Abstract
Objective: To evaluate the correlation of EGFR mutation and histological subtypes of lung adenocarcinoma based on the IASLC/ATS/ERS classification. Methods: EGFR exons 18-21 of 206 resected lung adenocarcinoma specimens were analyzed with pyrosequecing, then the differences between histological subtypes and EGFR mutation were compared. Results: EGFR mutation was detected in 123 specmens, most of which were papillary and acinar predominant adenocarcinoma. EGFR mutation rate of the specimens with papillary, acinar or lepidic component was higher than without these components (P < 0.05), and with solid or mucinous component was lower than that without the component (P < 0.05). EGFR mutation in solid predominant mixed other subtypes was more commonly found than that of pure solid component (P=0.018). Conclusions: The presence of well-differentiated components in lung adenocarcinoma, such as lepidic, papillary and acinar, indicates a higher EGFR mutation rate, while the solid and mucinous component indicate a lower EGFR mutation rate. There is heterogeneity of EGFR mutation in lung adenocarcinoma.
Keywords: Lung neoplasms, adenocarcinoma, receptor, epidermal growth factor
Introduction
Lung cancer is one of the most deadly diseases of the world, and the mortality rate has been stubbornly high. Appearance of tyrosine kinase receptor inhibitors (TKI) had brought a revolutionary change in non-small cell lung cancer therapy, especially in lung adenocarcinoma. We have been know that epidermal growth factor receptor (EGFR) mutation was closely related to the efficacy of TKI, and it was frequently found in women, non-smokers and adenocarcinoma [1]. However, the relationship of EGFR mutation with histologic subtypes of lung adenocarcinoma still have not a certain conclusion. Some researchers believed that lepidic subtype was relative to EGFR mutation [2-4]. There were also studies suggest that EGFR gene in papillary predominant [5-7], acinar predominant [8,9] and micropapillary predominant [4,5,9] adenocarcinoma had a higher mutation rate. The International Society for the Study of lung cancer (IASLC), the American Thoracic Society (ATS) and European Respiratory Society (ERS) published an international multidisciplinary classification of lung adenocarcinoma in 2011 [10] (we call this the new classification here). This article intends to explore the relationship between EGFR mutation and histologic subtypes of lung adenocarcinoma according to the new classification.
Materials and methods
Specimens
All the 206 primary resection specimens of lung adenocarcinoma (from 2006 to 2013) came from the First Affiliated Hospital of Medical College, Zhejiang University, and were confirmed by pathology. All the patients had not received preoperative chemotherapy.
Histological evaluation
Two pathologists evaluated all the specimens according to the new classification separately. Recording the percentage in 5% increments for each histologic component (Figure 1). The predominant pattern is defined as the pattern with the largest percentage of carcinoma cells, even through the percentage was smaller than 50%. Discordant results were reviewed together to determine a consensus. The staging was in accordance with the standards of the American Joint Committee on Cancer’s AJCC Cancer Staging Manual, 7th edition [11].
Figure 1.
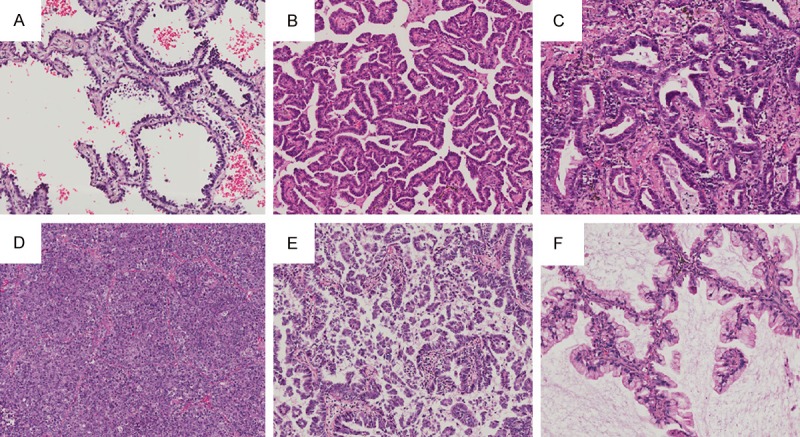
Histogical substypes of lung carcinoma: A. Lepidic substype; B. Papillary substype; C. Acinar substype; D. Solid substype; E. Micropapillary substype; F. Mucinous adenocarcinoma substype.
EGFR mutation analysis
All the specimens were formalin-fixed paraffin-embedded (FFPE) archival tissue blocks obtained during surgical excision of the tumors. 5-7 sections (5 μm thick) of the block of which the histologic subtype was preponderant in the whole tumor were provided to isolate DNA. The FFPE tissue DNA extraction kit came from Gene Tech Company Limited. Exon 18-21 of EGFR were analysised by using polymerase chain reaction (PCR) and Qiagen PyroMark 24 sequencer (Qiagen, Hilden, Germany).
Statistical analysis
The χ2 test and Fisher exact test for independence were used to compare frequencies of clinicopathologic variables. P values < 0.05 were considered statistically significant. Statistical analysis was performed with SPSS 13.0 software.
Results
Clinicopathologic characteristics
The clinicopathologic characteristics of patients were showed in Table 1 (parts of data missed). There were 97 men and 109 women. The mean age was 61 years (age range, 27 to 81 years).
Table 1.
Relationship between EGFR mutation and clinicopathologic characteristics in lung adenocarcinoma
| N | EGFR+ | P | |
|---|---|---|---|
| Gender | |||
| Male | 97 | 43 (44.3%) | 0.000 |
| Female | 109 | 80 (73.4%) | |
| Age | |||
| < 61 y | 94 | 53 (56.4%) | 0.373 |
| ≥ 61 y | 112 | 70 (62.5%) | |
| Smoking status | |||
| Never | 123 | 88 (71.5%) | 0.000 |
| Former/current | 62 | 25 (40.3%) | |
| Drinking status | |||
| Rare | 142 | 91 (64.1%) | 0.043 |
| Frequently | 39 | 18 (46.2%) | |
| Lymph node metastasis | |||
| No | 75 | 50 (66.7%) | 0.124 |
| Yes | 98 | 54 (55.1%) | |
| Lung membrane invasion | |||
| No | 111 | 64 (57.7%) | 0.197 |
| Yes | 59 | 40 (67.8%) | |
| Location | |||
| Central | 35 | 17 (48.6%) | 0.106 |
| Peripheral | 145 | 92 (63.4%) | |
| Differentiation | |||
| Well | 30 | 25 (83.3%) | 0.000 |
| Moderate | 97 | 63 (64.9%) | |
| Poor | 79 | 35 (44.3%) | |
| Tumor size | |||
| < 3 cm | 130 | 82 (63.1%) | 0.383 |
| ≥ 3 cm | 50 | 28 (56.0%) | |
| Pathological stage | |||
| I | 64 | 41 (64.1%) | 0.628 |
| II | 49 | 27 (55.1%) | |
| III/IV | 60 | 36 (60.0%) |
EGFR+, EGFR mutation.
Histologic features
All the specimens were invasive adenocarcinoma, in which 16 cases were lepidic predominant (7.8%), 58 cases were papillary predominant (28.2%), 82 cases were acinar predominant (39.8%), 40 cases were solid predominant (19.4%), 5 cases were micropapillary predominant subtype (2.4%), 4 cases were mucinous adenocarcinoma (1.9%), 1 case was fetal adenocarcinoma (0.5%). And the number of cases with lepidic, papillary, acinar, solid, micropapillary, and mucinous component was 47, 101, 133, 59, 44, and 6, respectively.
EGFR mutation and clinicopathologic characteristics
EGFR mutations were detected in 123 cases (59.7%), in which 1 case was G719S, 63 cases were 19-DEL, 57 cases were L858Q, and 2 cases were L858Q combined T790M. EGFR mutation rate in female patients was higher than males; in patients who never smoke was higher than smokers; in patients who rarely drink alcohol was higher than that that drinks frequently; in cases of well-differentiated adenocarcinoma was higher than that of poorly-differentiated. EGFR mutation was no correlated with age, lymph node metastasis, lung membrane invasion, tumor size or pathological stage (Table 1).
EGFR mutation and histologic subtypes of lung adenocarcinoma
The rates of EGFR mutation in lepidic, papillary, acinar, solid, micropapillary and mucinous predominant patterns were 68.8%, 70.7%, 69.5%, 22.5%, 80.0%, and 25.0%, respectively, and no mutation was detected in the case of fetal adenocarcinoma. The difference of EGFR mutation rate between the subtypes was significantly (P < 0.001). In males, EGFR mutation was frequently found in micropapillary predominant, acinar predominant and papillary predominant substypes. In females, EGFR mutation was frequently found in lepidic predominant, acinar predominant and papillary predominant subtypes (Table 2).
Table 2.
EGFR mutation in the predominant substypes of lung adenocarcinoma
| Predominant substypes | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | EGFR+ | % | N | EGFR+ | % | N | EGFR+ | % | |
| Lepidic | 16 | 11 | 68.8 | 6 | 2 | 33.3 | 10 | 9 | 90.0 |
| Papillary | 58 | 41 | 70.7 | 24 | 12 | 50.0 | 34 | 29 | 85.3 |
| Acinar | 82 | 57 | 69.5 | 34 | 22 | 64.7 | 48 | 35 | 72.9 |
| Solid | 40 | 9 | 22.5 | 27 | 4 | 14.8 | 13 | 5 | 38.5 |
| Micropapillary | 5 | 4 | 80.0 | 2 | 2 | 100 | 3 | 2 | 66.7 |
| Mucinous | 4 | 1 | 25.0 | 3 | 1 | 33.3 | 1 | 0 | 0 |
| Fetal | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
EGFR+, EGFR mutation.
Furthermore, EGFR mutation was more frequently found in the specimens with lepidic, papillary or acinar component than without these components (P < 0.05). However, EGFR mutation was more frequently found in the specimens without solid or mucinous component than with the component (P < 0.05). In males, EGFR mutation was more frequently found in the cases with acinar component than without it, and more frequently found in the specimens without solid component than with it no matter in males or females (Table 3, Figure 2).
Table 3.
Correlation of EGFR mutation and histological component in lung adenocarcinoma
| Histologic component | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | EGFR+ | P | N | EGFR+ | P | N | EGFR+ | P | |
| Lepidic | |||||||||
| Without | 168 | 94 | 82 | 34 | 86 | 60 | |||
| With | 38 | 29 | 0.021 | 15 | 9 | 0.184 | 23 | 20 | 0.117 |
| Papillary | |||||||||
| Without | 105 | 54 | 58 | 21 | 47 | 33 | |||
| With | 101 | 69 | 0.013 | 39 | 22 | 0.050 | 62 | 47 | 0.513 |
| Acinar | |||||||||
| Without | 73 | 30 | 39 | 7 | 34 | 23 | |||
| With | 133 | 93 | 0.000 | 58 | 36 | 0.000 | 75 | 57 | 0.361 |
| Solid | |||||||||
| Without | 147 | 104 | 61 | 36 | 86 | 68 | |||
| With | 59 | 19 | 0.000 | 36 | 7 | 0.000 | 23 | 12 | 0.010 |
| Micropapillary | |||||||||
| Without | 162 | 93 | 81 | 35 | 81 | 58 | |||
| With | 44 | 30 | 0.196 | 16 | 8 | 0.617 | 28 | 22 | 0.472 |
| Mucinous | |||||||||
| Without | 200 | 122 | 92 | 42 | 108 | 80 | |||
| With | 6 | 1 | 0.041 | 5 | 1 | 0.378 | 1 | 0 | 0.266 |
EGFR+, EGFR mutation.
Figure 2.
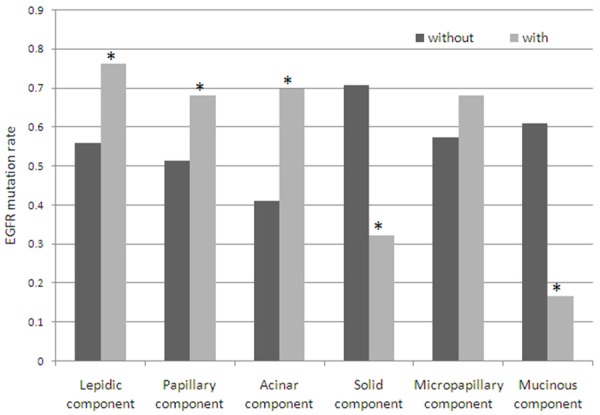
EGFR mutation in the histologic components of lung adenocarcinoma. EGFR mutation rate of the specimens with papillary, acinar or lepidic component was higher than without these components, and with solid or mucinous component was lower than without the component. “*” means that the difference of EGFR mutation rate between two groups was significant.
We also compared the difference of EGFR mutation between the predominant subtypes mixed with and without other components, the results were showed in Table 4.
Table 4.
Difference of EGFR mutation between the predominant subsype mixed and without other substype
| N | EGFR+ | P | |
|---|---|---|---|
| Lepidic | |||
| Pure | 2 | 0 | |
| Mixed | 14 | 11 | 0.083 |
| Papillary | |||
| Pure | 12 | 8 | |
| Mixed | 46 | 33 | 0.733 |
| Acinar | |||
| Pure | 32 | 23 | |
| Mixed | 45 | 31 | 0.778 |
| Solid | |||
| Pure | 24 | 2 | |
| Mixed | 16 | 7 | 0.018 |
EGFR+, EGFR mutation.
Discussion
Appearance of TKI has brought a revolutionary change in non-small cell lung cancer therapy, especially in lung adenocarcinoma. It has been well known that EGFR mutation was closely related to the efficacy of TKI [12]. We detected 59.7% of 206 primary lung adenocarcinoma specimens with EGFR mutation which was more frequently found in never-smokers and females. We aslo noted that EGFR mutation was more frequently found in patients who drunk alcohol rarely than those who drunk alcohol frequently. The possible reason is that 79.9% patients drunk frequently and smoke at the same time, or they neither drink nor smoke at the same time.
There were studies described that patients characterized by bronchioloalveolar carcinoma (BAC) were more sensitive to TKI [13]. Takayuki et al certified that non-mucious BAC carried EGFR mutation more frequently than other subtypes [14], and most cases with EGFR mutation were terminal respiratory unit (TRU) [15]. The IASLC/ATS/ERS published an international multidisciplinary histological classification of lung adenocarcinoma in 2011 [10]. The terms BAC and mixed subtype adenocarcinoma are no longer used according to the new classification which requests to record the percentage in 5% increments for each histological component. According to the new classification, Shim et al showed that EGFR mutation was significantly correlated with the presence of lepidic or micropapillary component through evaluating 107 cases of lung adenocarcinoma [3]. Sun et al showed that EGFR mutation was not correlated with micropapillary component but was correlated with the presence of lepidic or papillary component and the absence of solid or mucinous component [16]. However, Zhang et al showed that acinar predominant was an independent predictor of EGFR mutation through investigating 349 cases of lung adenocarcinoma who were females and never-smokers [8]. The results of other studies were not fully consensus [4,6,9].
The results of the present work showed that EGFR mutation was the most frequently found in micropapillary predominant, followed by papillary predominant, acinar predominant and lepidic predominant adenocarcinoma. EGFR mutation was rarely found in solid predominant and mucious adenocarcinoma. EGFR mutation was more frequently found in the cases with lepidic, papillary or acinar component than without these components and more frequently found in the cases without solid or mucinous component than with these components. Lung adenocarcinoma with micropapillary feature indicates aggressive, easily metastatic potential and poor prognosis [17,18]. Couples of studies showed that EGFR mutation was more frequently found in micropapillary adenocarcinoma than other subtypes [3-5]. Our results showed that althrough EGFR mutation rate of micropapillary predominant was the highest, the difference between specimens with and without micaropapillary component was not significant. It may be that micropapillary component of many tumors was so little that not enough for EGFR mutation test. However, when we defined the minimum percentage of containing micropapillary component as 15%, the EGFR mutation between with and without micropapillary component was not significantly different (data not shown).
We also compared the difference between predominant subtype mixed with other subtypes and without other subtypes for verifying the difference of EGFR mutation in different histological subtypes. The results showed that EGFR mutation in solid predominant mixed with other subtypes was significantly more frequently found than that in pure solid component. It indicates that solid adenocarcinoma may have a different mechanism in carcinogenesis. These required pathologists try to avoid solid component when choosing tissue blocks for EGFR mutation test.
Because of the difference of EGFR mutation in gender, we separated the males from females to see whether the histological subtype with EGFR mutation is also different. In males, EGFR mutation was frequently found in acinar predominant and papillary predominant adenocarcinoma. And EGFR mutation was more frequently existed in the specimens with acinar component than without it. While in females, EGFR mutation was frequently found in lepedic predominant, papillary predominant and acinar predominant adenocarcinoma. It was more frequently found in the specimens without solid component than with it no matter in males or females. So, the histological subtype with EGFR mutation in males and females was different, but the difference was not significant.
Because of the limited cases, we could not separate the various histological subtypes out to analyze the relation with EGFR mutation in a direct method. In conclusion, the presence of well-differentiated components in lung adenocarcinoma, such as lepidic, papillary and acinar, component indicates a higher EGFR mutation rate, while the presence of solid or mucinous component indicates a lower EGFR mutation rate. However, no matter which histological subtype of lung adenocarcinoma is, it can not fully predict the status of EGFR mutation, and therefore, the EGFR mutation test is necessary for any case of lung adenocarcinoma.
Disclosure of conflict of interest
None.
References
- 1.Gupta R, Dastane AM, McKenna R Jr, Marchevsky AM. The predictive value of epidermal growth factor receptor tests in patients with pulmonary adenocarcinoma: review of current“best evidence” with meta-analysis. Hum Pathol. 2009;40:356–365. doi: 10.1016/j.humpath.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Sonobe M, Manabe T, Wada H, Tanaka F. Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br J Cancer. 2005;93:355–363. doi: 10.1038/sj.bjc.6602707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim HS, Lee da H, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med. 2011;135:1329–1334. doi: 10.5858/arpa.2010-0493-OA. [DOI] [PubMed] [Google Scholar]
- 4.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol. 2013;30:645. doi: 10.1007/s12032-013-0645-1. [DOI] [PubMed] [Google Scholar]
- 5.Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, Rusch VW, Gerald WL, Travis WD. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 6.Jie L, Li XY, Zhao YQ, Liu RQ, Zhang JB, Ma J, Chen LJ, Hu XF. Genotype-phenotype correlation in Chinese patients with pulmonary mixed type adenocarcinoma: Relationship between histologic subtypes, TITF-1/SP-A expressions and EGFR mutations. Pathol Res Pract. 2014;210:176–181. doi: 10.1016/j.prp.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, Luo X, Ye T, Wang R, Hu H, Li H, Wang L, Pao W, Chen H. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell PA, Barnett SA, Walkiewicz M, Wainer Z, Conron M, Wright GM, Gooi J, Knight S, Wynne R, Liew D, John T. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol. 2013;8:461–468. doi: 10.1097/JTO.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lababede O, Meziane M, Rice T. Seventh edition of the cancer staging manual and stage grouping of lung cancer: quick reference chart and diagrams. Chest. 2011;139:183–189. doi: 10.1378/chest.10-1099. [DOI] [PubMed] [Google Scholar]
- 12.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Kris MG, Shah N, Patel J, Azzoli C, Gomez J, Krug LM, Pao W, Rizvi N, Pizzo B, Tyson L, Venkatraman E, Ben-Porat L, Memoli N, Zakowski M, Rusch V, Heelan RT. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 14.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 15.Yatabe Y, Kosaka T, Takahashi T, Mitsudomi T. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol. 2005;29:633–639. doi: 10.1097/01.pas.0000157935.28066.35. [DOI] [PubMed] [Google Scholar]
- 16.Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, Jheon S, Lee CT, Lee JS, Chung JH. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323–330. doi: 10.1097/JTO.0b013e3182381515. [DOI] [PubMed] [Google Scholar]
- 17.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya K, Hayashi Y, Douguchi J, Hashiguchi A, Yamada T, Izumi Y, Watanabe M, Kawamura M, Horinouchi H, Shimada N, Kobayashi K, Sakamoto M. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21:992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]


