicated that the levels of MMP-9 mRNA and protein were increased by Hb treatment in a time-dependent manner.
Figure 3.
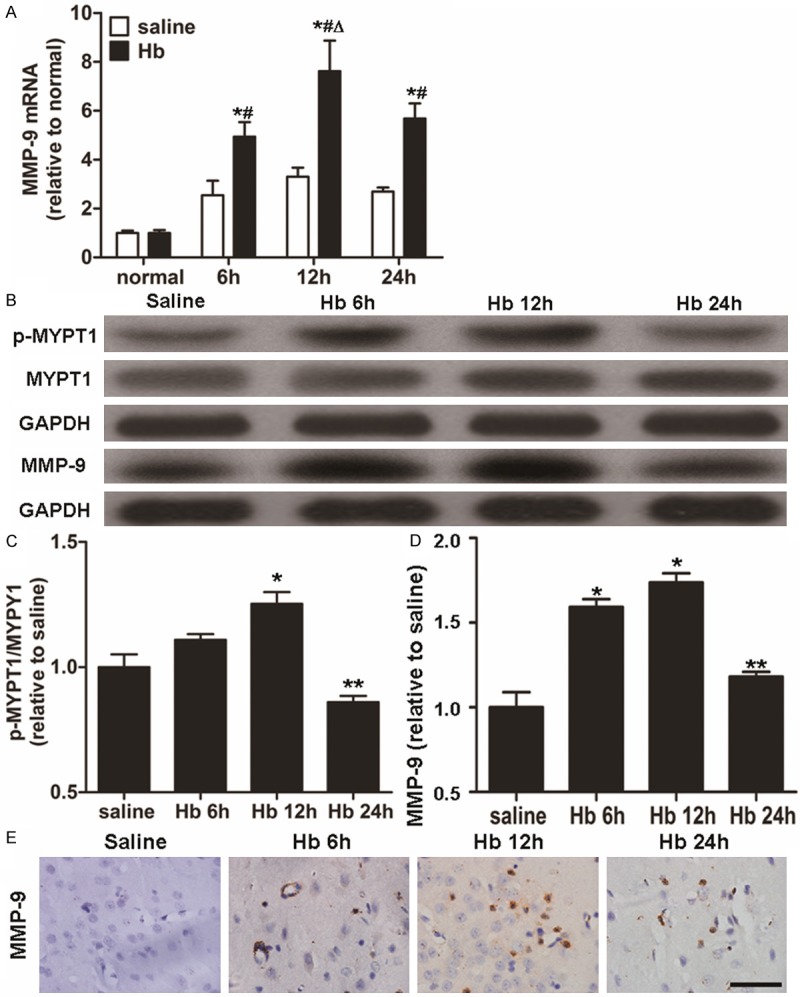
Alterations in the expression of MMP-9 and the activity of ROCK (p-MYPT1/MYPT1). (A) Quantification of mRNA level of MMP-9 in saline and Hb groups at 6, 12 and 24 h after injection. (B) Western blotting analysis of p-MYPT1, MYPT1 and MMP-9 protein expression. (C) Quantification of p-MYPT1/MYPT1 at 6, 12 and 24 h after injection. (D) Quantification of MMP-9 protein expression at 6, 12 and 24 h after injection. Data are means ± SD. *, P < 0.05 compared with normal control group; #, P < 0.05 compared with saline group at respective time points; Δ, P < 0.05 compared with Hb group at other time points; **, P < 0.05 compared with Hb group at 12 h after injection. (E) Immunohistochemical analysis of the expression of MMP-9 in Hb group at 6, 12 and 24 h. Scale bar = 100 μm.
Hb treatment augments ROCK activity at 12 h, with a positive correlation with MMP-9 protein expression
To detect ROCK activity, Western blotting was used. The data showed that Hb administration led to activation of ROCK with up-regulated expression of p-MLC and p-MYPT1 at 12 h (P < 0.05) (Figures 2A, 2D, 3B and 3C). Similar to alterations of ROCK2 expression and BBB permeability, the maximum activity of ROCK was observed at 12 h in Hb group. Pearson’s correlation analysis indicated that ROCK activity (p-MYPT1/MYPT1 and p-MLC/MLC) had a positive correlation with the protein expression of MMP-9 (r = 0.729, P = 0.007 and r = 0.640, P = 0.025, respectively). These data suggested that Hb treatment augmented ROCK activity at 12 h, with a positive correlation with MMP-9 protein expression.
Discussion
Our previous study showed a significant reduction in claudin-5 mRNA expression after Hb injection into the caudate nucleus of rats, accompanied by an increase in BBB permeability [4]. In this study, similar down-regulation of claudin-5 expression by Hb was detected using Western blotting and immunofluorescence. This result supports the notion that Hb-induced increases in BBB permeability can be attributed to injury to endothelial TJ proteins.
Our results showed a significantly elevated activity of the ROCK at 12 h in Hb group. The increased expression of RhoA and ROCK2 was consistent with this finding. Immunostaining showed stronger expression of p-MLC in endothelial cells after Hb injection. These data suggested strongly that ROCK was activated in endothelial cells by Hb after the occurrence of ICH.
Formation of stress fibers caused by the accumulation of p-MLC, together with actin-myosin contractility, are the main mechanisms of ROCK activation-induced BBB destabilization [10]. p-MLC is an important downstream effector of ROCK and the direct driving force for the formation of stress fibers [10]. It is regulated by MLC phosphatase and MLC kinase [10,20]. Activated ROCK phosphorylates MYPT1, the regulatory subunit of MLC phosphatase, to decrease the activity of MLC phosphatase. ROCK itself also causes phosphorylation of MLC. Both mechanisms lead to further increase in p-MLC levels [12]. Current data showed that p-MLC expression was increased after Hb injection, which in turn facilitated the formation of stress fibers and enhanced actin-myosin contractility [12]. The enhanced actin-myosin contractility produces a centripetal force that results in BBB leakage by increasing inter-endothelial gaps [20]. Meanwhile, contracted actin-myosin aggravates injury to endothelial TJ proteins [10,13], thereby leading to the disruption of BBB and subsequent cerebral edema. After inhibition of the ROCK, impaired TJ proteins can be repaired by the down-regulation of p-MLC expression to decrease the permeability of BBB [13]. In addition, activation of ROCK also results in phosphorylated modification of the conformation of claudin-5 [20], which is associated with the increased permeability and decreased “tightness” of BBB [21]. We demonstrated increased expression of p-MLC and significant degradation of endothelial TJ protein claudin-5, followed by an increase in BBB permeability after Hb injection. Moreover, immunostaining revealed stronger staining of p-MLC and weaker staining of claudin-5 in vascular walls. These findings suggest that the activation of endothelial ROCK accounts for Hb-induced increase in BBB permeability by up-regulating p-MLC expression and aggravating injuries to TJ proteins.
MMP-9 is highly expressed in the endothelia and astrocytes in multiple diseases of the CNS [19]. MMP-9 overexpression is closely associated with BBB disruption after the occurrence of ICH. MMP-9 degrades the vascular matrix and damages the basal lamina, leading to edema and hemorrhage in the brain [14]. One study indicates that Hb can increase MMP-9 activity and lead to BBB dysfunction in rats [7]. On the one hand, MMP-9 can degrade the basal lamina proteins that provide structural support for endothelia; on the other hand, attacks on the basal lamina expose TJ proteins that are hydrolyzed by MMP-9 in endothelial cells [22]. The reduction of the levels of TJ protein mRNAs suggests a generalized suppression of synthesis because of Hb treatment [4], or a decrease in the stability of TJ protein mRNAs [22]. Recently, increasing evidences suggest that the activation of ROCK may be related to the expression of MMP-9. In vitro MMP-9 expression is reduced after the administration of the inhibitor of Rho/ROCK pathway in human saphenous vein smooth muscle cells [17]. ROCK inhibitor fasudil suppresses the activity of MMP-9 in lung cancer cells [18]. In vivo increase in ROCK mRNA promotes microvascular damage by up-regulating MMP-9 expression in rats with cerebral ischemia reperfusion, and ROCK inhibitor fasudil protects the integrity of BBB by decreasing the mRNA level of MMP-9 [14]. Our results are partly in accordance with the evidence mentioned above, accompanied by the elevated expression and activity of ROCK. MMP-9 expression was also up-regulated, followed by increased BBB permeability. Statistical analyses showed a positive correlation between ROCK activity and protein expression of MMP-9. These results suggest that Hb-induced overexpression of MMP-9 may be partly attributed to the activation of the ROCK, thereby leading to BBB disruption. However, further investigation of the potential mechanism by which ROCK regulates MMP-9 expression, especially the effect of ROCK inhibitor on MMP-9, will be necessary to confirm our findings.
In conclusion, the present study suggested that ROCK was activated in endothelial cells by Hb. This may account for the early disruption of the BBB via up-regulation of p-MLC expression and aggravation of injuries to TJ proteins. The activation of ROCK may also increase MMP-9 expression, thereby leading to further BBB disruption. Our findings indicated that ROCK might be a promising therapeutic target for the treatment of early ICH.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81271314 and 30500526), Natural Science Foundation of Guangdong (No. 5300468), and Special Project on the Integration of Industry, Education and Research of Guangdong Province and Ministry of Education (No. 2012B091100154).
Disclosure of conflict of interest
None.
References
- 1.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;12:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Chen Y, Deng X, Jiang W, Li B, Fu Z, Du M, Ding R. Hemoglobin-induced nitric oxide synthase overexpression and nitric oxide production contribute to blood-brain barrier disruption in the rat. J Mol Neurosci. 2013;2:352–363. doi: 10.1007/s12031-013-9990-y. [DOI] [PubMed] [Google Scholar]
- 5.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 6.Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci. 1995;134:102–112. doi: 10.1016/0022-510x(95)00215-n. [DOI] [PubMed] [Google Scholar]
- 7.Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30:1939–1950. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao X, Wu G, Hu S, Huang F. Poly (ADP-ribose) polymerase activation and brain edema formation by hemoglobin after intracerebral hemorrhage in rats. Acta Neurochir Suppl. 2008;105:23–27. doi: 10.1007/978-3-211-09469-3_5. [DOI] [PubMed] [Google Scholar]
- 9.Saito M, Ohmasa F, Shomori K, Dimitriadis F, Ohiwa H, Shimizu S, Tsounapi P, Kinoshita Y, Satoh K. Rhos and Rho kinases in the rat prostate: their possible functional roles and distributions. Mol Cell Biochem. 2011;358:207–213. doi: 10.1007/s11010-011-0936-9. [DOI] [PubMed] [Google Scholar]
- 10.Spindler V, Schlegel N, Waschke J. Waschke, Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 11.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases what is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Nieuw Amerongen, Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 13.Huang XN, Fu J, Wang WZ. The effects of fasudil on the permeability of the rat blood-brain barrier and blood-spinal cord barrier following experimental autoimmune encephalomyelitis. J Neuroimmunol. 2011;239:61–67. doi: 10.1016/j.jneuroim.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Liu K, Li Z, Wu T, Ding S. Role of rho kinase in microvascular damage following cerebral ischemia reperfusion in rats. Int J Mol Sci. 2011;12:1222–1231. doi: 10.3390/ijms12021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60:327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma T, Liu L, Wang P, Xue Y. Evidence for involvement of ROCK signaling in bradykinin-induced increase in murine blood-tumor barrier permeability. J Neurooncol. 2012;106:291–301. doi: 10.1007/s11060-011-0685-3. [DOI] [PubMed] [Google Scholar]
- 17.Turner NA, O’Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J. 2005;19:804–806. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- 18.Zhu F, Zhang Z, Wu G, Li Z, Zhang R, Ren J, Nong L. Rho kinase inhibitor fasudil suppresses migration and invasion though down-regulating the expression of VEGF in lung cancer cell line A549. Med Oncol. 2011;28:565–571. doi: 10.1007/s12032-010-9468-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Rempe DA. Rempe, Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7:439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79:477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem. 2001;276:10423–10431. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]


